Abstract
Background
Triggered Purkinje ectopy can lead to the initiation of serious ventricular arrhythmias in post myocardial infarction (MI) patients. In the canine model, Purkinje cells from the subendocardial border of the healing infarcted heart can initiate ventricular arrhythmias. Intracellular Ca2+ abnormalities underlie these arrhythmias yet the subcellular reasons for these abnormalities remain unknown.
Methods and Results
Using 2D confocal microscopy, we directly quantify and compare typical spontaneous Ca2+ events in specific subcellular regions of normal Purkinje cells (NZPCs) with those Purkinje cells from the subendocardium of the 48hr infarcted canine heart (IZPCs). The Ca2+ event rate was higher in subsarcolemmal region (SSL) of IZPCs when compared to NZPCs, IZPC amplitudes were higher yet the spatial extents of these events were similar. The amplitude of Caffeine releasable Ca2+ in either the SSL or Core regions of IZPCs did not differ from NZPCs suggesting that Ca2+ overload was not related to the frequency change. In permeabilized Purkinje cells from both groups, the event rate was related to free [Ca2+] in both SSL and Core but in IZPCs this event rate was significantly increased at each free Ca2+ suggesting an enhanced sensitivity to Ca2+ release. Furthermore, decays of wide long lasting Ca2+ release events in IZPC's Core were significantly accelerated compared to those in NZPCs. JTV519 (K201) suppressed IZPC cell wide Ca2+ waves as well as normalized the enhanced event rate and its response to free Ca2+.
Conclusions
Increased spontaneous Ca2+ release events in IZPCs are due to uniform regionally increased Ca2+ release channel sensitivity to Ca2+ without a change in SR content. In addition, Ca2+ reuptake in IZPCs is accelerated. These properties would lower the threshold of Ca2+ release channels, setting the stage for the highly frequent arrhythmogenic cell wide Ca2+ waves observed in IZPCs.
Keywords: myocardial infarction, calcium, arrhythmias, Purkinje cells, Ca2+ waves
Introduction
Recently, several reports have appeared about the occurrence and ablation attempts of ventricular premature beats in patients with and without structural heart disease1, 2. In some, mapping has implicated the Purkinje system as the cause of these abnormal ventricular beats. In particular, the Haissaguerre group has reported about Purkinje ectopy initiating ventricular tachycardia (VT) in patients with Brugada syndrome, long QT and RVOT syndromes. In these studies ablation of the early Purkinje network activity has been successful. More recent reports, where similar approaches have been taken, suggest that for some clinically mapped ventricular premature beats in patients post MI, the earliest activation occurred in the Purkinje network at the so called endocardial “border zone” 3,4,5. Ablation at some of these sites successfully reduced electrical storm arrhythmias post MI as well as those VTs with a focal origin in the distal His Purkinje system4,3, 6. Thus triggered Purkinje ectopy can lead to initiation of serious storms of ventricular arrhythmias in post MI patients.
Our lab and others 7 have studied the mechanisms of these arrhythmias in the canine post MI model. In particular, we have developed the model of the isolated Purkinje cell aggregate from both the normal and 48hr infarcted myocardium (IZPCs) 8, 9. These Purkinje myocytes isolated from the subendocardial border zone are the cells that initiate these arrhythmias 10. Like ventricular myocytes, cardiac Purkinje cells respond to an action potential by an overall rise in intracellular Ca2+ ([Ca2+]i). However, in Purkinje cells, Ca2+ rises first just at the periphery of the cell below the membrane (subsarcolemmal, SSL). This is then followed by an elevation of [Ca2+] in the cell's Core. Such centripetal activation is uniform in normal Purkinje cells (NZPCs). In IZPCs, the same initial peripheral Ca2+ increase occurs in response to an action potential but often the subsequent large central Ca2+ elevation does not happen 11, 12. Importantly using global epi-fluorescent techniques, we found that such non-uniform Ca2+ activation increased the likelihood of IZPCs generating large cell wide Ca2+ waves (CWW) which underlie delayed afterdepolarizations (DADs), spontaneous action potentials and arrhythmias 11. However these studies did not provide precise information about the altered subcellular regional Ca2+ release events in Purkinje cells. One probable cause is a fundamental and regional change in sarcoplasmic reticulum (SR) Ca2+ release elements. Therefore, in this study we have examined using the enhanced spatial resolution of confocal microscopy, IZPCs to quantify directly the release of Ca2+ from the SR at sites in the SSL area as well as Core. Thus we compared the local Ca2+ release events in different subcellular regions of NZPCs and IZPCs using confocal microscopy and fluorescence Ca2+ imaging techniques.
Methods
Preparation of intact Purkinje cell aggregates
Myocardial infarction was induced in the dog 13 and Purkinje cells were prepared as described before 11, 12, 14. Aggregates of 2-6 cells were enzymatically dispersed from the Purkinje network of canine left ventricle and were placed in a perfusion chamber on the stage of inverted microscope equipped respectively with a rapid 2D confocal spinning disk system. Fluorescence was measured only in rod-shaped Purkinje cells with typical junctional ends, clear striations and membranes free of blebs 11, 15. Cells were superfused with Tyrode's solution ((mM) : 137 NaCl, 24 NaHCO3, 1.8 NaH2PO4, 0.5 MgCl2, 2CaCl2, 4 KCl, 5.5 dextrose, pH7.4, 24°C) for at least 15 minutes before experiment.
Preparation of permeabilized Purkinje cells
Intact cells were superfused with Tyrode's containing 2 mM Ca2+ for 10 min. Tyrode's solution was then replaced with a Ca2+ free ‘internal solution’ (see below) for 10 mins before cells were permeabilized by exposure to 0.01 % saponin in a Ca2+ free mock intracellular solution (60s). Fluo 4-K+ salt [Ca2+] containing intracellular solution was then superfused for at least 5 mins. The composition of the internal solution was (mM) : 100 potassium aspartate, 15 KCl, 5 KH2PO4, 0.5 EGTA, 0.75 MgCl2, 5 MgATP, 10 phosphocreatine, 10 Hepes, 5U/ml creatine phosphokinase, 8% dextran, pH7.2. Solutions with different free [Ca2+] were prepared by adding appropriate amounts of CaCl2. The free [Ca2+] and [Mg2+] were calculated using a computer program (MAXChelator 2.50), and the free [Mg2+] was adjusted to 1mM. The free [Ca2+] was verified using calcium calibration buffer kits (Molecular Probes).
Confocal Ca2+ imaging
Spatial and temporal characteristics of local Ca2+ events were measured in Purkinje cell aggregates by using 2D spinning disk (Nipkow) confocal microscopy15. Ca2+ concentration was monitored using the fluorescence of the Ca2+ indicator Fluo-4. The cells were loaded with 5μM Fluo-4AM for 30 min and washed for 15-20 min with Tyrode's solution. The intensity of the Ca2+-related fluorescence was captured from the illumination field and light intensity was collected using a fast CCD camera (ORCA-ER C4742-95, Hamamatsu Photonics KK, Japan). The system was attached to the video port of an inverted microscope equipped with 60× oil objective lens. In the 2DCM system, the fluorescence images were captured at 17 to 30 fps. [Ca2+]i was estimated from the pixel-to-pixel ratio F/Fo (F: intensity of instantaneous fluorescence; Fo: intensity of reference fluorescence) and analyzed using custom IDL programs (IDL 5.4, Research Systems, Boulder, CO, USA). In caffeine experiments, [Ca2+]i was given by Kd(F/F0)/[Kd/[Ca2+]i,rest +1-F/F0]. [Ca2+]i rest.
Data Analysis and Processing
Apparent non-propagating events were detected and selected for counting as follows. 20 consecutive frames of the 2D imaged intact cell or 33 frames of a permeabilized cell (F images) were divided by basal fluorescence image (F0 image) to make ratio images (F/F0)15 (Figure 1). The basal fluorescence image was made by averaging all frames. Ca2+ release events were defined as elevations of basal [Ca2+] equivalent to F/F0 3.4SD over F0 using a computer-based detection algorithm modified from Cheng H et al 16. Only frames free of Ca2+ waves were analyzed with the exception of some of the JTV519 (K201) studies. The events were counted, confirmed visually and compared to the results of the computer detection. The sensitivities were 95.3±2.3 % in intact cell (n=8) and 97.4±0.3 % in permeabilized cell (n=5). Probabilities of a detecting false event were 6.0±2.3 % in intact cell and 4.8±0.6% in permeabilized cell.
Figure 1.
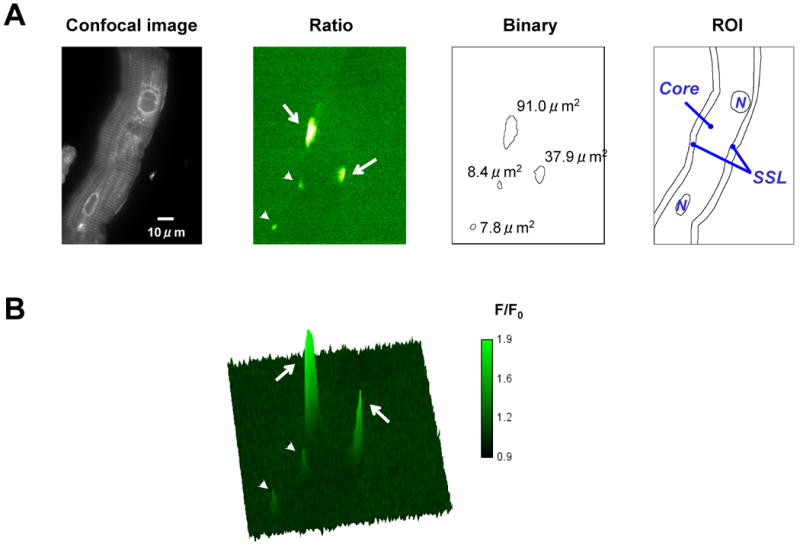
Typical 2D image of an isolated canine Purkinje cell. Amplitude of the event was calculated from ratio images. Spatial extent of the event and the event rate were calculated from binary images using IDL program (IDL6.0, Research Systems). In ratio images, arrows indicate wide long lasting event (WLE) and arrow heads indicate typical events (TEs) (Panels A, B). The amplitude and spatial extent of WLEs were markedly larger than those of typical events. A region of interest (ROI) was drawn on a confocal image and then superimposed on binary images to determine the subcellular location (SSL or Core) of events using ImageJ. N indicates nucleus.
Determination of event location and measurements
Regions of interest (ROIs) were drawn on a cell image and then superimposed on binary cell images to determine the subcellular location (SSL or Core) of events (ImageJ) (Figure 1). Amplitudes of events were calculated from ratio images. Spatial extent of an event and the event rate were calculated from binary images using IDL functions (IDL6.0, Research Systems). Typical Events (TEs) were defined as those nonpropagating events occurring at same site within one frame 15.
Caffeine experiments
SR-Ca2+ load was estimated in intact Purkinje cells by rapid exposure of a cell to Tyrode's solution containing 20mM Caffeine, 10mM BDM, and 30μM TTX. The regional SR-Ca2+ load was measured in the SSL and Core and was given by the peak of the caffeine induced Ca2+ transient. A similar protocol was used to estimate SR-Ca2+ load in a subset of saponin treated Purkinje cells at three different free [Ca2+]. In some experiments, solutions were supplemented with 1mM tetracaine to block Ca2+ loss through ryanodine receptors (RyR) 17.
JTV519 (K201) protocol
The events in the absence and presence of JTV519 (K201) (1μM) were determined by 2D imaging of aliquots of cells in the absence and after 10mins superfusion with JTV519 (K201) from each of several preparations. JTV519 (K201) was generously provided by Aetas Pharmaceuticals.
Statistics
For comparisons of data of this study, SPSS (ver.11.0.1J, SPSS Inc.) and R (GNU-style copyleft, http://www.r-project.org/) were used. Shapiro-Wilk test was performed to test normal distribution. When the distribution was normal distribution, the results were described as Mean ± S.E. When the distribution was not normal distribution, the results were described as quartile deviation (Median (25 percentile, 75 percentile)). Levene test was performed to test homoscedasticity. In Figure 2A, 2B, 3B, 4A, 4B, 5A, a Two-Way ANOVA was first completed and if appropriate, a post hoc test (Tukey's Post Hoc Test, Kruskal-Wallis test, Steel-Dwass test) was carried out (see legends). In Figure 2A, 2B, 3B, 4A, 4B, 5A, an interaction was not observed between cell types (NZPC, IZPC) and location (SSL, Core). In Figure 4B, an interaction was not observed between drug and location, drug and cell types.
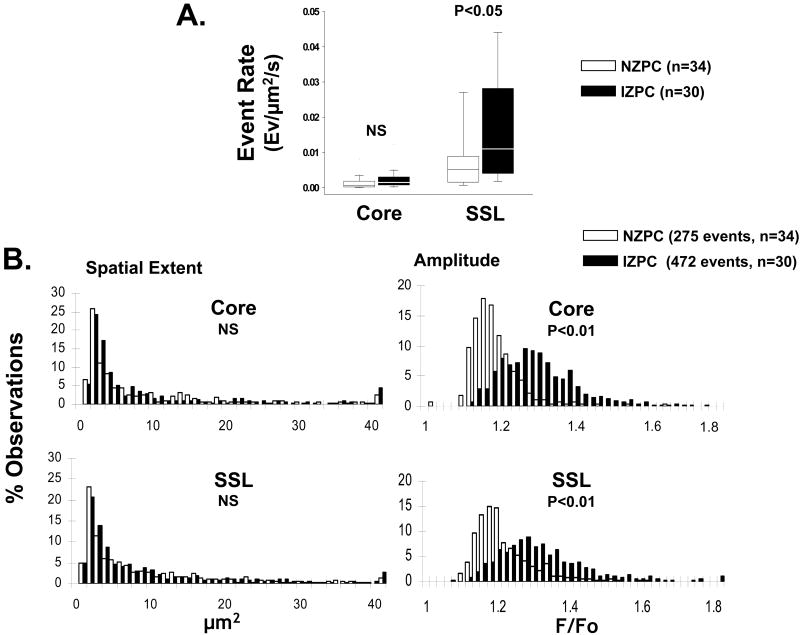
Figure 2.
Panel A shows event rate of TEs in NZPCs (white bars) and IZPCs (black bars) by region (Core and subsarcolemmal, SSL). The data are described as quartile deviation (Median, 25 percentile, 75 percentile). Panel B shows histograms of spatial extent and amplitude of TEs in NZPCs (white bars) and IZPCs (black bars) by region (Core, SSL). n= number of cells. Mann-Whitney test was used for event rate and spatial extent comparisons. Unpaired student-t test for statistics of the amplitude. NS indicates not significant.
Figure 3.
Panel A Typical caffeine induced Ca2+ release by region in an NZPC and IZPC. Right tracings are examples from two SSL regions and one Core region in each cell represented. Panel B. Average data are plotted. NS indicates not significant.
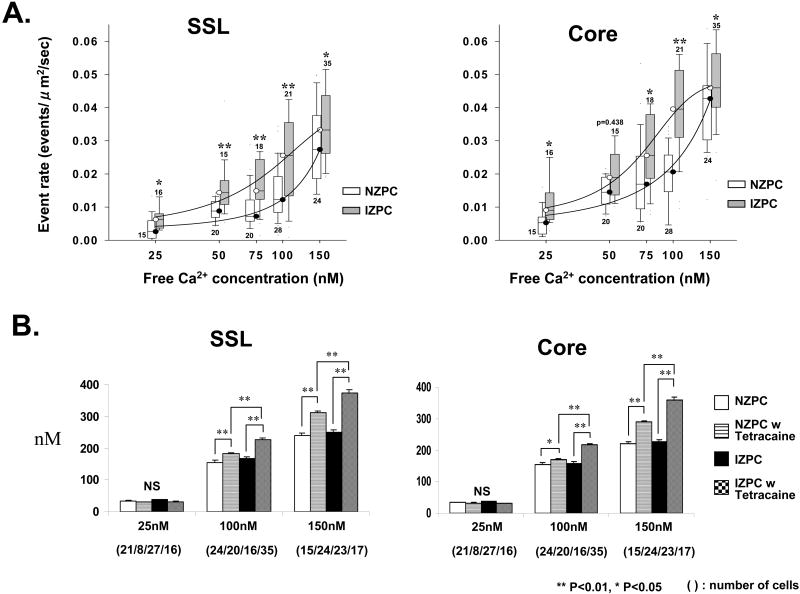
Figure 4.
Panel A Relationship between average TE rate and free Ca2+ concentration in the two cell groups by Region (SSL, Core). Numbers below/above each plotted average indicate number of cells used for average. Mann Whitney test was used at each free Ca2+ concentration. Asterisks indicate statistical difference. Panel B. Amplitude of caffeine induced Ca2+ transient with and without tetracaine (1mM) in saponin treated cells at 3 different free Ca2+. Asterisks indicate statistical difference. Unpaired student-t test was used if appropriate after two-way ANOVA. There was no significant difference in cell type (NZPC, IZPC) and location (SSL, Core) in Two-Way ANOVA test. An interaction was not observed between drug and location, drug and cell type. NS indicates not significant.
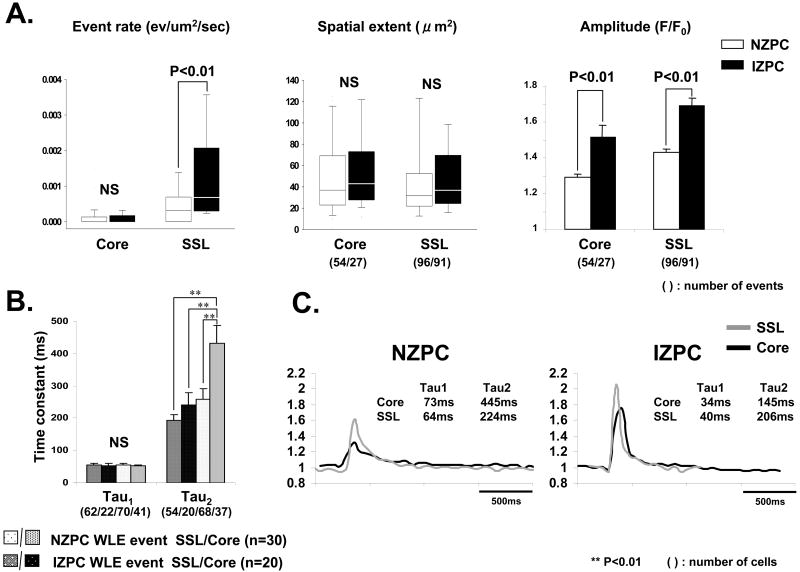
Figure 5.
Panel A shows event rate, spatial extent and amplitude (mean +/-SEM) of wide long lasting events (WLEs) in intact NZPCs (white bars) and IZPCs (black bars) by region (Core and SSL). Numbers in parentheses indicate actual number of events in each group. Mann-Whitney test was carried out for statistics of the WLE event rate. Unpaired t test was carried out for statistics of the amplitude. Panel B. Time constants of decay of WLE events in both intact NZPCs and IZPCs were determined in each event/cell using Clampfit. Average Tau1 and Tau2 values are shown. Note while Tau1 values did not differ between cell groups, Tau2 was significantly reduced in IZPCs. The One-Way ANOVA and Tukey's Post Hoc Test were carried out for the statistics. Panel C. Linear profiles to the right show typical NZPC and IZPC events from both the SSL and Core. NS indicates not significant.
Results
A. Intact Cell Data
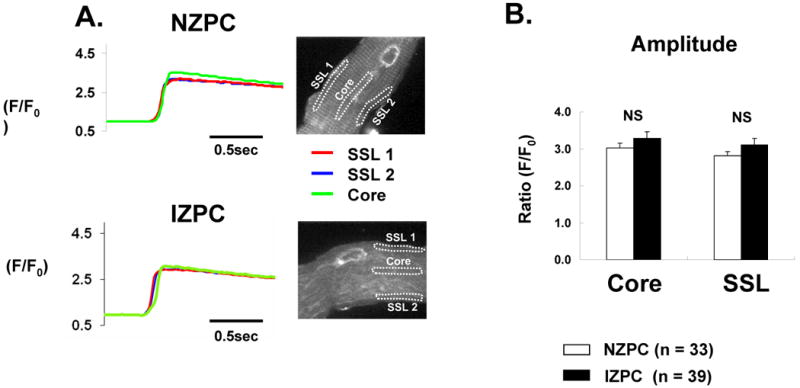
In intact cells at [Ca2+]o (2mM), the majority (94% NZPCs; 92% IZPCs) of spontaneous Ca2+ release events (typical events, TE) were detected within a single frame. The TE rate in all regions of IZPCs (0.00428 ev/μm2/s (0.00178, 0.00741)) was significantly higher than that in NZPC (0.00222 ev/μm2/s (0.00118,0.00590), P<0.05, Mann-Whitney test). The cell areas were not different between two cell groups (4414±170 μm2 vs 4888±268 μm2, P=0.142, unpaired t test). To investigate the subcellular regionality of the event rates, SSL and Core region were independently analyzed in both cell types (FIGURE 2A). The TE rates in Core were not different between NZPCs and IZPCs (0.00067 ev/μm2/s (0.00018, 0.00184) vs 0.00143 ev/μm2/s (0.00073, 0.00310), P=0.270, Mann-Whitney). The TE rate in SSL region of IZPC (1198 events, n cells=30, 0.01101 ev/μm2/s (0.00447, 0.02818) (black bars, FIGURE 2A)) was significantly higher than that in NZPC (747 events, n =34, 0.00512 ev/μm2/s (0.00152, 0.00889), P<0.05, Mann-Whitney). TE amplitude in IZPCs was also significantly higher (9-10%) than that of NZPCs in both SSL (1.32±0.004 vs 1.21±0.004 F/F0) and Core (1.30±0.006 vs 1.18±0.004 F/F0)(FIGURE 2B) whereas spatial extent did not differ (SSL: 9.35±0.35 vs 9.04±0.40 μm2, Core: 9.11±0.59 vs 8.99±0.68 μm2).
To determine whether the observed changes in the incidence of Ca2+ events were due to regional differences in the SR-Ca2+ load, we assessed Ca2+ transients evoked by rapid caffeine exposure (Figure 3). The Ca2+ level was calculated by integrating fluorescence over a selected ROI. The time course of [Ca2+] in the ROI was obtained from serial frames acquired at video rate during the caffeine response. Average [Ca2+] data for each ROI was obtained for every frame. The amplitude of the Ca2+ transient was measured in 3 different ROIs: two in the SSL, one in the Core (Figure 3A). No significant difference in the amount of Ca2+ released by caffeine was detected between the SSL and the Core and between NZPCs and IZPCs (Figure 3B). Thus changes detected in IZPC TE rates were not caused by a regional variation in the (caffeine-sensitive) SR Ca2+ content.
B. Saponin Cell Data
Cytosol and SR luminal Ca2+ levels are both critical factors that affect individual Ca2+ release sites as well as interactions between sites. Therefore a change in the Ca2+ sensitivity of SR Ca2+ release function could explain the augmented event rate in IZPCs. The Ca2+ sensitivity of SR Ca2+ release was tested by measuring the Ca2+ event rate in permeabilized NZPCs and IZPCs at various free Ca2+ concentrations ranging from 25 to 150 nM. This technique allows for control of cytoplasmic milieu (ionic composition) while maintaining SR structurally and functionally. TEs were counted in the SSL and Core of cells from both groups. Figure 4A shows that the relationship between TE rate and free Ca2+ is shifted leftward and upward in both the Core and the SSL of IZPCs suggesting a greater sensitivity of Ca2+ release function to cytosolic Ca2+.
To rule out that permeabilization of cells exposed to varying free [Ca2+] affected IZPCs differently than NZPCs, SR-Ca2+ content was estimated using a rapid caffeine (20mM) application to saponin treated cells (Figure 4B). Our analyses show that under these conditions, SR content varied depending on free [Ca2+] but did not differ between NZPCs and IZPCs (FIGURE 4B). However tetracaine clearly increased Caffeine induced Ca release, while reducing the Ca2+ event rate significantly (NZPCs: drug free (100nM; TE drug-free rate 0.0198+/- 0.01434 ev/μm2/s (n=28), plus tetracaine TE rate 0+/-0 (n=10); IZPCs; drug free (100nM; TE drug-free rate 0.04041+/- 0.0246 ev/μm2/s (n=21), plus tetracaine TE rate 0+/-0 (n=10)).
Other Ca2+ Release Events
Events which lasted over several frames (120-300ms) and had a greater spatial extent and yet failed to propagate within the confocal plane were called “wide, long lasting events” (WLEs) 15(Figure 5). The amplitude and event rate of WLEs in SSL of IZPCs were significantly greater than those in NZPCs (FIGURE 5A), while these events had similar spatial extents. Core and SSL WLE Ca2+ transients decayed with multiple time constants (FIGURE 5B, 5C). However in IZPCs, the decay of Core WLEs was significantly accelerated compared to that of NZPCs.
In sum, increased spontaneous Ca2+ release TEs in IZPCs appear to result from a uniform increase of Ca2+ release channel sensitivity without a change in SR content. In addition, Ca2+ reuptake in IZPCs is accelerated consistent with other data 18. These properties could set the stage for the arrhythmogenic cell wide Ca2+ waves observed in IZPCs11.
Effects of JTV519 (K201)
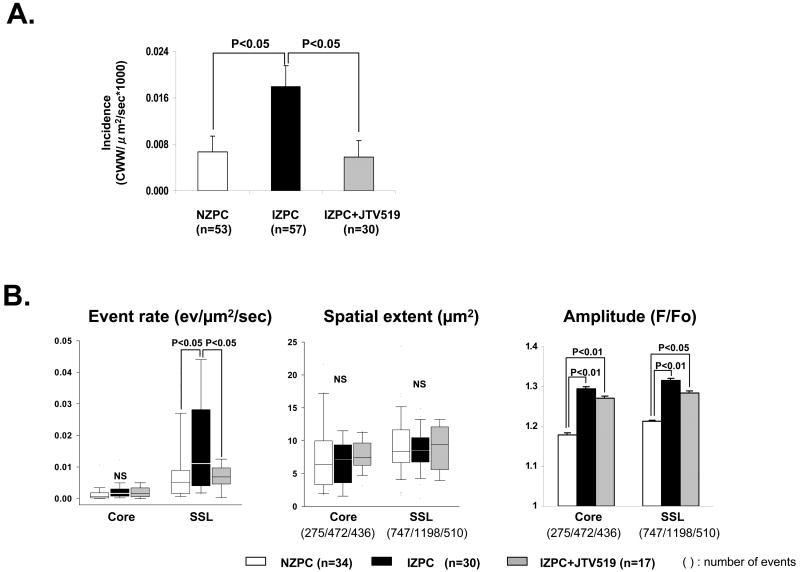
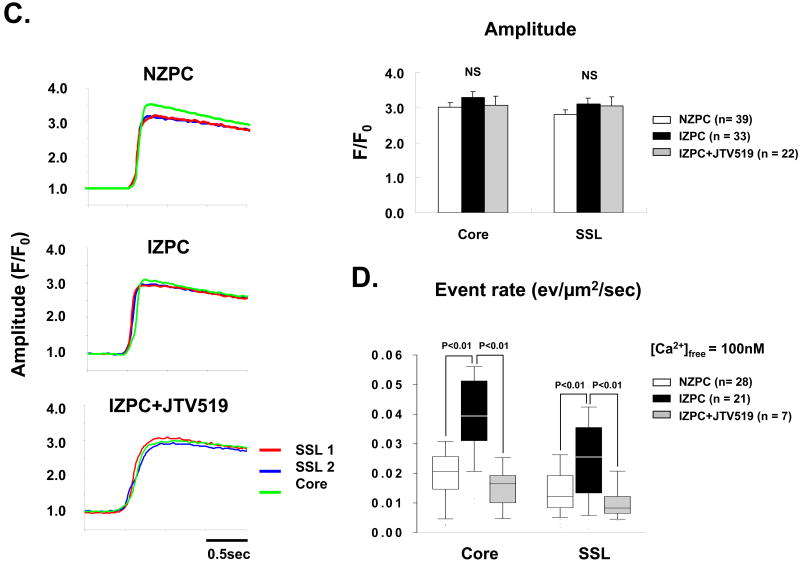
We previously reported that JTV519 (K201) can suppress the incidence of cell wide Ca2+ waves in arrhythmogenic IZPCs 19 (FIGURE 6A). Since our results above suggest that there is an enhanced Ca2+ sensitivity to Ca2+ release events in IZPCs, we tested whether JTV519 (K201)(1μM) was effective in altering the regional Ca2+ event rate in both intact and saponin treated IZPCs. JTV519 (K201) significantly reduced TE rate of intact IZPCs without changing either their spatial extent or the amplitude of Caffeine releasable pools of Ca2+ (FIGURE 6B, 6C). Furthermore, the TE rate in response to [Ca2+] in saponin skinned IZPCs was normalized to NZPC values (free [Ca2+] =100nM) by JTV519 (K201) (Figure 6D).
Figure 6.
Panel A. Graph shows the incidence of cell wide Ca2+ waves (CWW) in NZPCs and in IZPCs in the absence and presence of JTV519 (K201) 1μM (grey bar). The One-Way ANOVA and Tukey's Post Hoc Test were carried out for the statistics. Panel B shows TE event rate, spatial extent and amplitude in IZPCs in the absence and presence of JTV519 (K201) (grey bars). Total number of events used is shown in parentheses. Kruskal-Wallis test and Steel-Dwass test were carried out for the statistics of the event rate and spatial extent. The One-Way ANOVA and Tukey's post hoc test were carried out for the statistics of the amplitude. Panel C shows typical caffeine induced Ca2+ release transients by region in an intact NZPC and an IZPC in the absence and presence of JTV519 (K201). The One-Way ANOVA was used. Panel D shows the effects of JTV519 (K201) on event rate in saponin treated permeabilized IZPCs. Note in the presence of JTV519 (K201) IZPC event rate was normalized to that of NZPCs (white bars) (free Ca2+ =100nM). Kruskal-Wallis and Steel-Dwass tests were carried out for the statistics. NS indicates not significant.
Discussion
The major findings of this study are that typical Ca2+ events in intact Purkinje cells from the subendocardium of the 48hr infarcted heart (IZPCs) are larger in amplitude and more frequent in the SSL, but not in the Core when compared to the same in NZPCs. This increase reflects a distribution of events to a population with a larger amplitude and occurs without a change in regional caffeine releasable Ca2+ pools. It is unlikely to result from a larger membrane Ca2+ influx since global ICaT and ICaL are reduced by 55% in IZPCs 9. Core TE Ca2+ events in IZPCs varied but most were larger and decayed faster than their NZPC counterparts. Wide long lasting Ca2+ events (WLEs) in IZPCs were also more frequent, larger in amplitude and decayed faster (Figure 5).
Clearly, fundamental Ca2+ events change in IZPCs without an altered SR content. Hence, we went on to determine whether the threshold for Ca2+ release events was changed in IZPCs using saponin skinned Purkinje cells under conditions where we manipulated free [Ca2+] bathing the cell ([Ca]cyto). We found that there was a leftward and upward shift in the relation between Ca2+ event frequency and free [Ca2+] in IZPCs (Figure 4A). Again we determined that under these conditions Caffeine releasable Ca2+ pools in IZPCs were not lower than those in NZPCs (Figure 4B). However, tetracaine significantly increased these pools, after reducing the event rate. These findings imply that a difference in SR Ca-content is probably not the cause of the IZPC increased event rate. On the other hand, the difference in event rate between IZPCs and NZPCs does not seem to have a large effect on the SR Ca-content. It is possible that the SR depleting effect of spontaneous SR Ca2+ release may have been offset by faster SR Ca2+ uptake as is witnessed by the faster decay of the IZPC Ca2+ transients (Figure 5B). JTV519 (K201) normalized the IZPC increased sensitivity to Ca2+ release to NZPC values as it also reduced incidence of the arrhythmogenic CWWs (Figure 6A).
An important limitation is that varying [Ca]cyto did not induce Ca2+ release directly but changing [Ca]cyto could increase luminal [Ca]SR which then could elicit Ca release by SOICR (store operated induced Ca release) 20. We did show that the SR- Ca2+ content is similar in IZPCs compared to NZPCs (Figure 3). However it is still reasonable to conclude that IZPCs are more sensitive to [Ca]cyto and/or the Ca content of the SR than NZPCs, implying that the cytosolic and/or luminal Ca2+ sensitivity of the Ca2+ release channels in the SR of IZPCs may be increased.
Comparison with other studies regarding sparks in cells from diseased hearts
Most subcellular Ca2+ release studies have been completed using ventricular cells from diseased hearts 21-25 and most findings are consistent with a diseased induced reduced global cellular Ca2+ transient. Most have suggested that a reduced SR load may be a reason for the reduced spark amplitude in CHF cells and only one study suggests that Ryanodine sensitivity (single channel recordings) to Ca2+ is enhanced in heart failure cells 26. This latter finding can account for the increase in spark rate as well as the reported reduced SR content in heart failure cells.
To our knowledge no one has described spontaneous Ca2+ sparks in Purkinje cells from diseased hearts. Our data suggest that typical Ca2+ events in IZPCs differ from those of normal Purkinje cells in that they are larger, and more frequent than NZPC sparks/events, particularly in the SSL region. In addition IZPCs sparks occur in a substrate that shows no change in SR content, but still has enhanced sensitivity to Ca2+ release. Further, wide long lasting Core events in IZPCs show an acceleration in decay suggesting enhanced SR uptake. Thus defective Ca2+ regulation in IZPCs leads to increased frequency of Ca2+ events however SR content is maintained due to enhanced rate of Ca2+ uptake to SR. Clearly our findings using these diseased Purkinje cells differ from those of ventricular cells and suggest that specific therapeutic modalities could be developed for Purkinje cell Ca2+ dependent arrhythmias.
When we used a drug (JTV519 (K201)) to ameliorate the disorder of defective Ca2+ regulation of the SR in intact IZPCs (similar to that shown for expression systems 20), we found that this drug not only reversed the enhanced sensitivity to Ca2+ in saponin treated IZPCs, but also reduced the incidence of cell wide Ca2+ waves in the intact IZPCs (Figure 6). An action to reduce the incidence of cell wide Ca2+ waves would be antiarrhythmic and is consistent with JTV519 (K201) being protective in models of ischemia reperfusion 27-29 where a disorder of Ca2+ handling has been shown. Also consistent with findings in ventricular cells, we found that JTV 519(K201) has no effect on Purkinje SR Ca2+ load 30 yet suppresses excess Ca2+ release events (as inferred from “noise analysis”) and cell wide Ca2+ waves in Purkinje cells 19. JTV519 (K201)'s effect in these diseased Purkinje cells is consistent with the theory that this drug binds to specific domains of the RyR protein 31 to stabilize this Ca2+ release channel's mediated Ca2+ release.
Limitations
No PCR or protein analysis was completed on these small Purkinje cell samples thus we cannot at this time detail whether there is profound structural remodeling of proteins in IZPCs. However since we found JTV519 (K201) to quickly reverse the observed IZPC functional abnormalities, it is unlikely that profound structural remodeling occurs.
We used saponin in some of our studies so we could then “equilibrate” cytosolic Ca2+(Cacyto) and SR Ca2+ and test SR dependence on Cacyto. This required exposing Purkinje cells to saponin to form 30 nm pores in the sarcolemma and expose the SR directly to the superfusion solutions. This technique has distinct advantages over the isolated lipid bilayer RyR preparation that studies Ca2+ release channel activity in isolation. We as well as others show that Ca2+ release activity increases in all cell regions under these conditions 32 presumably due to the loss of unknown protein/ protein interactions. Nevertheless, we saponin treated NZPCs and IZPCs the same way in order to complete Ca2+ dependent release experiments and found a difference between the two cell types.
Studies here were completed on isolated Purkinje cells and not intact Purkinje tissues. Nevertheless our findings of abnormal intracellular Ca2+ regulation underlying arrhythmias in Purkinje cells that have survived in the infarcted heart are consistent with those showing the antiarrhythmic effects of agents that alter intracellular Ca2+ release on the triggerability of intact Purkinjes that survive in the infarcted subendocardium 10, 19.
Acknowledgments
Funding sources: Supported by grant HL58860 from the NHLBI; grants 81150(BDS), 135573&104907(HtK) from the Canadian Institute of Health Research and AHFMR grant (20040163), Canada (HtK).
All authors had full access and take responsibility for the integrity of these data. All authors have read and agree to the manuscript as written.
Footnotes
Clinical Summary: Recently, premature ventricular contractions (PVCs) originating from Purkinje system have been shown to be responsible for the initiation of VF in patients with early post-myocardial infarction (MI), and a radio frequency ablation of the triggering PVCs for those patients can prevent VF. Although the precise mechanisms of the triggering PVC generation had not been shown, our current work on Purkinje cells that have survived in the infarcted heart elucidate one of the cellular mechanisms for these arrhythmias. Also we show here that a drug which stabilize Ca2+ release channels, e.g. JTV-519 (K201), is effective in correcting the abnormality and as such, may also be effective for Ca2+ dependent arrhythmias in post MI patients
Disclosures: For all authors there are no potential conflicts of interest such as relationships with pharmaceutical companies, biomedical device manufacturers, or other corporations whose products or services are related to the subject matter of the article. Nothing to disclose.
Reference List
- 1.Aiba T, Suyama K, Aihara N, Taguchi A, Shimizu W, Kurita T, Kamakura S. The role of Purkinje and pre-Purkinje potentials in the reentrant circuit of verapamil sensitive idiopathic LV tachycardia. PACE. 2001;24:333–44. doi: 10.1046/j.1460-9592.2001.00333.x. [DOI] [PubMed] [Google Scholar]
- 2.Ouyang F, Cappato R, Ernst S, Goya M, Volkmer M, Hebe J, Antz M, Vogtmann T, Schaumann A, Fotuhi P, Hoffmann-Riem M, Kuck KH. Electroanatomic substrate of idiopathic LV tachycardia: unidirectional block and macroreentry within the Purkinje network. Circ. 2002;105:462–9. doi: 10.1161/hc0402.102663. [DOI] [PubMed] [Google Scholar]
- 3.Marrouche NF, Verma A, Wazni O, Schweikert R, Martin DO, Saliba W, Kilicaslan F, Cummings J, Burkhardt JD, Bhargava M, Bash D, Brachmann J, Guenther J, Hao S, Beheiry S, Rossillo A, Raviele A, Themistoclakis S, Natale A. Mode of initiation and ablation of ventricular fibrillation storms in patients with ischemic Cardiomyopathy. JACC. 2004;43:1715–1720. doi: 10.1016/j.jacc.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Bansch D, Oyang F, Antz M, Arentz T, Weber R, Val-Mejias JE, Ernst S, Kuck KH. Successful catheter ablation of electrical storm after myocardial infarction. Circ. 2003;108:3011–6. doi: 10.1161/01.CIR.0000103701.30662.5C. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi M, Kobayashi Y, Iwasaki Y, Morita N, Miyauchi Y, Kato T, Takano T. Novel mechanism of postinfarction ventricular tachycardia originating in surviving left posterior Purkinje fibers. Heart Rhythm. 2006;3:908–18. doi: 10.1016/j.hrthm.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Lopera G, Stevenson WG, Soejima K, Maisel WH, Koplan B, Sapp JL, Satti SD, Epstein LM. Identification and ablation of three types of ventricular tachycardia involving the HIs-Purkinje system in Patients with heart disease. J Cardiovasc Electr. 2004;15:52–8. doi: 10.1046/j.1540-8167.2004.03189.x. [DOI] [PubMed] [Google Scholar]
- 7.Wit AL, Janse MJ. The Ventricular Arrhythmias of Ischemia and Infarction. Electrophysiological Mechanisms. Mount Kisco, NY: Futura Publishing Co,Inc.; 1993. [Google Scholar]
- 8.Boyden PA, Albala A, Dresdner K. Electrophysiology and ultrastructure of canine subendocardial Purkinje cells isolated from control and 24 hour infarcted hearts. Circ Res. 1989;65:955–70. doi: 10.1161/01.res.65.4.955. [DOI] [PubMed] [Google Scholar]
- 9.Boyden PA, Pinto JMB. Reduced calcium currents in subendocardial Purkinje myocytes that survive in the 24 and 48 hour infarcted heart. Circulation. 1994;89:2747–59. doi: 10.1161/01.cir.89.6.2747. [DOI] [PubMed] [Google Scholar]
- 10.Janse MJ, Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989;69:1049–169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]
- 11.Boyden PA, Barbhaiya C, Lee T, Ter Keurs HEDJ. Nonuniform Ca2+ Transients in Arrhythmogenic Purkinje Cells that survive in the infarcted canine heart. Cardiovasc Res. 2003;57:681–93. doi: 10.1016/s0008-6363(02)00725-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stuyvers BD, Dun W, Matkovich SJ, Sorrentino V, Boyden PA, Ter Keurs HEDJ. Ca2+ sparks and Ca2+ waves in Purkinje Cells: A Triple Layered System of Activation. Circ Res. 2005;97:35–43. doi: 10.1161/01.RES.0000173375.26489.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris AS. Delayed development of ventricular ectopic rhythms following experimental coronary occlusion. Circulation. 1950;1:1318–28. doi: 10.1161/01.cir.1.6.1318. [DOI] [PubMed] [Google Scholar]
- 14.Boyden PA, Pu J, Pinto JMB, Ter Keurs HEDJ. Ca2+ Transients and Ca2+ waves in Purkinje Cells. Role in action potential initiation. Circ Res. 2000;86:448–55. doi: 10.1161/01.res.86.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirose M, Stuyvers BD, Dun W, Ter Keurs HEDJ, Boyden PA. Wide long lasting Perinuclear Ca2+ release events generated by an Interaction between Ryanodine and IP3 receptors in Canine Purkinje cell. JMCC. 2008 doi: 10.1016/j.yjmcc.2008.05.008. online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng H, Song LS, Shirokova N, González A, Lakatta EG, Ríos E, Stern MD. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophys J. 1999;76:606–17. doi: 10.1016/S0006-3495(99)77229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venetucci LA, Trafford AW, Diaz ME, O'Neill SC, Eisner DA. Reducing Ryanodine Receptor Open Probability as a Means to Abolish Spontaneous Ca2+ Release and Increase Ca2+ Transient Amplitude in Adult Ventricular Myocytes. Circ Res. 2006;98:1299–305. doi: 10.1161/01.RES.0000222000.35500.65. [DOI] [PubMed] [Google Scholar]
- 18.Stuyvers BD, Boyden PA, Hirose M, Ter Keurs HEDJ. Ca2+ activity in cardiac Purkinje Cells; a source of arrhythmogenicity in the infarcted heart. Biophysical J. 2007;92:S344a. [Google Scholar]
- 19.Boyden PA, Dun W, Barbhaiya C, Ter Keurs HEDJ. 2APB- and JTV519 (K201) sensitive micro Ca2+ waves in arrhythmogenic Purkinje cells that survive in infarcted canine heart. Heart Rhythm. 2004;1:218–26. doi: 10.1016/j.hrthm.2004.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt DJ, Jones PP, Wang R, Chen W, Bolstad J, Chen K, Shimoni Y, Chen SR. K201 (JTV519) suppresses spontaneous Ca2+ release and [H3] ryanodine binding to RyR2 irrespective of FKBP12.6 association. Biochem J. 2007;404:431–8. doi: 10.1042/BJ20070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–6. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- 22.Balijepalli RC, Lokuta AJ, Maertz NA, Buck JM, Haworth RA, Valdivia HH, Kamp TJ. Depletion of t-tubules and specific subcellular changes in sarcolemmal proteins in tachycardia induced heart failure. Cardiovasc Res. 2003;59:67–77. doi: 10.1016/s0008-6363(03)00325-0. [DOI] [PubMed] [Google Scholar]
- 23.Gomez AM, Guatimosim S, Dilly KW, Vassort G, Lederer WJ. Heart failure after myocardial infarction: altered excitation- contraction coupling. Circulation. 2001;104:688–93. doi: 10.1161/hc3201.092285. [DOI] [PubMed] [Google Scholar]
- 24.Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. PNAS. 2006;103:4305–10. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shorofsky SR, Aggarwal R, Corretti M, Baffa JM, Strum JM, Al-Seikhan BA, Kobayashi YM, Jones LR, Wier WG, Balke CW. Cellular mechanisms of altered contractility in the hypertrophied heart: big hearts, big sparks. Circ Res. 1999;84:424–34. doi: 10.1161/01.res.84.4.424. [DOI] [PubMed] [Google Scholar]
- 26.Kubalova Z, Terentyev D, Viatchenko-Karpinski S, Nishijima Y, Györke I, Terentyeva R, da Cuñha DN, Sridhar A, Feldman DS, Hamlin RL, Carnes CA, Györke S. Abnormal intrastore calcium signaling in chronic heart failure. PNAS. 2005;102:14104–9. doi: 10.1073/pnas.0504298102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura J, Kawahara M, Sakai E, Yatabe J, Nakanishi H. Effects of a novel cardioprotective drug,JTV-519 on membrane currents of guinea pig ventricular myocytes. Japn J Pharmacol. 1999;79:275–81. doi: 10.1254/jjp.79.275. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko N. New 1,4-benzothiazepine derivative,K201, demonstrates cardioprotective effects against sudden cardiac death and intracellular calcium blocking action. Drug Dev Res. 1994;33:429–38. [Google Scholar]
- 29.Kohno M, Yano M, Kobayashi S, Doi M, Oda T, Tokuhisa T, Okuda S, Ohkusa T, Kohno M, Matsuzaki M. A new cardioprotective agent, JTV519, improves defective channel gating of ryanodine receptor in heart failure. Am J Physiol. 2003;284:H1035–H1042. doi: 10.1152/ajpheart.00722.2002. [DOI] [PubMed] [Google Scholar]
- 30.Loughrey CM, Otani N, Seidler T, Craig MA, Matsuda R, Kaneko N, Smith GL. K201 modulates excitation-contraction coupling and spontaneous Ca2+ release in normal adult rabbit ventricular cardiomyocytes. Cardiovasc Res. 2007;76:236–46. doi: 10.1016/j.cardiores.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto T, Yano M, Xu X, Uchinoumi H, Tateishi H, Mochizuki M, Oda T, Kobayashi S, Ikemoto N, Matsuzaki M. Identification of Target Domains of the Cardiac Ryanodine Receptor to Correct Channel Disorder in Failing Hearts. Circulation. 2008;117:762–72. doi: 10.1161/CIRCULATIONAHA.107.718957. [DOI] [PubMed] [Google Scholar]
- 32.Sheehan KA, Zima AV, Blatter LA. Regional differences in spontaneous Ca2+ spark activity and regulation in cat atrial myocytes. The Journal of Physiology. 2006;572:799–809. doi: 10.1113/jphysiol.2005.103267. [DOI] [PMC free article] [PubMed] [Google Scholar]