Abstract
Colorectal cancer is one of the leading causes of cancer mortality and morbidity worldwide. Previous studies indicate that the zinc finger-containing transcription factor Krüppel-like factor 5 (KLF5) positively regulates proliferation of intestinal epithelial cells and colorectal cancer cells. Importantly, inhibition of KLF5 expression in intestinal epithelial cells and colorectal cancer cells by pharmacologic or genetic means reduces their rate of proliferation. To identify additional and novel small molecules that inhibit KLF5 expression and thus colorectal cancer proliferation, we developed a reporter assay using colorectal cancer cell line (DLD-1) that stably expressed a luciferase reporter gene directed by 1,959 bp of the human KLF5 promoter upstream of the ATG start codon and performed a cell-based high-throughput screen with the Library of Pharmacologically Active Compounds that contains 1,280 biologically active compounds. The screen identified 8 potential inhibitors and 6 potential activators of the KLF5 promoter. Three potential inhibitors, wortmannin, AG17, and AG879, were further evaluated by secondary analyses. All three significantly reduced both KLF5 promoter-luciferase activity and protein level in DLD-1 cells in a dose- and time-dependent manner when compared with controls. They also significantly reduced the rate of proliferation of DLD-1 and two other colorectal cancer cell lines, HCT116 and HT29. Our results show the principle of using high-throughput screening to identify small-molecule compounds that modulate KLF5 activity and consequently inhibit colorectal cancer proliferation.
Introduction
Colorectal cancer is the third most common cancer diagnosed in men and women in the United States. The American Cancer Society estimates that ~148,810 new cases of colorectal cancer will be diagnosed in 2008, which will cause ~49,600 deaths, making colorectal cancer the seconding leading cause of death from cancer (American Cancer Society, Cancer Facts & Figures 2008). These figures render colorectal cancer a significant health concern in the United States.
Significant progress has been made in understanding the molecular mechanisms responsible for the pathogenesis of colorectal cancer in the last two decades (1–5). In 1990, Fearon and Vogelstein proposed a genetic model for colorectal carcinogenesis, stating that colorectal cancer is the cumulative result of stepwise genetic changes (mutations) in key genes with important functions in the control of cell growth and proliferation (6). Thus, both mutational activation of oncogenes and inactivation of tumor suppressor genes are involved in this process (6). The identification of genes involved in colorectal cancer was facilitated by investigating genetic changes that occur in individuals with familial predisposition to colorectal cancer such as familial adenomatous polyposis and hereditary nonpolyposis colorectal cancer (7). Recent advances in genomic technology have further facilitated the identification of the genomic landscape and critical mutations in colorectal cancer (8).
Our group has been investigating the biological functions of Krüppel-like factors (KLF), which are a subfamily of zinc finger-containing transcription factors, in the intestinal epithelium (9– 11). KLF5 is highly expressed in the proliferating crypt epithelial cells throughout the gastrointestinal tract and exhibit important functions in regulating proliferation (11). Previous studies have shown that ectopic expression of KLF5 results in increased rates of proliferation and anchorage-independent growth in both 3T3 fibroblasts (12) and intestinal epithelial cells (13). KLF5 expression is induced by the mitogen-activated protein kinases, MEK/extracellular signal-regulated kinase, through activation of the transcription factor, EGR1 (14). As a downstream target of the mitogen-activated protein kinase pathway, we showed that KLF5 mediates the transforming effect of oncogenic HRAS in 3T3 fibroblasts (15, 16) and oncogenic KRAS in intestinal epithelial cells (17). Here, induction of KLF5 in response to HRAS- or KRAS-induced mitogen-activated protein kinases leads to the transcriptional activation of genes encoding several cell cycle-promoting proteins including cyclin D1 and B1 and Cdk1/Cdc2 (15, 16). In addition, increased KLF5 expression is observed in intestinal tumors from transgenic mice expressing oncogene KRAS under the control of an intestine-specific promoter and in human colon cancer harboring activating mutations of KRAS (17). Importantly, inhibition of KLF5 expression by small interfering RNA, MEK inhibitors, or all-trans retinoic acid in oncogenic RAS-transformed fibroblasts or intestinal epithelial cells and human colon cancer cell lines containing mutated KRAS results in the reduction of rate of proliferation of treated cells (13, 15, 16), indicating that KLF5 is essential for oncogenic RAS signaling and may be a potential target for therapy in colorectal cancer harboring mutated RAS.
Expression of KLF5 is often regulated at the level of transcription. Thus, the promoter of KLF5 has been shown to be activated by diverse signaling processes elicited by the phorbol ester, phorbol 12-myristate 13-acetate, fetal bovine serum, fibroblast growth factor, and oncogenic HRAS (12, 14, 17) and inhibited by all-trans retinoic acid and mitogen-activated protein kinase inhibitors such as PD98059 and U0126 (13, 16). Here, we used an unbiased high-throughput screening (HTS) approach as a means to identify novel small-molecule compounds that modulate KLF5 promoter activity with the hope of identifying novel compounds that may modulate colorectal cancer proliferation.
Materials and Methods
Reagents and Cell Lines
Cell culture medium, fetal bovine serum, and geneticin were purchased from Invitrogen. Steady-Glo luciferase assay mixtures were purchased from Promega. Cell proliferation reagent WST-1 was purchased from Roche. Wortmannin, tyrphostin A9(AG17), tyrphostin AG879, and mouse monoclonal antibody against β-actin were purchased from Sigma-Aldrich and LY294002 from Calbiochem. A rabbit polyclonal antibody generated against 95 to 111 amino acids of the mouse KLF5 protein was manufactured by QCB (17, 18). Rabbit antibodies against AKT, pAKT, and Egr1 were purchased from Cell Signaling. The Cell Extraction Buffer was acquired from Biosource Invitrogen. The Library of Pharmacologically Active Compounds (LOPAC1280) was purchased from Sigma-Aldrich.
Human colorectal cancer cell lines DLD-1, HCT116, and HT29 were purchased from the American Type Culture Collection. DLD-1 cells were maintained in RPMI 1640 and HCT116 and HT29 were maintained in McCoy’s medium. All media were supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. The DLD-1/pGL4.18hKLF5p cell line (see below) was maintained in RPMI 1640 with 10% fetal bovine serum and 1% penicillin/streptomycin supplemented with 800 µg/mL geneticin.
Generation of Stable Reporter Cell Lines
A 1,959-bp fragment of the human KLF5 promoter upstream of the ATG start codon (19) was amplified by PCR on the RPCI-11-505F4 BAC clone (Resgen Invitrogen). The amplified fragment contains HindIII overhangs and was subcloned into the HindIII cloning site of the pGL4.18 firefly luciferase reporter plasmid (Promega) to generate pGL4.18hKLF5p (for human KLF5 promoter). Following verification of the sequence by restriction and sequencing, the pGL4.18hKLF5p construct (which contains a neomycin resistance cassette) was transfected into DLD-1 cells followed by selection in neomycin (geneticin)-containing medium for 2 weeks. Sixty neomycin-resistant clones were pooled for further analysis. We validated this reporter cell line based on three criteria: (a) stability of luciferase expression over time, (b) response of the reporter activity to known stimuli, and (c) compatibility of the reporter cell line with HTS in a 96-well format (data not shown).
HTS of LOPAC1280
The DLD-1/pGL4.18hKLF5p cells were seeded at a density of 3 × 104 per well in 96-well plates in 100 µL RPMI 1640 containing 10% fetal bovine serum. Medium was changed to RPMI 1640 only on the following day and incubated for an additional 24 h at which time compounds from LOPAC1280 (Sigma-Aldrich) were added at a final concentration of 10 µmol/L in 1% DMSO using the liquid handling system Sciclone ALH3000A Workstation (Caliper Life Sciences) available at the Emory Chemical Biology Discovery Center. After 8 h, 100 µL Steady-Glo luciferase assay mixture was added to each well and luciferase activity was determined using the Analyst HT (Molecular Devices). The data were validated by two independent variables: signal-to-background (S/B) ratio and Z′ factor. Briefly, S/B ratio is the mean of the signal divided by the mean of the background and Z′ is calculated using the following equation: 1- [(3σs + 3σb) / (μs − μb)], where σ is the SD of signal (σ s) or background (σb) and μ is the mean (20, 21). In addition, we calculated the coefficient of variation for the entire sample of the microtiter plates. The coefficient of variation is defined as the ratio between the SD of the sample and the mean of the sample. Usually, this is multiplied by 100 and presented as a percentage.
Western Blot Analysis
Protein extracts were prepared with the Cell Extraction Buffer according to the manufacturer’s protocol. Total cellular proteins (30 µg) were resolved by SDS-PAGE gels and transferred to nitrocellulose membrane (Bio-Rad). Membranes were blocked with blocking solution containing 5% nonfat dried milk in 1× Tween 20-TBS for 1 h at room temperature and then incubated overnight with the primary antibodies in the blocking solution. After two 15-min washes in 1× Tween 20-TBS, membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibody for 2 h. Membranes were then washed and developed with Immobilon Western Horseradish Peroxidase Substrate (Millipore).
Cell Proliferation Assay
DLD-1 cells were seeded in 96-well plates at the density 104 per well in 100 µL medium. At the same time, various concentrations of LY294002, wortmannin, AG17, and AG879 suspended in DMSO were added to the wells. After 48 h incubations, 10 µL Cell Proliferation Reagent WST-1 was added to each well, and 4 h after the addition, absorption was measured throughout the plates. The measurement of the control (cells with medium and DMSO) was defined as 100% and the results from other measurements were calculated accordingly. Each experiment was done in triplicates.
Cell Cycle Profile Analysis
DLD-1 cells were seeded in 100 cm plates at a density of 2 × 106 per plate in 10 mL medium. The following day, LY294002, wortmannin, AG17, and AG879 suspended in DMSO were added to the plates to reach final concentration of 10 µmol/L. After 24 h incubation, cells were collected, washed twice with cold PBS, and fixed overnight with 70% ethanol in −20°C. The next day, cells were stained with propidium iodide (8) solution (200 µmol/L propidium iodide, 0.5 mg/L RNase, 100 µmol/L EDTA, and 1 µL/mL Triton X-100) and subjected to flow cytometry analysis.
Results
LOPAC1280 HTS
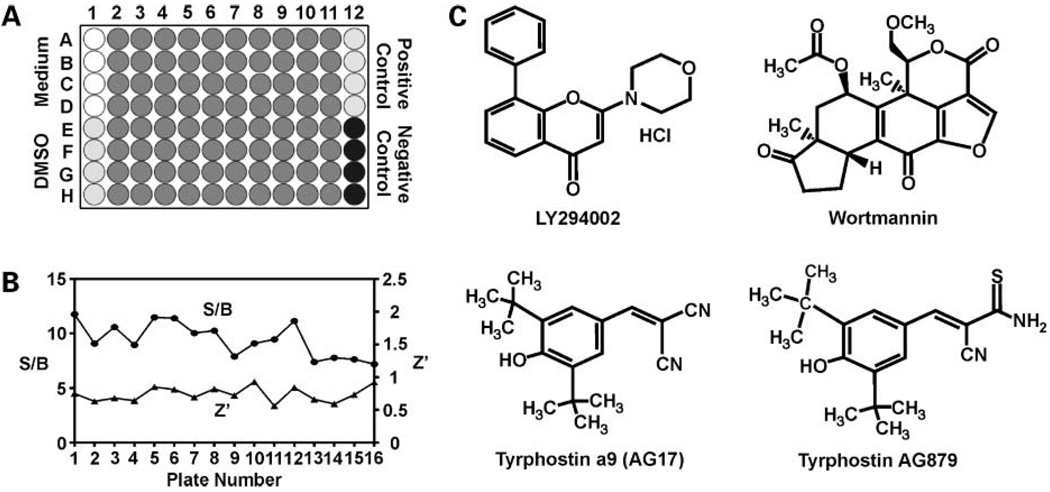
As an initial step to identify novel small-molecule compounds that modify KLF5 expression and with which to aid in the development of therapeutic agents for treating colorectal cancer, we screened the LOPAC1280 library using the DLD-1/pGL4.18hKLF5p reporter cell line. The LOPAC screen was done as described in Materials and Methods and the 96-well plate layout used in this screen is shown in Fig. 1A. The controls were phorbol 12-myristate 13-acetate for activating compounds and LY294002 for inhibitory compounds. The assay was validated using two variables: S/B ratio and Z′ factor (described in Materials and Methods). Variations of S/B ratios and Z′ factors among the different plates are shown in Fig. 1B. As seen, all of the S/B ratios were >5 and all of the Z′ factors were >0.5, indicating that the assays were robust and reproducible. In addition, we calculated the coefficient of variation for the entire sample. In our case, the coefficient of variation for this experiment was 8%, again validating the HTS strategy.
Figure 1.
Results of LOPAC1280 HTS and chemical structures of the hit compounds. A, 96-well plate layout for HTS of LOPAC1280. Column 1, rows A to D, medium only without cells; rows E to H, cells treated with DMSO; columns 2 to 11, rows A to H, cells treated with LOPAC1280 compounds with a final concentration of 10 µmol/L in 1% DMSO; column 12, rows A to D, cells treated with an activating control (100 ng/mL phorbol 12-myristate 13-acetate); rows E to H, cells treated with an inhibitory control (20 µmol/L LY294002). B, variations in S/B ratios and Z′ factors across plates in HTS of LOPAC1280. S/B ratios and Z′ factors were calculated for each plate and plotted in the graphs. Left, y axis, S/B ratios; right, Z′ factors. C, chemical structures of LY294002, wortmannin, AG17, and AG879.
To identify positive hits, we adopted the traditional hit threshold selection of μ-3σ, where μ is the mean value and σ is the SD of the entire assay (20). Analysis of the HTS results revealed eight potential hit compounds that significantly inhibited the KLF5 promoter activity. In addition to the inhibitors, the screen yielded six apparent activators of the KLF5 promoter. Both sets of potential inhibitors and activators are listed in Table 1. It is gratifying from the results that phorbol 12-myristate 13-acetate, a known activator of the KLF5 promoter (12, 14), was identified in our screen (Table 1). Moreover, the screen identified dequalinium analogue (C-14 linker), a protein kinase C-α inhibitor, as an inhibitor of KLF5 promoter activity, again validating the screen as an effective means to identify compounds that modulate KLF5 expression. The hit compounds from our primary screen belong to several classes of agents: neurotransmission, ion pump, phosphorylation, cell cycle, and apoptosis. We decided to focus on three potential inhibitory molecules: wortmannin and AG17 that were actual hits from the screen as well as AG879 that was slightly below the threshold for a hit. Wortmannin was selected because, like the control LY294002, it inhibits phosphoinositide 3-kinase (PI3K) that is often overreactive in cancer (8, 22). AG17 and AG879 were selected because they belong to the class of receptor tyrosine kinase inhibitors or tyrphostins, which have shown promises as cancer therapeutics [ref. 23; AG17 inhibits platelet-derived growth factor receptor (PDGFR) tyrosine and AG879 inhibits ERRB2 tyrosine kinase]. The chemical structures of the compounds subjected to further analysis are depicted in Fig. 1C.
Table 1.
Potential hit compounds from LOPAC1280 HTS
| Effect on KLF5 promoter |
Name of compound* | Action | Selectivity | Description |
|---|---|---|---|---|
| Activator | SB204741 | Antagonist | 5-HT2B | 5-HT2B serotonin receptor inhibitor |
| 6-Methyl-2-(phenylethynyl)pyridine hydrochloride | Antagonist | mGluR5 | Highly selective, noncompetitive mGluR5 metabotropic glutamate receptor antagonist | |
| GW9662 | Inhibitor | Peroxisome proliferator-activated receptor-γ | Irreversible peroxisome proliferator-activated receptor-γ inhibitor | |
| 1-Phenyl-3-(2-thiazolyl)-2-thiourea | Inhibitor | β-Hydroxylase | Dopamine β-hydroxylase inhibitor | |
| Piceatannol | Inhibitor | Syk/Lck | Nonreceptor kinase Syk and Lck inhibitor | |
| Phorbol 12-myristate 13-acetate | Activator | Protein kinase C | Activates protein kinase C in vivo and in vitro | |
| Inhibitor | Dihydrooubain | Inhibitor | Na+/K+ Pump | Sodium potassium pump inhibitor |
| Dequalinium analogue, C-14 linker | Inhibitor | Protein kinase C-α | Protein kinase C-α inhibitor | |
| Emetine dihydrochloride hydrate | Activator | Apoptosis inducer, RNA-protein translation inhibitor | ||
| NSC95397 | Inhibitor | Cdc25 | Selective, irreversible Cdc25 dual-specificity phosphatase inhibitor | |
| Minocycline hydrochloride | Inhibitor | Basement membrane protease inhibitor, inhibits endothelial cell proliferation | ||
| Oubain | Inhibitor | Na+/K+ ATPase | Blocks movement of the H5/H6 trans-membrane domains of Na+/K+ ATPase | |
| Wortmannin | Inhibitor | PI3K | Potent and specific PI3K inhibitor | |
| AG17 | Inhibitor | PDGFR | Selective PDGFR tyrosine kinase inhibitor |
Detailed information on the action, selectivity, and description of the compounds can be obtained from the manufacturer (Sigma-Aldrich).
Validation of Hit Compounds
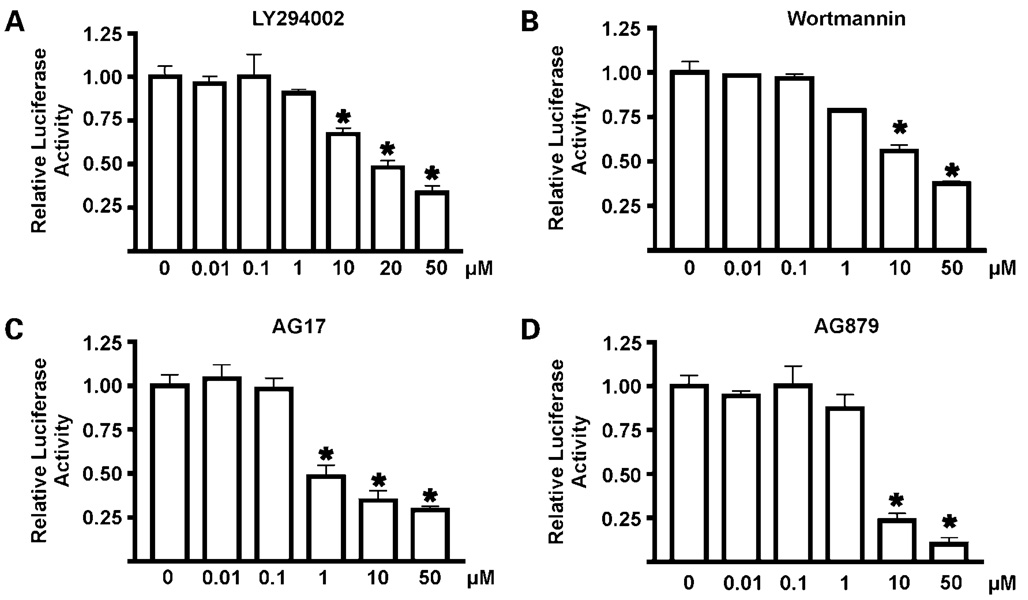
Next, we performed a dose-response and time-response evaluation of the potential hit compounds in the DLD-1/pGL4.18hKLF5p cell line to confirm the results obtained from the LOPAC1280 primary screen. The concentration of the compounds used in the primary screen was 10 µmol/L (see Materials and Methods). Here, we used a concentration range between 0.01 and 50 µmol/L and measured the reporter activity at 2, 4, 6, 8, and 24 h. Figure 2 shows the results of luciferase assays after 24 h treatment with the four different compounds. Results of the time-course study with various concentrations of compounds are shown in Supplementary Fig. S1.4 As seen, all four compounds show a dose- and time-dependent inhibitory effect on the luciferase activity. However, the degrees of inhibition varied among the tested agents, with AG17 and AG879 being more potent than LY294002 and wortmannin in reducing the luciferase signal at the 24 h time point (Fig. 2).
Figure 2.
Dose-response studies of the effect of hit compounds on the luciferase activity in DLD-1/pGL4.18hKLF5p cells. Twenty-four hours after seeding, various concentrations of LY294002 (A), wortmannin (B), AG17 (C), and AG879 (D) suspended in 1% DMSO were added to the wells. Cells were harvested 24 h later for luciferase activity determination as well as protein concentration. All luciferase activities were standardized to the amount of proteins. Relative luciferase activity of the control (cells with medium and DMSO only) was defined as 1 and results from other measurements were calculated accordingly. Relative luciferase activity is shown as fold change. Each experiment was done in triplicate. Bars, SD. *, P < 0.01, compared with control (column 1) by two-tailed t test.
Effects of Hit Compounds on the Endogenous KLF5 Protein Levels
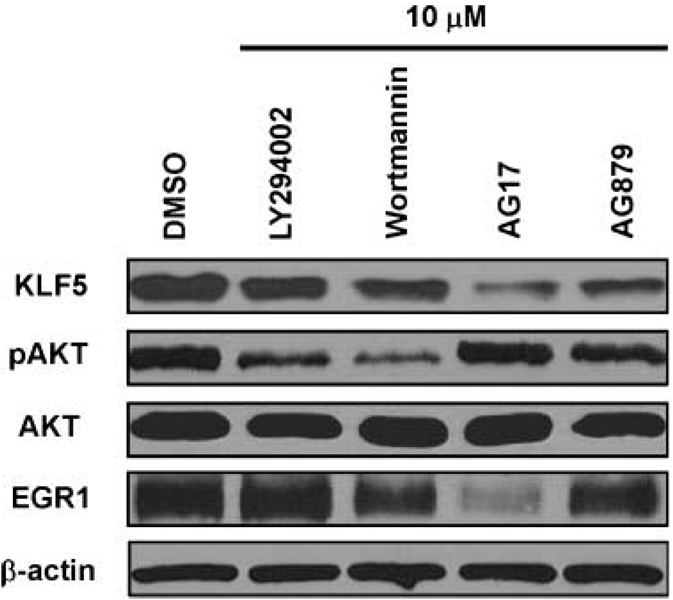
Following validation of the effect of the hit compounds in inhibiting KLF5 promoter activity, we examined their effect on the endogenous KLF5 levels in DLD-1 cells. Results in Fig. 3 show that, following 24 h treatment with 10 µmol/L of the compounds, KLF5 level was lower when compared with control (DMSO). Similar to the results of luciferase reporter assays in Fig. 2, the inhibitory effect of AG17 and AG879 was more pronounced than LY294002 and wortmannin. We then examined the effect of the compounds on cellular pAKT and AKT levels. As seen in Fig. 3, LY294002 and wortmannin, both known PI3K inhibitors, significantly decreased the levels of pAKT but not total AKT, indicating effective inhibition of AKT phosphorylation. In contrast, AG17 and AG879 failed to influence either pAKT or AKT levels, suggesting that the two compounds function in a PI3K-independent manner. AG17 significantly reduced the level of EGR1, an established transcriptional activator of KLF5 (14). The mechanism by which AG879 inhibits KLF5 remains to be determined. Additionally, we examined the effects of the hit compounds on the KLF5 protein levels in HCT116 and HT29 colon cancer cell lines. Results from these experiments are provided in Supplementary Figs. S2E4 and S3E4 for HCT116 and HT29 cells, respectively. As seen and similar to the findings in DLD-1 cells, AG17 and AG879 decreased KLF5 protein levels in both HCT116 and HT29cells more profoundly than LY294002 and wortmannin in both HCT116 and HT29cells.
Figure 3.
Western blot analyses of the effects of hit compounds on protein levels in DLD-1 cells. DLD-1 cells were treated with 10 µmol/L of the hit compounds in 1% DMSO or DMSO alone for 24 h before extraction of proteins for Western blotting against KLF5, AKT, pAKT, EGR1, and β-actin.
Effects of Hit Compounds on Proliferation of Colorectal Cancer Cells
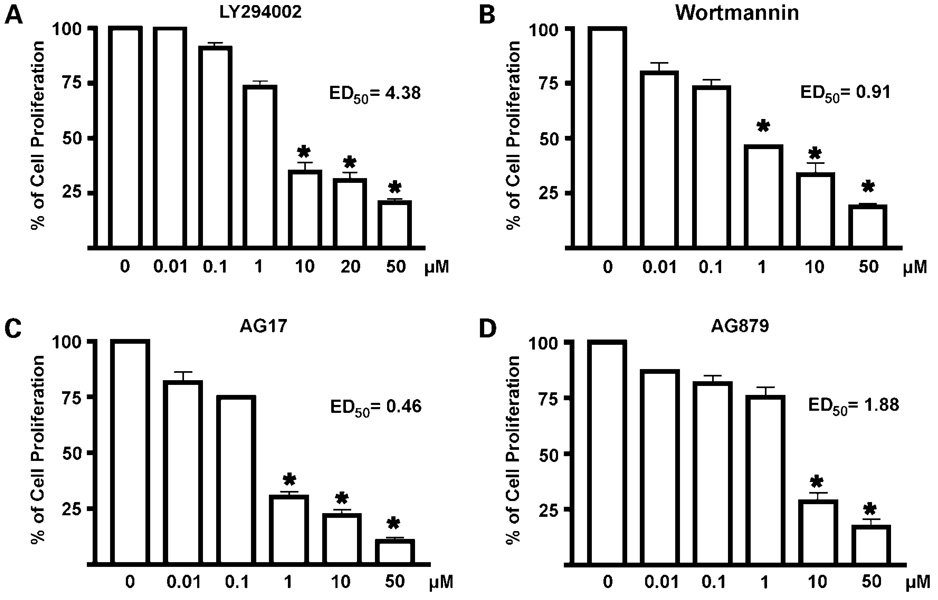
KLF5 is a proproliferative factor in intestinal epithelial cells and colorectal cancer cells (13, 17). To determine whether the hit compounds affect proliferation of colorectal cancer cells, we treated DLD-1, HCT116, and HT29 cells with the hit compounds for 48 h followed by the WST-1 cell proliferation assays. Results of the effects of various concentrations of the hit compounds were shown in Fig. 4 for DLD-1 and in Supplementary Figs. S24 and S34 for HCT116 and HT29, respectively. As seen, each of the four compounds tested inhibited cell proliferation in a dose-dependent manner. The two AG compounds, AG17 and AG879, with ranges of ED50 from 0.46 to 1.77 µmol/L and 1.88 to 3.62 µmol/L, respectively, were more effective in inhibiting proliferation of the three colon cancer cell lines when compared with LY294002 and wortmannin. The three cell lines also exhibited different sensitivities to the compounds, with DLD-1 being the most sensitive for all drugs tested.
Figure 4.
Cell proliferation assays of DLD-1 cells treated with the hit compounds. Cells were seeded in 96-well plates with medium containing DMSO or increasing concentrations of the stated compounds: LY294002 (A), wortmannin (B), AG17 (C), and AG879 (D) for 2 d before the measurement of absorbance by WTS-1 assays. Control (cells with medium containing DMSO) was defined as 100% and results from other measurements were calculated accordingly. ED50 was calculated for each compound. Each experiment was done in triplicates. Bars, SD. *, P < 0.001, compared with DMSO (column 1).
We performed flow cytometry of DLD-1 cells treated with the various compounds to determine their effects of the cell cycle profiles. The results of an experiment done in triplicates are shown in Table 2. As seen, all four compounds caused a statistically significant reduction in the population of cells in the S phase of the cell cycle when compared with control, suggesting that these compounds inhibit DNA synthesis. Two compounds, wortmannin and AG879, led to a statistically significant increase in cells in the G0-G1 phase of the cell cycle, suggesting that they induce a G1-S cell cycle arrest. In contrast, treatment with AG17 caused a statistically increase in the sub-G1 population of the cells compared with control, suggesting that AG17 was able to induce apoptosis. Wortmannin, in addition to causing a G1-S arrest, also increased apoptosis.
Table 2.
Cell cycle profile analysis
| Treatment | % Cells in sub-G1 | % Cells in G0-G1 | % Cells in S | % Cells in G2-M |
|---|---|---|---|---|
| DMSO | 0.50 ± 0.14 | 56.92 ± 3.49 | 16.10 ± 2.30 | 26.74 ± 3.47 |
| LY294002 | 0.57 ± 0.28 | 63.06 ± 4.08 | 11.95 ± 1.85* | 24.66 ± 3.96 |
| Wortmannin | 1.12 ± 0.17† | 75.97 ± 3.40† | 7.71 ± 1.83† | 15.61 ± 2.11† |
| AG17 | 1.90 ± 0.47† | 62.81 ± 3.49 | 6.44 ± 3.24† | 27.89 ± 4.11 |
| AG879 | 0.87 ± 0.25 | 69.35 ± 2.52† | 7.32 ± 0.41† | 22.65 ± 2.70 |
NOTE: DLD-1 cells were seeded in 100 mm plates at a density of 2 × 106 per plate in 10 mL medium. On the following day, LY294002, wortmannin, AG17, and AG879 suspended in DMSO were added to the plates to a final concentration of 10 µmol/L. DMSO was used as a control. After 24 h incubation, cells were collected and processed for flow cytometry as described in Materials and Methods. The experiment was done in triplicates. Data are percentages of total cells.
P < 0.05, compared with DMSO control (two-tailed t test).
P < 0.01, compared with DMSO control (two-tailed t test).
Discussion
HTS is a relatively cost- and time-effective method that allows the simultaneous screening of hundreds to thousands of small-molecule compounds. In the current study, we attempted to identify compounds that modulate activity of the human KLF5 promoter. The rationale behind this screen lies with the documented proproliferative effect of KLF5 in colon cancer cells (17). Any identified molecules that inhibit KLF5 expression may potentially inhibit colon cancer cell proliferation, thus aiding in the development of potentially novel therapeutic agents for colorectal cancer. Indeed, a primary screen of the LOPAC1280 library using our cell-based reporter system identified eight novel compounds that down-regulated KLF5 promoter activity (Table 1). Some of these were validated for their ability to inhibit KLF5 expression and further characterized for their ability to inhibit proliferation of colon cancer cells.
Previous studies have established several inhibitors of KLF5 expression including all-trans retinoic acid (13) and mitogen-activated protein kinase inhibitors such as PD98059 and U0126 (16). In this screen, we used LY294002 as a control, which also effectively inhibits KLF5 expression (Fig. 2).5 LY294002 is a reversible inhibitor of PI3K by competitively blocking the ATP-binding site of PI3K (24). The inhibition of PI3K abrogates phosphorylation of AKT, a downstream effector of the PI3K pathway (25). In our screen, we identified another PI3K inhibitor, wortmannin, as an inhibitor of KLF5 expression (Table 1). Wortmannin irreversibly inhibits PI3K by covalently binding to the p110 subunit of PI3K, which then leads to a decrease in AKT phosphorylation (26, 27). Results of our study confirmed that both LY294002 and wortmannin reduce pAKT levels in DLD-1 cells, which is accompanied by a reduction in KLF5 levels (Fig. 3).
There is a growing body of evidence to suggest that PI3K/AKT activation is often associated with colorectal cancer and promotes colorectal cancer development (8, 22). Thus, inhibition of PI3K/AKT by LY294002 or wortmannin has been shown to inhibit growth of colorectal cancer cells (28, 29). Results of our study also show that both LY294002 and wortmannin inhibit proliferation of several colon cancer cell lines in a dose-dependent fashion. The extent of the inhibitory effect of these two molecules varies between the tested cell lines, which could be attributed to genetic differences among the cells. Notably, our study links reduced KLF5 promoter activity and KLF5 protein levels to decreased rates of proliferation of treated colon cancer cells. The identified compounds are known to interfere with several signaling pathways, which may then cause a decrease in cell proliferation. Although KLF5 may not be the only target of these compounds, it is part of the signaling pathways that those compounds modulate. Thus, KLF5 may be an excellent indicator of the proliferative state of these cells. It is also of interest to note that overexpression of KLF5 in a human bladder cancer cell line leads to increased level of pAKT (30). It is therefore possible that PI3K/AKT and KLF5 form an autoregulatory loop with which to promote proliferation of colon cancer cells. Reduction of KLF5 by either pharmacologic inhibition of PI3K/AKT (this study) or genetic knockdown (17) results in a favorable therapeutic response in the colon cancer cell lines examined.
Receptor tyrosine kinases have been implicated in the development and progression of various cancers (31). Recently, many of the receptor tyrosine kinases have become targets of anticancer therapy (32, 33). Tyrphostins are a group of compounds that inhibit receptor tyrosine kinase activity, some of which have been used to treat tumors (23). Of the eight hit compounds that we identified in our primary screen, one was a tyrphostin (AG17; Table 1). A second tyrphostin, AG879, although did not meet our criteria for a hit, was identified under less stringent conditions and was selected for further characterization because of its relatedness to AG17.
AG17 is a known potent inhibitor of the PDGFR (34). It has been shown to induce apoptosis and inhibit Cdk2 in lymphoma cells (35) and perturb mitochondria and the Golgi complex to block cell proliferation (36, 37). Our study shows that AG17 reduces KLF5 promoter activity and protein level in a dose- and time-dependent manner. Importantly, AG17 efficiently decreases the rate of proliferation of three different colon cancer cell lines, DLD-1, HCT116, and HT29. Cell cycle profile analysis indicates that AG17 causes more apoptosis than control and a trend toward G1-S cell cycle arrest (Table 2), a finding that is consistent with published studies in other cell types (35–37). Among all compounds tested in this study, AG17 causes the greatest reduction in KLF5 protein level and is the most potent inhibitor of cell proliferation (Fig. 3 and Fig 4; Supplementary Figs. S2 and S3). The reduction in KLF5 protein level after AG17 treatment is correlated with a significant reduction in the level of EGR1 (Fig. 3), previously implicated in the transcriptional activation of KLF5 expression (14, 16). It is of interest to note that KLF5 is known to activate expression of the PDGF-A chain (38, 39). This raises the possibility that KLF5 is involved in an autoregulatory loop of PDGFR and PDGF-A during cell proliferation.
AG879 is an inhibitor of the ERBB2 receptor (40), which belongs to the larger family of epidermal growth factor receptors (41). Previous studies indicate that ERBB2 level is increased in colon cancer and that treatment of colon cancer cells with AG879 prevents cell cycle reentry (40). Our study also shows that AG879 is an inhibitor of cell proliferation in colon cancer cells and does so by blocking the G1-S progression of the cell cycle (Table 2). Like AG17, AG879 treatment of DLD-1 cells leads to a reduction in KLF5 protein level (Fig. 3). However, unlike AG17, AG879 does not cause a significant decrease in EGR1 level (Fig. 3). The mechanism by which AG879 inhibits KLF5 expression is currently under investigation.
In summary, we have conducted a “proof-of-principle” HTS of a small-molecule library for compounds that inhibit proliferation of colon cancer cells using the proproliferative KLF5 promoter activity as readout. We successfully identified several compounds that inhibit KLF5 expression and consequently colon cancer cell proliferation. Despite these shared effects, these compounds function to interfere with different signaling pathways, which shed lights on the mechanism by which KLF5 is regulated. Additional screening of a much larger library is likely to yield novel compounds with different chemical scaffolds that modulate KLF5 expression and with which to develop potential therapeutic agents for treating cancers that rely on KLF5 as a crucial mediator of their proliferation.
Supplementary Material
Acknowledgments
We thank Amr Ghaleb for help with cell cycle profile analysis experiment.
Grant support: NIH grants DA26215, DK52330, DK64399, and CA84197.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary material for this article is available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
Ngwenya and Yang, unpublished observations.
References
- 1.de Lau W, Barker N, Clevers H. WNT signaling in the normal intestine and colorectal cancer. Front Biosci. 2007;12:471–491. doi: 10.2741/2076. [DOI] [PubMed] [Google Scholar]
- 2.Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. 2005;19:877–890. doi: 10.1101/gad.1295405. [DOI] [PubMed] [Google Scholar]
- 3.Jass JR. Colorectal cancer: a multipathway disease. Crit Rev Oncog. 2006;12:273–287. doi: 10.1615/critrevoncog.v12.i3-4.50. [DOI] [PubMed] [Google Scholar]
- 4.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 5.Worthley DL, Whitehall VL, Spring KJ, Leggett BA. Colorectal carcinogenesis: road maps to cancer. World J Gastroenterol. 2007;13:3784–3791. doi: 10.3748/wjg.v13.i28.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 7.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 8.Wood LD, Parsons DW, Jones S, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 9.Dang DT, Pevsner J, Yang VW. The biology of the mammalian Kruppel-like family of transcription factors. Int J Biochem Cell Biol. 2000;32:1103–1121. doi: 10.1016/s1357-2725(00)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghaleb AM, Yang VW. The pathobiology of Kruppel-like factors in colorectal cancer. Curr Colorectal Cancer Rep. 2008;4:59–64. doi: 10.1007/s11888-008-0011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McConnell BB, Ghaleb AM, Nandan MO, Yang VW. The diverse functions of Kruppel-like factors 4 and 5 in epithelial biology and pathobiology. Bioessays. 2007;29:549–557. doi: 10.1002/bies.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun R, Chen X, Yang VW. Intestinal-enriched Kruppel-like factor (Kruppel-like factor 5) is a positive regulator of cellular proliferation. J Biol Chem. 2001;276:6897–6900. doi: 10.1074/jbc.C000870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chanchevalap S, Nandan MO, Merlin D, Yang VW. All-trans retinoic acid inhibits proliferation of intestinal epithelial cells by inhibiting expression of the gene encoding Kruppel-like factor 5. FEBS Lett. 2004;578:99–105. doi: 10.1016/j.febslet.2004.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawai-Kowase K, Kurabayashi M, Hoshino Y, Ohyama Y, Nagai R. Transcriptional activation of the zinc finger transcription factor BTEB2 gene by Egr-1 through mitogen-activated protein kinase pathways in vascular smooth muscle cells. Circ Res. 1999;85:787–795. doi: 10.1161/01.res.85.9.787. [DOI] [PubMed] [Google Scholar]
- 15.Nandan MO, Chanchevalap S, Dalton WB, Yang VW. Kruppel-like factor 5 promotes mitosis by activating the cyclin B1/Cdc2 complex during oncogenic Ras-mediated transformation. FEBS Lett. 2005;579:4757–4762. doi: 10.1016/j.febslet.2005.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nandan MO, Yoon HS, Zhao W, et al. Kruppel-like factor 5 mediates the transforming activity of oncogenic H-Ras. Oncogene. 2004;23:3404–3413. doi: 10.1038/sj.onc.1207397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nandan MO, McConnell BB, Ghaleb AM, et al. Kruppel-like factor 5 mediates cellular transformation during oncogenic KRAS-induced intestinal tumorigenesis. Gastroenterology. 2008;134:120–130. doi: 10.1053/j.gastro.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McConnell BB, Klapproth JM, Sasaki M, Nandan MO, Yang VW. Kruppel-like factor 5 mediates transmissible murine colonic hyperplasia caused by Citrobacter rodentium infection. Gastroenterology. 2008;134:1007–1016. doi: 10.1053/j.gastro.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C, Zhou Y, Zhou Z, et al. Regulation of KLF5 involves the Sp1 transcription factor in human epithelial cells. Gene. 2004;330:133–142. doi: 10.1016/j.gene.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Gagarin A, Makarenkov V, Zentilli P. Using clustering techniques to improve hit selection in high-throughput screening. J Biomol Screen. 2006;11:903–914. doi: 10.1177/1087057106293590. [DOI] [PubMed] [Google Scholar]
- 21.Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 22.Michl P, Downward J. Mechanisms of disease: PI3K/AKT signaling in gastrointestinal cancers. Z Gastroenterol. 2005;43:1133–1139. doi: 10.1055/s-2005-858638. [DOI] [PubMed] [Google Scholar]
- 23.Levitzki A, Mishani E. Tyrphostins and other tyrosine kinase inhibitors. Annu Rev Biochem. 2006;75:93–109. doi: 10.1146/annurev.biochem.75.103004.142657. [DOI] [PubMed] [Google Scholar]
- 24.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzo-pyran- 4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 25.Hemmings BA. Akt signaling: linking membrane events to life and death decisions. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- 26.Geltz NR, Augustine JA. The p85 and p110 subunits of phosphatidylinositol 3-kinase-α are substrates, in vitro, for a constitutively associated protein tyrosine kinase in platelets. Blood. 1998;91:930–939. [PubMed] [Google Scholar]
- 27.Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 28.Semba S, Itoh N, Ito M, Harada M, Yamakawa M. The in vitro and in vivo effects of 2-(4-morpholinyl)-8-phenyl-chromone (LY294002), a specific inhibitor of phosphatidylinositol 3′-kinase, in human colon cancer cells. Clin Cancer Res. 2002;8:1957–1963. [PubMed] [Google Scholar]
- 29.Itoh N, Semba S, Ito M, et al. Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer. 2002;94:3127–3134. doi: 10.1002/cncr.10591. [DOI] [PubMed] [Google Scholar]
- 30.Chen C, Benjamin MS, Sun X, et al. KLF5 promotes cell proliferation and tumorigenesis through gene regulation and the TSU-Pr1 human bladder cancer cell line. Int J Cancer. 2006;118:1346–1355. doi: 10.1002/ijc.21533. [DOI] [PubMed] [Google Scholar]
- 31.Arteaga CL. Epidermal growth factor receptor dependence in human tumors: more than just expression? Oncologist. 2002;7 Suppl 4:31–39. doi: 10.1634/theoncologist.7-suppl_4-31. [DOI] [PubMed] [Google Scholar]
- 32.Faivre S, Djelloul S, Raymond E. New paradigms in anticancer therapy: targeting multiple signaling pathways with kinase inhibitors. Semin Oncol. 2006;33:407–420. doi: 10.1053/j.seminoncol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Zwick E, Bange J, Ullrich A. Receptor tyrosine kinases as targets for anticancer drugs. Trends Mol Med. 2002;8:17–23. doi: 10.1016/s1471-4914(01)02217-1. [DOI] [PubMed] [Google Scholar]
- 34.Levitzki A, Gilon C. Tyrphostins as molecular tools and potential antiproliferative drugs. Trends Pharmacol Sci. 1991;12:171–174. doi: 10.1016/0165-6147(91)90538-4. [DOI] [PubMed] [Google Scholar]
- 35.Palumbo GA, Yarom N, Gazit A, et al. The tryphostin AG17 induces apoptosis and inhibition of cdk2 activity in a lymphoma cell line that overexpresses bcl-2. Cancer Res. 1997;57:2434–2439. [PubMed] [Google Scholar]
- 36.Burger AM, Kaur G, Alley MC, et al. Tyrphostin AG17, [(3,5-di-tert-butyl-4-hydroxybenzylidene)-malononitrile], inhibits cell growth by disrupting mitochondria. Cancer Res. 1995;55:2794–2799. [PubMed] [Google Scholar]
- 37.Thyberg J. Tyrphostin A9 and wortmannin perturb the Golgi complex and block proliferation of vascular smooth muscle cells. Eur J Cell Biol. 1998;76:33–42. doi: 10.1016/S0171-9335(98)80015-0. [DOI] [PubMed] [Google Scholar]
- 38.Aizawa K, Suzuki T, Kada N, et al. Regulation of platelet-derived growth factor-A chain by Kruppel-like factor 5: new pathway of cooperative activation with nuclear factor-κB. J Biol Chem. 2004;279:70–76. doi: 10.1074/jbc.M306621200. [DOI] [PubMed] [Google Scholar]
- 39.Shindo T, Manabe I, Fukushima Y, et al. Kruppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nat Med. 2002;8:856–863. doi: 10.1038/nm738. [DOI] [PubMed] [Google Scholar]
- 40.Venkateswarlu S, Dawson DM, St Clair P, et al. Autocrine heregulin generates growth factor independence and blocks apoptosis in colon cancer cells. Oncogene. 2002;21:78–86. doi: 10.1038/sj.onc.1205011. [DOI] [PubMed] [Google Scholar]
- 41.Mendelsohn J, Baselga J. Epidermal growth factor receptor targeting in cancer. Semin Oncol. 2006;33:369–385. doi: 10.1053/j.seminoncol.2006.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.