Summary
Disease progression in EAE is regulated by PD-1 and its ligands, B7-H1 (PD-L1) and B7-DC (PD-L2). B7-H1 and B7-DC, have negative regulatory affects upon binding PD-1 on activated T cells and B7-H1 deficiency increases severity of both diabetes and EAE. However the role of PD-L expression on different APC in the CNS in regulating local T cell function during relapsing EAE (R-EAE) has not been examined. Our data show that the majority of CNS CD4+ T cells isolated during acute EAE are PD-1+, and T cells specific for relapse-associated epitopes express PD-1 upon antigen-stimulation in the CNS. B7-H1 and B7-DC are differentially expressed on discrete APC populations in the inflamed CNS. B7-H1 and PD-1 have mainly inhibitory functions on CNS T cells. B7-H1 negatively regulates the stimulation of activated PD-1+ TH cells, in co-cultures with microglia and different CNS-infiltrating APC presenting endogenously processed peptides. The preponderance of IFN-γ+ versus IL-17+ T cells in the CNS of B7-H1−/− mice suggests that B7-H1 more selectively suppresses TH-1 than TH-17 responses in vivo. In contrast, blockade of B7-DC has less pronounced regulatory effects. Overall, the results demonstrate that B7-H1 expressed by CNS myeloid APC negatively regulates T cell activation during acute R-EAE.
Keywords: antigen presenting cells, autoimmunity, costimulatory molecules, dendritic cells, inhibitory receptors
Introduction
EAE is a widely employed model of multiple sclerosis (MS) [1]. Multiple lines of evidence indicate that autoreactive CD4+ TH-1 cells mediate inflammatory damage in the CNS characterized by demyelination of nerve fibers and manifested clinically by paralysis [2–4]. However, mice lacking critical components of the TH-1–IFN-γ pathway remain highly susceptible to EAE [5, 6] consistent with recent studies show that a distinct subset of IL-17-producing effector CD4+ T cells (TH-17) are also crucial for EAE progression [7, 8].
Inflammatory cytokine production by T cells infiltrating the CNS requires activation by myelin peptides presented by resident APC [9, 10]. CD11c+ DC have been implicated as local inducers of CNS autoimmunity [11]. Epitope spreading, the induction of reactivity to epitopes distinct from the disease-inducing epitope, has been characterized in R-EAE and recent studies indicate that naïve T cells enter the inflamed CNS and are activated by local myeloid DC (mDC) presenting endogenous myelin peptides [12, 13]. Tolerance experiments indicate that T cells specific for spread epitopes are the major functional cause of disease progression [14]. Short-term blockade of the CD28/CD80(B7–1)CD86(B7–2) or CD40/CD154 costimulatory pathway in animals with established R-EAE prevents disease relapse by inhibiting epitope spreading [15– 17]. CTLA-4, a negative regulatory homologue of CD28, that binds B7 molecules with higher affinity, down-regulates epitope spreading and is involved in mediating remission in R-EAE [18].
B7-H1 (also termed PD-L1, CD274) is not found or found at low levels on the cell surface of resting T cells, B cells, DC, macrophages, bone-marrow derived mast cells and diverse parenchymal cell types, but can be upregulated to high levels on these cell types [19– 27]. In contrast, expression of B7-DC (also termed PD-L2, CD273) is much more restricted to DC and a subset of macrophages, as well as bone marrow-derived mast cells [23, 28, 29]. Both ligands bind to PD-1 (CD279), found on activated T and B cells, as well as monocytes [30, 31]. The amount of PD-1 expression and the extent of engagement of PD-1 by its ligands regulate the threshold for T cell activation and quantities of cytokines produced (reviewed in [32]). Accumulation of PD-1 at the immunological synapse can be induced by both B7-H1 and B7-DC on DC [33]. Disruption of PD-1 results in autoimmune cardiomyopathy, lupus-like glomerulonephritis [34, 35], progressive arthritis [36], rapid onset of diabetes in NOD mice [37], and exacerbated EAE [38, 39] suggesting that PD-1 is critical for regulating peripheral T cell tolerance and autoimmunity.
Whether B7-H1 and B7-DC have overlapping or distinct functions is uncertain. Some studies have suggested that B7-H1 and B7-DC inhibit T cell responses [19, 28], whereas others support a stimulatory role for the PD-Ls under certain conditions of in vitro and in vivo T cell stimulation [20, 29, 40, 41]. One explanation for these conflicting results is that positive effects are the result of inhibition of negative signaling [42]. The existence of a second receptor that binds B7-H1 and B7-DC has been postulated, but not proven [41, 43, 44]. However, a recent study shows that B7–1 could inhibit T cell function by interacting with B7-H1 on T cells [45], supporting an additional receptor for B7-H1. Studies of mouse models of autoimmunity also emphasize important immunoregulatory functions for PD-1 ligands. Blocking the PD-1/B7-H1 pathway in NOD mice results in rapid and exacerbated diabetes with PD-1/B7-H1 interactions within the pancreas critical for limiting autoreactivity [37, 46]. Comparing mice lacking B7-H1 and B7-DC (B7-H1/B7-DC−/− mice) to mice individually lacking either PD-L, revealed that B7-H1 and B7-DC had overlapping functions in inhibiting IL-2 and IFN-γ production during T cell activation. Remission in new-onset NOD mice, induced by insulin-coupled ECDI-fixed APC, and long-term tolerance are also maintained by the PD-1-B7-H1 pathway [47]. In contrast, studies of transgenic (Tg) overexpression of B7-H1 in pancreatic β cells have yielded discordant results, demonstrating increased diabetes incidence and a costimulatory function of B7-H1 [48]. PD-1 and its ligands also regulate EAE [38, 39, 49, 50]. Studies suggest both anti-B7-H1 and anti-B7-DC can exacerbate EAE depending on the genetic background [38, 50]. Such differences between mouse strains did not correlate with altered amounts of B7-H1 or B7-DC on conventional APC [50]. B7-H1 deficiency converted the 129S4/SvJae strain from an EAE-resistant to EAE-susceptible strain and transfer of encephalitogenic T cells from wild-type mice into B7-H1−/− recipients led to exacerbated disease [49]. On the 129svEv background, PD-1−/− and B7-H1−/− mice developed more severe EAE than wild type and B7-DC−/− mice [39]. Based on these studies, it is unclear whether the regulatory effects of PD-1/PD-L blockade operated in the periphery or in the CNS target organ.
To determine the effects of the PD-1/PD-L on activation of naïve and effector T cells in the CNS target organ, we compared the expression levels and functional significance of PD-Ls on CNS cells during the acute phase of R-EAE in SJL/J mice. We focused on the acute disease phase for several important reasons. First, recent work from our lab established that epitope spreading to PLP139–151 develops shortly after the peak of acute PLP178–191-induced R-EAE [12, 13] and regulation of epitope spreading spread can prevent disease progression in this model [14, 16]. Secondly, the late acute phase was also of particular interest to study mechanisms that mediate clinical remission by down-regulation of the primary PLP178–191 T cell response. Lastly, during the acute disease we were able to recover enough APC and T cells to accurately assess their phenotype and function. We demonstrate that increased expression of B7-H1 in the CNS was attributable to its induction on resident microglia and infiltration of B7-H1+ MHC class IIhigh myeloid APC, whereas B7-DC was mainly found on infiltrating CD11c+ DC and macrophages. Bone marrow chimera studies indicated that the majority of CD4+ T cells isolated from the CNS during acute EAE expressed PD-1, and the majority of T cells specific for relapse-associated epitopes expressed PD-1 upon antigen stimulation in the CNS. Antibody blocking studies showed that the B7-H1/PD-1 interaction inhibited the function of T cells in the inflamed CNS. Most interestingly, B7-H1 negatively regulated the stimulation of IFN-γ-producing TH-1 cells, but not IL-17-producing TH-17 cells, in co-cultures with different CNS-infiltrating APC and microglia presenting endogenously processed myelin peptides. In contrast, blockade of B7-DC had less pronounced effects on CNS T cell activation.
Results
Expression of B7-H1 and B7-DC on microglia and different CNS-infiltrating APC subsets in the inflamed CNS
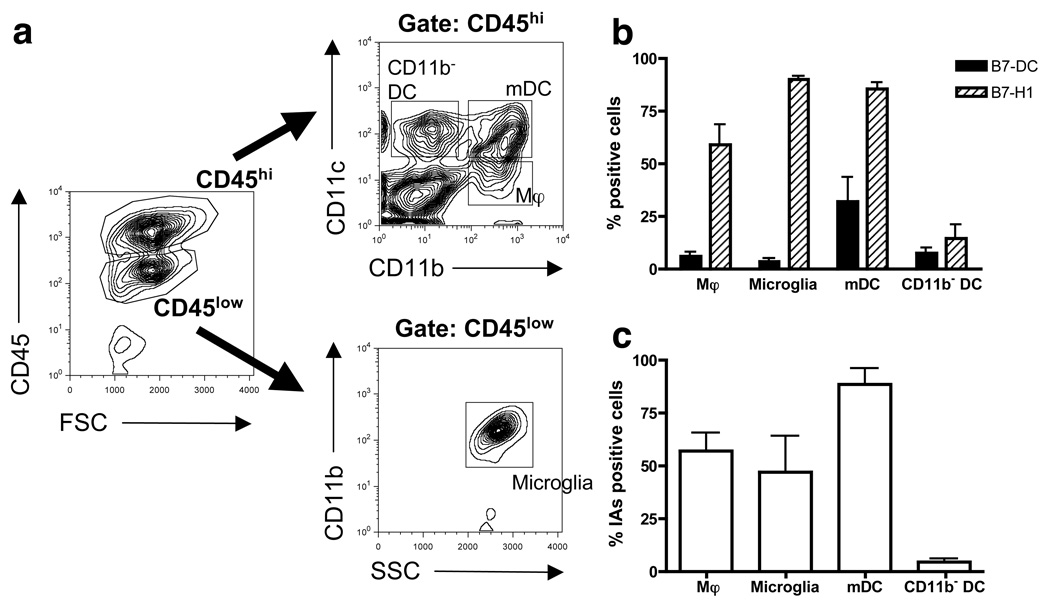
During EAE, expression of PD-1 ligands in the brain is increased and can largely be found in inflammatory foci [38, 50–52]. B7-H1 and B7-DC expression levels on microglia and different CNS-infiltrating APC at the peak acute phase of R-EAE were compared on CD45lowCD11b+ microglia, CD45highCD11c−CD11b+ macrophages, CD45highCD11c+CD11b+ myeloid DC (mDC), and CD45highCD11c+CD11b− DC (CD11b− DC) isolated from the CNS (Fig. 1a). Microglia cells were largely negative for CD11c (data not shown). CD11b− DC contained two subpopulations, the major population are B220+PDCA-1+CD8α+/− pDC and the minor B220−CD8α+CD8 DC [13] (data not shown). Flow cytometric analysis demonstrated that B7-H1 was highly expressed on all three myeloid cells (microglia, macrophages, mDC), whereas the percentage of B7-H1 expressing CD11b− DC was lower (14.7%; Fig. 1b). The proportion of B7-H1 positive CNS-resident microglia (89.9%) and infiltrating mDC (85.4%) were similar and approximately 59.0% of macrophages expressed B7-H1. B7-DC expression was lower and restricted mainly to mDC (31.9%) and less on CD11b− DC (7.7%) and macrophages (5.9%). Only 3.3% of microglia cells were positive for B7-DC. All myeloid APC subsets (mDC, 88.5% > macrophages, 57.1% > microglia, 47.2%) expressed MHC class II molecules (I-As), required for antigen-presentation to CD4+ T cells (Fig. 1c). In contrast, MHC class II expression on pooled CD11b− DC was low (4.6%). These findings suggest that during the acute phase of EAE, increased expression of B7-H1 in the CNS is attributable to upregulation on resident microglia and infiltrating myeloid B7-H1+ MHC class IIhigh APC while B7-DC is mainly found on a subset of infiltrating mDC and macrophages.
Figure 1.
Expression of B7-H1 and B7-DC on microglia and different CNS-infiltrating APC subsets in the inflamed CNS. (a) FACS purification of microglia and CNS-infiltrating APC populations. Cells were isolated from the pooled CNS of 20 PLP178–191-primed mice at the peak acute phase of R-EAE (day 14–15 PI) and stained with anti mouse CD45-PECy7, CD11b-Allophycocyanin and CD11c-FITC. The cells were sorted into the following four APC populations: CD45low gate (31.9%): CD45lowCD11b+ microglia (96.7%); CD45high gate (60.5%): CD45highCD11c−CD11b+ macrophages (Mφ; 7.3%), CD45highCD11c+CD11b− DC (CD11b− DC; 14.7%) and CD45highCD11c+CD11b+ DC (mDC; 18.9%). (b) B7-H1, B7-DC or (c) I-As molecule expression on CNS APC subsets during the acute phase of CNS inflammation. Data is plotted as mean percentage ± SEM of (b) B7-DC+ (black bars), B7-H1+ (shaded bars) or (c) I-As+ cells of gated microglia and CNS-infiltrating APC from at least 3 independent experiments. Gates were set with respective to isotype controls.
PD-1 expression on CNS-infiltrating, acute phase- and relapse-associated T cells
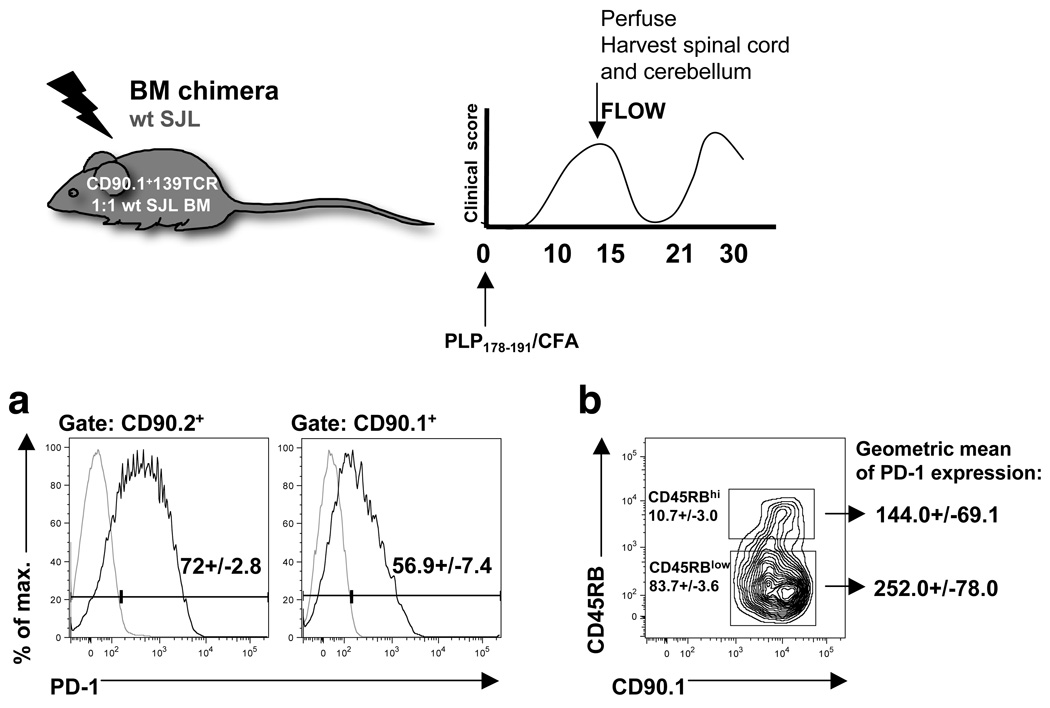
We utilized mixed bone marrow chimeras composed of wildtype (WT) CD90.2+ and 139TCR CD90.1+ cells primed with PLP178–191 to assess PD-1 expression on CNS-infiltrating T cells specific for both the initiating (PLP178–191) and spread (PLP139–151) epitopes. During the acute phase of active PLP178–191-induced R-EAE in WT SJL mice (day 16–18 post-immunization), PLP178–191-specific T cells constitute the vast majority of T cells as determined by IFN-γ ELISPOT or [3H]-thymidine incorporation assays [12, 16]. Most acute disease phase-associated CNS CD90.2+ CD4+ T cells expressed PD-1 (72%; Fig. 2a). The percentage of relapse-associated CD90.1+ 139TCR Tg T cells in the CNS, that showed positive staining for PD-1, was slightly lower (56.9%; Fig. 2a). Using mixed bone marrow chimeras and CFSE-labeled CD90.1+ 139TCR Tg T cells, we have recently shown that activation of naïve PLP139–151-specific Tg T cells in PLP178–191-induced R-EAE occurs directly in the CNS detectable approximately 15–16 days post-immunization [12]. Accordingly, further analysis revealed, that the majority (83.7%) of relapse-associated CD90.1+ 139TCR Tg T cells sorted from the acutely inflamed CNS were activated (CD45RBlow, Fig. 2b) and had up-regulated PD-1 upon antigen-recognition in the CNS (geometric mean of PD-1 expression 144 versus 252; Fig. 2b). As anticipated, T cell numbers recovered from the CNS of OVA323–339-primed control mice were low and therefore we were unable to make reliable conclusions about PD-1 expression (data not shown).
Figure 2.
PD-1 expression on CNS-infiltrating, acute phase- and relapse-associated T cells. SJL chimeras (1:1 mix of CD90.2+ WT SJL and CD90.1+ 139TCR Tg bone marrow; BM) were primed with PLP178–191. At the peak of acute disease, cells were isolated from the CNS, and stained for CD90.1, CD4, CD45RB and PD-1. CNS samples of 4–5 mice per experiment (n=2) were analyzed individually, and (a) PD-1+ expression of CD90.1+CD4+ or CD90.2+CD4+ gated T cells (numbers in histograms represent mean ± SD of the percentage of PD-1+ cells (grey line, isotype staining). (b) The activation level of CD90.1+139TCR Tg cells was determined by CD45RB expression, and the averaged geometric mean ± SD of PD-1 expression between CD45RBhigh/low was compared.
Inhibition of T cells from the inflamed CNS by B7-H1 and its receptor PD-1
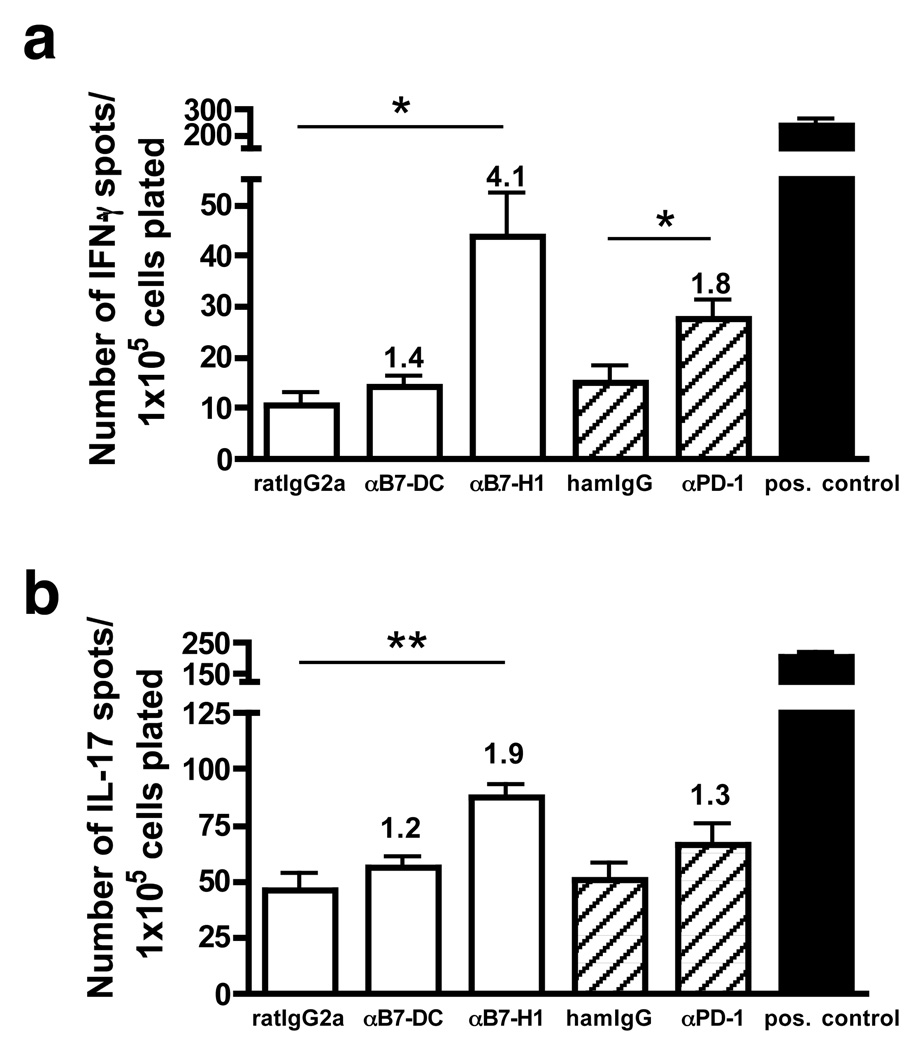
We next investigated the effect of individual blockade of the B7-DC/PD-1 or B7-H1/PD-1 pathways on endogenous antigen presentation (in the absence of added peptide) by pooled infiltrating CNS APC and microglia to CNS-infiltrating T cells at the acute phase of EAE using ELISPOT assays. Blocking the inhibitory receptor PD-1 resulted in an 1.8 fold increase in the frequency of IFN-γ(p = 0.03; by unpaired t-test) and to a lesser extent IL-17 producing cells (1.3 fold; Fig. 3). Moreover, in the presence of blocking anti-B7-H1 antibody, the frequency of both IFN-γ+ (4.1 fold; p = 0.006; by unpaired t-test) and IL-17+ (1.9 fold; p = 0.002; by unpaired t-test) producing cells were significantly increased, suggesting an inhibitory effect of B7-H1, at least partially via the PD-1 pathway. In contrast, addition of blocking anti-B7-DC antibody to CNS infiltrates, only moderately enhanced IFN-γ (1.4 fold) and IL-17 (1.2 fold) responses. Stimulation with PLP178–191 or PLP139–151 peptides confirmed that most of the T cells isolated from the CNS during the early acute phase (day 14–15) were specific for the priming peptide (data not shown).
Figure 3.
Inhibition of acute phase-associated CNS T cells by B7-H1 and its receptor PD-1. The frequency of IFN-γ- (a) or IL-17-producing (b) T cells was determined by ELISPOT from the pooled CNS of PLP178–191-primed SJL mice (n=5–10) at the peak acute phase of R-EAE (day 14–15 PI). Endogenous presentation (in the absence of exogenously added PLP178–191 peptide) was determined in the presence of blocking anti-mouse B7-DC, B7-H1 (white bars) or PD-1 (hatched bars). Blocking/stimulatory indices, based on respective isotype controls, are shown above the bars. CNS cells incubated with anti-CD3 (black bars) were used as positive control. Data are representative of three (IL-17) or four (IFN-γ) separate experiments. Mean number of IL-17 or IFN-γ ELISPOTS significantly higher than isotype controls, **p< 0.01, *p< 0.05.
Blockade of B7-H1 or B7-DC in co-cultures of microglia and CNS-infiltrating APC subsets and activated or naïve CD4+ T cells
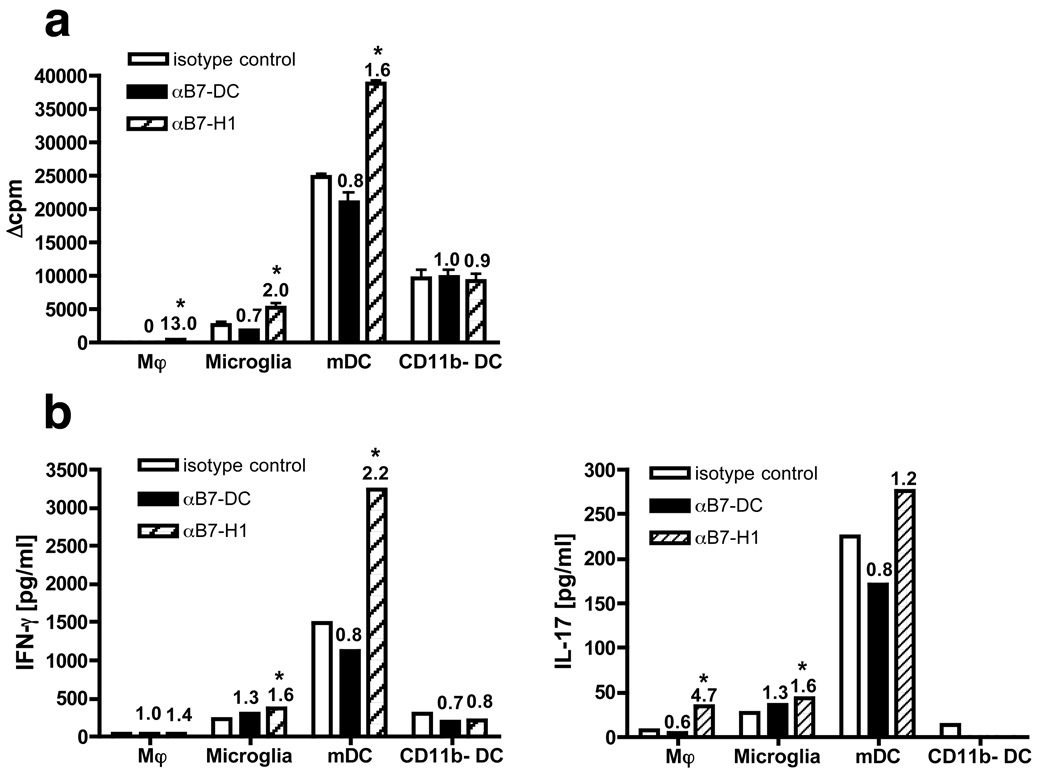
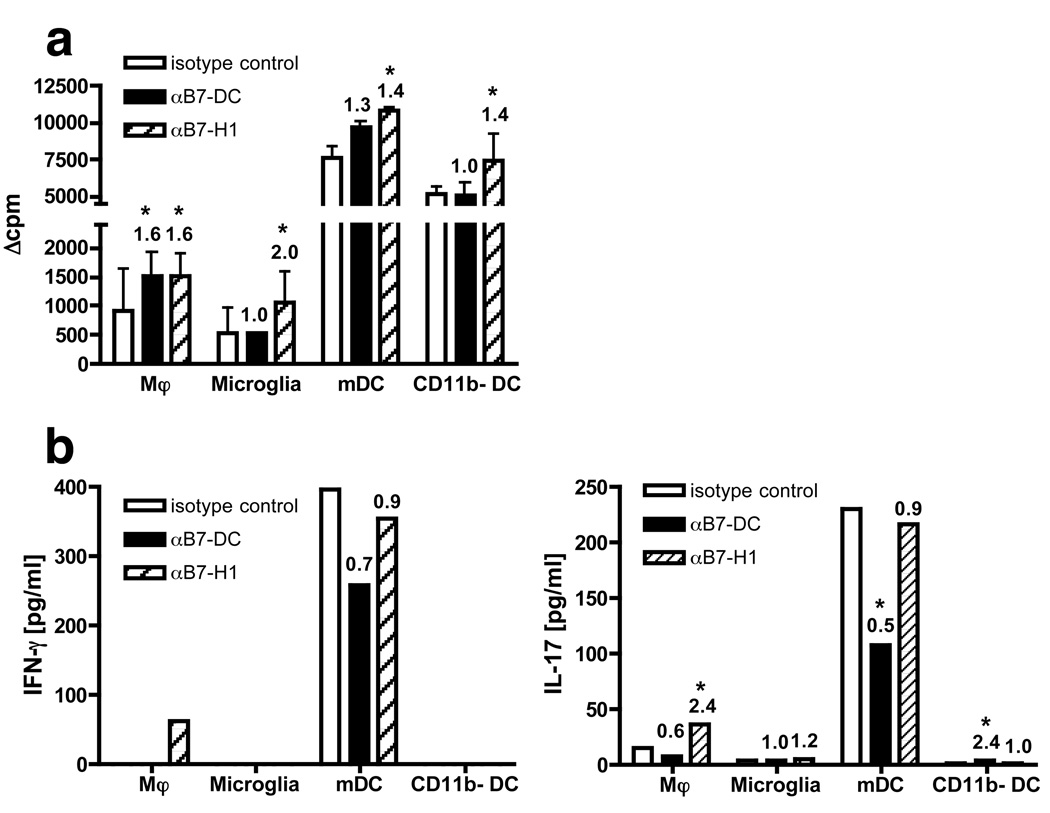
To test the functional role of the PD-1 ligands, microglia and individual CNS-infilrating APC subsets were FACS purified from the CNS of mice with EAE and analyzed for their ability to stimulate myelin-reactive T cells in the presence or absence of B7-H1 or B7-DC blocking mAbs. Sorted microglia and CNS-infiltrating APC populations were first co-cultured with a short-term PLP139–151-specific CD4+ T cell line in the absence (to assess endogenous presentation) of PLP139–151 peptide at an APC:T cell ratio of 1:2 (Fig.4). Previous data from our laboratory have demonstrated that endogenous activation of naïve PLP139–151-specific TCR Tg cells induced by CNS DC subpopulations was MHC II-restricted and CD80/86-dependent [13]. In accordance with recently published studies from our laboratory [12, 13], CNS DC, particularly mDC, were far more efficient than macrophages and microglia in inducing proliferation (Fig. 4a; isotype controls). The addition of blocking anti-B7-H1 antibody significantly increased T cell proliferation in cultures with myeloid CNS APC subsets (1.6-fold), but not CD11b− DC (0.9-fold) (Fig. 4a). The relative increase in proliferation was higher in cultures with macrophages (13-fold) and microglia (2-fold), which have weak stimulatory capacity, compared to mDC. However, even in the presence of blocking anti-B7-H1 antibody, neither macrophage- nor microglia-induced T cell proliferation reached levels close to those found in cultures stimulated with mDC in the presence of isotype control antibody. Upon addition of exogenous PLP139–151 peptide, microglia could induce comparably high proliferation by the T helper cell line (on average 45.4-fold compared to isotype control cultures without peptide in 2 separate experiments - data not shown). In contrast, addition of blocking anti-B7-DC antibody did not change or slightly decreased T cell proliferation (0.7- to 1.0-fold).
Figure 4.
Blockade of B7-H1 or B7-DC in co-cultures of microglia and CNS-infiltrating APC subsets and activated CD4+ T cells. FACS-purified Mφ, microglia, mDC and CD11b− DC isolated from the pooled CNS of 20 PLP178–191-primed SJL mice at the peak of acute EAE were co-cultured with activated PLP139–151-specific CD4+ T cells. Control Ig (open bars), anti-mouse B7-DC (black bars) or B7-H1 (shaded bars) were added to cultures. Peptide was omitted to determine endogenous presentation. (a) Proliferation was analyzed by [3H]-thymidine uptake of duplicate or triplicate wells per group. (b) Supernatants from duplicate or triplicate wells of each group in (a) were pooled after 72 hrs and IFN-γ and IL-17 levels determined. Blocking/stimulatory indices, based on respective isotype controls, are shown above the bars. Data are representative of three separate experiments. *An increase/decrease of >30% as compared to isotype control levels was considered to be significant.
As previously reported [12], CNS microglia and macrophages induced only minimal proliferation of naïve PLP139–151-specific 139TCR cells (Fig. 5a). DC, in particular mDC, were the most efficient stimulators, and even under B7-H1 blockade, neither microglia nor macrophages could stimulate T cell proliferation comparable with the level stimulated by DC in cultures containing isotype control antibodies. Addition of blocking anti-B7-H1 antibody to cultures with mDC significantly enhanced T cell proliferation (1.4-fold, Fig. 5a), less pronounced than the enhancement observed in co-cultures with the activated T helper cell line. Blockade of B7-DC did not affect or only modestly increased the proliferation of the naïve T cells. We confirmed by flow cytometry, that PD-1 was highly expressed on CD4+ T helper cells which showed an activated phenotype (CD44highCD45RBlowCD62Llow). In contrast, PD-1 was absent or only expressed at low levels on CD45RBhigh lymph node T cells from naïve 139 TCR Tg mice (data not shown). Collectively these results indicate that the different efficiencies of microglia and CNS-infiltrating APC subsets to induce T cell proliferation do not solely depend on differential expression profiles of B7-H1 as the poorest (microglia and macrophages) and best stimulators (mDC) are myeloid cells that highly express B7-H1 (Fig. 1). However, our data demonstrate that in co-cultures with microglia and CNS-infiltrating APC presenting endogenously processed peptides, B7-H1 negatively regulates the stimulation of T cells, in particular activated T helper cells, expressing the inhibitory PD-1 receptor.
Figure 5.
Blocking B7-H1 or B7-DC in co-cultures with microglia and CNS-infiltrating APC subsets and naïve 139TCR Tg T cells. Experiments were performed as in Fig.4, except that sorted microglia and CNS-infiltrating APC subsets were co-cultured with naïve CD4+CD62Lhigh PLP139–151-specific T cells. Results are representative of two separate experiments. *An increase/decrease of >30% as compared to isotype control levels was considered to be significant.
We also analyzed the culture supernatants from the activated and naïve T cells for levels of the pro-inflammatory cytokines IFN-γ and IL-17 (Fig. 4b and 5b). PLP139–151-specific T helper cells activated with mDC presenting endogenous peptide produced the highest amounts of IFN-γ (1494 pg/ml) and IL-17 (225 pg/ml; isotype controls in Fig. 4b). Addition of blocking B7-H1 antibodies, resulted in production of significantly elevated amounts of IFN-γ (2.2-fold), but interestingly IL-17 was only slightly increased (1.2-fold). mDC were also the most potent stimulators of IFN-γ (396 pg/ml) and IL-17 (230 pg/ml) production in cultures with naïve PLP139–151-specific CD4+ 5B6 T cells (isotype controls in Fig. 5b). In contrast to co-cultures with T helper cells, addition of B7-H1 antibody did not enhance IFN-γ and IL-17 production by naïve 139TCR Tg T cells (both 0.9-fold) and blockade of B7-DC inhibited secretion of primarily IL-17 (0.5-fold), and to a lesser extent IFN-γ (0.7-fold). Microglia and CNS-infiltrating APC and myelin-reactive T cells were FACS-sorted to high purity, and the levels of IFN-γ and IL-17 were found to be below the level of detection by LiquiChip assay in supernatants of sorted microglia and CNS-infiltrating APC cultured for 4 days in the absence of peptide suggesting an absence of contaminating cells in the ex vivo APC assay capable of producing IL-17 and IFN-γ [13]. Thus, B7-H1 has mainly inhibitory function on IFN-γ secretion of activated PD-1+ TH-1 cells, but interestingly has little regulatory effect on TH-17 cells, and B7-DC seems to be slightly stimulatory on mDC driving naïve CD4+ TH-1 and TH-17 activation.
Loss of B7-H1 results in increased numbers of IFN-γ+ T cells in the inflamed CNS
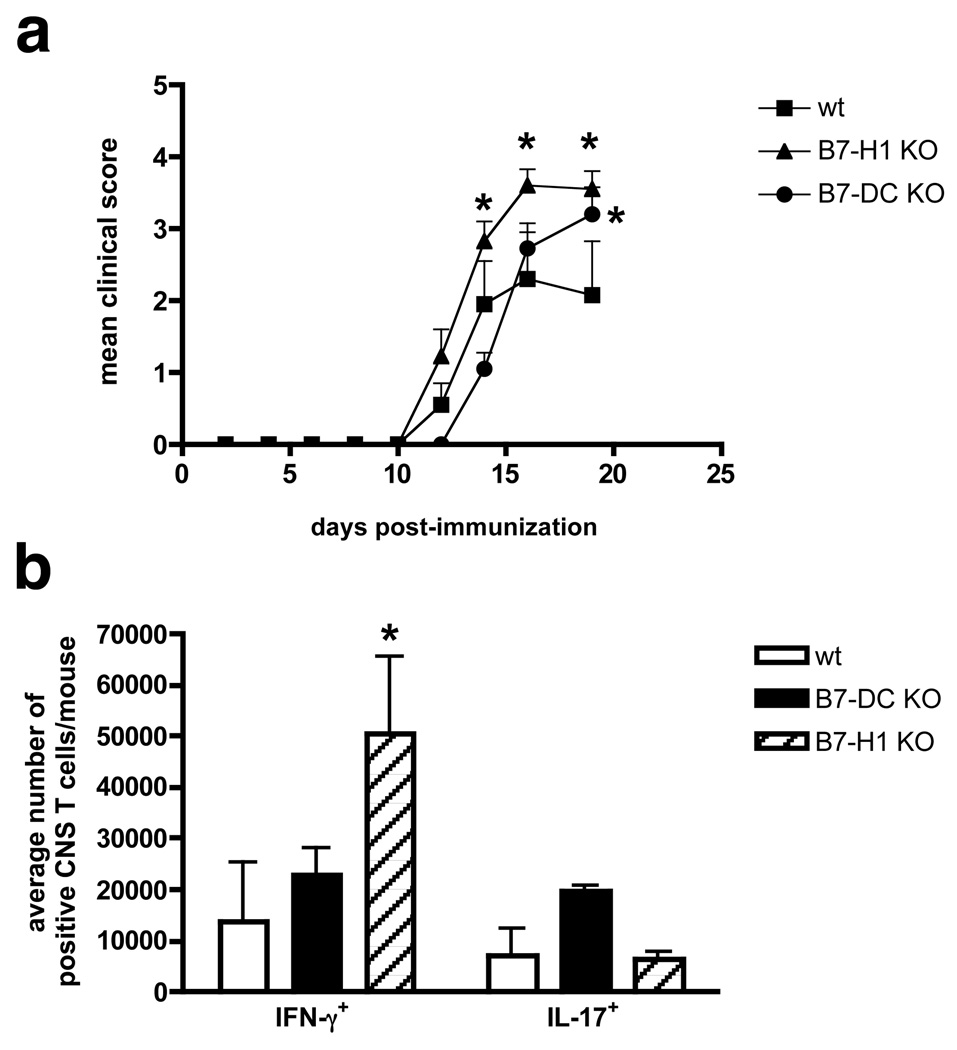
Down-regulation of IFN-γ responses of activated T cells by B7-H1 was the predominant effect of PD-1/PD-L interaction observed in our in vitro analyses. Therefore, we tested whether the numbers of IFN-γ+ or IL-17+ effector T cells were different in the CNS of WT vs. PD-L−/− C57BL/6 mice with EAE (Fig. 6). Intracellular FACS analysis of CNS-derived T cells was employed following a short-term (3 h) stimulation with PMA/ionomycin in the presence of golgi stop to allow estimation of the number of the Th subsets, an accepted technique that we have previously employed [13]. By virtue of specifically gating on CD90.2+ T cells, we excluded non-T cells potentially capable of producing IL-17 and IFN-γ, such as NK cells. There is some evidence that genetic background is important for the function of the PD-1/PD-L pathway [50], however distribution of PD-Ls on C57BL/6 microglia and CNS-infiltrating APC and blockade in CNS ELISPOTS yielded similar results compared to the SJL strain (data not shown). We confirmed earlier reports [39, 49] that B7-H1−/− mice developed exacerbated clinical EAE compared to WT controls (Fig. 6a). Interestingly and supporting the above in vitro analyses, the vast majority of T cells in the inflamed CNS of PLP178–191 primed B7-H1−/− mice were IFN-γ+ and significantly outnumbered IL-17+ cells, whereas in WT and B7-DC−/− mice frequencies of TH-1 and TH-17 cells were comparable (Fig. 6b). These data indicate that in vivo B7-H1 also seems to predominantly regulate TH-1 as opposed to TH-17 responses in the inflamed CNS of mice with EAE.
Figure 6.
Production of IFN-γ and IL-17 in the inflamed CNS in the absence of B7-H1 or B7-DC. (a) Wildtype (WT), B7-DC or B7-H1 KO mice were immunized with PLP178–191/CFA and monitored for clinical disease. The data are expressed as the mean clinical score versus days post-immunization (n=10/group). *Mean disease score significantly higher than WT control mice, p<0.05. Results are representative of three separate experiments. (b) CNS mononuclear cells were recovered on days 20–21, pooled (WT: n=3; KO: n=7) and stained for CD90.2 and intracellular IL-17 and IFN-γ Mean numbers of IL-17+ or IFN-γ+CD90.2+ T cells per mouse ± SEM compiled from 2 separate experiments are shown. *Mean number of IFN-γ+ cells significantly higher than in WT controls, p< 0.01.
Discussion
The goal of this study was to examine the expression pattern and functional significance of PD-1/PD-L on CNS inflammatory cells during the acute phase of R-EAE. Previous studies have documented that PD-1 and its ligands regulate disease progression in EAE [38, 39, 49, 50, 53]. However none of these studies directly addressed the question how PD-Ls expressed on microglia and different CNS-infiltrating APC, might participate in regulation of acute phase or relapse-associated T cell function.
During the acute phase of EAE, we found increased expression of B7-H1 in the CNS on infiltrating myeloid MHC class IIhigh APC and on resident microglia. B7-DC was mainly found on infiltrating DC and macrophages (Fig. 1). The distribution of PD-Ls in the CNS of C57BL/6 mice with MOG35–55 induced chronic EAE was similar (data not shown) [50]. Our quantitative analysis is consistent with recent immunohistochemical studies demonstrating B7-H1 expression on macrophages, microglial cells, astrocytes and endothelial cells of vessels close to inflammatory foci in the CNS, and the absence of B7-DC on CNS-resident cells [38, 51, 52]. Up-regulation of B7-H1 mRNA on human microglial cells by TH-1-supernatants in vitro has also been shown [52]. B7-H1 and PD-1 expression in the CNS parallels that of clinical disease in mice with EAE [38, 52]. Our results further demonstrate, that in PLP178–191-induced R-EAE most activated PLP178–191-specific CD4+ T cells sorted from the inflamed CNS had up-regulated PD-1 and surprisingly, at the same time point, the majority of relapse-associated 139TCR Tg T cells were also PD-1 positive (Fig.2). We have shown that activation of naïve 139TCR Tg T cells in PLP178–191 induced R-EAE in the CNS already occurs during the late acute phase [12] and T cells are able to express high levels of surface PD-1 by the time of their first cell division [54].
Recent studies have implicated PD-1 and its ligands in regulating both disease susceptibility and progression in EAE [38, 39, 49, 50]. In adoptive transfer EAE, injection of MOG35–55-specific T cells led to exacerbated disease in B7-H1−/− compared to WT recipients [49]. Because MHC class II-restricted antigen-recognition and reactivation of MOG35–55-specific T cells in the CNS is necessary for clinical EAE induction in this model [9–11], it is likely suppressed by locally expressed B7-H1. In relapsing EAE in SJL/J mice immunized with PLP139–151, B7-H1 blockade during clinical remission precipitated EAE relapses [38, 50]. However, the potency of blockade of PD-1 ligands in the CNS by systemic administration of antibodies and therefore, the site where encephalitogenic T cell responses may be inhibited at this time point remains unclear. Locally expressed PD-1 ligands in the CNS might regulate EAE by suppressing either acute phase and/or relapse-associated T cell function.
IL-17 and IFN-γ producing cells can be detected in the inflamed CNS at peak of acute EAE (Fig. 3) [13]. By using blocking monoclonal antibodies [21, 37, 38], we observed an increase in the frequency of both IFN-γ and IL-17 producing cells in pooled isolates from the inflamed CNS (Fig. 3). As B7-H1 is also expressed on T cells [21, 51], it is possible that this increase is a result of blockade of B7-H1 on microglia and CNS infiltrating APC, T cells, or both. In this context, recent in vitro studies indicated that loss of B7-H1 on T cells (and not on B cells) removes an inhibitory signal [45, 49]. In contrast, B7-DC has not been observed on T cells [24, 51]. IL-17 expression is generally thought to be restricted to T cells [55, 56], but other cells, e.g. natural killer (NK) cells can produce IFN-γ. NK cells also have been shown to express B7-H1 in vitro [57] and to infiltrate the CNS in EAE [58] therefore possibly contributing to increased numbers of IFN-γ positive cells in CNS ELISPOTS. However, the high frequency of T cells reactive to priming peptide (Fig. 3, pos. control with added peptide not shown), which mostly express PD-1 (Fig. 2), and the suppressive effect of PD-1 (Fig. 3) argue for an inhibitory role of B7-H1 on microglia and CNS-infiltrating APC down-regulating TH-1 (IFN-γ) and TH-17 (IL-17) T cell responses. Participation of B7-DC in down-regulation of acute-phase associated T cell functions seems to be less critical. A second receptor for PD-Ls has been postulated, after a number of studies had demonstrated that B7-H1 and B7-DC can stimulate T cell proliferation and cytokine production under certain conditions of in vitro stimulation [20, 29, 41, 43]. Although a stimulatory receptor for PD-Ls has yet to be identified, a recent study revealed B7-1 as binding partner for B7-H1. B7-1/B7-H1 interactions inhibited T cell proliferation and cytokine production [45]. Our finding that blockade of B7-H1 had a greater effect on the number of cytokine producing cells compared to PD-1, supports the notion that B7-H1 might act via a second (inhibitory) receptor.
The outcome of PD-L/PD-1 interactions on T stimulation might depend on the microenvironment and the type of APC that interacts with the T cell. Microglia and different CNS-infiltrating APC subsets are distinct in their ability to present myelin antigens and (re−) stimulate activated or naïve CD4+ T cells. Consistent with previous works from our laboratory [12, 13], macrophages and microglia were far less efficient than CNS DC, particularly mDC, in driving activation of myelin-reactive cells (Fig. 4 and 5). It has been reported that the down-regulatory effect of PD-Ls may be greatest on weakly activated CD4+ T cells [22, 28], and indeed the relative increase in T cell proliferation was slightly higher when B7-H1 was blocked in cultures with macrophages/microglia. However, our data indicate that in the inflamed CNS, the reduced ability of CNS macrophages and microglia to present myelin antigens is primarily not the result of active CD4+ T cell suppression via PD-Ls/PD-1, but alternatively may rather be due to a relative “lack” of available MHC class II:peptide complexes on the cell surface. CNS cells (mainly macrophages) have been shown to be producers of NO, which suppresses T cell proliferation [59]. In a study by Yamazaki et al. [42], anti-B7-H1 or anti–B7-DC monoclonal antibody increased IFN-γ and IL-2 production by T cells but, paradoxically, inhibited their proliferation when macrophages were used as APC. This inhibition was caused by the IFN-γ-dependent induction of NO production. We employed aminoguanidine to block NO production in our in vitro assays, but cannot exclude contribution of such indirect effects of PD-Ls in the CNS in vivo. Thus, it is interesting that in diabetes, PD-Ls on pancreatic β cells have been suggested to regulate autoreactive T cells, although bone marrow chimera/islet transplantation and Tg overexpression yielded discordant results [46, 48].
With regard to CNS mDC that present endogenously processed peptides [13], B7-H1 negatively regulated the stimulation of T cells, in particular activated T helper cells, that expressed the inhibitory PD-1 receptor. B7-H1 had mainly inhibitory function on IFN-γ secretion of T helper cells both in vitro (Fig. 4) and in CNS responses in vivo (Fig. 6). Increased IFN-γ production after B7-H1 blockade seems to be a common result reported in various experimental settings [37, 38, 46, 60, 61]. In our system, B7-DC seemed to be slightly stimulatory on mDC driving naïve CD4+ TH-17 and TH-1 activation (Fig. 5). Studies on B7-DC show conflicting results [28, 46], but several observations support a partial costimulatory function of B7-DC [29, 41, 62]. Our ex vivo data (Fig. 3, 4, 5) and also results of in vitro experiments analyzing the effect of PD-Ls on TH-1 or TH-17 driving (data not shown), do not demonstrate a strongly selective TH-1 or TH-17 skewing by either of the PD-Ls but rather similar down-regulation. In contrast, the preponderance of IFN-γ+ versus IL-17+ T cells in the CNS of B7-H1−/− mice (Fig. 6) suggests that B7-H1 more selectively suppresses TH-1 than TH-17 responses in vivo. Future, complementary experiments e.g. using bone marrow chimera from WT and B7-H1−/− animals should elucidate if this in vivo observation is specific to the CNS environment.
In summary our data support the notion that PD-Ls expressed in the CNS during the acute phase of R-EAE participate in local regulation of CNS inflammation. Overall, a suppressive effect of B7-H1 expressed by myeloid CNS APC on acute-phase associated T cell function seems to predominate. Our observations and the finding, that transfer of genetically modified DC over-expressing MOG peptide/MHC class II and B7-H1 suppressed EAE [63] support the idea that immunotherapies targeting PD-Ls could be beneficial for the treatment of EAE, or MS.
Materials and Methods
Mice
Female SJL/J mice were purchased from Harlan Sprague Dawley or Taconic. Female C57BL/6 mice were obtained from Jackson. SJL Thy1.1+ 5B6 PLP139–151-specific TCR transgenic (Tg) mice (139TCR) have been described elsewhere [12, 64]. B7-H1−/− and B7-DC −/− mice on the C57BL/6 background have been described previously [41, 65]. All mice were housed and cared for according to Northwestern University ACUC approved protocols.
Mixed Bone Marrow chimeras
Mice were irradiated with 2 doses of 350 rads, 4 hours apart, and reconstituted intravenously with a 1:1 mix of CD90.2+ SJL and CD90.1+ 139TCR bone marrow as described previously [12].
Antibodies
Directly conjugated antibodies specific for mouse antigens CD4 (RM4–5), CD11b (M1/70), CD11c (N418), CD45 (30-F11), CD45RB (C363.16A), CD62L (MEL-14), CD90.1 (HIS51), CD90.2 (53–2.1), B7-H1 (M1H5), B7-DC (TY25), PD-1 (J43) and mouse IFN-γ and IL-17 were purchased from BD Pharmingen or eBioscience. Biotinylated anti-I-As (clone MKS4) was detected with anti-biotin:PE (Miltenyi).
Peptides
PLP139–151 (HSLGKWLGHPDKF), OVA323–339 (ISQAVHAAHAEINEAGR), MOG35–55 (MEVGWYRSPFSRVVHLYRNGK) were synthesized by Genemed Synthesis, Inc. PLP178–191 (NTWTTCQSIAFPSK) was obtained from Peptides International.
Induction of EAE
Female SJL mice were immunized s.c. with 100 µl of emulsified incomplete Freund’s adjuvant (Difco) supplemented with 200 µg Mycobacterium tuberculosis H37Ra (Difco) and 100 µg of either PLP178–191, or 50 µg PLP139–151. Female or male C57BL/6 WT or PD-L−/− mice were immunized s.c. with 200 µg of PLP178–191 or 200 µg of MOG35–55/CFA, and received i.p. injections of 200 ng pertussis toxin (Sigma) at the time of immunization and 48 h later. Clinical disease was assessed as previously described [66].
Isolation of CNS-infiltrating leukocytes
CNS cells were isolated from perfused mice as described previously [12, 13].
Flow cytometric analysis and gating
Cells for analysis were blocked with 10% rat serum, 10% hamster serum and antiCD16/32 for at least 10 minutes on ice before staining with multicolor antibody cocktails. Data was acquired on an LSR II cytometer (BD), and analyzed using FACS DIVA (BD) or Flow Jo (Treestar Inc.) software.
CNS ELISPOT
ELISPOT assays were performed as reported previously [9]. CNS cells were isolated from perfused PLP178–191-primed SJL mice at the peak acute phase of R-EAE (day 14–15) and pooled (n=5–10). We plated 1×105 cells/well. Medium contained 1 mM aminoguanidine and 20 µM indomethacin to suppress nitric oxide synthetase and prostaglandin production. Functional grade purified blocking B7-DC, B7-H1, PD-1 or isotype control antibodies (10 µg/ml), but no peptides were added. CNS cells incubated with anti-CD3 were used as positive control.
CNS antigen presentation assay
Antibody stained CNS leukocytes resuspended in R10 media (RPMI with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 0.1 mM nonessential amino acids, 50 µM 2-mercaptoethanol, 1 mM aminoguanidine, and 20 µM indomethacin) were sorted on a MoFlo (DakoCytomation). Cell populations were gated as described in Fig. 1. Sorted populations were >95% pure. APC were co-cultured at a fixed 1:2 ratio with CD4+ T cells from a PLP139–151–specific line or CD62LhighCD4+CD90.1.1+ cells from 5B6 Tg mice (139TCR) cells for 96 h. T cell lines were generated as described previously [67]. Control Ig, anti mouse B7-DC or B7-H1 (10 µg/ml) were added to cultures. 16 h incorporation of [3H]-thymidine (1 µCi/well; ICN) was analyzed with a TopCount-NXT (Packard). Cytokines in the supernatants after 72 hours were assessed by cytokine bead array for levels of IL-17 and IFN-γ according to manufacturers instruction (Upstate).
CNS intracellular cytokine staining
In PLP178–191-induced EAE in C57BL/6 WT or PD-L−/− mice (d20/21), mice were perfused, and the CNS mononuclear cells isolated as described above. Cells were activated with 5 ng/ml PMA and 500 ng/ml ionomycin in the presence of golgi stop for 3 hours before staining for CD90.2 and intracellular IL-17 and IFN-γ.
Statistical analysis
Differences between groups were determined using the unpaired t-test.
Acknowledgments
This work was supported in part by U.S. Public Health Service, National Institutes of Health Grants NS-030871 and NS-026543, the National Multiple Sclerosis Society (RG 3793-A-7), and the Myelin Repair Foundation. SLB was supported by NMSS Postdoctoral Fellowship Grant FG 1563 A-1, and BS by a fellowship from the Deutsche Forschungsgemeinschaft.
Abbreviations
- MS
multiple sclerosis
- PD-1
programmed death receptor 1
- PD-L
programmed death ligand
- PLP
proteolipid protein
- R-EAE
relapsing experimental autoimmune encephalomyelitis
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–1971. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- 2.Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;1:893–895. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- 3.Zamvil S, Nelson P, Trotter J, Mitchell D, Knobler R, Fritz R, Steinman L. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. Nature. 1985;317:355–358. doi: 10.1038/317355a0. [DOI] [PubMed] [Google Scholar]
- 4.Renno T, Taupin V, Bourbonniere L, Verge G, Tran E, De Simone R, Krakowski M, et al. Interferon-gamma in progression to chronic demyelination and neurological deficit following acute EAE. Mol. Cell. Neurosci. 1998;12:376–389. doi: 10.1006/mcne.1998.0725. [DOI] [PubMed] [Google Scholar]
- 5.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J. Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 6.Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J. Clin. Invest. 2002;110:493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tompkins SM, Padilla J, Dal Canto MC, Ting JP, Van Kaer L, Miller SD. De novo central nervous system processing of myelin antigen is required for the initiation of experimental autoimmune encephalomyelitis. J. Immunol. 2002;168:4173–4183. doi: 10.4049/jimmunol.168.8.4173. [DOI] [PubMed] [Google Scholar]
- 10.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 11.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 12.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat. Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 13.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides 'preferentially' polarize CD4(+) T(H)-17 cells in relapsing EAE. Nat. Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 14.McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J. Exp. Med. 1995;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller SD, Vanderlugt CL, Lenschow DJ, Pope JG, Karandikar NJ, Dal Canto MC, Bluestone JA. Blockade of CD28/B7-1 interaction prevents epitope spreading and clinical relapses of murine EAE. Immunity. 1995;3:739–745. doi: 10.1016/1074-7613(95)90063-2. [DOI] [PubMed] [Google Scholar]
- 16.Vanderlugt CL, Eagar TN, Neville KL, Nikcevich KM, Bluestone JA, Miller SD. Pathologic role and temporal appearance of newly emerging autoepitopes in relapsing experimental autoimmune encephalomyelitis. J. Immunol. 2000;164:670–678. doi: 10.4049/jimmunol.164.2.670. [DOI] [PubMed] [Google Scholar]
- 17.Howard LM, Miga AJ, Vanderlugt CL, Dal Canto MC, Laman JD, Noelle RJ, Miller SD. Mechanisms of immunotherapeutic intervention by anti-CD40L (CD154) antibody in an animal model of multiple sclerosis. J. Clin. Invest. 1999;103:281–290. doi: 10.1172/JCI5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karandikar NJ, Eagar TN, Vanderlugt CL, Bluestone JA, Miller SD. CTLA-4 downregulates epitope spreading and mediates remission in autoimmune disease. J. Neuroimmunol. 2000;109:173–180. doi: 10.1016/s0165-5728(00)00322-2. [DOI] [PubMed] [Google Scholar]
- 19.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, et al. Expression of programmed death 1 ligands by murine T cells and APC. J. Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 22.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 23.Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J. Immunol. 2006;176:2238–2248. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- 24.Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur. J. Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 25.Petroff MG, Chen L, Phillips TA, Azzola D, Sedlmayr P, Hunt JS. B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol. Reprod. 2003;68:1496–1504. doi: 10.1095/biolreprod.102.010058. [DOI] [PubMed] [Google Scholar]
- 26.Schoop R, Wahl P, Le Hir M, Heemann U, Wang M, Wuthrich RP. Suppressed T-cell activation by IFN-gamma-induced expression of PD-L1 on renal tubular epithelial cells. Nephrol. Dial. Transplant. 2004;19:2713–2720. doi: 10.1093/ndt/gfh423. [DOI] [PubMed] [Google Scholar]
- 27.Nakazawa A, Dotan I, Brimnes J, Allez M, Shao L, Tsushima F, Azuma M, et al. The expression and function of costimulatory molecules B7H and B7-H1 on colonic epithelial cells. Gastroenterol. 2004;126:1347–1357. doi: 10.1053/j.gastro.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 29.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 31.Ishida M, Iwai Y, Tanaka Y, Okazaki T, Freeman GJ, Minato N, Honjo T. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1,in the cells of lymphohematopoietic tissues. Immunol. Lett. 2002;84:57–62. doi: 10.1016/s0165-2478(02)00142-6. [DOI] [PubMed] [Google Scholar]
- 32.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 33.Pentcheva-Hoang T, Chen L, Pardoll DM, Allison JP. Programmed death-1 concentration at the immunological synapse is determined by ligand affinity and availability. Proc. Natl. Acad. Sci. U. S. A. 2007;104:17765–17770. doi: 10.1073/pnas.0708767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 35.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 36.Lin SC, Yen JH, Tsai JJ, Tsai WC, Ou TT, Liu HW, Chen CJ. Association of a programmed death 1 gene polymorphism with the development of rheumatoid arthritis, but not systemic lupus erythematosus. Arthritis Rheum. 2004;50:770–775. doi: 10.1002/art.20040. [DOI] [PubMed] [Google Scholar]
- 37.Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, Yamazaki T, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salama AD, Chitnis T, Imitola J, Ansari MJ, Akiba H, Tushima F, Azuma M, et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J. Exp. Med. 2003;198:71–78. doi: 10.1084/jem.20022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter LL, Leach MW, Azoitei ML, Cui J, Pelker JW, Jussif J, Benoit S, et al. PD-1/PD-L1, but not PD-1/PD-L2, interactions regulate the severity of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2007;182:124–134. doi: 10.1016/j.jneuroim.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Shin T, Kennedy G, Gorski K, Tsuchiya H, Koseki H, Azuma M, Yagita H, et al. Cooperative B7-1/2 (CD80/CD86) and B7-DC costimulation of CD4+ T cells independent of the PD-1 receptor. J. Exp. Med. 2003;198:31–38. doi: 10.1084/jem.20030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin T, Yoshimura K, Shin T, Crafton EB, Tsuchiya H, Housseau F, Koseki H, et al. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J. Exp. Med. 2005;201:1531–1541. doi: 10.1084/jem.20050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamazaki T, Akiba H, Koyanagix A, Azuma M, Yagita H, Okumura K. Blockade of B7-H1 on macrophages suppresses CD4+ T cell proliferation by augmenting IFN-gamma-induced nitric oxide production. J. Immunol. 2005;175:1586–1592. doi: 10.4049/jimmunol.175.3.1586. [DOI] [PubMed] [Google Scholar]
- 43.Wang S, Bajorath J, Flies DB, Dong H, Honjo T, Chen L. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J. Exp. Med. 2003;197:1083–1091. doi: 10.1084/jem.20021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu X, Gao JX, Wen J, Yin L, Li O, Zuo T, Gajewski TF, et al. B7DC/PDL2 promotes tumor immunity by a PD-1-independent mechanism. J. Exp. Med. 2003;197:1721–1730. doi: 10.1084/jem.20022089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, Yagita H, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J. Exp. Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Subudhi SK, Zhou P, Yerian LM, Chin RK, Lo JC, Anders RA, Sun Y, et al. Local expression of B7-H1 promotes organ-specific autoimmunity and transplant rejection. J. Clin. Invest. 2004;113:694–700. doi: 10.1172/JCI19210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, et al. PD-L1-deficient mice show that PD-L1 on T cells, ntigen-presenting cells, and host tissues negatively regulates T cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu B, Guleria I, Khosroshahi A, Chitnis T, Imitola J, Azuma M, Yagita H, et al. Differential role of programmed death-ligand 1 and programmed death-ligand 2 in regulating the susceptibility and chronic progression of experimental autoimmune encephalomyelitis. J. Immunol. 2006;176:3480–3489. doi: 10.4049/jimmunol.176.6.3480. [DOI] [PubMed] [Google Scholar]
- 51.Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur. J. Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 52.Magnus T, Schreiner B, Korn T, Jack C, Guo H, Antel J, Ifergan I, et al. Microglial expression of the B7 family member B7 homolog 1 confers strong immune inhibition: implications for immune responses and autoimmunity in the CNS. J. Neurosci. 2005;25:2537–2546. doi: 10.1523/JNEUROSCI.4794-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ortler S, Leder C, Mittelbronn M, Zozulya AL, Knolle PA, Chen L, Kroner A, et al. B7-H1 restricts neuroantigen-specific T cell responses and confines inflammatory CNS damage: Implications for the lesion pathogenesis of multiple sclerosis. Eur. J. Immunol. 2008;38:1734–1744. doi: 10.1002/eji.200738071. [DOI] [PubMed] [Google Scholar]
- 54.Goldberg MV, Maris CH, Hipkiss EL, Flies AS, Zhen L, Tuder RM, Grosso JF, et al. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186–192. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J. Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 56.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saudemont A, Jouy N, Hetuin D, Quesnel B. NK cells that are activated by CXCL10 can kill dormant tumor cells that resist CTL-mediated lysis and can express B7-H1 that stimulates T cells. Blood. 2005;105:2428–2435. doi: 10.1182/blood-2004-09-3458. [DOI] [PubMed] [Google Scholar]
- 58.Huang D, Shi FD, Jung S, Pien GC, Wang J, Salazar-Mather TP, He TT, et al. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. 2006;20:896–905. doi: 10.1096/fj.05-5465com. [DOI] [PubMed] [Google Scholar]
- 59.Juedes AE, Ruddle NH. Resident and infiltrating central nervous system APCs regulate the emergence and resolution of experimental autoimmune ncephalomyelitis. J. Immunol. 2001;166:5168–5175. doi: 10.4049/jimmunol.166.8.5168. [DOI] [PubMed] [Google Scholar]
- 60.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 61.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J. Exp. Med. 2003;198:39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsumoto K, Inoue H, Nakano T, Tsuda M, Yoshiura Y, Fukuyama S, Tsushima F, et al. B7-DC regulates asthmatic response by an IFN-gamma-dependent mechanism. J. Immunol. 2004;172:2530–2541. doi: 10.4049/jimmunol.172.4.2530. [DOI] [PubMed] [Google Scholar]
- 63.Hirata S, Senju S, Matsuyoshi H, Fukuma D, Uemura Y, Nishimura Y. Prevention of experimental autoimmune encephalomyelitis by transfer of embryonic stem cell-derived dendritic cells expressing myelin oligodendrocyte glycoprotein peptide along with TRAIL or programmed death-1 ligand. J. Immunol. 2005;174:1888–1897. doi: 10.4049/jimmunol.174.4.1888. [DOI] [PubMed] [Google Scholar]
- 64.Waldner H, Whitters MJ, Sobel RA, Collins M, Kuchroo VK. Fulminant spontaneous autoimmunity of the central nervous system in mice transgenic for the myelin proteolipid protein-specific T cell receptor. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3412–3417. doi: 10.1073/pnas.97.7.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 66.Turley DM, Miller SD. Peripheral tolerance Induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J. Immunol. 2007;178:2212–2220. doi: 10.4049/jimmunol.178.4.2212. [DOI] [PubMed] [Google Scholar]
- 67.Katz-Levy Y, Neville KL, Padilla J, Rahbe SM, Begolka WS, Girvin AM, Olson JK, et al. Temporal development of autoreactive Th1 responses and endogenous antigen presentation of self myelin epitopes by CNS-resident APCs in Theiler's virus-infected mice. J. Immunol. 2000;165:5304–5314. doi: 10.4049/jimmunol.165.9.5304. [DOI] [PubMed] [Google Scholar]