Abstract
Background
Argyrophilic grains (AG) are silver-positive spindle shaped lesions found at postmortem. Their significance is controversial.
Objective
To determine clinical correlates of AG and MRI signature patterns of atrophy that could allow premortem recognition of this pathology.
Methods
Cases with AG were identified from a longitudinal study of aging and dementia. Clinical features were compared between subjects with and without dementia. Voxel-based morphometry (VBM) was used to assess patterns of grey matter atrophy in subjects compared to controls. Whole brain volumes (WBV) were compared across groups.
Results
Twenty-two cases (14 females; median age at death of 90 years; range: 70–101) with AG were identified. Eight of the 22 were demented. Those with dementia had higher median Braak (p=0.02) and lower MMSE (p=0.002). VBM demonstrated hippocampal atrophy in those with dementia (N=3) but no atrophy in those without (N=9). There was no difference in WBV between groups.
Conclusion
AG is a feature of old age commonly occurring in non-demented subjects. In this age group, the presence of AG may reduce the threshold for dementia.
Keywords: Voxel based morphometry, total intracranial volume, argyrophilic, MRI, volume loss, Alzheimer’s disease
INTRODUCTION
Pathologically, argyrophilic grains (AG) are characterized by the finding of spindle-shaped lesions in neuronal processes, and coiled bodies in oligodendrocytes in the neuropil of the hippocampus, entorhinal cortex and other limbic areas[5, 6]. The significance of finding AG during a postmortem evaluation is controversial. Since the original description of AG almost two decades ago[5], only a few series have described clinical features that may correlate with the presence of this pathology [3, 17, 20, 26, 35, 36]. Some of these reports have suggested that the presence of AG correlates with dementia characterized by behavioral disturbances including agitation and violence, personality changes, and later by forgetfulness.
Controversy arises because of two main reasons. First, although AG have been found in subjects with cognitive impairment and dementia, large clinicopathological studies have found AG in subjects without any cognitive impairment[26, 36]. Secondly, AG is rarely the sole pathological finding in cognitively impaired subjects, and is most commonly found to co-exist with other pathologies [4, 6, 20, 26, 34], especially neurofibrillary tangles (NFT)[19], one of the hallmark lesions of Alzheimer’s disease. Currently there are no prospective studies that have assessed clinicopathological and MRI correlates of AG.
The primary aims of this study were therefore to determine what clinical features correspond to the presence of AG, and to determine if there is an MRI signature pattern of volume loss that would allow premortem recognition of this pathology. Given prior evidence that AG is found in cognitively normal, as well as demented individuals, we also set out to compare demographic and imaging features between cases with AG, with and without dementia.
METHODS
Subjects and Recruitment
In order to best address the aims of this study we needed to attend to two important concerns. First, all subjects would have to have been clinically well characterized, and second, only subjects without any additional pathology that could account for cognitive impairment, would be included in the study. Based on these two concerns we developed our inclusion and exclusion criteria:
Inclusion Criteria
All subjects must have been enrolled in our Alzheimer’s Disease Research Center (ADRC) or Alzheimer’s Diseases Patient Registry (ADPR), Mayo Clinic, Rochester, MN. Both the ADRC and ADPR are longitudinal studies with well characterized clinical histories, serial yearly head MRI scans, and pathological material. All subjects must have had autopsy examination by at least one of our neuropathologists with expertise in degenerative neuropathology (JEP or DWD) and confirmation of the presence of AG. In order to be included in the imaging component of this study all subjects must have had one volumetric head MRI scan.
Exclusion Criteria
We excluded any subject in which in addition to the presence of AG, there was another pathology that could have account for, or is associated with cognitive impairment. Therefore, we excluded any case in which there was pathological features of high probability for Alzheimer’s disease as defined by the NIA Reagan criteria [16], hippocampal sclerosis [10], frontotemporal lobar degeneration [28], progressive supranuclear palsy [14], corticobasal degeneration[9], or Lewy body disease[27]. In addition, we excluded any case that did not meet NIA Reagan criteria for Alzheimer’s disease[16], but had a Braak Stage of V, or VI [7] (e.g. tangle dominant cases). We also excluded any subject in which the historical records were incomplete. For the imaging component of this study we excluded any subject in which the volumetric MRI was of poor quality which could have affected the analysis
Pathological analysis
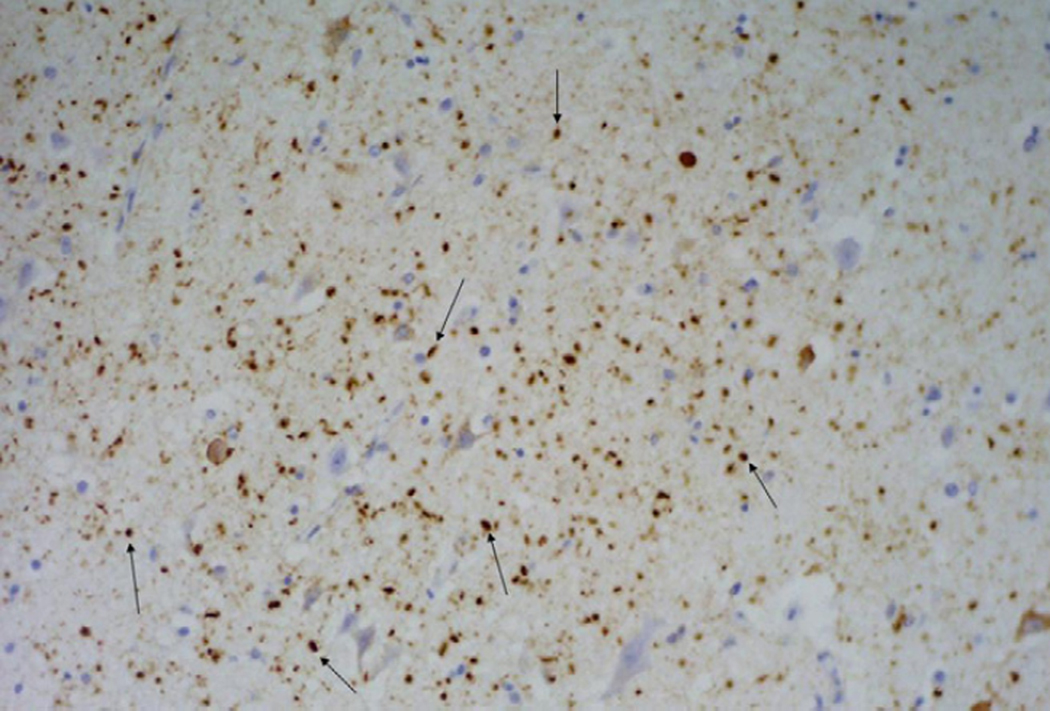
All subjects underwent a standard battery of routine, silver and immunohistochemical analysis as have been previously described in detail[24].Tau immunohistochemistry was completed with antibodies that recognize hyperphosphorylated tau (AT8, 1/1000 dilution: Endogen, Woburn, MA). All subjects were diagnosed as having AG if on histological sections there was the finding of silver and tau-positive spindle-shaped lesions in transentorhinal and entorhinal cortex, amygdala or temporal allocortex (Figure 1).[19] The density of NFT was also assessed and all subjects were given a Braak stage according to published criteria[7]. The presence of diffuse and neuritic plaque density was also document according to both Khachaturian [23] and CERAD criteria[29]. Khachaturian criteria was determined with modified Bielschowsky, while CERAD criteria was determined with tau immunohistochemistry as previously reported.[25]
Figure 1.
Tau immunohistochemistry demonstrating the presence of typical AG (arrows highlight a few examples) in the entorhinal cortex. The AG are seen as spindle shaped dot-like structures of varying size throughout the brain parenchyma
We also identified a control group for the MRI analysis only, which consisted of a pathologically defined cohort of individuals without pathological finding of AG, or any neurodegenerative or vascular disease who had been followed longitudinally in our ADRC or ADPR and had one volumetric head MRI scan.
Imaging Analysis
Patterns of regional grey matter atrophy in the groups of subjects with AG were compared with the normal control group using voxel-based morphometry (VBM). T1-weighted volumetric MRI scans were acquired at 1.5T (TE = minimum full 5ms, TR = 23ms, 22×16.5cm FOV, 25° flip angle, 124 contiguous 1.6mm thick coronal slices). An optimized method of VBM was applied [1, 32], implemented using SPM2 (http://www.fil.ion.ucl.ac.uk/spm). In order to reduce any potential normalization bias across the disease groups’ customized templates and prior probability maps were created from all subjects in the study. To create the customized template and priors all images were registered to the Montreal Neurological Institute (MNI) template using a 12 degrees of freedom (dof) affine transformation and segmented into grey matter (GM), white matter (WM) and CSF using MNI priors. GM images were normalized to the MNI GM prior using a nonlinear discrete cosine transformation (DCT). The normalization parameters were applied to the original whole head and the images were segmented using the MNI priors. Average images were created of whole head, GM, WM and CSF, and smoothed using 8mm full-width at half-maximum (FWHM) smoothing kernel. All images were then registered to the customized whole brain template using a 12dof affine transformation and segmented using the customized priors. The GM images were normalized to the custom GM prior using a nonlinear DCT. The normalization parameters were then applied to the original whole head and the images were segmented once again using the customized priors. All images were modulated and smoothed with an 8mm FWHM smoothing kernel. Two-sided t-tests were used to analyze the smoothed modulated images from the AG groups with and without dementia compared to controls and to each other.
In addition, whole brain volume and total intracranial volume (TIV) were estimated from the raw T1 weighted MRI using MIDAS image analysis software as previously described[13, 38]. Whole-brain volumes were normalized to TIV and the median normalized volumes in the AG group and control group were compared.
Statistical analysis
Statistical analyses were performed utilizing the JMP computer software (JMP Software, version 5.1.2; SAS Institute Inc, Cary, NC) with statistical significance set at p < 0.05. Mann-Whitney-U test was used to compare the ages of death, ages of scan, Braak stages, and TIV-corrected whole brain volumes in subjects with and without dementia and in subjects versus controls. A comparison of gender ratios and whether or not a subject met CERAD or Khachaturian criteria and Braak stages between cases of AG with and without dementia were analyzed using a Chi-squared test. Fisher’s Exact test was used for cells with small numbers. The Spearman Rank test was used to correlate Mini-Mental State Examination (MMSE) scores and Braak scores. A linear regression model was used to determine if Braak scores predicted MMSE scores. The Hochberg method was used to adjust for multiple comparisons by grouping the analysis into clinical comparisons, NFT pathology comparisons, and plaque pathology comparisons. [15]
RESULTS
From a review of 359 cases of autopsy confirmed subjects from our ADRC and ADPR we identified 57 cases (16%) with AG. Thirty five of these were excluded from subsequent analysis because of the presence of at least one additional pathology, leaving 22 cases with AG and a Braak stage of IV or less,[7] for analysis (Table 1).
Table 1.
Demographics, clinical and pathological features of all 22 cases with AG
| Cases | Sex | Age at death | Last MMSE prior to death | Khachaturian | CERAD | Braak stage |
|---|---|---|---|---|---|---|
| AG with dementia | ||||||
| 1 | F | 91 | 18 | No | A | IV |
| 2 | M | 83 | 19 | No | A | III–IV |
| 3 | M | 92 | 23 | Yes | A | IV |
| 4 | F | 96 | 20 | Yes | B | IV |
| 5 | F | 100 | 21 | Yes | A | IV |
| 6 | F | 90 | 5 | Yes | A | IV |
| 7 | M | 88 | 28 | Yes | B | IV |
| 8 | M | 77 | 22 | No | 0 | III |
| AG without dementia | ||||||
| 9 | F | 78 | 29 | No | 0 | II |
| 10 | M | 80 | 29 | Yes | 0 | I |
| 11 | F | 90 | 28 | Yes | A | IV |
| 12 | F | 94 | 30 | Yes | A | IV |
| 13 | F | 89 | 28 | No | 0 | III |
| 14 | M | 74 | 30 | No | 0 | II |
| 15 | M | 92 | 22 | Yes | A | IV |
| 16 | F | 101 | NT | Yes | A | III |
| 17 | F | 91 | 26 | Yes | A | II |
| 18 | F | 85 | 26 | Yes | 0 | III |
| 19 | F | 90 | 29 | Yes | 0 | III–IV |
| 20 | F | 86 | 23 | No | 0 | III |
| 21 | F | 91 | 29 | No | 0 | 0 |
| 22 | M | 86 | 26 | No | 0 | IV |
Features of all AG subjects
Of the 22 subjects with AG, 64% were female and the median age at death was 90 years old. Only eight subjects (36%) of all 22 cases with AG had been given a diagnosis of dementia by a behavioral neurologist prior to death (Table 2). Of the 64% without a diagnosis of dementia, the majority were recruited into the ADRC or ADPR as a normal control and remained cognitively normal. There was however a subset of the non-demented subjects (less than a third) in which cognitive impairment was considered, however was always mild, and in most cases non-progressive. None of these cases were ever diagnosed as demented.
Table 2.
Detailed clinical information on the 8 cases of AG with dementia
| Cases | Age at onset | Illness duration | Presenting symptoms | Prominent symptoms that developed later in the disease course |
|---|---|---|---|---|
| 1 | 84 | 7 | Memory loss, fatigue, hypersomnolence | Parkinsonism and difficult with gait |
| 2 | 74 | 9 | Memory loss, problems with directions | Worsening of cognitive impairment |
| 3 | 85 | 7 | Memory loss | Worsening of cognitive impairment |
| 4 | 90 | 6 | Memory loss | Apathy and gait impairment with ataxia of gait |
| 5 | 97 | 3 | Behavioral changes, short tempered, decreased interactions, memory loss | Depression-like episodes including crying spells |
| 6 | 87 | 3 | Paranoid, frightened after dark, hallucinations, memory loss | None reported |
| 7 | 87 | 1 | Memory loss | Wandering |
| 8 | 73 | 4 | Memory loss | Gait impairment and visual hallucinations |
All of the 22 subjects had a Braak score of IV or less,[7] which was by design. None of the subjects met CERAD criteria-C for Alzheimer’s disease[29], that is, histological evidence of neuritic plaques density indicating a diagnosis of Alzheimer’s disease, and only two subjects met CERAD criteria-B[29], that is, evidence suggesting a diagnosis of Alzheimer’s disease. These two subjects had a clinical diagnosis of dementia. The remaining subjects met CERAD criteria 0 (N=9; no evidence of Alzheimer’s disease) or CERAD criteria-A (N=11; findings uncertain). Fifty nine percent of the subjects had diffuse plaques and did meet Khachaturian criteria for Alzheimer’s disease.[23]
Features of the subjects with dementia
Of the 22 subjects with AG, eight had been given a dementia diagnosis prior to death (Table 2). All eight subjects met DSM IV criteria[2] for dementia as all had memory loss, impairment in at least one other cognitive domain and impairment of their activities of daily living secondary to their cognitive dysfunction. The median age at onset of the dementia was 87 years old with disease duration of approximately five years. The most common presenting symptom was forgetfulness in six of the eight subjects. One subject presented with predominant behavioral changes and some memory loss, while the eighth subject presented with memory loss, paranoia and visual hallucinations. The median MMSE[12] score prior to death for the subjects with dementia was 20.5/30 (range: 5–28/30).
A comparison between the demented cases with AG and the non-demented cases with AG is shown in Table 3. There was no difference in gender, or age at death, however the MMSE was significantly lower in the subjects with dementia at the last examination prior to death (P=0.002). There was no difference between the time from MMSE to death, between the two groups (P=0.3). The median Braak score[7] was higher in those with dementia (p=0.02) compared to those without dementia. The MMSE scores correlated with the Braak scores (r = −0.36; P=0.03) and predicted the Braak scores in a linear regression model (p=0.03).
Table 3.
A Comparison of subjects with AG with and without dementia
| AG with dementia (N=8) | AG without dementia (N=14) | P value | |
|---|---|---|---|
| Female gender | 4 (50%) | 10 (71%) | ns |
| *Age at onset of dementia | 87 years (73–97) | n/a | n/a |
| *MMSE score prior to death | 20.5 (5–28) | 28 (22–30) | 0.002 |
| *Age at death | 90.5 years (77–100) | 89.5 years (74–101) | ns |
| *Braak stage | 4 (3–4) | 3 (0–4) | 0.02 |
| Number of subjects with Braak stage ≤ 2 | 0 (0%) | 5 (36%) | ns |
| Number of subjects that fulfilled Khachaturian criteria | 5 (63%) | 8 (57%) | ns |
| Number of subjects that fulfilled CERAD criteria - C | 0 (0%) | 0 (0%) | ns |
| Number of subjects that fulfilled CERAD criteria – B | 2 (25%) | 0 (0%) | ns |
| Number of subjects that fulfilled CERAD criteria - A | 5 (63%) | 5 (38%) | ns |
| βNumber of subjects that fulfilled CERAD criteria - 0 | 1 (13%) | 9 (64%) | 0.03 |
| Number of subjects that fulfilled NIA-Reagan criteria for AD | 0 (0%) | 0 (0%) | ns |
ns = not significant, n/a = not applicable; MMSE = Mini-mental State Examination
Values shown are medians (range)
Not significant after adjusting for multiple comparisons
Imaging findings
Volumetric MRI was available in 12 subjects with AG and seven subjects without AG who met our inclusion and exclusion criteria and had adequate volumetric MRI. There was no difference between these groups in terms of gender (AG = 8F:4M, controls = 7F:1M), median Braak score[7] (AG =3, controls =3), or age at scan, although there was a trend for a younger median age at scan in the AG group (AG = 85 (range 71–91), controls = 89 (73–96)). Of the 12 subjects with AG that had a volumetric MRI scan, only three (2F:1M) had been given a dementia diagnosis prior to death. The median age at scan in these three subjects was 84 (81–91). The AG subjects with dementia had a significantly higher Braak score than the AG subjects without dementia (4 vs. 2, p=0.02).
The VBM comparison of all 12 subjects with AG compared to the seven controls without AG showed no regions of significant grey matter volume loss in the AG subjects at a threshold of p<0.001 (uncorrected for multiple comparisons). Similarly, in the reverse comparison no regions showed greater volume loss in the control group than the AG group (p<0.001, uncorrected).
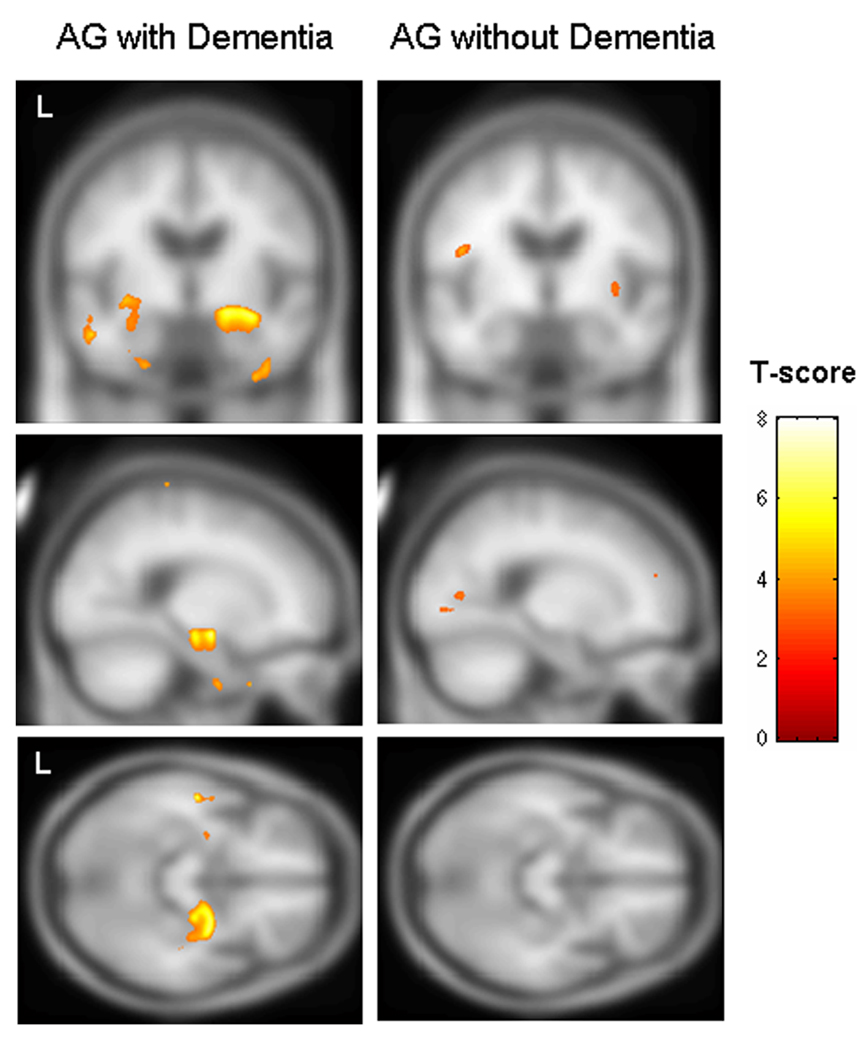
The three AG subjects that had been given a dementia diagnosis prior to death were also compared to the control group. In this comparison, regions of grey matter volume loss were identified bilaterally in the anterior hippocampus/amygdala complex and inferior temporal lobes (p<0.005, uncorrected, Figure 2). Volume loss in the right hippocampus remained at a more stringent threshold of p<0.001. A comparison of the remaining nine AG subjects without dementia to controls showed only a few tiny scattered regions of loss p<0.005 (Figure 2) which completely disappeared at a threshold of p<0.001. A direct comparison between the subjects with AG with and without dementia again confirmed greater hippocampal/amygdala volume loss in the demented subjects (p<0.001, uncorrected). No regions showed greater loss in the subjects without dementia compared to those with dementia (p<0.001, uncorrected). No regions in any of the above comparisons survived a correction for multiple comparisons over the whole brain volume (p<0.05).
Figure 2.
Voxel-based morphometry results showing bilateral volume loss of the anterior hippocampus/amygdala complex and inferior temporal lobes in the group of subjects with AG and dementia (n=3) compared to the control group (left panel), and scattered insignificant loss in the group of AG subjects without dementia (n=9) compared to controls (right panel) (p<0.005, uncorrected for multiple comparisons). Results have been overlaid on the smoothed customized template.
There was no significant difference in TIV-corrected whole brain volumes between the 12 subjects with AG and the seven controls. In addition, there was no significant difference in corrected whole brain volume between the AG subjects with and without dementia.
DISCUSSION
In this study of prospectively collected clinical data, the majority of subjects with AG did not have a dementia. We also found hippocampal atrophy in the three demented subjects, a feature that was absent in the group without dementia.
Argyrophilic grains were found in demented and non-demented subjects similar to reports by other groups.[26, 36] The dementia versus non-dementia diagnoses were supported by the significant difference in MMSE scores across the two groups. Presenting features of those with dementia always included a complaint of forgetfulness, which was most commonly, the only initial complaint. In two subjects there were additional complaints, in one behavioral dyscontrol and in the other paranoia and hallucinations. These findings differ from what has been typically reported in other clinicopathological series of AG. While Parkinson’s disease has been reported as the premortem diagnosis in two subjects with AG[36], the features reported by Ikeda and colleagues[17] are most often associated with the presence of AG. These include emotional and personality changes with aggression and ill temper. It should be noted however, although not frequently cited, that memory disturbance was found in all four of the subjects in that series. Therefore, based on this study and another[35] memory loss seems to be the most common presentation in subjects with AG, similar to Alzheimer’s disease.
The presence of AG mostly in non-demented subjects has also been reported by others,[26, 36] however there is a difference in opinion in the interpretation. Some have interpreted this as AG being a disease, and have argued that the findings of AG in non-demented subjects suggest a preclinical stage of so-called ‘argyrophilic grain disease’. One study found an increased density of AG in the posterior CA1 subfield of the hippocampus in demented subjects compared to non-demented subjects[36] while another study found that demented subjects had more atrophy of the ambient gyrus of the antero-medial temporal lobe compared to non-demented subjects.[30] This latter study subsequently resulted in a proposed staging scheme for AG. [31] Both these authors have argued for the concept of argyrophilic grain disease independently contributing to dementia. Others have argued against this concept.[26]
Even with our very stringent criteria to obtain the most ‘pure’ cases of AG, we still found more Alzheimer’s-type pathology in those with dementia. In fact the Braak scores correlated with the MMSE. Furthermore, we demonstrated that the hippocampus was atrophic only in those with dementia, even though the total corrected brain volumes were no different between those with and without dementia. The results of the VBM analysis are therefore useful in the antemortem clinical prediction of the presence of AG; specifically, in those patients with dementia. In patients with dementia in which there is MRI evidence of hippocampal and amygdala atrophy, the differential diagnosis should include Alzheimer’s disease,[8, 18] frontotemporal dementia, [37, 39]and now AG with Alzheimer’s type pathology. In the context of a very old patient however, the differential should be narrowed to Alzheimer’s disease and AG with Alzheimer’s-type pathology, since frontotemporal dementia is predominantly a presenile dementia.[21] Differentiating Alzheimer’s disease from AG may even be possible as atrophy of the medial temporal was demonstrated to be less severe in AG compared to Alzheimer’s disease. [35]
The combination of forgetfulness, higher Braak score and hippocampal atrophy in our demented group is highly reminiscent of Alzheimer’s disease, yet none of these subjects met pathological criteria for Alzheimer’s disease.[16] There are three possible explanations for these findings. First, AG is a distinct disease process sufficient to cause a dementia and the concept of argyrophilic grain disease is valid. Second, the presence of AG by itself is insufficient to cause dementia but in the presence of neurofibrillary tangle pathology, even if the second pathology is minimal, can result in dementia. And third, the finding of AG is secondary to the aging process only, and dementia is associated with the presence of the Alzheimer’s-type pathology. The fact that the VBM analysis did not show any signature pattern of volume loss in the 22 subjects with AG is not a sufficient argument against the presence of AG being a distinct disease process causing dementia. Voxel based morphometric studies have revealed an absence of atrophy in subjects with dementia syndromes including pathologically confirmed frontotemporal dementia.[22] The presence of AG mainly in the non-demented subjects may argue against AG being a distinct disease process, but does not imply that AG is a benign pathology, as Lewy bodies, neuritic plaques, and neurofibrillary tangles have also been reported in non-demented subjects. [25] The presence of a higher Braak score however in those with dementia suggests that AG may lower the threshold for dementia. Given the age of onset of the demented cohort (almost 90 years old), it is likely that at this old age, there is less cognitive reserve, and hence dementia occurs in the presence of AG plus less severe Alzheimer’s-type pathology. The presence of AG as an additive pathology resulting in dementia is a view that has been supported by others.[33]
We chose to limit our cases to those with a Braak stage of IV or less, which according to consensus criteria excludes subjects with a high probability of Alzheimer’s disease,[16] as this could be a confounder. Furthermore, when we limited our analysis to cases of Braak stages 0-II[7] (results not shown), a similar trend was noted, although not significant. It should also be pointed out that in our eight cases with dementia none had a Braak stage of 0, I or II. Therefore of our 22 cases with AG we did not find any case with dementia and a low Braak score. This is similar to another report looking at the impact of Alzheimer’s disease pathology on dementia in subjects with AG where the authors state that all their AG cases with dementia were found to have neurofibrillary tangles.[33] And while subjects with AG and dementia and low Braak scores have been described, secondary pathologies that could account for the dementia, for example hippocampal sclerosis, vascular lesions, Lewy body disease, or progressive supranuclear palsy, were not necessarily excluded. In one study describing three subjects of ‘pure’ AG with dementia, one subject had Lewy body disease, and another, severe white matter lesions.[11] The third subject did not have any ‘significant’ secondary pathologies but was graded as a Braak score II[7] and also had mild white matter lesions. At least 80 % of cases of AG have a secondary pathology.[20]
The strengths of our study are that the subjects were prospectively studied; pathological diagnosis was limited to only cases of relatively pure AG without any secondary pathology, the control group was also pathologically diagnosed, and the application of the technique of VBM which is automated and unbiased. Limitations include the small number of subjects with dementia having had a volumetric MRI, and the small number of subjects with Braak scores of 0 or I, although at this age range of 90, cases with such low Braak scores are rare.
We were unable to demonstrate a distinct clinical syndrome associated with the presence of AG. Actually, most of our subjects with this pathology were not demented. Of those who met criteria for dementia, we found a very old age at onset, a high prevalence of memory loss as a presenting symptom, a higher Braak score, and hippocampal atrophy. These findings challenge the concept of argyrophilic grain disease as a distinct clinicopathological entity, but do not suggest that AG is a benign pathology. The findings validate the concept that AG may lower the threshold for dementia, and therefore dementia in the very old may be occurring as a result of AG plus milder Alzheimer-type pathology.
ACKNOWLEDGEMENTS
This study was supported by the NIH Roadmap Multidisciplinary Clinical Research Career Development Award Grant (K12/NICHD)-HD49078, by grants P50 AG16574, U01 AG06786 and R01 AG11378 from the National Institute on Aging, Bethesda MD and the generous support of the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program of the Mayo Foundation, U.S.A.
We would like to acknowledge Professor Nick Fox at the Dementia Research Centre, London, UK, for providing the MIDAS image analysis software.
Footnotes
DISCLOSURE STATEMENT
There are no conflicts on interest, or financial disclosures. The manuscript is not being considered for publication by any other journal. The corresponding author takes full responsibility of the integrity of the entire data. All authors have seen and agree to the final submitted version
References
- 1.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 2.Association AP. Washington DC: American Psychiatric Association; 1994. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 3.Botez G, Schultz C, Ghebremedhin E, Bohl J, Braak E, Braak H. [Clinical aspects of “argyrophilic grain disease”] Nervenarzt. 2000;71:38–43. doi: 10.1007/s001150050005. [DOI] [PubMed] [Google Scholar]
- 4.Braak H, Braak E. Argyrophilic grain disease: frequency of occurrence in different age categories and neuropathological diagnostic criteria. J Neural Transm. 1998;105:801–819. doi: 10.1007/s007020050096. [DOI] [PubMed] [Google Scholar]
- 5.Braak H, Braak E. Argyrophilic grains: characteristic pathology of cerebral cortex in cases of adult onset dementia without Alzheimer changes. Neurosci Lett. 1987;76:124–127. doi: 10.1016/0304-3940(87)90204-7. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Braak E. Cortical and subcortical argyrophilic grains characterize a disease associated with adult onset dementia. Neuropathol Appl Neurobiol. 1989;15:13–26. doi: 10.1111/j.1365-2990.1989.tb01146.x. [DOI] [PubMed] [Google Scholar]
- 7.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 8.Chan D, Fox NC, Scahill RI, Crum WR, Whitwell JL, Leschziner G, Rossor AM, Stevens JM, Cipolotti L, Rossor MN. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer's disease. Ann Neurol. 2001;49:433–442. [PubMed] [Google Scholar]
- 9.Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, Jellinger K, Lantos PL, Lippa CF, Mirra SS, Tabaton M, Vonsattel JP, Wakabayashi K, Litvan I. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61:935–946. doi: 10.1093/jnen/61.11.935. [DOI] [PubMed] [Google Scholar]
- 10.Dickson DW, Davies P, Bevona C, Van Hoeven KH, Factor SM, Grober E, Aronson MK, Crystal HA. Hippocampal sclerosis: a common pathological feature of dementia in very old (> or = 80 years of age) humans. Acta Neuropathol (Berl) 1994;88:212–221. doi: 10.1007/BF00293396. [DOI] [PubMed] [Google Scholar]
- 11.Ding ZT, Wang Y, Jiang YP, Yoshida M, Mimuro M, Inagaki T, Iwase T, Hashizume Y. Argyrophilic grain disease: frequency and neuropathology in centenarians. Acta Neuropathol (Berl) 2006;111:320–328. doi: 10.1007/s00401-006-0043-2. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Freeborough PA, Fox NC. The boundary shift integral: an accurate and robust measure of cerebral volume changes from registered repeat MRI. IEEE Trans Med Imaging. 1997;16:623–629. doi: 10.1109/42.640753. [DOI] [PubMed] [Google Scholar]
- 14.Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Neurology. 1994;44:2015–2019. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- 15.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrica. 1988;75:800–802. [Google Scholar]
- 16.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda K, Akiyama H, Arai T, Matsushita M, Tsuchiya K, Miyazaki H. Clinical aspects of argyrophilic grain disease. Clin Neuropathol. 2000;19:278–284. [PubMed] [Google Scholar]
- 18.Jack CR, Jr, Petersen RC, O'Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- 19.Jellinger KA. Dementia with grains (argyrophilic grain disease) Brain Pathol. 1998;8:377–386. doi: 10.1111/j.1750-3639.1998.tb00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jicha GA, Petersen RC, Knopman DS, Boeve BF, Smith GE, Geda YE, Johnson KA, Cha R, Delucia MW, Braak H, Dickson DW, Parisi JE. Argyrophilic grain disease in demented subjects presenting initially with amnestic mild cognitive impairment. J Neuropathol Exp Neurol. 2006;65:602–609. doi: 10.1097/01.jnen.0000225312.11858.57. [DOI] [PubMed] [Google Scholar]
- 21.Johnson JK, Diehl J, Mendez MF, Neuhaus J, Shapira JS, Forman M, Chute DJ, Roberson ED, Pace-Savitsky C, Neumann M, Chow TW, Rosen HJ, Forstl H, Kurz A, Miller BL. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Arch Neurol. 2005;62:925–930. doi: 10.1001/archneur.62.6.925. [DOI] [PubMed] [Google Scholar]
- 22.Josephs KA, Whitwell JL, Jack CR, Jr, Parisi JE, Dickson D. Frontotemporal lobar degeneration without lobar atrophy. Arch Neurol. doi: 10.1001/archneur.63.11.1632. In Press. [DOI] [PubMed] [Google Scholar]
- 23.Khachaturian ZS. Diagnosis of Alzheimer's disease. Arch Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 24.Knopman DS, Boeve BF, Parisi JE, Dickson DW, Smith GE, Ivnik RJ, Josephs KA, Petersen RC. Antemortem diagnosis of frontotemporal lobar degeneration. Ann Neurol. 2005;57:480–488. doi: 10.1002/ana.20425. [DOI] [PubMed] [Google Scholar]
- 25.Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Lage P, Munoz DG. Prevalence and disease associations of argyrophilic grains of Braak. J Neuropathol Exp Neurol. 1997;56:157–164. doi: 10.1097/00005072-199702000-00006. [DOI] [PubMed] [Google Scholar]
- 27.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 28.McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 29.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 30.Saito Y, Nakahara K, Yamanouchi H, Murayama S. Severe involvement of ambient gyrus in dementia with grains. J Neuropathol Exp Neurol. 2002;61:789–796. doi: 10.1093/jnen/61.9.789. [DOI] [PubMed] [Google Scholar]
- 31.Saito Y, Ruberu NN, Sawabe M, Arai T, Tanaka N, Kakuta Y, Yamanouchi H, Murayama S. Staging of argyrophilic grains: an age-associated tauopathy. J Neuropathol Exp Neurol. 2004;63:911–918. doi: 10.1093/jnen/63.9.911. [DOI] [PubMed] [Google Scholar]
- 32.Senjem ML, Gunter JL, Shiung MM, Petersen RC, Jack CR., Jr Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. Neuroimage. 2005;26:600–608. doi: 10.1016/j.neuroimage.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thal DR, Schultz C, Botez G, Del Tredici K, Mrak RE, Griffin WS, Wiestler OD, Braak H, Ghebremedhin E. The impact of argyrophilic grain disease on the development of dementia and its relationship to concurrent Alzheimer’s disease-related pathology. Neuropathol Appl Neurobiol. 2005;31:270–279. doi: 10.1111/j.1365-2990.2005.00635.x. [DOI] [PubMed] [Google Scholar]
- 34.Togo T, Cookson N, Dickson DW. Argyrophilic grain disease: neuropathology, frequency in a dementia brain bank and lack of relationship with apolipoprotein E. Brain Pathol. 2002;12:45–52. doi: 10.1111/j.1750-3639.2002.tb00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Togo T, Isojima D, Akatsu H, Suzuki K, Uchikado H, Katsuse O, Iseki E, Kosaka K, Hirayasu Y. Clinical features of argyrophilic grain disease: a retrospective survey of cases with neuropsychiatric symptoms. Am J Geriatr Psychiatry. 2005;13:1083–1091. doi: 10.1176/appi.ajgp.13.12.1083. [DOI] [PubMed] [Google Scholar]
- 36.Tolnay M, Schwietert M, Monsch AU, Staehelin HB, Langui D, Probst A. Argyrophilic grain disease: distribution of grains in patients with and without dementia. Acta Neuropathol (Berl) 1997;94:353–358. doi: 10.1007/s004010050718. [DOI] [PubMed] [Google Scholar]
- 37.van de Pol LA, Hensel A, van der Flier WM, Visser PJ, Pijnenburg YA, Barkhof F, Gertz HJ, Scheltens P. Hippocampal atrophy on MRI in frontotemporal lobar degeneration and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2006;77:439–442. doi: 10.1136/jnnp.2005.075341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitwell JL, Crum WR, Watt HC, Fox NC. Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. AJNR Am J Neuroradiol. 2001;22:1483–1489. [PMC free article] [PubMed] [Google Scholar]
- 39.Whitwell JL, Sampson EL, Watt HC, Harvey RJ, Rossor MN, Fox NC. A volumetric magnetic resonance imaging study of the amygdala in frontotemporal lobar degeneration and Alzheimer's disease. Dement Geriatr Cogn Disord. 2005;20:238–244. doi: 10.1159/000087343. [DOI] [PubMed] [Google Scholar]