Abstract
MHC class I molecules (MHC-I) have been implicated in nervous system development in the mouse. In this study we present evidence for the interaction of MHC-I with the NK cell receptor Ly49 in primary cortical neuronal cultures. We show that MHC-I and Ly49 are expressed on neuronal soma and axon surfaces, with Ly49 also present on dendrites. Anti-MHC-I Abs reduce synapsin-I expression and enhance neurite outgrowth and neuronal death. Conversely, anti-Ly49 mAbs increase synapsin-I expression, reduce neurite outgrowth, and promote neuron viability. Because we show that Ly49 genes are selectively expressed in the adult brain, these findings suggest an unsuspected role for the MHC-I-Ly49 interaction in the development and function of the brain.
Major histocompatibility complex class I (MHC-I)2 molecules play a key role in immunosurveillance by TCD8 + and NK cells. In mice, many NK receptors belong to the Ly49 gene family (1, 2). MHC-I is a ligand for many Ly49 gene products, with some interactions leading to NK activation and others to NK inhibition. The CNS has been classically considered a “privileged site” with limited MHC-I expression. Recent findings, however, suggest a role for MHC-I in neuronal biology in establishing and maintaining neuronal synapses (3–9).
The function of class I molecules in the CNS implies a specific interaction between MHC-I and a receptor. Because class I genes are typically the most variable genes expressed by vertebrates, class I receptors in the CNS should be molecules that interact with MHC-I in an allomorph-independent manner. One such group of molecules consists of the Ly49 gene products. In this study, we examine the function of MHC-I in the growth and synapse formation of embryonic neuronal cells ex vivo and provide the initial evidence for the potential role of Ly49 gene products as class I receptors in the CNS.
Materials and Methods
Primary cortical neuronal cultures
Primary E16 cortical neuronal cultures from C57BL/6J (B6) or BALB/cJ (Jax mice; The Jackson Laboratory) were prepared as described (10). Neurobasal growth medium was supplemented with the proliferation inhibitor fluorideoxyuridine (100 µM) to minimize glial cells.
Chemicals and antibodies
We used the following Abs: B22.249 (H-2Db), HB.176 (H-2Kb), D7 and F9 (anti-H-2b Fabs; Ref. 11), TW2.3 (vaccinia virus), 4D11 (Ly49), G7 (Thy1) (both BD Pharmingen), AB5852 (Shank1a), AB5220 (GAP-43) (both Millipore), A6442 (synapsin-I) (Invitrogen), FITC-conjugated SIINFEKL, and nonconjugated RGYVYQGL (Biological Resources Branch, National Institute of Allergy and Infectious Diseases, Bethesda, MD; Ref. 12).
In situ hybridization
cDNA corresponding to Ly49A mRNA (provided by Dr. W. Yokoyama) was Cy3-cytidine 5′-triphosphate-labeled using a pCRII-TOPO vector (Invitrogen) (forward primer: 5′-TAACTGCAGCACCACGCAAAGTGACGT CAA-3′; reverse primer: 5′-TTCTGTTATGGTATAAAATGTT-3′) as described (13). Cy3-labeled Ly49A probe was hybridized with paraformaldehydefixed brain slices (12 µm) and imaged (5-µm section resolution; GenePix 4100A, Molecular Devices). No signal was detected from control hybridizations with either sense riboprobe or slices pretreated with RNase before antisense hybridization.
Treatment of cells, immunohistochemistry, and microscopy
Neurons were incubated with the following Abs for 1, 5, or 13 days: H-2Db H-2Kb (2 or 4 µg/ml in B6 and 4 µg/ml in BALB mice), H-2Db and H-2Kb together (2 or 4µg/ml in B6), Ly49G2 (0.1, 0.2, or 1µg/ml in B6), vaccinia (4 µg/ml in B6), Thy1 (4 µg/ml in B6), and D7 or F9 (4 µg/ml in B6) for 5 days only. Abs were added every 3 to 4 days starting the day after seeding. Cells were either permeabilized with acetone/methanol (50:50%; v/v) for 2 min or gently fixed with 3% paraformaldehyde in PBS for 10 min. Permeabilized neurons were incubated with Zenon-Alexa Fluor 488-labeled (Invitrogen) GAP-43 and their nuclei were stained with benzimide (1 µM; Sigma-Aldrich). Fixed neurons were incubated with appropriate Abs and neuronal distribution was revealed using the following secondary Abs (The Jackson Laboratory): F9- Cy3 labeled goat anti-human, Ly49G2/A-Cy2-labeled goat anti rat, synapsin-I, and Shank1a-Cy5-labeled goat anti-rabbit Abs. To localize the peptide receptive H-2Kb molecules, cultures were incubated with human β2-microglobulin (5 µg/ml) overnight before 0.5 h of incubation with SIINFEKL-FITC (1µM) on ice followed by paraformaldehyde fixation.
Coverslips were slide mounted and imaged using confocal microscopy (Pathway HT; BD Biosciences). Cell survival and neurite outgrowth were analyzed from 4 × 4 montages acquired from each coverslip (2.15 mm2 each; ×20 original magnification). Gap-43, Ly49G2, Shank1a, synapsin-I, and MHC-I distribution were determined using Plan Apo ×63 objective (Zeiss). Cell survival was determined by comparing the average number of nuclei per frame for each treatment to untreated neurons and to other concentrations of the same Ab (192 frames/treatment/day; Student’s t test.) Neurite outgrowth was assessed by a modified Sholl method (14) in which three horizontal lines were drawn at 25, 50, and 75% of each frame height and the numbers of neurites crossing these lines were counted and summed per the frame. The crossing number per frame was averaged for each treatment and normalized for cell number by dividing the line crossing number by the nuclei number. Statistical analysis was done as above (192 frames/treatment/day).
Results and Discussion
We cultured primary embryonic cortical neurons from B6 mice for 1–5 days and localized MHC-I molecule with the F9 Fab specific for Kb and Db. F9 bound to cell bodies and axons of 1-day-old neurons, suggesting a possible role in differentiation (red in Fig. 1A). A similar staining pattern was observed using the well-characterized mAbs specific for H-2Db (B22.249) or H-2Kb (HB.176) (Refs. 15 and 16 and data not shown). Neuronal Kb expression was further verified using the Kb binding peptide SIINFEKL-FITC (Fig. 1B). Staining was specifically inhibited by a competing Kb binding peptide. Notably, axonal staining is punctate in a pattern similar to that of the staining of synaptic components. In 5-day-old neurons, Ab stains showed similar pattern but with more variable stain intensity (not shown).
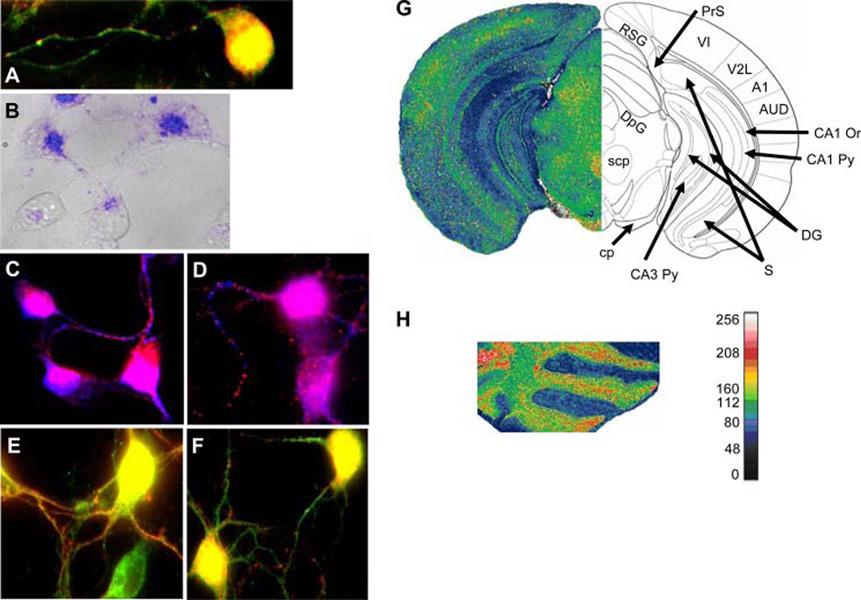
FIGURE 1.
MHC-I and Ly49 distribution and expression in cultured cortical neurons and their mRNA expression in the adult mouse brain. A, Codistribution of Abs specific for MHC I (red) and NK receptor Ly49G2 (green). In 1-day neurons MHC-I and Ly 49 colocalize (yellow) in somas and in some axonal regions. MHC-I primarily stains neurites in a clear punctate manner while Ly49 stains axons and dendrites. B, Distribution of SIINFEKL-FITC (blue; overlaid on the transmitted light image of the neurons in gray) in 1-day cell bodies and axons. C, MHC-I (blue) and synapsin I (red) show colocalization in cell bodies (purple) whereas in the axons they show alternate punctate distribution. D, MHC-I (blue) and Shank (red) also show colocalization in the cell bodies (purple) and alternating punctate distribution in axons. E, Ly49 (green) and synapsin-I (red) show colocalization (yellow) in cell bodies and in neurites. F, Ly49 (green) and Shank (red) show co localization (yellow) in cell bodies and alternating punctate distribution in axons. G, Cy3-cytidine 5′-triphosphate-labeled specific Ly49a probe was used to reveal its mRNA expression in adult mouse brain by in situ hybridization (see side bar next to H for intensities in pseudocolor.) Ly49a was expressed in the cerebral sensory cortex (VI, primary visual cortex; V2L, secondary visual cortex; A1, primary auditory cortex; AUD, secondary visual cortex), the hippocampus (CA1 Py, CA1 pyramidal cell layer; CA1 Or, CA1 oriens layer; CA3 Py, CA1 pyramidal cell layer; DG, dentate gyrus) and some of the pathways connecting the cortex and the hippocampus (RSG, retrosplenial cortex; PrS, presubiculum; S, subiculum). In the diencephalon expression was observed in areas containing en passent (scp and cp) or terminating (DpG) cerebellar axons, and also axons from other origins. H, Ly49a mRNA expression in the cerebellum was high in the Purkinje cell layer and moderate in the molecular and granule cell layer.
MHC-I interact in an allomorph-independent manner with the Ly49 family of NK receptors, which includes 23 known members (1). We stained fixed neuronal cultures with the 4D11 mAb specific for Ly49A and Ly49G2 (17). 4D11 decorated 1-day-cultured soma axons and dendrites (green in Fig. 1A) in a pattern that colocalized with F9 staining in cell bodies and some neurites (yellow in Fig. 1A). In cell bodies, both anti-MHC-I and anti-Ly49 staining colocalizes with synapsin, a presynaptic marker, and Shank, a postsynaptic density complex member (Fig. 1, C–F). In neurites however, anti-MHC-I alternates with either synapsin or Shank while anti-Ly49 colocalizes with synapsin and alternates with Shank.
Expression of Ly49 mRNA in mouse brain was verified by in situ hybridization (because B6 mice encode numerous Ly49 genes that exhibit 79–95% identity with the probe, we cannot be certain of its hybridization partner). Ly49 mRNA was detected the cerebral cortex, hippocampus, hypothalamus (Fig. 1G), and cerebellum (Fig. 1H).
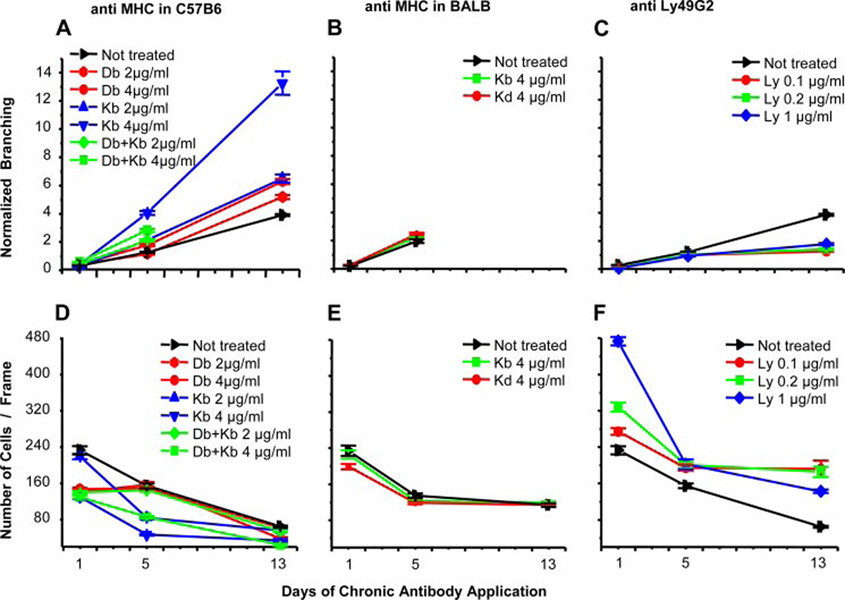
The functional significance of neuronal MHC-I expression was revealed by incubating cultures with MHC-I-specific Abs for 1, 5, or 13 days. Five-day treatment with anti-Kb mAb increased neurite growth by 3-fold, whereas anti-Db increased neurite growth by 1.5-fold (Fig. 2 and Fig 3A). Treatment with anti-Kb and Db together increased the 5-day neurite growth by 2-fold and was lethal in longer treatments. Similarly, 5-day incubation with D7 or F9 Fabs increased neurite growth by 2-fold (not shown). Because the Fabs are synthesized as monovalent Ab fragments, Ab-mediated cross-linking of MHC-I is probably not required for their neuritogenic activity. Control experiments demonstrate the specificity of the effects in three different ways. First, anti-H-2b specific Abs had no effect on BALB/c neurites, demonstrating that their activity is based on binding their target epitopes on class I molecules and not other effects. Second, a mAb specific for a viral protein did not affect B6 neurons, demonstrating that Ab alone does not affect B6 neurites. Third, anti-Thy-1 mAb did not affect B6 neurites despite the abundant expression of Thy-1 on neuron surfaces, demonstrating that simple Ab binding to neurons does not affect neurite growth (Fig. 3B).
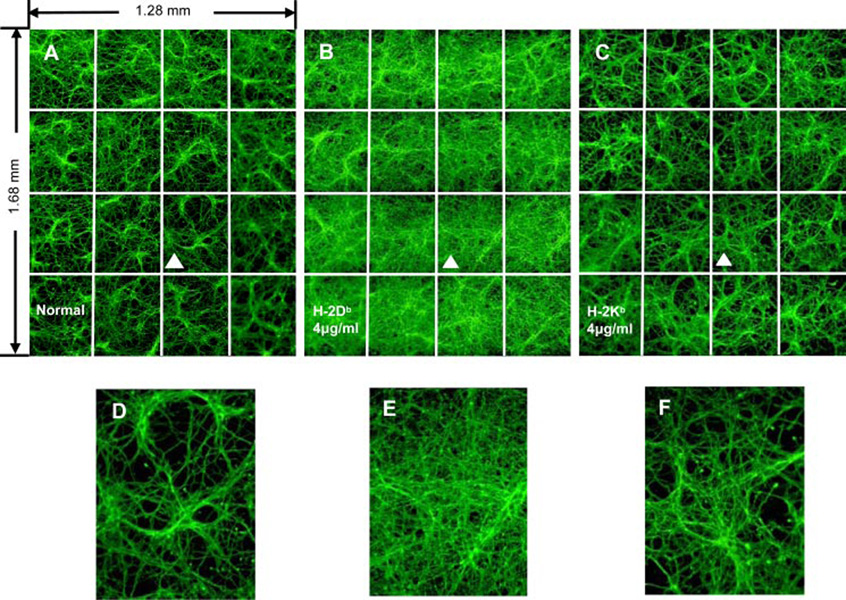
FIGURE 2.
Anti-MHC I induction of neurite branching. Montages (4 × 4) of neurite branching in 5-day-old neurons incubated with no Ab (A) or Abs specific for Db (B) or Kb (C) as revealed by GAP43 staining. A, Montage (4×4) of normal neuronal culture. B and C, Incubation with either H-2Db (B) or H-2Kb (C) Abs induced extensive neurites growth. D, E, and F show magnification of the frames marked by white triangles in A, B, or C, respectively.
FIGURE 3.
Effects of anti-MHC or Ly49 Abs on neurite branching and neuron survival. Abs of indicated specificity were incubated at the indicated concentration with neurons from B6 (A and C–F) or BALB/c mice (B). Please note that because our treatment had also affected cell survival (D–F), branching was normalized for cell number.
Anti-MHC-I Abs also had a significant effect on cultured neuronal survival. Treatment of B6 cultures with anti-Kb or Db mAbs decreased neuronal cell numbers (Fig. 3D). Similar results were obtained using D7 and F9 Fabs (not shown). Importantly, culture with anti-Ly49 mAb suppressed neurite growth (Fig. 3C) and enhanced cell survival (Fig. 3F).
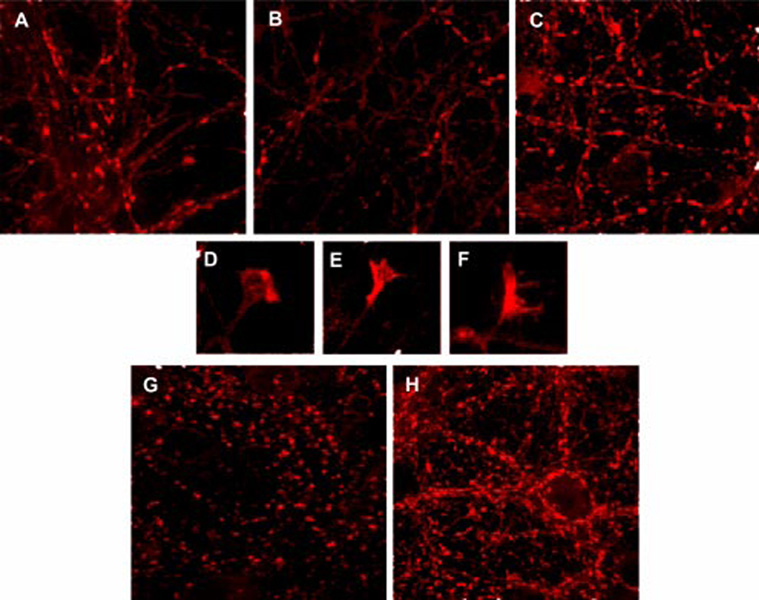
Consistent with these effects, synapsin expression of cultured neurons was modulated in opposite directions by treatment with anti-MHC-I vs anti-Ly49 Abs. Although anti-MHC-I drastically diminished the number of synapsin-I-positive synapses (Fig. 4, A and B) (despite the increased neurite growth), anti-Ly49 dramatically increased the number and size of the synapsin-I positive puncta (Fig. 4, C, G, andH). Anti-Ly49 also increased synapsin-I stain throughout growth cones (Fig. 4, E and F), whereas normal growth cones showed more constricted synapsin-I stain (Fig. 4D). None of the Abs affected Shank distribution.
FIGURE 4.
Effects of anti-MHC or Ly49 Abs on neuronal architecture. Synapsin-I was located in 5-day (A–F) or 13-day (G and H) neuronal cultures (all images obtained with ×63 objective). A and G, Untreated neurons. B, Incubation with anti-Kb (4 µg/ml) Ab. C and H, Incubation with anti-Ly49 (1 µg/ml) Ab. D, Growth cone in untreated neurons. E and F, Growth cone of neurons incubated with Ly49G2.
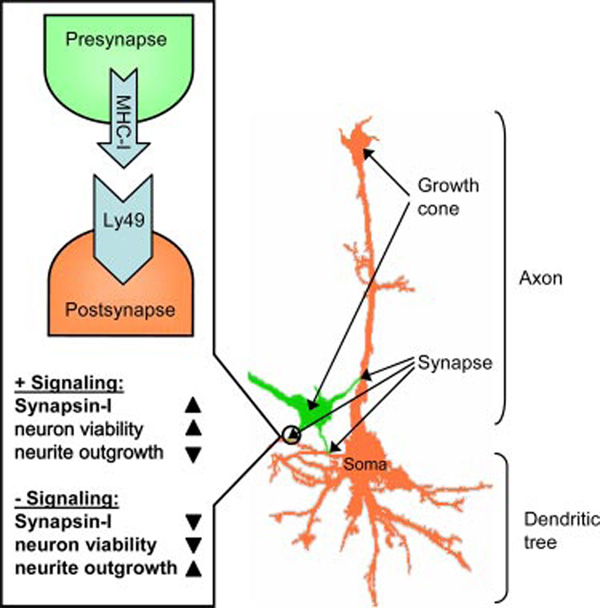
Our findings support an unanticipated role for the MHC-I/NK receptor recognition system in the generation of neural synapses based on the interaction of axon-associated MHC-I with axon- or dendrite-associated Ly49A. Though we have not rigorously demonstrated the direct interaction of Ly49A with MHC-I, the following model (Fig. 5) provides a parsimonious explanation for our observations: 1) MHC-I specific Abs disturb the association of synapse-seeking axons with Ly49 on prospective postsynaptic sites, causing neurites to continue elongating as they seek synapse formation; and 2) Ly49-specific Abs bind to synapse-ready neuronal sites, mimicking the interaction of Ly49 with MHC-I and thus retarding their growth and enhancing the expression of proteins involved in the synapse formation.
FIGURE 5.
Model for MHC-I/NK receptor signaling in neurons. In neurons, MHC-I/NK interactions initiate synapse formation and signal for assembly of synaptic proteins such as synapsin-I, which in turn, result in end point formation and increase neuronal viability. Lack of these interactions result in the opposite effects.
This model explains why MHC-I Abs induce neuronal growth and suppress synapsin-I expression while Ly49 Abs have the opposite effect. Further, because death generally awaits neurons that fail to establish synaptic connections, this model is consistent with the respective complementary neuron death-enhancing and -inhibiting effects of anti-MHC-I and Ly49 Abs.
Our findings strongly suggest that the mouse nervous system adopts the immunological MHC-I Ly49A receptor system for cell-cell recognition by growing neuronal networks. The expression of Ly49 mRNA in adult brains in a region-specific manner suggests that MHC I-Ly49 interaction also functions in the mature brain. We speculate that this system is tightly controlled by the nervous system and is up-regulated during periods of plastic changes that require new synaptic connections. Given the extreme polymorphism of MHC-I genes, we predict that the MHC genotype will influence neuronal development and function. Why use MHC-I in this manner? Because class I molecules preferentially display peptides derived from newly synthesized proteins (18), they could possibly function as a “translatometers” that enable other cells to accurately monitor translational activity. This information may be very useful for neurons in particular, because translation in neurons is independently regulated at individual synapses and is intimately related to synaptic function. Because humans and other mammals lack Ly49, it is an open question as to whether MHC I plays a similar role in neuronal development and function in other species and, if so, whether NK-associated receptors or other class I-interacting gene products are used for synaptic signaling. Finally, the broadest implication of our findings is to raise the possible involvement of MHC-I/NK receptors in intercellular communication in other “nonimmune” organs and tissues.
Acknowledgments
We thank Elizabeth Waffarn for outstanding technical assistance, Dr. Wayne Yokoyama for LY49A specific cDNA and Dr. Michael Edidin for helpful suggestions.
Footnotes
Disclosures
The authors have no financial conflict of interest.
Abbreviations used in this paper: MHC-I, MHC class I; B6, C57BL/6J.
References
- 1.Anderson SK, Ortaldo JR, McVicar DW. The ever-expanding Ly49 gene family: repertoire and signaling. Immunol. Rev. 2001;181:79–89. doi: 10.1034/j.1600-065x.2001.1810106.x. [DOI] [PubMed] [Google Scholar]
- 2.Natarajan K, Dimasi N, Wang J, Mariuzza RA, Margulies DH. Structure and function of natural killer cell receptors: multiple molecular solutions to self, nonself discrimination. Annu. Rev. Immunol. 2002;20:853–885. doi: 10.1146/annurev.immunol.20.100301.064812. [DOI] [PubMed] [Google Scholar]
- 3.Kornguth S, Mack KJ, Bersu E. Relationship between the neural dysgenesis and increased production of class I MHC H-2Kk mRNA and protein in neurons of murine trisomy 16 fetuses. Biochem. Biophys. Res. Commun. 1991;179:102–107. doi: 10.1016/0006-291x(91)91340-i. [DOI] [PubMed] [Google Scholar]
- 4.Neumann H, Schmidt H, Cavalie A, Jenne D, Wekerle H. Major histocompatibility complex (MHC) class I gene expression in single neurons of the central nervous system: differential regulation by interferon (IFN)-γ and tumor necrosis factor (TNF)-α. J. Exp. Med. 1997;185:305–316. doi: 10.1084/jem.185.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corriveau RA, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 6.Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveira AL, Thams S, Lidman O, Piehl F, Hokfelt T, Karre K, Linda H, Cullheim S. A role for MHC class I molecules in synaptic plasticity and regeneration of neurons after axotomy. Proc. Natl. Acad. Sci. USA. 2004;101:17843–17848. doi: 10.1073/pnas.0408154101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- 9.Goddard CA, Butts DA, Shatz CJ. Regulation of CNS synapses by neuronal MHC class I. Proc. Natl. Acad. Sci. USA. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaschke AJ, Weiner JA, Chun J. Programmed cell death is a universal feature of embryonic and postnatal neuroproliferative regions throughout the central nervous system. J. Comp. Neurol. 1998;396:39–50. doi: 10.1002/(sici)1096-9861(19980622)396:1<39::aid-cne4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 11.Lev A, Denkberg G, Cohen CJ, Tzukerman M, Skorecki KL, Chames P, Hoogenboom HR, Reiter Y. Isolation and characterization of human recombinant antibodies endowed with the antigen-specific, major histocompatibility complex-restricted specificity of T cells directed toward the widely expressed tumor T-cell epitopes of the telomerase catalytic subunit. Cancer Res. 2002;62:3184–3194. [PubMed] [Google Scholar]
- 12.Day PM, Esquivel F, Lukszo J, Bennink JR, Yewdell JW. Effect of TAP on the generation and intracellular trafficking of peptide-receptive major histocompatibility complex class I molecules. Immunity. 1995;2:137–147. doi: 10.1016/s1074-7613(95)80014-x. [DOI] [PubMed] [Google Scholar]
- 13.Parenti R, Paratore S, Torrisi A, Cavallaro S. A natural antisense transcript against Rad18, specifically expressed in neurons and upregulated during β-amyloid-induced apoptosis. Eur. J. Neurosci. 2007;26:2444–2457. doi: 10.1111/j.1460-9568.2007.05864.x. [DOI] [PubMed] [Google Scholar]
- 14.Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- 15.Joyce S, Woods AS, Yewdell JW, Bennink JR, De Silva AD, Boesteanu A, Balk SP, Cotter RJ, Brutkiewicz RR. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science. 1998;279:1541–1544. doi: 10.1126/science.279.5356.1541. [DOI] [PubMed] [Google Scholar]
- 16.Brutkiewicz RR, Bennink JR, Yewdell JW, Bendelac A. TAP-independent, β2-microglobulin-dependent surface expression of functional mouse CD1.1. J. Exp. Med. 1995;182:1913–1919. doi: 10.1084/jem.182.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makrigiannis AP, Etzler J, Winkler-Pickett R, Mason A, Ortaldo JR, Anderson SK. Identification of the Ly49L protein: evidence for activating counterparts to inhibitory Ly49 proteins. J. Leukocyte Biol. 2000;68:765–771. [PubMed] [Google Scholar]
- 18.Yewdell JW. Plumbing the sources of endogenous MHC class I peptide ligands. Curr. Opin. Immunol. 2007;19:79–86. doi: 10.1016/j.coi.2006.11.010. [DOI] [PubMed] [Google Scholar]