Abstract
Restorative cell-based and pharmacological therapies for experimental stroke substantially improve functional outcome. These therapies target several types of parenchymal cells (including neural stem cells, cerebral endothelial cells, astrocytes, oligodendrocytes, and neurons), leading to enhancement of endogenous neurogenesis, angiogenesis, axonal sprouting, and synaptogenesis in the ischaemic brain. Interaction between these restorative events probably underpins the improvement in functional outcome. This Review provides examples of cell-based and pharmacological restorative treatments for stroke that stimulate brain plasticity and functional recovery. The molecular pathways activated by these therapies, which induce remodelling of the injured brain via angiogenesis, neurogenesis, and axonal and dendritic plasticity, are discussed. The ease of treating intact brain tissue to stimulate functional benefit in restorative therapy compared with treating injured brain tissue in neuroprotective therapy might more readily help with translation of restorative therapy from the laboratory to the clinic.
Introduction
Stroke is a major cause of morbidity and mortality worldwide. Thrombolytic therapy with alteplase is effective when given within 4·5 h after stroke.1,2 However, fewer than 5% of patients with ischaemic stroke in the USA receive this treatment. Even with effective thrombolysis, most patients will have neurological deficits.3,4 Therefore, development of therapies for ischaemic stroke designed specifically to reduce neurological deficits is crucial.
Preclinical data indicate that cell-based and pharmacological therapies that enhance brain-repair processes substantially improve functional recovery when given 24 h or later after stroke or brain injury.5-13 Cell-based therapies under investigation include use of bone-marrow mesenchymal cells, cord blood cells, fetal cells, and embryonic cells.12,14-20 Pharmacological treatments include drugs that increase cGMP (eg, phosphodiesterase 5 inhibitors, such as sildenafil and tadalafil), statins, erythropoietin, granulocyte-colony stimulating factor, nicotinic acid, and minocycline.6,7,10,21-25 The common restorative characteristic of these therapies is that they target many types of parenchymal cells (including neural stem cells, cerebral endothelial cells, astrocytes, oligodendrocytes, and neurons), leading to enhancement of endogenous neurogenesis, angio genesis, axonal sprouting, and synaptogenesis in ischaemic brain tissue. These events collectively improve neurological function after stroke. Furthermore, in addition to providing enhanced cerebral tissue perfusion, angiogenic vessels produce neurotrophic compounds, which create a suitable microenvironment within the injured brain that attracts endogenous stem cells and promotes integration of these cells within the parenchyma. Together with parenchymal astrocytes, angiogenic vessels contribute to enhancement of synaptogenesis and axonal sprouting.
In this Review, we describe the mechanisms by which cell-based and pharmacological treatments stimulate endogenous brain remodelling after stroke, particularly neurogenesis, angiogenesis, axonal plasticity, and white-matter change. We also briefly outline the potential of MRI to view these restorative events. Finally, we discuss the challenges of translating these therapies into the clinic and ongoing clinical trials.
Enhancement of neurogenesis
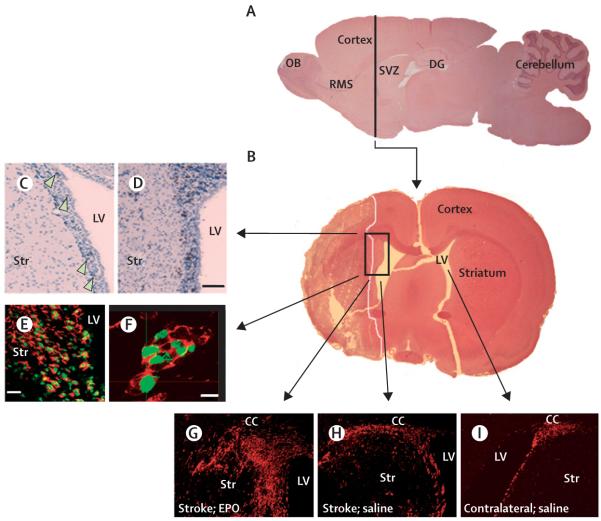
The subventricular zone (SVZ) of the lateral ventricle and the dentate gyrus of the hippocampus of adult rodent brains contain neural stem cells that produce neuroblasts.26,27 Under physiological conditions, neuroblasts in the SVZ travel via the rostral migratory stream to the olfactory bulb where they differentiate into granule and periglomerular neurons throughout adult life.27,28 In the SVZ of adult human brains, neural stem cells are present in a band of astrocytes separated from the ependyma.29-31 In experimental stroke, focal cerebral ischaemia increases neurogenesis in the ipsilateral SVZ (figure 1) and neuroblasts emigrate from the SVZ to the ischaemic boundary regions of the striatum and cortex where they have the phenotypes of mature neurons.32-37 Stroke-induced neurogenesis also takes place in the SVZ and ischaemic boundary of adult human brains, even in elderly patients aged 60-87 years.38-40
Figure 1. Neurogenesis in the SVZ of the lateral ventricle of adult rodent non-ischaemic and ischaemic brain tissue.
A and B show sagittal and coronal views, respectively, of rat brain tissue in the SVZ. SVZ cells in the non-ischaemic brain are proliferating, as shown by BrdU-positive cells (C, arrows, brown dots). After stroke, the number of these proliferating cells increased (D, brown). Confocal microscopic images (E and F) show that BrdU-positive cells (green) are positive for doublecortin (red), indicating that these are newly generated neuroblasts. G to I show doublecortin-positive cells (red) in the ischaemic (G and H) and non-ischaemic (I) hemispheres of rats treated with erythropoietin (G) and saline (H and I). Treatment of erythropoietin substantially increased doublecortin-positive cells in the SVZ and ischaemic striatum (G). Bars are 50 μm (D), 20 μm (E), and 10 μm (F). BrdU=5-bromo-2′-deoxyuridine. CC=corpus callosum. DG=dentate gyrus. EPO=erythropoietin. LV=lateral ventricle. OB=olfactory bulb. RMS=rostral migratory stream. Str=striatum. SVZ=subventricular zone.
Neurogenesis induced by stroke involves proliferation of neural stem and progenitor cells, differentiation of neural progenitor cells, and migration of neuroblasts to the ischaemic boundary where neuroblasts mature into resident neurons and integrate into the parenchymal tissue. In adult mice, gene-profile analysis of neural progenitor cells from the SVZ that were isolated by laser-capture microdissection has shown that these cells share more than 70% of all expressed genes with embryonic cortical neural progenitor cells.41 In murine neural progenitor cells from the SVZ, stroke activates many genes involved in neurogenesis during embryonic development.42 The most upregulated genes after stroke are those in the transforming growth factor β superfamily, such as bone morphogenetic protein 8, bone morphogenetic protein type I receptors, and growth differentiation factor 2.42 After stroke, adult neural progenitor cells seem to recapture embryonic molecular signals, which probably mediate neuroblast migration and stroke-induced proliferation and differentiation of neural progenitor cells.
In vivo analysis of the cytokinetics of neural progenitor cells has suggested that stroke might trigger actively proliferating neural progenitor cells from the SVZ in adult rodents to repeat the cell-cycle kinetics of the embryonic form of these cells.43 During cortical neurogenesis, cell-cycle length is associated with progression of neural progenitor cells from proliferation to neurogenic division, and lengthening of the G1 phase of the neuroepithelial cell cycle activates neuronal differentiation.44-47 In rats, studies done in vivo that used cumulative and single S-phase labelling with 5-bromo-2′-deoxyuridine (BrdU)48 showed that dynamic changes in cell-cycle kinetics of neural progenitor cells correlated with the proportion of daughter cells that remained within and left the cell cycle over a period of 2 to 14 days after stroke.49 Decreasing the length of the G1 phase of the cell cycle at 2 to 4 days after stroke was associated with an increase in dividing daughter cells that remained within the cell cycle to expand the SVZ progenitor pool rapidly. By contrast, lengthening the G1 phase at 4 to 14 days after stroke was accompanied by an increased number of daughter cells that left the cell cycle to differentiate into neurons.49 These data indicate that stroke triggers dynamic changes in the G1 phase of the actively dividing SVZ cell cycle, resulting in early expansion of a neural progenitor pool and subsequent neuronal differentiation, which leads to increased neurogenesis.49 Neuroblasts in the ischaemic boundary have the phenotypes of mature neurons;35,50 by use of the patch-clamp technique, new neurons in the ischaemic boundary were shown to have the electrophysiological characteristics of mature neurons. These finding suggest that neuroblasts mature into resident neurons and integrate into local neuronal circuitry.51 However, neurogenesis is diminished after stroke and many newly formed neurons die.35
Cell-based and pharmacological therapies increase neurogenesis in the ischaemic brain (figure 1). These therapies activate the phosphatidylinositol 3-kinase (PI3K)-Akt signalling pathway in neural progenitor cells.7,10 The PI3K-Akt pathway affects several cellular functions such as cell survival, proliferation, differentiation, and migration.52,53 Akt regulates proliferation of neural stem cells and neuronal differentiation in embryonic mice,53,54 and blockage of Akt activation with a selective PI3K inhibitor decreases proliferation of neural progenitor cells.55 Therefore, the PI3K-Akt signalling pathway seems to be important in the regulation of neuro genesis enhanced by restorative therapies. However, initiation of the PI3K-Akt signalling pathway could differ with individual therapies. Treatment with bone-marrow mesenchymal cells stimulates brain parenchymal cells to secrete an array of neurotrophic factors, including basic fibroblast growth factor and brain-derived neurotrophic factor, which are known to activate Akt.56,57 Erythropoietin activates the PI3K-Akt pathway by interaction with its receptor in neural progenitor cells, whereas phosphodiesterase 5 inhibitors and statins are thought to activate Akt via increased concentrations of cGMP.7,10,58,59
Mammalian achaete-scute homolog 1 (Mash1) and neurogenin 1 (Neurog1; also known as Ngn1) are pro-neuronal basic helix-loop-helix (bHLH) transcription factors that mediate differentiation of neural progenitor cells into neurons.60,61 Akt regulates the assembly and activity of bHLH-coactivator complexes to promote this differentiation.53 Inhibition of the PI3K-Akt pathway in neural progenitor cells suppresses expression of Mash1 and Ngn1. As a result, neuronal differentiation induced by erythropoietin and statins is prevented.62,63 Small interfering RNA in neural progenitor cells also attenuates expression of endogenous Mash1 and Ngn1, which further minimises the rise in the neuronal population caused by erythropoietin and statins.62,63 These findings indicate that the PI3K-Akt signalling pathway activated by these restorative therapies can trigger pro-neuronal bHLH transcription factors in neural progenitor cells, leading to neuronal, but not astrocytic, differentiation.10,62-66
Neurogenesis in the adult brain is associated with neurological function.67 Ionising radiation applied to the subgranular zone of the dentate gyrus where there are neural progenitor cells reduces neurogenesis and impairs functional recovery after global ischaemia.68 Neurogenesis enhanced by cell-based and pharmacological therapies might drive functional improvement during stroke recovery. A substantial improvement in neurological function and enhancement of neurogenesis has been observed even 1 year after stroke in animals treated with bone-marrow mesenchymal cells.69 However, currently, there are no data on the mechanisms of endogenous neurogenesis in functional recovery after stroke. In a recent genetic study in adult mice, conditional ablation of newly formed neurons in the olfactory bulb resulted in shrinkage of the olfactory bulb, and removal of new neurons in the dentate gyrus caused impairment of memory.51 This transgenic mouse line could provide insight into the direct effect of neurogenesis on functional outcome during stroke recovery.
Enhancement of cerebral angiogenesis
The cerebral vascular system mainly develops through angiogenesis.70 Although proliferation of cerebral endothelial cells ceases in the adult brain, angiogenesis in adult human and rodent brains can take place under pathophysiological conditions.71,72 In the rodent brain, capillary sprouting is initiated at the border of the infarct and new vessels develop in the ischaemic boundary between 2 and 28 days after the onset of stroke,73,74 whereas angiogenesis takes place in the penumbra of human ischaemic brains 3 to 4 days after stroke.72 Angiogenic vessels are permeable during the early stages of development and new vessels become less leaky as they mature.71,75 We have used this transient increase in vascular permeability as a signal to identify formation of new blood vessels.16 Vascular permeability can be quantified and detected with MRI T1 indices of brain-to-blood transfer constants of extrinsic-contrast agents, such as gadolinium DTPA (diethylene triamine pentaacetic acid), as well as intrinsic magnetisation-contrast techniques.16,76 Cerebral blood flow can be measured by perfusion-weighted MRI. With these MRI indices, we found that a transient increase in vascular permeability in the ischaemic boundary 2 to 3 weeks after stroke led to increased cerebral blood flow 6 weeks after stroke.16 Histological measurement of vascular density showed notable correlation between increased cerebral blood flow and rises in vascular density indicative of angiogenesis.16 These findings show that stroke induces new functional vessels in the ischaemic boundary and that angiogenesis can be monitored with MRI.
Angiogenesis is a multi-step process that involves endothelial-cell proliferation, migration, tube formation, branching, and anastomosis (figure 2).77,78 Vascular endothelial growth factor (VEGF) and VEGF receptor 2 (VEGFR2) initiate angiogenesis, and angiopoietins 1 and 2 and their receptor, Tie2, are involved in maturation, stabilisation, and remodelling of vessels.15,79 In rodent ischaemic brain tissue, upregulation of VEGF and VEGFR2 and of angiopoietins and Tie2 lasts for at least 28 days.80,81 Patients with stroke have high serum concentrations of VEGF 7 days after acute stroke, and these concentrations remain high for 14 days after stroke.82 The VEGF and VEGFR2 and the angiopoietin and Tie2 pathways mediate angiogenesis in the ischaemic boundary.71,74,83,84 VEGF and VEGFR2 upregulated by stroke promote cerebral-vessel sprouting to form new permeable vessels, whereas upregulation of angiopoietin 1 and Tie2 leads to maturation of the vessels to functional cerebral vessels.71,74,83,84 Treatment with VEGF 24 h after stroke enhances angiogenesis.71,85 In rodents, cell-based and pharmacological therapies increase angiogenesis in the ischaemic boundary by regulating expression of VEGF and VEGFR2, as well as angiopoietins 1 and 2 and Tie2.86-88 In preclinical studies, drugs such as recombinant human erythropoietin, statins, and phosphodiesterase 5 inhibitors increase concentrations of VEGF in the ischaemic boundary. In a tube-formation assay, blockage of VEGFR2 in endothelial cells suppressed angiogenesis promoted by these drugs.5,10,59,88,89 In mice, endothelial nitric oxide synthase mediated statin-induced angiogenesis.90 Bone-marrow mesenchymal cells stimulated parenchymal cells in rats to express VEGF, angiopoietin 1, and Tie2,86 leading to increased angiogenesis and maturation of newly formed vessels by reducing vascular permeability and increasing the expression of tight-junction proteins.88
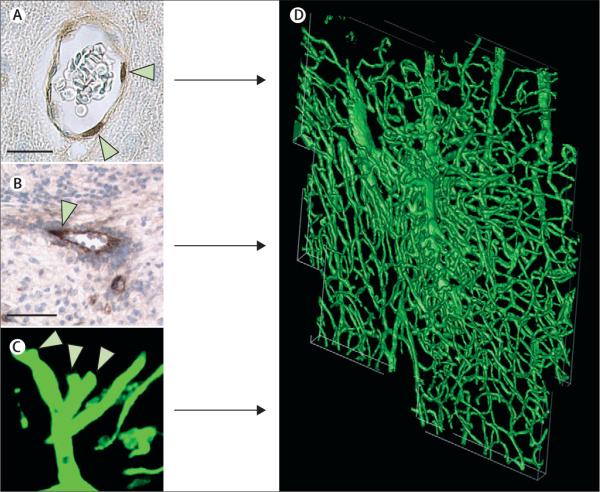
Figure 2. Stroke induces angiogenesis within the ischaemic boundary.
Immunostaining with antibodies against BrdU shows proliferative endothelial cells of cerebral blood vessels (A, arrowheads). Sprouting cerebral vessels are detected with immunoreactive von Willebrand factor (B, arrowhead) and shown on three-dimensional images obtained from confocal microscopy (C, arrowheads). Proliferating endothelial cells and sprouting vessels contribute to angiogenesis seen at the ischaemic boundary region (D). D is a three-dimensional image of angiogenesis at the cortical ischaemic boundary of a rat 14 days after stroke. Bars are 10 μm (A) and 50 μm (B). BrdU=5-bromo-2'-deoxyuridine.
The effect of cell-based and pharmacological therapies on angiogenesis has been non-invasively monitored with MRI indices, including susceptibility-weighted imaging and T2*-weighted imaging. These techniques use susceptibility differences in cerebral tissues and are sensitive to blood in cerebral veins because of blood oxygenation level dependent (BOLD) effects.16,91-96 Treatment of stroke with sildenafil or erythropoietin in rats substantially increases angiogenesis detected by susceptibility-weighted and T2*-weighted imaging in the ischaemic boundary, and enhanced angiogenesis lasts for at least 6 weeks after stroke (figure 3).94-96
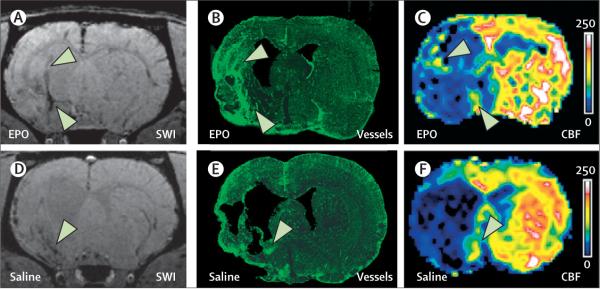
Figure 3. Cerebral angiogenesis identified with MRI and confocal images.
SWI of brain coronal sections shows dark areas at the ischaemic boundary (A and D, arrowheads), which match angiogenic areas detected with confocal microscopy (B and E, arrowheads). These areas show increased CBF measured with perfusion-weighted MRI (C and F, arrowheads). All images were acquired 6 weeks after stroke from rats treated with EPO (A to C) and saline (D to F). CBF=cerebral blood flow. EPO=erythropoietin. SWI=susceptibility-weighted imaging.
Angiogenesis is essential for ischaemic brain repair as this event stimulates blood flow and metabolism in the ischaemic boundary. In patients with stroke, there was a significant correlation between the number of cerebral blood vessels in the cortical rim and survival times.72,82,97 Patients who have a high density of blood vessels seem to survive longer than patients with low vascular density.72,82,97 In ischaemic brain tissue of animals treated with cell-based and pharmacological therapies, angiogenesis was increased, which was associated with improvements in functional outcome.5,10,59,88,89 These findings suggest that, in addition to neurogenesis, angiogenesis increased by these restorative therapies also improves functional recovery.
Coupling of neurogenesis and angiogenesis
Stroke induces angiogenesis and neurogenesis, two processes that are linked together.71,74,81,84,85,98-102 Cerebral blood vessels mainly provide nutritive blood flow. However, cerebral endothelial cells secrete factors that regulate the biological activity of neural progenitor cells. Under physiological conditions, neurogenesis in the subgranular zone of the dentate gyrus takes place within an angiogenic microenvironment.103 The laminin receptor α6β1 integrin expressed by neural stem and progenitor cells interacts with laminin-containing vessels in the SVZ of adult mice: blockage of this interaction increases the proliferation of neural stem cells and progenitor cells.104,105 After stroke, neuroblasts formed in the SVZ migrate to the ischaemic boundary where angiogenesis takes place and, during migration, these cells are closely associated with cerebral vessels.16,100,102 Suppression of angiogenesis either with endostatins or with a neutralising antibody against Tie2 substantially reduces migration of newly formed neuroblasts to the ischaemic region.100 Activated endothelial cells in angiogenic areas secrete many factors, among which are stromal-derived factor 1α and matrix metalloproteinases (MMPs).103,106,107 Stromal-derived factor 1α is a CXC chemokine that mediates neuroblast migration in the developing brain.107 In adult rodent brains, stromal-derived factor 1α released by activated endothelial cells in the ischaemic boundary attracts neuroblasts in the SVZ to the boundary by interacting with its receptor CXCR4 expressed in neurob lasts.37,100,108,109 Blocking CXCR4 inhibits stroke-induced neuroblast migration.37,109,110 Treatment with bone-marrow mesenchymal cells increases concentrations of stromal-derived factor 1α and promotes migration of neuroblasts to the ischaemic boundary.111-113
MMPs degrade the extracellular matrix, which enables cells to penetrate the extracellular matrix.114,115 MMP2 and MMP9 facilitate neuroblast migration to the ischaemic boundary.114,115 In vitro, erythropoietin stimulates cerebral endothelial cells to secrete active forms of MMP2 and MMP9.114 Co-culture of cerebral endothelial cells activated by erythropoietin with neural progenitor cells promotes neuroblast migration, and MMP2 and MMP9 mediate cell motility.114 These data indicate that MMPs regulate the relation between erythropoietin-enhanced angiogenesis and neurogenesis.10,114
In addition to guiding neuroblast migration, activated endothelial cells secrete VEGF to increase neurogenesis.101 Co-culture of cerebral endothelial cells from the ischaemic boundary with neural progenitor cells from the nonischaemic SVZ substantially increases the number of neurons.101 Blockage of VEGFR2 with a VEGFR2 antagonist suppresses the effect of endothelial cells on neurogenesis.101 VEGF is an angiogenic and a neurogenic growth factor.116 Intraventricular infusion of VEGF increases neurogenesis in the SVZ and dentate gyrus of adult mice.85 Therefore, VEGF and VEGFR2 could be common factors involved in the promotion of angiogenesis and neurogenesis.
Neural progenitor cells also enhance angiogenesis.89,101 In a microarray analysis of neural progenitor cells isolated by laser-capture microdissection, ischaemic neural progenitor cells in the SVZ expressed several angiogenic factors, including angiopoietin 2, VEGFR2, and fibroblast growth factor.42 These neural progenitor cells promote angiogenesis in vitro, as measured by a capillary-like tube-formation assay.89,101 Transplantation of neural progenitor cells into ischaemic brains also promoted angiogenesis.117
Collectively, these data provide insight into the molecular mechanisms that underlie the coupling of angiogenesis and neurogenesis enhanced by cell-based and pharmacological therapies. These findings suggest that, in vivo, neurogenesis and angiogenesis are highly interdependent and work together to promote brain remodelling and subsequent improvement of neurological function after stroke.
Effects on astrocytes, oligodendrocytes, and axons
Axons in ischaemic brains have little capability to sprout.118 Astrocytes form glial scars along ischaemic lesions and produce proteoglycans that inhibit axonal growth and that act as physical and biochemical barriers to axonal regeneration.119 In experimental stroke, treatment with bone-marrow mesenchymal cells substantially increases axonal density around the ischaemic lesion, extends axonal fibres, and orients these fibres parallel to the boundary of a coronal section of an ischaemic lesion.120,121 The increased axonal density is maintained for at least 1 year after stroke.69 Bone-marrow mesenchymal cells substantially reduce expression of axonal-growth inhibitory proteins, such as reticulon (Rtn4; also known as Nogo), enabling axonal and neurite outgrowth.121,122 Real-time RT-PCR analysis of astrocytes isolated by laser-capture microdissection showed that transplantation of bone-marrow mesenchymal cells notably downregulated neurocan (Ncan), a proteoglycan that inhibits axonal growth.122 Co-culture of bone-marrow mesenchymal cells with astrocytes also substantially reduced expression of Ncan in astrocytes activated by deprivation of oxygen-glucose.122 These findings suggest that, in addition to induction of many growth factors within astrocytes, bone-marrow mesenchymal cells suppress inhibitory genes for axonal regeneration, which could contribute to facilitation of axonal remodelling. Suppression of inhibitory proteoglycans by cell-based therapies also leads to neurite outgrowth and axonal remodelling in the spinal cord and ipsilateral and contralateral hemispheres, which significantly correlate with improved functional outcome after stroke.123
Mature oligodendrocytes form myelin sheaths for sprouting axons in ischaemic brain tissue.124-126 These oligodendrocytes are derived from non-myelinating oligodendrocyte progenitor cells that are present in the corpus callosum, striatum, and SVZ of adult rodent brains.125-127 Transplantation of bone-marrow mesenchymal cells substantially increased the number of oligodendrocyte progenitor cells in these areas of the ischaemic hemisphere and the number of mature oligodendrocytes in the ischaemic boundary adjacent to myelinated axons.120 Treatment of stroke with erythropoietin or sildenafil also notably enhanced myelinated axons adjacent to the ischaemic boundary.128,129 Therefore, cell-based and pharmacological therapies might promote generation of oligodendrocyte progenitor cells in the ischaemic brain, which migrate to target axons where they extend their processes and myelinate axons. In addition to erythropoietin, statins promote neurite outgrowth in vitro and increase synaptogenesis around the ischaemic boundary.7,10
Diffusion-tensor imaging enables delineation of the anatomical connectivity of white-matter pathways. Water in white matter moves more easily in the direction parallel to the tract than perpendicular to it.130-132 This diffusional directionality is known as fractional anisotropy,130-132 and can be used to detect changes in white-matter structure in the ischaemic brain. Fractional anisotropy is directly correlated with histological markers of myelination (figure 4).128 Diffusion-tensor imaging measurements have shown that treatment of stroke with sildenafil or erythropoietin substantially increases fractional anisotropy measurements around the ischaemic boundary starting 2 weeks after stroke; these increases lasted for at least 6 weeks after stroke.129,133 Histological analysis verified that axons in areas with high fractional anisotropy measurements were myelinated.129,133 Angiogenesis detected with T2*-weighted imaging was shown to take place 1 week earlier than increased fractional anisotropy measurements, suggesting that angiogenesis is closely associated with axonal remodelling.129,133
Figure 4. Diffusion tensor imaging measurements of FA and fibre tracking.
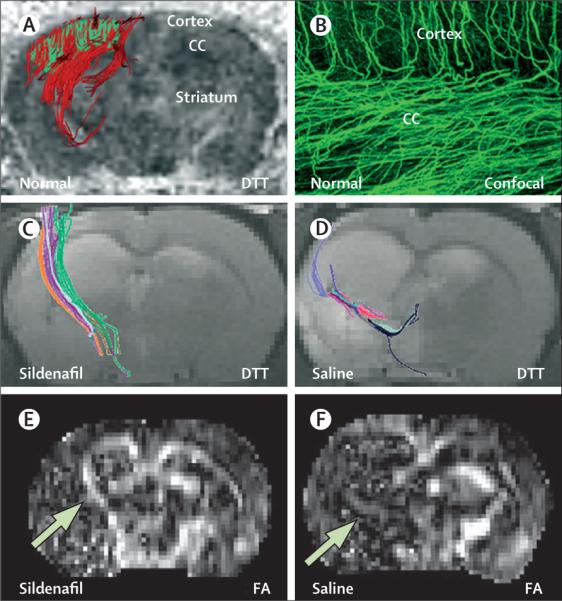
(A) A three-dimensional DTT image shows tracking of axonal projections (red) in a selected area of the CC and cortex (green). (B) A confocal image shows similar patterns of axonal projections in the same area. Treatment of stroke with sildenafil increases axonal projections (C) and FA concentrations (E, arrow) at the ischaemic boundary compared with animals treated with saline (D and F, arrow). CC=corpus callosum. DTT=diffusion tensor tractography. FA=fractional anisotropy.
Translation to the clinic
Apart from treatment with alteplase, translation of therapies for stroke to the clinic from those in the laboratory has not been successful.134 These attempts all aimed to develop neuroprotective treatments of stroke with early intervention to reduce the volume of cerebral infarction. Reasons for failure include the short time window required to intervene to salvage cerebral tissue. Many of the drugs tested in the laboratory were given immediately after or within the first hours after onset of stroke. Translation to the clinic frequently involved extending the therapeutic window to 6 h or longer after stroke: times at which animals showed no benefit. Doses used in animals often could not be used in human beings because of adverse effects.134 Furthermore, without adequate tissue perfusion, neuroprotective drugs cannot target the compromised tissue.
The essential difference between neuroprotective and neurorestorative treatments is that the former treat the lesion and the latter, whether they are cell-based or pharmacological therapies, treat the intact tissue.135,136 The therapeutic window and treatment protocols will thus be very different. Restorative therapies are effective when initiated 1 month after stroke onset111 and cerebral perfusion is not problematic because the therapeutic target is cerebral tissue with normal perfusion. Restorative treatments are expected to reduce some of the impediments to the translation of laboratory-proven therapies to patients. However, restorative treatments have their own sets of complicating factors. The treatments must be clearly proven to be safe in patients; this is particularly challenging for cell-based therapies. A further complication is that patients who have had stroke are commonly not in a controlled environment. Moreover, patients often have various types and conditions of rehabilitation and home and social environments, which can affect functional response.135 The interactions between restorative interventions and different environments, comorbidities, and rehabilitation strategies must be taken into account. More extensive and specific neurological outcome measures, beyond the National Institutes of Health stroke scale, Barthel index, and European stroke scale, need to be developed and implemented for restorative treatments.137 Recommendations and guidelines for translation of laboratory stroke studies with stem cells to patients have been published after the Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS) conference.138
MRI could have an important role in the management of patients with stroke who receive neurorestorative treatment. The focus of MRI should not be on the ischaemic lesion volume and cerebral oedema, but on the restructuring of white matter, angiogenesis, and, possibly, neurogenesis and synaptic activity. These changes form the biological basis of functional improvement and can be non-invasively monitored with MRI and magnetic resonance spectroscopy, which could be used to monitor response to treatment and, possibly, to predict therapeutic response.
Clinical trials
Approaches to enhance recovery of function after stroke in the laboratory and in clinical trials extend beyond the use of drugs and cell-based treatments and include electromagnetic stimulation, device-based strategies, repetitive training, and task-oriented strategies.136 The recent Extremity Constraint Induced Therapy Evaluation (EXCITE) trial reported significantly positive results for distal and proximal arm motor function in response to constraint-induced therapy.139,140 Here, we focus on cell-based and pharmacological approaches and how these approaches change brain structure and neural plasticity to promote functional recovery; few such restorative therapies tested in the laboratory have moved to clinical trials (table).141-145 Patients with ischaemic stroke treated with autologous bone-marrow mesenchymal cells had no adverse effects and showed functional improvement.143 A dose-tiered phase I safety trial of sildenafil in patients with stroke is in progress,145 with patients receiving treatment 3-7 days after stroke. In a case of compassionate use, sildenafil caused notable recovery in a patient with locked-in syndrome.146 Plasticity of human and animal brains is increased after stroke, with clear induction of angiogenesis and neurogenesis. The available preclinical data show functional improvement through brain remodelling. Many of the therapies under consideration for restorative treatment of stoke are in clinical use for other indications; therefore, assuring the safety of these compounds for patients with stroke might not be difficult. Because most patients with stroke could be treated with restorative therapy, and the clinical need to promote recovery in patients with stroke is great, efforts to translate laboratory studies into the clinic safely and quickly are needed.
Table.
Clinical studies of cell-based and pharmacological restorative therapies
| Patients (n) | Interventions | Results | |
|---|---|---|---|
| Phase I | 12 | NT2N cells; parenchymal implantation | No cell-associated adverse effects 12-18 months after cell transplantation141 |
| Phase II | 18 | NT2N cells; parenchymal implantation | Safety and feasibility of neuron transplantation but no evidence of a substantial benefit on motor function142 |
| Phase I/II | 30 | Autologous bone-marrow mesenchymal cells; intravenous injection | No adverse effects and functional improvement seen 1 year after cell transplantation143 |
| Pilot | 36 | Granulocyte-colony stimulating factor 1-10 μg/kg for one or five doses; subcutaneous injection | Safety and feasibility 90 days after treatment144 |
| Phase I | Ongoing | Sildenafil 150 mg; oral treatment | Not yet available145 |
NT2N cells=Ntera2/D1 neuron-like cells.
Conclusions
The cell-based and pharmacological therapies described in this Review target multiple types of parenchymal cells in ischaemic brain tissue to increase neurogenesis, angiogenesis, and axonal outgrowth during recovery. Potential mechanisms underlying these beneficial therapies are emerging. Future studies must investigate mechanisms that temporally and spatially coordinate these events.
Brain remodelling after stroke and subsequent improvement of functional outcome probably result from several restorative events that are enhanced by restorative therapies. Induction of angiogenesis couples with and promotes neurogenesis and neuroblast migration to the lesion. These interlinked remodelling events could create a microenvironment within the injured brain through their interaction with astrocytes and oligodendrocytes, which then promote neurite outgrowth and plasticity within the brain and spinal cord. These restorative events enhanced by restorative cell-based and pharmacological therapies lead to improved functional outcome.
One main difference between cell-based and pharmacological treatments is that transplanted cells actively interact with parenchymal cells depending on their microenvironment, whereas drugs interact with brain cells depending on their pharmacokinetic profiles. Understanding the mechanisms underlying the beneficial effects of these therapies will greatly enhance translation of these treatments to clinical use.
Search strategy and selection criteria.
References for this Review were identified through searches of PubMed with the search terms “cell-based and pharmacological therapies”, “experimental stroke”, “restorative therapies”, “neurogenesis”, “angiogenesis”, “MRI”, from January, 1975, to January, 2009. Papers for cell-based and pharmacological therapies were only included if treatments were initiated 24 h or longer after stroke. Only papers published in English were reviewed.
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke grants PO1 NS42345, P01 NS23392, and RO1 HL64766.
Footnotes
Conflicts of interest We have no conflicts of interest.
References
- 1.NINDS Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–87. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 3.Kawamata T, Speliotes EK, Finklestein SP. The role of polypeptide growth factors in recovery from stroke. In: Freund H-J, Sabel BA, Witte OW, editors. Brain Plasticity. Lippincott-Raven; Philadephia: 1997. pp. 377–82. [PubMed] [Google Scholar]
- 4.Cramer SC, Chopp M. Recovery recapitulates ontogeny. Trends Neurosci. 2000;23:265–71. doi: 10.1016/s0166-2236(00)01562-9. [DOI] [PubMed] [Google Scholar]
- 5.Zhang R, Wang L, Zhang L, et al. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92:308–13. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- 6.Zhang R, Wang Y, Zhang L, et al. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33:2675–80. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Zhang ZG, Li Y, et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–51. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 8.Lu D, Goussev A, Chen J, et al. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J Neurotrauma. 2004;21:21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- 9.Shyu WC, Lin SZ, Yang HI, et al. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004;110:1847–54. doi: 10.1161/01.CIR.0000142616.07367.66. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–37. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 11.Jin K, Sun Y, Xie L, Childs J, Mao XO, Greenberg DA. Post-ischemic administration of heparin-binding epidermal growth factor-like growth factor (HB-EGF) reduces infarct size and modifies neurogenesis after focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 2004;24:399–408. doi: 10.1097/00004647-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–38. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willing AE, Vendrame M, Mallery J, et al. Mobilized peripheral blood cells administered intravenously produce functional recovery in stroke. Cell Transplant. 2003;12:449–54. doi: 10.3727/000000003108746885. [DOI] [PubMed] [Google Scholar]
- 14.Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Sanberg PR, Li Y, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–88. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Q, Zhang ZG, Ding GL, et al. Investigation of neural progenitor cell induced angiogenesis after embolic stroke in rat using MRI. Neuroimage. 2005;28:698–707. doi: 10.1016/j.neuroimage.2005.06.063. [DOI] [PubMed] [Google Scholar]
- 17.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders—how to make it work. Nat Med. 2004;10:S42–50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 18.Zhang RL, Zhang ZG, Chopp M. Neurogenesis in the adult ischemic brain: generation, migration, survival, and restorative therapy. Neuroscientist. 2005;11:408–16. doi: 10.1177/1073858405278865. [DOI] [PubMed] [Google Scholar]
- 19.Borlongan CV, Lind JG, Dillon-Carter O, et al. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain Res. 2004;1010:108–16. doi: 10.1016/j.brainres.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 20.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25:2896–902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 21.Zhang R, Zhang L, Zhang Z, et al. A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann Neurol. 2001;50:602–11. doi: 10.1002/ana.1249. [DOI] [PubMed] [Google Scholar]
- 22.Hossmann KA, Buschmann IR. Granulocyte-macrophage colony-stimulating factor as an arteriogenic factor in the treatment of ischaemic stroke. Expert Opin Biol Ther. 2005;5:1547–56. doi: 10.1517/14712598.5.12.1547. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Zhang Z, Zhang RL, et al. Tadalafil, a long-acting type 5 phosphodiesterase isoenzyme inhibitor, improves neurological functional recovery in a rat model of embolic stroke. Brain Res. 2006;1118:192–98. doi: 10.1016/j.brainres.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Cui X, Zacharek A, et al. Niaspan increases angiogenesis and improves functional recovery after stroke. Ann Neurol. 2007;62:49–58. doi: 10.1002/ana.21160. [DOI] [PubMed] [Google Scholar]
- 25.Zhao LR, Berra HH, Duan WM, et al. Beneficial effects of hematopoietic growth factor therapy in chronic ischemic stroke in rats. Stroke. 2007;38:2804–11. doi: 10.1161/STROKEAHA.107.486217. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–34. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–61. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luskin MB, Zigova T, Soteres BJ, Stewart RR. Neuronal progenitor cells derived from the anterior subventricular zone of the neonatal rat forebrain continue to proliferate in vitro and express a neuronal phenotype. Mol Cell Neurosci. 1997;8:351–66. doi: 10.1006/mcne.1996.0592. [DOI] [PubMed] [Google Scholar]
- 29.Curtis MA, Kam M, Nannmark U, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–49. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 30.Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494:415–34. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- 31.Sanai N, Tramontin AD, Quinones-Hinojosa A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–44. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 32.Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 33.Jin K, Minami M, Lan JQ, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–15. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–13. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 35.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 36.Zhang R, Zhang Z, Wang L, et al. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–48. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Thored P, Arvidsson A, Cacci E, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–47. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- 38.Jin K, Wang X, Xie L, et al. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci USA. 2006;103:13198–202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macas J, Nern C, Plate KH, Momma S. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J Neurosci. 2006;26:13114–19. doi: 10.1523/JNEUROSCI.4667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minger SL, Ekonomou A, Carta EM, Chinoy A, Perry RH, Ballard CG. Endogenous neurogenesis in the human brain following cerebral infarction. Regen Med. 2007;2:69–74. doi: 10.2217/17460751.2.1.69. [DOI] [PubMed] [Google Scholar]
- 41.Abramova N, Charniga C, Goderie SK, Temple S. Stage-specific changes in gene expression in acutely isolated mouse CNS progenitor cells. Dev Biol. 2005;283:269–81. doi: 10.1016/j.ydbio.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 42.Liu XS, Zhang ZG, Zhang RL, et al. Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells. J Cereb Blood Flow Metab. 2007;27:564–74. doi: 10.1038/sj.jcbfm.9600371. [DOI] [PubMed] [Google Scholar]
- 43.Zhang RL, Zhang ZG, Chopp M. Ischemic stroke and neurogenesis in the subventricular zone. Neuropharmacology. 2008;55:345–52. doi: 10.1016/j.neuropharm.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–88. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 45.Calegari F, Haubensak W, Haffner C, Huttner WB. Selective lengthening of the cell cycle in the neurogenic subpopulation of neural progenitor cells during mouse brain development. J Neurosci. 2005;25:6533–38. doi: 10.1523/JNEUROSCI.0778-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huttner WB, Kosodo Y. Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr Opin Cell Biol. 2005;17:648–57. doi: 10.1016/j.ceb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi T, Nowakowski RS, Caviness VS. The leaving or Q fraction of the murine cerebral proliferative epithelium: a general model of neocortical neuronogenesis. J Neurosci. 1996;16:6183–96. doi: 10.1523/JNEUROSCI.16-19-06183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nowakowski RS, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol. 1989;18:311–18. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- 49.Zhang RL, Zhang ZG, Roberts C, et al. Lengthening the G phase of neural progenitor cells is concurrent with an increase of symmetric neuron generating division after stroke. J Cereb Blood Flow Metab. 2008;28:602–11. doi: 10.1038/sj.jcbfm.9600556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamashita T, Ninomiya M, Hernandez Acosta P, et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–36. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hou SW, Wang YQ, Xu M, et al. Functional integration of newly generated neurons into striatum after cerebral ischemia in the adult rat brain. Stroke. 2008;39:2837–44. doi: 10.1161/STROKEAHA.107.510982. [DOI] [PubMed] [Google Scholar]
- 52.Katakowski M, Zhang ZG, Chen J, et al. Phosphoinositide 3-kinase promotes adult subventricular neuroblast migration after stroke. J Neurosci Res. 2003;74:494–501. doi: 10.1002/jnr.10775. [DOI] [PubMed] [Google Scholar]
- 53.Vojtek AB, Taylor J, DeRuiter SL, et al. Akt regulates basic helix-loop-helix transcription factor-coactivator complex formation and activity during neuronal differentiation. Mol Cell Biol. 2003;23:4417–27. doi: 10.1128/MCB.23.13.4417-4427.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sinor AD, Lillien L. Akt-1 expression level regulates CNS precursors. J Neurosci. 2004;24:8531–41. doi: 10.1523/JNEUROSCI.1470-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang R, Zhang Z, Tsang W, Wang L, Chopp M. Down-regulation of p27kip1 increases proliferation of progenitor cells in adult rats. Neuroreport. 2004;15:1797–800. doi: 10.1097/01.wnr.0000135693.81613.cc. [DOI] [PubMed] [Google Scholar]
- 56.Chaudhary LR, Hruska KA. The cell survival signal Akt is differentially activated by PDGF-BB, EGF, and FGF-2 in osteoblastic cells. J Cell Biochem. 2001;81:304–11. [PubMed] [Google Scholar]
- 57.Alessi DR, Andjelkovic M, Caudwell B, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. Embo J. 1996;15:6541–51. [PMC free article] [PubMed] [Google Scholar]
- 58.Wang L, Gang Zhang Z, Lan Zhang R, Chopp M. Activation of the PI3-K/Akt pathway mediates cGMP enhanced-neurogenesis in the adult progenitor cells derived from the subventricular zone. J Cereb Blood Flow Metab. 2005;25:1150–58. doi: 10.1038/sj.jcbfm.9600112. [DOI] [PubMed] [Google Scholar]
- 59.Chen J, Zhang C, Jiang H, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–90. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kageyama R, Ohtsuka T, Hatakeyama J, Ohsawa R. Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res. 2005;306:343–48. doi: 10.1016/j.yexcr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 61.Parras CM, Galli R, Britz O, et al. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. Embo J. 2004;23:4495–505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang L, Zhang ZG, Zhang RL, et al. Neurogenin 1 mediates erythropoietin enhanced differentiation of adult neural progenitor cells. J Cereb Blood Flow Metab. 2006;26:556–64. doi: 10.1038/sj.jcbfm.9600215. [DOI] [PubMed] [Google Scholar]
- 63.Wang L, Zhang ZG, Gregg SR, et al. The Sonic hedgehog pathway mediates carbamylated erythropoietin-enhanced proliferation and differentiation of adult neural progenitor cells. J Biol Chem. 2007;282:32462–70. doi: 10.1074/jbc.M706880200. [DOI] [PubMed] [Google Scholar]
- 64.Wang L, Zhang Z, Zhang R, et al. Erythropoietin up-regulates SOCS2 in neuronal progenitor cells derived from SVZ of adult rat. Neuroreport. 2004;15:1225–29. doi: 10.1097/01.wnr.0000127636.15181.c1. [DOI] [PubMed] [Google Scholar]
- 65.Iwai M, Cao G, Yin W, Stetler RA, Liu J, Chen J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38:2795–803. doi: 10.1161/STROKEAHA.107.483008. [DOI] [PubMed] [Google Scholar]
- 66.Gonzalez FF, McQuillen P, Mu D, et al. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev Neurosci. 2007;29:321–30. doi: 10.1159/000105473. [DOI] [PubMed] [Google Scholar]
- 67.Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: a possible role in learning. Trends Cogn Sci. 1999;3:186–92. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- 68.Raber J, Fan Y, Matsumori Y, et al. Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Ann Neurol. 2004;55:381–89. doi: 10.1002/ana.10853. [DOI] [PubMed] [Google Scholar]
- 69.Shen LH, Li Y, Chen J, et al. One-year follow-up after bone marrow stromal cell treatment in middle-aged female rats with stroke. Stroke. 2007;38:2150–56. doi: 10.1161/STROKEAHA.106.481218. [DOI] [PubMed] [Google Scholar]
- 70.Risau W. Development and differentiation of endothelium. Kidney Int Suppl. 1998;67:S3–6. doi: 10.1046/j.1523-1755.1998.06701.x. [DOI] [PubMed] [Google Scholar]
- 71.Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–38. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–98. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 73.Garcia J, Cox J, Hudgins W. Ultrastructure of the microvasculature in experimental cerebral infarction. Acta Neuropathol. 1971;18:273–85. doi: 10.1007/BF00688441. [DOI] [PubMed] [Google Scholar]
- 74.Zhang ZG, Zhang L, Tsang W, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–92. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 75.Plate KH. Mechanisms of angiogenesis in the brain. J Neuropathol Exp Neurol. 1999;58:313–20. doi: 10.1097/00005072-199904000-00001. [DOI] [PubMed] [Google Scholar]
- 76.Li L, Jiang Q, Zhang L, et al. Ischemic cerebral tissue response to subventricular zone cell transplantation measured by iterative self-organizing data analysis technique algorithm. J Cereb Blood Flow Metab. 2006;26:1366–77. doi: 10.1038/sj.jcbfm.9600288. [DOI] [PubMed] [Google Scholar]
- 77.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–74. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 78.Carmeliet P. VEGF gene therapy: stimulating angiogenesis or angioma-genesis? Nat Med. 2000;6:1102–03. doi: 10.1038/80430. [DOI] [PubMed] [Google Scholar]
- 79.Yancopoulos GD, Klagsbrun M, Folkman J. Vasculogenesis, angiogenesis, and growth factors: ephrins enter the fray at the border. Cell. 1998;93:661–64. doi: 10.1016/s0092-8674(00)81426-9. [DOI] [PubMed] [Google Scholar]
- 80.Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23:166–80. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Z, Chopp M. Vascular endothelial growth factor and angiopoietins in focal cerebral ischemia. Trends Cardiovasc Med. 2002;12:62–66. doi: 10.1016/s1050-1738(01)00149-9. [DOI] [PubMed] [Google Scholar]
- 82.Slevin M, Krupinski J, Slowik A, Kumar P, Szczudlik A, Gaffney J. Serial measurement of vascular endothelial growth factor and transforming growth factor-beta1 in serum of patients with acute ischemic stroke. Stroke. 2000;31:1863–70. doi: 10.1161/01.str.31.8.1863. [DOI] [PubMed] [Google Scholar]
- 83.Beck H, Acker T, Wiessner C, Allegrini PR, Plate KH. Expression of angiopoietin-1, angiopoietin-2, and tie receptors after middle cerebral artery occlusion in the rat. Am J Pathol. 2000;157:1473–83. doi: 10.1016/S0002-9440(10)64786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin TN, Wang CK, Cheung WM, Hsu CY. Induction of angiopoietin and Tie receptor mRNA expression after cerebral ischemiareperfusion. J Cereb Blood Flow Metab. 2000;20:387–95. doi: 10.1097/00004647-200002000-00021. [DOI] [PubMed] [Google Scholar]
- 85.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–50. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen J, Zhang ZG, Li Y, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–99. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 87.Chen J, Li Y, Zhang R, et al. Combination therapy of stroke in rats with a nitric oxide donor and human bone marrow stromal cells enhances angiogenesis and neurogenesis. Brain Res. 2004;1005:21–28. doi: 10.1016/j.brainres.2003.11.080. [DOI] [PubMed] [Google Scholar]
- 88.Zacharek A, Chen J, Cui X, et al. Angiopoietin1/Tie2 and VEGF/ Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007;27:1684–91. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang L, Chopp M, Gregg SR, et al. Neural progenitor cells treated with EPO induce angiogenesis through the production of VEGF. J Cereb Blood Flow Metab. 2008;28:1361–68. doi: 10.1038/jcbfm.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen J, Zacharek A, Zhang C, et al. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005;25:2366–75. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reichenbach JR, Venkatesan R, Schillinger DJ, Kido DK, Haacke EM. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology. 1997;204:272–77. doi: 10.1148/radiology.204.1.9205259. [DOI] [PubMed] [Google Scholar]
- 92.Fazekas F, Kleinert R, Roob G, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999;20:637–42. [PMC free article] [PubMed] [Google Scholar]
- 93.Arnould MC, Grandin CB, Peeters A, Cosnard G, Duprez TP. Comparison of CT and three MR sequences for detecting and categorizing early (48 hours) hemorrhagic transformation in hyperacute ischemic stroke. AJNR Am J Neuroradiol. 2004;25:939–44. [PMC free article] [PubMed] [Google Scholar]
- 94.Li L, Jiang Q, Zhang L, et al. Angiogenesis and improved cerebral blood flow in the ischemic boundary area detected by MRI after administration of sildenafil to rats with embolic stroke. Brain Res. 2007;1132:185–92. doi: 10.1016/j.brainres.2006.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ding G, Jiang Q, Li L, et al. Magnetic resonance imaging investigation of axonal remodeling and angiogenesis after embolic stroke in sildenafil-treated rats. J Cereb Blood Flow Metab. 2008;28:1440–48. doi: 10.1038/jcbfm.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ding G, Jiang Q, Li L, et al. Angiogenesis detected after embolic stroke in rat brain using magnetic resonance T2*WI. Stroke. 2008;39:1563–68. doi: 10.1161/STROKEAHA.107.502146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krupinski J, Kaluza J, Kumar P, Wang M, Kumar S. Prognostic value of blood vessel density in ischaemic stroke. Lancet. 1993;342:742. doi: 10.1016/0140-6736(93)91734-4. [DOI] [PubMed] [Google Scholar]
- 98.Morris DC, Davies K, Zhang Z, Chopp M. Measurement of cerebral microvessel diameters after embolic stroke in rat using quantitative laser scanning confocal microscopy. Brain Res. 2000;876:31–36. doi: 10.1016/s0006-8993(00)02543-9. [DOI] [PubMed] [Google Scholar]
- 99.Zhang ZG, Tsang W, Zhang L, Powers C, Chopp M. Up-regulation of neuropilin-1 in neovasculature after focal cerebral ischemia in the adult rat. J Cereb Blood Flow Metab. 2001;21:541–49. doi: 10.1097/00004647-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 100.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–16. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Teng H, Zhang ZG, Wang L, et al. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab. 2008;28:764–71. doi: 10.1038/sj.jcbfm.9600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–39. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- 103.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 104.Shen Q, Wang Y, Kokovay E, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tavazoie M, Van der Veken L, Silva-Vargas V, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–88. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–64. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- 107.Bajetto A, Bonavia R, Barbero S, Florio T, Schettini G. Chemokines and their receptors in the central nervous system. Front Neuroendocrinol. 2001;22:147–84. doi: 10.1006/frne.2001.0214. [DOI] [PubMed] [Google Scholar]
- 108.Robin AM, Zhang ZG, Wang L, et al. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–34. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- 109.Imitola J, Raddassi K, Park KI, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101:18117–22. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hill WD, Hess DC, Martin-Studdard A, et al. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 2004;63:84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- 111.Shen LH, Li Y, Chen J, et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27:6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- 112.Chen J, Li Y, Katakowski M, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778–86. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- 113.Wang Y, Deng Y, Zhou GQ. SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008;1195:104–12. doi: 10.1016/j.brainres.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 114.Wang L, Zhang ZG, Zhang RL, et al. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci. 2006;26:5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee SR, Kim HY, Rogowska J, et al. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006;26:3491–95. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carmeliet P, Moons L, Dewerchin M, et al. Insights in vessel development and vascular disorders using targeted inactivation and transfer of vascular endothelial growth factor, the tissue factor receptor, and the plasminogen system. Ann N Y Acad Sci. 1997;811:191–206. doi: 10.1111/j.1749-6632.1997.tb52002.x. [DOI] [PubMed] [Google Scholar]
- 117.Roitbak T, Li L, Cunningham LA. Neural stem/progenitor cells promote endothelial cell morphogenesis and protect endothelial cells against ischemia via HIF-1alpha-regulated VEGF signaling. J Cereb Blood Flow Metab. 2008;28:1530–42. doi: 10.1038/jcbfm.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–42. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- 119.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–27. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Y, Chen J, Zhang CL, et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–17. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 121.Shen LH, Li Y, Chen J, et al. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006;137:393–99. doi: 10.1016/j.neuroscience.2005.08.092. [DOI] [PubMed] [Google Scholar]
- 122.Shen LH, Li Y, Gao Q, Savant-Bhonsale S, Chopp M. Down-regulation of neurocan expression in reactive astrocytes promotes axonal regeneration and facilitates the neurorestorative effects of bone marrow stromal cells in the ischemic rat brain. Glia. 2008;56:1747–54. doi: 10.1002/glia.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu Z, Li Y, Qu R, et al. Axonal sprouting into the denervated spinal cord and synaptic and postsynaptic protein expression in the spinal cord after transplantation of bone marrow stromal cell in stroke rats. Brain Res. 2007;1149:172–80. doi: 10.1016/j.brainres.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gregersen R, Christensen T, Lehrmann E, Diemer NH, Finsen B. Focal cerebral ischemia induces increased myelin basic protein and growth-associated protein-43 gene transcription in peri-infarct areas in the rat brain. Exp Brain Res. 2001;138:384–92. doi: 10.1007/s002210100715. [DOI] [PubMed] [Google Scholar]
- 125.Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–18. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 127.Roy NS, Wang S, Harrison-Restelli C, et al. Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J Neurosci. 1999;19:9986–95. doi: 10.1523/JNEUROSCI.19-22-09986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jiang Q, Zhang ZG, Ding GL, et al. MRI detects white matter reorganization after neural progenitor cell treatment of stroke. Neuroimage. 2006;32:1080–89. doi: 10.1016/j.neuroimage.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 129.Ding G, Jiang Q, Li L, et al. Magnetic resonance imaging investigation of axonal remodeling and angiogenesis after embolic stroke in sildenafil-treated rats. J Cereb Blood Flow Metab. 2008;28:1440–48. doi: 10.1038/jcbfm.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 131.Mori S, van Zijl PC. Fiber tracking: principles and strategies— technical review. NMR Biomed. 2002;15:468–80. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- 132.Watanabe T, Honda Y, Fujii Y, Koyama M, Matsuzawa H, Tanaka R. Three-dimensional anisotropy contrast magnetic resonance axonography to predict the prognosis for motor function in patients suffering from stroke. J Neurosurg. 2001;94:955–60. doi: 10.3171/jns.2001.94.6.0955. [DOI] [PubMed] [Google Scholar]
- 133.Li L, Jiang Q, Ding G, et al. MRI identification of white matter reorganization enhanced by erythropoietin treatment in a rat model of focal ischemia. Stroke. 2009;40:936–41. doi: 10.1161/STROKEAHA.108.527713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cheng YD, Al-Khoury L, Zivin JA. Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx. 2004;1:36–45. doi: 10.1602/neurorx.1.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cramer SC. Repairing the human brain after stroke—I— mechanisms of spontaneous recovery. Ann Neurol. 2008;63:272–87. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- 136.Cramer SC. Repairing the human brain after stroke—II— restorative therapies. Ann Neurol. 2008;63:549–60. doi: 10.1002/ana.21412. [DOI] [PubMed] [Google Scholar]
- 137.Cramer SC, Koroshetz WJ, Finklestein SP. The case for modality-specific outcome measures in clinical trials of stroke recovery-promoting agents. Stroke. 2007;38:1393–95. doi: 10.1161/01.STR.0000260087.67462.80. [DOI] [PubMed] [Google Scholar]
- 138.Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40:510–15. doi: 10.1161/STROKEAHA.108.526863. [DOI] [PubMed] [Google Scholar]
- 139.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 140.Wolf SL, Winstein CJ, Miller JP, et al. Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: the EXCITE randomised trial. Lancet Neurol. 2008;7:33–40. doi: 10.1016/S1474-4422(07)70294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kondziolka D, Wechsler L, Goldstein S, et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000;55:565–69. doi: 10.1212/wnl.55.4.565. [DOI] [PubMed] [Google Scholar]
- 142.Kondziolka D, Steinberg GK, Wechsler L, et al. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J Neurosurg. 2005;103:38–45. doi: 10.3171/jns.2005.103.1.0038. [DOI] [PubMed] [Google Scholar]
- 143.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–82. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 144.Sprigg N, Bath PM, Zhao L, et al. Granulocyte-colony-stimulating factor mobilizes bone marrow stem cells in patients with subacute ischemic stroke: the Stem cell Trial of recovery EnhanceMent after Stroke (STEMS) pilot randomized, controlled trial (ISRCTN 16784092) Stroke. 2006;37:2979–83. doi: 10.1161/01.STR.0000248763.49831.c3. [DOI] [PubMed] [Google Scholar]
- 145.Sildenafil (Viagra) treatment of subacute ischemic stroke. NCT00452582. http://clinicaltrials.gov/ct2/show/NCT00452582? term=NCT00452582&rank=1 (accessed March 9, 2009)
- 146.Silver B, Grover KM, Arcila X, Mitsias PD, Bowyer SM, Chopp M. Recovery in a patient with locked-in syndrome. Can J Neurol Sci. 2006;33:246–49. doi: 10.1017/s0317167100005084. [DOI] [PubMed] [Google Scholar]