Abstract
Background
Anapalsia is rare in childhood rhabdomyosarcoma and has not been included in the International Classification of Rhabdomyosarcoma (ICR). A recent review of cases from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group (COG) suggests that anaplasia might be more common than previously reported and may impact clinical outcome.
Materials and Methods
The prevalence of anaplasia (focal or diffuse) was prospectively assessed in 546 eligible cases who were registered in an Intergroup Rhabdomyosarcoma Study Group (IRSG) or COG therapeutic trial from 1995–1998. The incidence of anaplasia in tumor samples and its impact in predicting clinical outcome was assessed.
Results
Overall 71 (13%) of all samples analyzed had anaplasia. Anaplasia was more common in patients with tumors in favorable sites and was less commonly seen in younger patients and in those with stage 2, 3 or clinical Group III disease. Regardless of its distribution (focal or diffuse), on univariate analysis the presence of anaplasia had a significant negative impact for both failure-free survival (FFS: 63% vs 77% at 5 years) and survival (S: 68% vs 82% at 5 years) in patients with embryonal rhabdomyosarcoma. This effect was most pronounced in children with intermediate risk disease. Using multivariate analysis, the hazard ratio was 1.6 for FFS (p=0.085) and 1.7 for overall survival (p=0.081). Anaplasia did not affect outcome in patients with alveolar tumors.
Conclusion
The incidence of anaplasia in rhabdomyosarcoma is higher than previously described and may be of prognostic significance in children with intermediate risk embryonal rhabdomyosarcoma.
Keywords: Rhabdomyosarcoma, anaplasia, soft tissue sarcoma
Introduction
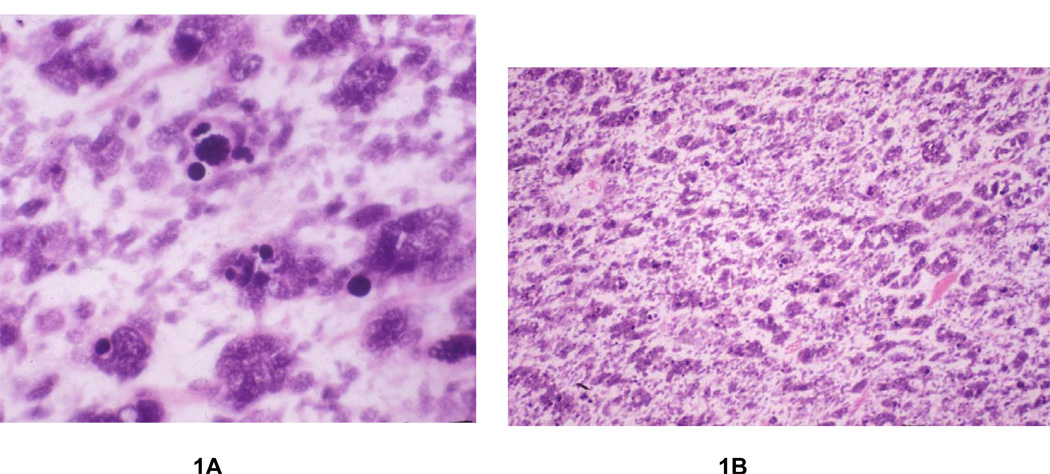
Rhabdomyosarcoma is the most common soft tissue sarcoma in children under the age of 15 years; approximately 300 new cases are diagnosed in the United States each year 1,2. Since 1972, the Intergroup Rhabdomyosarcoma Study Group (IRSG) (now the Soft Tissue Sarcoma Committee of the Children’s Oncology Group) has conducted 5 consecutive clinical trials for the treatment of childhood rhabdomyosarcoma and has identified robust prognostic factors that strongly correlate with clinical outcome3–10. These factors include clinical group, stage, age, and histologic subtype. The presence of alveolar and undifferentiated histology has been associated with a worse clinical outcome than embryonal or botryoid histology4,10. Palmer first noted that the anaplastic cellular pattern originally described by Beckwith and him in Wilms’ tumor 11,12 13 was also present in rhabdomyosarcoma and that this subtype had a similarly poor prognosis. Anaplastic tumor cells in either Wilms’ tumor or rhabdomyosarcoma contain large, lobate hyperchromatic nuclei (at least 3 times the size of neighboring nuclei) with or without large atypical (obvious, multipolar) mitotic figures (Figure 1A). In 1993, Kodet et al14 retrospectively identified 110 randomly sampled cases of embryonal and alveolar rhabdomyosarcoma with anaplastic cells. These cases accounted for 3% of all cases of rhabdomyosarcoma studied in the first three Intergroup Rhabdomyosarcoma Studies (IRS I-III). The degree of anaplasia was further defined not just by relative quantity but also apparent clonal expansion of the anaplastic nuclei in the tumor13. Type I tumors as defined by Kodet included anaplastic cells loosely scattered among non-anaplastic cells (so called focal anaplasia), and type II tumors included those with anaplastic cells that were aggregated in clusters or formed continuous sheets (figure 1B)14 In this report, patients with type II tumors (so called diffuse anaplasia) had a worse clinical outcome. Despite the suggestion that anaplasia could significantly affect outcome, its relative rarity and lack of reproducibility on multi-reviewer studies precluded incorporation of this feature as a morphologic criteria for assessment in the International Classification of Rhabdomyosarcoma15.
Figure 1.
Figure 1A and 1B. A The tumor cells possess enlarged, hyperchromatic, irregular nuclei and aberrant mitotic figures, representative of anaplasia. B Rhabdomyosarcoma with diffuse anaplasia, containing confluent sheets of anaplastic tumor cells.
In this report, we have expanded the observations by Kodet and describe the prevalence and clinical impact of anaplasia in a well defined prospective cohort of patients with rhabdomyosarcoma who were enrolled in two consecutive IRSG studies from 1995–1998.
Materials and Methods
Patients
Among the 655 eligible patients enrolled in an IRSG therapeutic trial from January 1, 1995 through December 31, 1998, 546 patients (83%) had sufficient pathologic material submitted for central pathologic review. Cases were categorized by the International Classification of Childhood Sarcomas 15, and anaplasia was defined as focal and diffuse. Clinical, pathologic, and treatment variables were correlated with clinical outcome and the presence or absence of anaplasia. Variables analyzed included age, sex, race, primary tumor site, histologic subtype (embryonal, alveolar, other), presence or absence of diffuse and focal anaplasia, IRSG Group (I-IV), and TNM pretreatment stage (1–4).8,16
Statistical analysis
Failure-free survival (FFS) was defined as the time from study entry to disease recurrence, second cancer, or death as a first event. Overall survival (S) was defined as the time from study entry to death from any cause. FFS and S for patients not experiencing an event were censored at the patients’ last contact date. FFS and S were estimated using the Kaplan-Meier method.17 To determine if anaplastic morphology was an independent prognostic factor in patients with RMS, multivariate analysis was performed. The Cox proportional hazards18 regression model was used with a stepwise selection procedure to identify independent prognostic factors from among the patient population characteristics enumerated above.
Cytogenetics
Utilizing standard culture and harvest procedures,19 cytogenetic analysis was performed on sterile, representative tissue from eleven anaplastic tumors, In brief, the tissue was mechanically and enzymatically disaggregated and then cultured for 3 to 7 days in RPMI 1640 media supplemented with 20% fetal bovine serum. Cells were exposed overnight to Colcemid (0.02 g/mL). After subsequent hypotonic treatment (0.7% sodium citrate for 20 minutes), the preparations were fixed three times with methanol and glacial acetic acid (3:1). Metaphase cells were banded with Giemsa trypsin, and the karyotypes described according to the International System for Human Cytogenetic Nomenclature (ISCN 2005).20
Results
The clinical characteristics of the 546 eligible patients are depicted in Table 1. The majority of patients had tumors in unfavorable sites (see Table 1 for definition) and presented with Group III disease. Two hundred sixty-eight patients had embryonal tumors and 154 had alveolar tumors (Table 4). Anaplasia was identified in 72 patients (13%) (Table 2). Forty (7%) of these patients had focal anaplasia and 32 (6%) had diffuse anaplasia. The distribution of anaplasia was similar among patients with tumors of embryonal or alveolar histology (see table). Anaplasia was less common in patients with younger age (no cases in patients under 1 year of age; p = 0.045) and Group III (p = 0.013) and Stage 2/3 disease (p=0.03). Patients with anaplasia (n=72) were more likely to have tumors in favorable sites, Group IV disease, and tumor size greater than 5 cm. Distributions of age, sex, race, histology, primary size and other variables were comparable between cases with and without anaplasia.
Table 1.
Clinicopathologic Characteristics of Rhabdomyosarcoma With and Without Anaplasia. (IRSG/COG studies – 1995–1998)
| Anaplasia | |||
|---|---|---|---|
| None (n=474) | Focal (n=40) | Diffuse (n=32) | |
| AGE (YEARS) | |||
| <1 | 18 | 3 (14%) | - |
| 1–9 | 296 | 19(6%) | 26 (8%) |
| 10+ | 157 | 18(10%) | 6 (3%) |
| Unknown | 3 | - | - |
| RACE | |||
| White | 333 | 24 (6%) | 23 (6%) |
| Non-white | 137 | 16 (10%) | 9 (6%) |
| SEX | |||
| Male | 300 | 26 (8%) | 19 (6%) |
| Female | 174 | 14 (7%) | 13 (7%) |
| CLINICAL GROUP | |||
| I | 86 | 10 (10%) | 10 (10%) |
| II | 50 | 7 (12%) | 3 (5%) |
| III | 249 | 16 (6%) | 8 (3%) |
| IV | 84 | 7 (7%) | 11 (11%) |
| Unknown | 4 | - | 1 |
| STAGE | |||
| 1 | 155 | 20 (11%) | 11 (6%) |
| 2 | 76 | 3 (2%) | 5 (6%) |
| 3 | 158 | 10 (6%) | 5 (3%) |
| 4 | 84 | 7 (7%) | 11 (11%) |
| Unknown | 1 | - | - |
| PRIMARY SITE | |||
| All Favorable Sites | 163 | 24 (12%) | 13 (7%) |
| Orbit | 49 | 4 (7%) | 3 (5%) |
| Head and neck/non-PM | 32 | 4 (11%) | 2 (5%) |
| GU, non-bladder/prostate | 82 | 16 (15%) | 8 (8%) |
| All Unfavorable Sites | 311 | 16 (5%) | 19 (5%) |
| Parameningeal | 97 | 2 (2%) | 2 (2%) |
| Parameningeal extension | 11 | 1 (8%) | 0 |
| Bladder/prostate | 49 | 2 (4%) | 2 (4%) |
| Extremity | 63 | 4 (5%) | 10 (13%) |
| Other | 91 | 7 (7%) | 5 (5%) |
| Tumor Invasiveness | |||
| T1 | 219 | 21 (8%) | 17 (7%) |
| T2 | 247 | 19 (7%) | 15 (5%) |
| Unknown | 8 | - | - |
| Nodal involvement | |||
| N0 | 351 | 28 (7%) | 25 (6%) |
| N1 | 93 | 7 (7%) | 6 (6%) |
| Unknown | 30 | 5 | 1 |
| Tumor size | |||
| ≤ 5cm | 216 | 19 (7%) | 19 (7%) |
| > 5 cm | 249 | 21 (7%) | 13 (5%) |
| Unknown | 9 | - | - |
Table 4.
Cytogenetic Findings in Anaplastic Rhabdomyosarcoma.
| Case | Age/Sex | Final Diagnosis | Karyotype |
|---|---|---|---|
| 1 | 2/M | Anaplastic RMS | 46,XY |
| 2 | 12/M | Alveolar RMS w/focal anaplasia | 46~48,XY,−3,?add(12)(p12),+mar1,1dmin,inc[12]/94~96,idemx2,+8,+8,+mar2×2[8] |
| 3 | 3/F | Embryonal RMS w/diffuse anaplasia | 69~70,X,-X,del(X)(q21),add(1)(p36.3),del(1)(q21),−4,+der(5)t(2;5)(p11.2;q33),−6,t(8;20)(p11.2;q13.3),der(9)t(1;9)(q21;q12),−10,del(11)(q12),add(12)(q13),+13,+del(13)(q22q33),−15,−16,i(17)(q10),+19,+der(19)t(19;21)(p13.2;q11.2),+der(19)del(19)(p13.2)t(11;19)(?;q13.2),+21,−22,+0~1mar, 5~25dmin[20].ishdel(X)(wcpX+),der(5)(wcp5+,wcp2+),t(8;20)(wcp8+,wcp20+;wcp20+,wcp8+),der(9)(wcp9+,wcp1+),del(11)(wcp11+),del(13)(wcp13+),i(17)(wcp17+),der(19)(wcp19+,wcp21+),der(19)(wcp19+, wcp11+).nuc ish13q14.1(RP11-89L15×3~6,RP11-181D10×3~6)[112]/13q14.1(RP11-89L15×2,RP11-81D10×2)[32] |
| 4 | 3/M | Mixed Alveolar/Embryonal RMS w/focal anaplasia | 78~87,XXY,-Y,i(1)(q10),+2,+2,−3,−4,−6,−9,−12,−13,−15,−15,+16,−18,−19,+20,−21,−21,−22,+mar,1dmin[cp10] |
| 5 | 4/M | Mixed Alveolar/Embryonal RMS w/focal anaplasia | Culture Failure |
| 6 | 2/F | Alveolar RMS w/diffuse anaplasia | 46,X,-X,t(2;13)(q35;q14),+22[3]/92,idemx2[2]/46,XX[10] |
| 7 | 11/F | Alveolar RMS w/diffuse anaplasia | 106~110,XXXXX,t(2;13)(q35;q14)x2,−17,+20, +20,1dmin,inc[2]/46,XX[24] |
| 8 | 9/F | Alveolar RMS w/diffuse anaplasia | 120,XXXXX,+15mar,2dmin,inc[1]/46,XX[15] |
| 9 | 14/M | Mixed Embryonal/Spindle Cell RMS w/focal anaplasia | 69~71,XXY,+1,−4,−7,−9,+add(12)(q22),−13,−16,−17, +del(20)(q13.1)x2,+mar1,+1~3mar[cp3]/70~72, idem,del(1)(p13),+8[cp2]/58~72,XXY,+add(1)(p32), +mar1,+mar2,+3~5mar,inc[cp4]/120~130,XXXYY, +mar1×2,+mar2×2,+10~20mar,0~4dmin,inc[cp3] |
| 10 | 2/M | Alveolar RMS w/diffuse anaplasia | 90,YY,-X,-X,+1,dic(1;2)(p13;q23)x2,+2,+2,+2,+2,−3,+7, +8,−10,−11,+12,−13,−14,−17,−17,+18,+19,−22[cp7]/46,XY[7] |
| 11 | 1/F | Embryonal RMS w/ diffuse anaplasia | 172,XXXXXXXX,dic(1;8)(q32;q24.3)x2,−3,−3, del(5)(q11.2)x2,−6,−6,add(8)(p12)x2,−9,−9,add(9) (q34.3)x2,−10,−10,−11,−11,add(12)(p12.3)x2,−16,−16, +17,t(17;18)(q10;q10)x2,del(18)(q12)x2,−19, +2mar[1]/46,XX[26] |
Table 2.
Prevalence of Anaplasia Amongst RMS Subtypes by ICR Classification.
| Anaplasia | |||
|---|---|---|---|
| Histology | None | Focal | Diffuse |
| Alveolar | 139 | 12 (8%) | 5 (3%) |
| Embryonal | 223 | 23 (9%) | 22 (8%) |
| Botryoid | 36 | 1 (3%) | 2 (3%) |
| Spindle cell | 17 | 2 (11%) | 0 |
| Rhabdomyosarcoma NOS | 32 | 0 | 2 (6%) |
| Undifferentiated sarcoma | 11 | 1 (8%) | 1 (8%) |
| Sarcoma, not classifiable | 11 | 0 | 0 |
| Other | 4 | 0 | 0 |
| Unknown | 1 | 1 | 0 |
| Total | 474 | 40 (7%) | 32 (6%) |
NOS; not otherwise specified
Follow-up information was available for the 379 patients alive at last contact and ranged from 48 days to 10.2 years (median 6.9 years).
Embryonal RMS
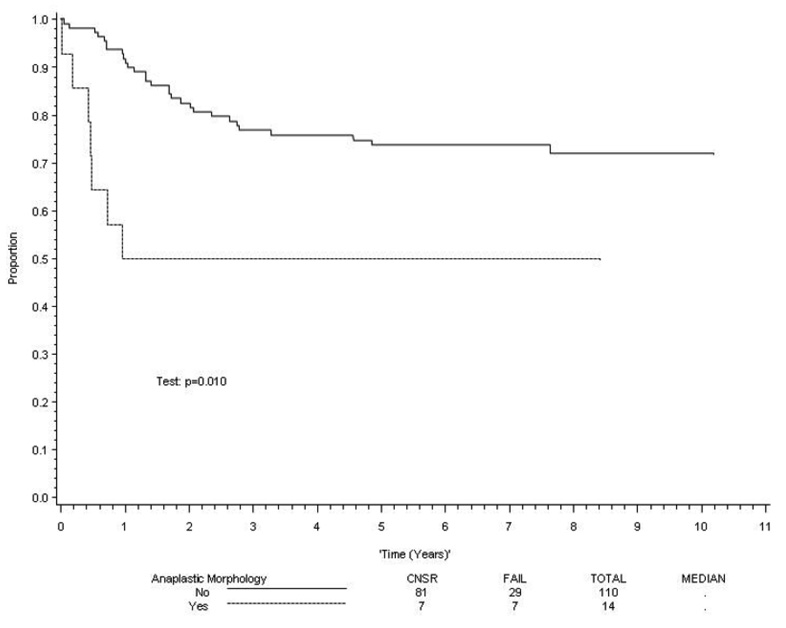
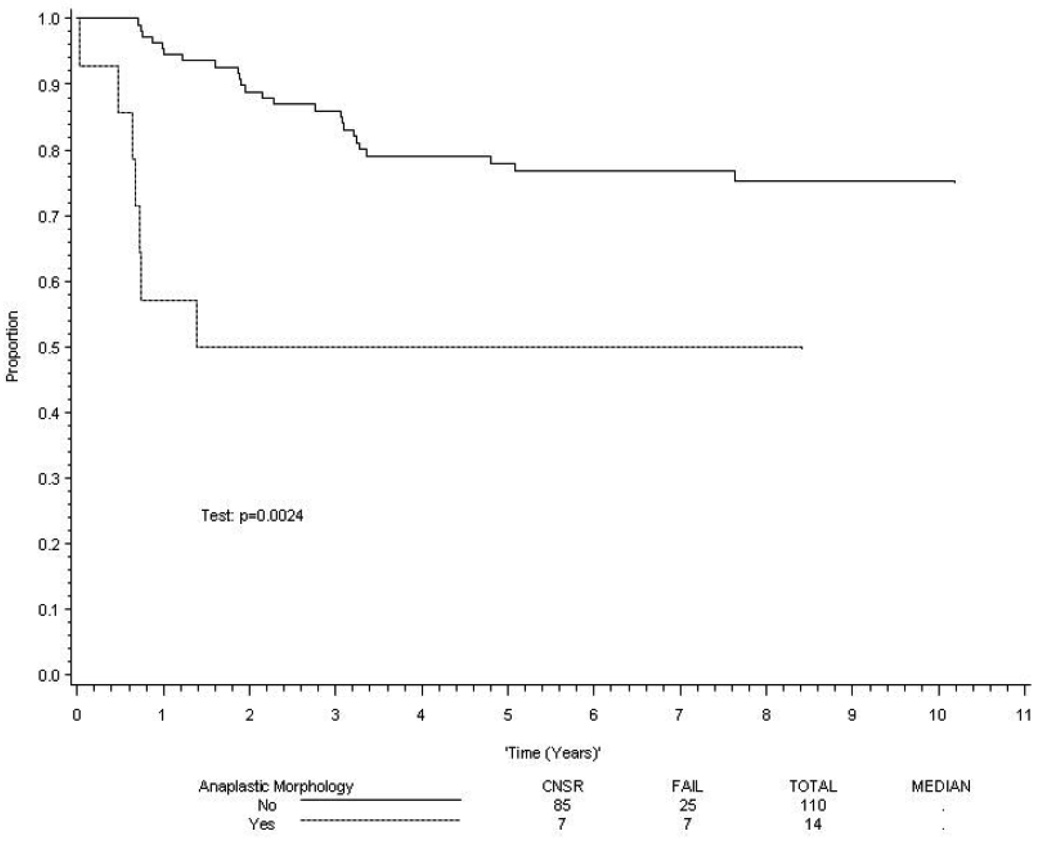
There was a statistically significant association between the presence of anaplasia and clinical outcome in patients with embryonal histology. However, there was no difference in outcome between patients with focal or diffuse anaplasia. The estimated 5-year survival rates for patients with and without anaplasia were 68% RMS (95% confidence interval [CI] 55%, 81%) and 82% (95% CI 77%, 87%) respectively (p=0.01). Similarly, the 5 year failure-free survival rates for patients with and without anaplasia were 63% (95% CI 50%, 76%) and 77% (95% CI 72%, 82%) respectively (p=.02). When stratified by risk group (low risk being Stage 1 Group I-III; Stage 2, Group 1 or 2; or Stage 3, Group 1 or 2 and high risk representing metastatic tumors), anaplasia was not predictive of clinical outcome in patients with low risk or high risk tumors, but it was significantly associated with a poorer clinical outcome in patients with intermediate risk disease (p = 0.01 for failure-free survival and p = 0.002 for overall survival; Figure 2 and Figure 3).To determine if anaplastic morphology was an independent prognostic factor in patients with embryonal disease, the Cox proportional hazards regression model was employed, using a stepwise selection procedure to identify independent prognostic factors. Anaplastic morphology showed a trend that did not reach significance (p=0.08) for both failure-free and overall survival (Table 3). A second set of multivariate analyses using the same modeling strategy for patients with loco-regional (Group I-III) and metastatic (Group IV) disease revealed that anaplasia was not an independent predictor of either failure-free or overall survival (p>0.1).
Figure 2. FFS in patients with intermediate risk embryonal rhabdomyosarcoma.
Figure 3. Survival in patients with intermediate risk embryonal rhabdomyosarcoma.
Table 3.
Multivariate analysis of prognostic factors in childhood embryonal rhadomyosarcoma
| Failure-Free Survival | |||
|---|---|---|---|
| HR | 95% CI | P value | |
| Intermediate Risk | 2.3 | 1.4, 4.0 | < 0.01 |
| High Risk | 6.5 | 3.6, 11.6 | < 0.0001 |
| Age >10 yrs | 2.2 | 1.4, 3.4 | < 0.001 |
| Anaplastic morphology | 1.6 | 0.9, 2.7 | 0.085 |
| Overall Survival | |||
| Intermediate Risk | 4.0 | 2.1, 7.8 | < 0.0001 |
| High Risk | 12.1 | 6.0, 24.3 | < 0.0001 |
| Age > 10 yrs | 1.9 | 1.2, 3.2 | < 0.01 |
| Anaplastic morphology | 1.7 | 10.9, 3.1 | 0.081 |
Alveolar Tumors
There was no association in univariate analysis between anaplasia and clinical outcome in patients with alveolar tumors (p = 0.29 for failure-free survival and p = 0.41 for overall survival). Even after adjustment for the statistically significant independent prognostic factors (data not shown), anaplastic morphology was not significantly related to either failure-free survival (p = 0.70) or overall survival (p = 0.46) in alveolar tumors.
Cytogenetics
Karyotypically abnormal cells were identified in nine of the eleven rhabdomyosarcomas with focal or diffuse anaplasia (Table 4). The modal numbers ranged from near-diploid to near-heptaploid. Two of the alveolar rhabdomyosarcoma cases with anaplasia (Cases 6 and 7) showed the characteristic t(2;13)(q35;q14) translocation. Recognition of the ARMS-associated translocations [t(2;13) or t(1;13)] in Cases 2 and 8 may be precluded by poor chromosomal morphology. Double minutes were seen in six of nine karyotypically abnormal cases.
Discussion
In this report we have documented that the prevalence of focal or diffuse anaplasia in childhood rhabdomyosarcoma is significantly higher than previously reported14 and was seen in 13% of pathologic specimens of childhood rhabdomyosarcoma. These findings are different from those previously reported by Kodet in which only 3% of the samples analyzed contained anaplasia. The differences between these two reports are likely explained by the fact that in the Kodet study, pathologic material was randomly selected from the IRS pathology center files, whereas our study uses a well defined denominator that allows a better calculation of the prevalence of this entity. The presence of anaplastic features has been known to correlate with poor clinical outcome in various pediatric malignancies including Wilms’ tumor and medulloblastoma. 13,21–24 Furthermore, the presence of anaplasia in these two malignancies correlates with unique genetic abnormalities. For example, in medulloblastoma, the presence of large anaplastic cells is associated with a higher level of ERBBB2 expression and disruption of the p53-ARF tumor suppressor pathway. 25 Children with anaplastic Wilms’ tumor often harbor p53 gene mutations and have abnormally short telomeres with abnormal mitotic segregation . 26–28 Specifically for anaplastic rhabdomyosarcoma, cytogenetic studies indicate that these tumors often contain gene amplifications in the form of double minutes. Our previous comparative genomic hybridization analyses29 also indicate that gene amplification are shared by embryonal and alveolar tumors. The candidate genes involved (eg. IGF1R, MYCN ) may explain their poor outcome and may provide fertile ground for future studies of this phenomenon. While no studies have been performed, it would be interesting to test for disruption of the p53 family of genes (including p63 and p73), which are required for appropriate RB gene function and transcription of muscle specific genes. 30
In our study, the presence of focal or diffuse anaplasia in rhabdomyosarcoma correlated with an inferior clinical outcome by univariate analysis, particularly in patients with intermediate risk disease, a subgroup that accounts for 38% of all cases of embryonal rhabdomyosarcoma. However, these findings did not reach statistical significance in the multivariate analysis, although the absolute difference in failure free and overall survival seen was sizable (14%).
Our results show that anaplasia is a pathologic feature that is more common than previously described, and its presence should be prospectively annotated in pathology reports. This will facilitate future research on archival samples and may identify novel mechanisms of rhabdomyosarcoma tumorigenesis. It is unclear from our study if larger trials will confirm anaplasia as an independent prognostic factor for patients with intermediate risk embryonal histology disease.
References
- 1.Ries L, Smith M, Gurney J, et al. Bethesda, MD: National Cancer Institute; 1999. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. SEER program Pub No 99–4649. [Google Scholar]
- 2.Qualman SJ, Bowen J, Parham DM, et al. Protocol for the examination of specimens from patients (children and young adults) with rhabdomyosarcoma. Arch Pathol Lab Med. 2003;127:1290–1297. doi: 10.5858/2003-127-1290-PFTEOS. [DOI] [PubMed] [Google Scholar]
- 3.Raney RB, Anderson JR, Barr FG, et al. Rhabdomyosarcoma and undifferentiated sarcoma in the first two decades of life: a selective review of intergroup rhabdomyosarcoma study group experience and rationale for Intergroup Rhabdomyosarcoma Study V. J Pediatr Hematol Oncol. 2001;23:215–220. doi: 10.1097/00043426-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Qualman SJ, Coffin CM, Newton WA, et al. Intergroup Rhabdomyosarcoma Study: update for pathologists. Pediatr Dev Pathol. 1998;1:550–561. doi: 10.1007/s100249900076. [DOI] [PubMed] [Google Scholar]
- 5.Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J.Clin.Oncol. 2001;19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 6.Crist W, Gehan EA, Ragab AH, et al. The Third Intergroup Rhabdomyosarcoma Study. J.Clin.Oncol. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 7.Maurer HM, Gehan EA, Beltangady M, et al. The Intergroup Rhabdomyosarcoma Study-II. Cancer. 1993;71:1904–1922. doi: 10.1002/1097-0142(19930301)71:5<1904::aid-cncr2820710530>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Maurer HM, Beltangady M, Gehan EA, et al. The Intergroup Rhabdomyosarcoma Study-I. A final report. Cancer. 1988;61:209–220. doi: 10.1002/1097-0142(19880115)61:2<209::aid-cncr2820610202>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 9.Pappo AS, Lyden E, Breitfeld P, et al. Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: the Children’s Oncology Group. J Clin Oncol. 2007;25:362–369. doi: 10.1200/JCO.2006.07.1720. [DOI] [PubMed] [Google Scholar]
- 10.Meza JL, Anderson J, Pappo AS, et al. Analysis of prognostic factors in patients with nonmetastatic rhabdomyosarcoma treated on intergroup rhabdomyosarcoma studies III and IV: the Children’s Oncology Group. J Clin Oncol. 2006;24:3844–3851. doi: 10.1200/JCO.2005.05.3801. [DOI] [PubMed] [Google Scholar]
- 11.Palmer NFM. Histopathology and prognosis in the second Intergroup Rhabdomyosarcoma Study (IRS II) Proc Amer Soc Clin Oncol. 1983:229. [Google Scholar]
- 12.Palmer NSN, Foulkes M. Histology and prognosis in rhabdomyosarcoma (IRS-I) Proc Amer Soc Clin Oncol. 1982;1:170. [Google Scholar]
- 13.Faria P, Beckwith JB, Mishra K, et al. Focal versus diffuse anaplasia in Wilms tumor--new definitions with prognostic significance: a report from the National Wilms Tumor Study Group. Am J Surg Pathol. 1996;20:909–920. doi: 10.1097/00000478-199608000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Kodet R, Newton WA, Jr, Hamoudi AB, et al. Childhood rhabdomyosarcoma with anaplastic (pleomorphic) features. A report of the Intergroup Rhabdomyosarcoma Study. Am J Surg Pathol. 1993;17:443–453. doi: 10.1097/00000478-199305000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Newton WA, Jr, Gehan EA, Webber BL, et al. Classification of rhabdomyosarcomas and related sarcomas. Pathologic aspects and proposal for a new classification--an Intergroup Rhabdomyosarcoma Study. Cancer. 1995;76:1073–1085. doi: 10.1002/1097-0142(19950915)76:6<1073::aid-cncr2820760624>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence W, Jr, Anderson JR, Gehan EA, et al. Pretreatment TNM staging of childhood rhabdomyosarcoma: a report of the Intergroup Rhabdomyosarcoma Study Group. Children's Cancer Study Group. Pediatric Oncology Group. Cancer. 1997;80:1165–1170. [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Cox DR. Regression models and life tables (with discussion) JRSS (B) 1972;74:187–220. [Google Scholar]
- 19.Althof PA, Ohmori K, Zhou M, et al. Cytogenetic and molecular cytogenetic findings in 43 aneurysmal bone cysts: aberrations of 17p mapped to 17p13.2 by fluorescence in situ hybridization. Mod Pathol. 2004;17:518–525. doi: 10.1038/modpathol.3800090. [DOI] [PubMed] [Google Scholar]
- 20.International System for Human Cytogenetic Nomenclature. Basel: Karger; 1995. [Google Scholar]
- 21.Gajjar A, Hernan R, Kocak M, et al. Clinical, histopathologic, and molecular markers of prognosis: toward a new disease risk stratification system for medulloblastoma. J Clin Oncol. 2004;22:984–993. doi: 10.1200/JCO.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 22.Dome JS, Cotton CA, Perlman EJ, et al. Treatment of Anaplastic Histology Wilms' Tumor: Results From the Fifth National Wilms' Tumor Study. J Clin Oncol. 2006;24:2352–2358. doi: 10.1200/JCO.2005.04.7852. [DOI] [PubMed] [Google Scholar]
- 23.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 24.Eberhart CG, Kepner JL, Goldthwaite PT, et al. Histopathologic grading of medulloblastomas: a Pediatric Oncology Group study. Cancer. 2002;94:552–560. doi: 10.1002/cncr.10189. [DOI] [PubMed] [Google Scholar]
- 25.Frank AJ, Hernan R, Hollander A, et al. The TP53-ARF tumor suppressor pathway is frequently disrupted in large/cell anaplastic medulloblastoma. Brain Res Mol Brain Res. 2004;121:137–140. doi: 10.1016/j.molbrainres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Lahoti C, Thorner P, Malkin D, et al. Immunohistochemical detection of p53 in Wilms' tumors correlates with unfavorable outcome. Am J Pathol. 1996;148:1577–1589. [PMC free article] [PubMed] [Google Scholar]
- 27.Bardeesy N, Falkoff D, Petruzzi MJ, et al. Anaplastic Wilms' tumour, a subtype displaying poor prognosis, harbours p53 gene mutations. Nat Genet. 1994;7:91–97. doi: 10.1038/ng0594-91. [DOI] [PubMed] [Google Scholar]
- 28.Stewenius Y, Jin Y, Ora I, et al. Defective chromosome segregation and telomere dysfunction in aggressive Wilms' tumors. Clin Cancer Res. 2007;13:6593–6602. doi: 10.1158/1078-0432.CCR-07-1081. [DOI] [PubMed] [Google Scholar]
- 29.Bridge JA, Liu J, Qualman SJ, et al. Genomic gains and losses are similar in genetic and histologic subsets of rhabdomyosarcoma, whereas amplification predominates in embryonal with anaplasia and alveolar subtypes. Genes Chromosomes. Cancer. 2002;33:310–321. doi: 10.1002/gcc.10026. [DOI] [PubMed] [Google Scholar]
- 30.Cam H, Griesmann H, Beitzinger M, et al. p53 family members in myogenic differentiation and rhabdomyosarcoma development. Cancer Cell. 2006;10:281–293. doi: 10.1016/j.ccr.2006.08.024. [DOI] [PubMed] [Google Scholar]