Abstract
Puberty marks the advent of adolescence and plays an important role in many changes and adjustments that adolescents must face. Pubertal maturation is advanced by sex hormones, yet it is not clear how best to measure puberty and how well existing measures capture hormone levels. We compared multiple indices of puberty to determine their interrelationships, including the Pubertal Development Scale (PDS), a picture-based interview about puberty (PBIP) and a physical exam. We also examined how physical pubertal measures were associated with basal hormones responsible for advancing pubertal development. Participants included 160 early adolescents (82 boys, 78 girls), 9-14 years of age. Basal hormones were derived using hierarchical linear modeling from 32 repeated saliva samples of testosterone and dehydroepiandrosterone (DHEA) in both sexes and 5 repeated measures of estradiol in girls. The two self-report measures were moderately concordant with the exam and with each other, with approximately half of the adolescents self-reporting the same stage as the physical exam. The different indices of puberty were highly correlated with each other, suggesting that self-report may be adequate when precise agreement is not necessary. Nevertheless, adolescents who were substantially more or less physically developed than their same-aged peers were most likely to self-report a stage that was different from the physical exam. The physical exam stages correlated well with boys' and girls' testosterone and DHEA, and less so with girls' estradiol. With a few exceptions, the PDS and PBIP were generally related to basal hormones in parallel with the exam. Multiple measures of pubertal development are viable options, each with respective strengths.
Introduction
Adolescence constitutes a transition between childhood and adulthood whose onset includes pubertal maturation. Puberty has important implications for the development of regulatory competence and many aspects of physical, emotional, cognitive and social development, including decision-making and mental health (Steinberg et al., 2006). For these reasons, biobehavioral researchers increasingly seek to examine measures of puberty to clarify studies of emotion-related neural circuitry (Nelson, Leibenluft, McClure, & Pine, 2005; Sisk & Foster, 2004), psychopathology (Angold & Worthman, 1993; Cyranowski, Frank, Young, & Shear, 2000), cognition (Steinberg, 2005), and behavioral changes (Carskadon, Acebo, Jenni, Dahl, & Spear, 2004; Steinberg, 2000). Yet, it is not clear how to best evaluate pubertal development (Brooks-Gunn, Warren, Rosso, & Gargiulo, 1987). Here, we compare several measures of pubertal maturation, including hormonal indices. The main hormones responsible for advancing secondary sexual characteristics were captured by measuring testosterone and dehydroepiandrosterone (DHEA), two androgens which facilitate masculine development, and estradiol, an estrogen which facilitates feminine development. We evaluated agreement between physical exam and different methods of self-report; the associations between hormones and the physical exam; and the extent to which self-report methods led to parallel relationships with hormonal measures as did the physical exam.
Physical Measures of Puberty
Nearly five decades ago, Tanner (1962) described five stages of puberty, ranging from 1 (no development) to 5 (adult development). These stages capture visible secondary sexual characteristics such as breast/genital development and pubic hair growth. Since its introduction, the gold standard for measuring pubertal status has been a physical exam conducted by a clinician employing Tanner's methods (Dorn, Dahl, Woodward, & Biro, 2006). Yet, researchers often find it difficult to integrate physical exams into non-clinical settings and the Tanner stages only measure one dimension of development-- external signs of physical development. To address these problems, the Pubertal Development Scale (PDS) asks adolescents to answer less invasive questions about puberty without mapping directly onto Tanner stages (Petersen, Crockett, Richards, & Boxer, 1988). The Kappa (κ) concordance between the physical exam and PDS is only .24 (Brooks-Gunn et al., 1987). An alternative self-report method maps directly onto Tanner stages. Adolescents examine photographs or line drawings of models at each Tanner stage and indicate which image they most closely resemble (Morris & Udry, 1980). Although easy to administer, there is only moderate agreement between the physical exam and various versions of self-reported Tanner Stage, with an average κ around .50 (see review by Coleman & Coleman, 2002; as well as more recent work by Desmangles, Lappe, Lipaczewski, & Haynatzki, 2006; Hergenroeder, Hill, Wong, Sangi-Haghpeykar, & Taylor, 1999; Schmitz et al., 2004). A few frequently cited studies, however, report excellent agreement (κs above .70) (Boas, Falsetti, Murphy, & Orenstein, 1995; Carskadon et al., 1980; Norris & Richter, 2005). Agreement between self-reported PDS and self-reported Tanner Stage was also moderate, κ=.50 (Bond et al., 2006). In addition to examining the agreement between multiple puberty measures, we also explored whether certain demographic factors predicted which adolescents had low agreement between puberty measures.
Hormonal Measures of Puberty
Although steroid hormones advance pubertal maturation, there is an imperfect match of hormones with physical measures of puberty for several reasons. Hormone levels change across the day; there are individual differences in hormone concentrations necessary to advance puberty; and there is overlap in hormone levels across each pubertal stage (Dawes et al., 1999). Nevertheless, knowledge about underlying hormonal processes provides information about pubertal maturation not available from overt physical measures alone.
The earliest peripheral sign of puberty occurs when androgens begin to be released gradually from the adrenal gland (Palmert et al., 2001). DHEA and other adrenal androgens cause pubic hair growth, body odor, acne, and pre-pubertal growth (Havelock, Auchus, & Rainey, 2004; Lucky, Biro, Simbartl, Morrison, & Sorg, 1997). Adrenal androgens increase two-fold in boys from when they show no pubertal development to when they reach adult-like development (Biro, Lucky, Huster, & Morrison, 1995). Puberty shows moderate correlations with DHEA in both sexes (Shirtcliff, Zahn-Waxler, Klimes-Dougan, & Slattery, 2007), although another study failed to detect a relationship in boys or girls (Maskarinec et al., 2005). Testosterone, the primary androgen released from the gonads (Rubinow & Schmidt, 1996), causes genital development in males (Hiort, 2002). Boys with delayed puberty show rapidly advancing pubertal maturation when administered testosterone (Finkelstein et al., 1999; Geller, Rogol, & Knitter, 1983). Testosterone is approximately 45 times higher by adulthood as compared to pre-pubertal development in boys (Biro et al., 1995), but the rise is smaller in girls (Legro, Lin, Demers, & Lloyd, 2000). Puberty and testosterone are highly correlated in boys, but no clear association is evident in girls (Granger et al., 2003; Maskarinec et al., 2005). Estradiol is the primary estrogen released from the gonads and other peripheral tissues (Fernandez-Garcia et al., 2002). Estradiol causes breast development, encourages female-typical fat distributions and long bone fusion during growth spurts, and helps stimulate ovulation and menstruation (Frank, 2003; MacGillivray, Morishima, Conte, Grumbach, & Smith, 1998). Girls with delayed puberty show advancing pubertal maturation when administered estradiol (Finkelstein et al., 1999; Rosenfield et al., 2005). Estradiol is 4-9 times higher in late adolescent girls as compared to childhood (Ikegami et al., 2001). Although more hormonal signals are involved, measuring these three hormones should provide converging information about gonadal and adrenal hormonal signals of puberty, two distinct components of maturation in early adolescents.
Methods
Participants were 82 boys and 78 girls recruited from the community through an existing laboratory registry and local advertisements. Adolescents ranged from 9 through 14 years of age (M=11.2 years), capturing early adolescence when pubertal stage is most variable. Exclusion criteria included use of allergy or asthma medication. Participants were from diverse backgrounds; Hollingshead scores spanned the full socioeconomic gradient (M=41.9, SD=15.6, range=5-66). Forty-eight percent of participants were White; 26% were Black; 26% were Asian, Hispanic, Mixed or unspecified. Body Mass Index (BMI) was 21.8 on average (SD=9.3), with 21% of the sample above the 95th percentile of BMI for age (overweight) and 2% below 5th percentile (underweight).
Procedures
Adolescents and their parent(s) provided informed assent and consent, respectively. Adolescents completed the self-report measures, and then a pediatric nurse practitioner (PNP) conducted the physical exam. Participants provided saliva throughout the laboratory day and were sent home with supplies for additional saliva collection. Procedures were approved by the University of Wisconsin Institutional Review Board.
Measures
Pubertal Development Scale (PDS)
Adolescents completed the five PDS questions about physical development, scored from 1 (no) to 4 (development seems complete) (Petersen et al., 1988). Reliability of the PDS was high (α=0.77 for boys, α=.81 for girls). Few (3%) adolescents had missing PDS scores. We developed a coding system to convert the PDS to a 5-point scale in order to parallel the physical exam Tanner stages (available upon request). Although inter-related, puberty is not a single event. Therefore, our coding system differentially captured gonadal and adrenal hormonal signals of physical development. In girls, growth spurt, breast development, and menarche are associated with gonadal hormonal signals. In boys, growth spurt, deepening of voice and facial hair growth are associated with gonadal hormones. For both sexes, pubic/body hair and skin changes are associated with adrenal hormones.
Picture-Based Interview about Puberty (PBIP)
In a comfortable room, a research assistant spoke with adolescents about “changes that happen when you grow up” with the assistance of a script and photographs (Dorn & Susman, 2002). Following this discussion, researchers left the room while adolescents reported their assessment of pubertal stage. Female researchers interviewed girls and male researchers interviewed half of the boys. There was no difference in accuracy of staging based on the sex of the interviewer, p>.29.
Physical Exam
Adolescents were given the option to wear a hospital gown or loose clothing for the exam. Height and weight were measured and later used to calculate BMI (age-corrected using CDC guidelines). Experienced pediatric nurse practitioners were trained to conduct exams for research purposes by the second author. PNPs inspected breast development with brief palpation for girls, and visually examined pubic hair. An orchidometer measured testicular size in boys (Genentech, 1997) along with visual inspection of genitals and pubic hair. Inter-observer reliability (N=10 exams, 6.3%) was good, κ=0.88. Thirteen percent of participants refused the exam, but assented to self-report measures. Those who refused the exam did not differ in age, race, BMI, or stage; there was a trend for boys (N=14) to refuse more than girls (N=6), χ2(1)=3.2, p=.07.
Hormonal Measures
Adolescents provided eight saliva samples across the laboratory day, beginning immediately after providing informed consent (M=9:38, SD=1:27h) through bedtime (M=21:04, SD=1:32h). Saliva was collected by passive drool (Shirtcliff, Granger, Schwartz, & Curran, 2001). At the time of each sample, participants completed a short diary which has been used previously (Granger et al, 2003). Samples were immediately frozen at -80°C until they were aliquotted to minimize freeze/thaw cycles. Participants were also sent home with supplies for home collection. To capture the full day, participants collected six samples on each of four days at pre-specified times between waking (M=7:45, SD=1:19h) and bedtime (M=21:17, SD=1:13h), prior to mealtimes. To ensure compliance, cryuvials were stored in time-locked caps (Aardex, Zug, Switzerland), which recorded collection time and date. Samples were stored in home-freezers until all samples were collected, then the batch was shipped overnight on ice and stored at -80°C (Dabbs, 1991). All 32 samples were assayed for testosterone and DHEA. One morning sample from each day of saliva collection was assayed for estradiol in girls (M=9:39, SD=0:51h). Salivary estradiol is not valid in boys (Shirtcliff et al., 2000).
Hormone Determination
Enzymeimmunoassays were completed by Madison Biodiagnostics (Madison, WI) using Salimetrics kits (State College, PA). Samples were measured in duplicate; duplicates that varied by more than 7% were repeat-tested. For DHEA, the range of sensitivity was from 5-1000 pg/mL. The average intra-assay coefficient of variation (CV) was 5.6% and the average inter-assay CV was 8.2%. For testosterone, the range of sensitivity was from 1-600 pg/mL. Average intra- and inter-assay CVs were 4.6% and 8.3%, respectively. For estradiol, the range of sensitivity was 1-32 pg/mL. Average intra- and inter-assay CVs were 7.1 and 7.5%, respectively.
A Basal Hormone measure was calculated using Hierarchical Linear Modeling which separates within-the-day and day-to-day variation in hormone levels (N=3704) from individual basal levels (N=160), thereby allowing removal of the effects of several important control variables and accurate aggregation across repeated measures of each hormone to a single basal level. At the within-the-day level, we controlled for linear and quadratic time since waking (in minutes) and time of day to account for the individual's intrinsic and extrinsic rhythm. These values were allowed to vary so that each individual had their own rhythm removed from the basal estimate. An Empirical Bayes estimate of each log-transformed hormone was extracted after accounting for additional control variables at both within-the-day and day-to-day levels (e.g., flow rate, response to awakening, location, medication usage, exercise, emotion, who the child was with). Basal DHEA comprised 73.8% of the total variation in DHEA, p<.0001. Basal Testosterone comprised 80.0% of the variation in testosterone, p<.0001. For estradiol, an average across the 5 samples/individual was calculated as analyses revealed no day-to-day predictors of estradiol (including controls above as well as menstrual cycle day-count, cycle regularity, menarcheal status). Basal estradiol comprised 36% of the variation in estradiol, p<.0001. Less variation in estradiol was basal than the other hormones, perhaps due to the reduced number of samples.
Statistical Analyses
Kappas (κ) and % accuracy described precise agreement between the three puberty measures. Pearson correlations examined whether measures were associated, without necessitating precision. To examine which adolescents were inaccurate informants, we calculated the discrepancy between the exam and the PDS and PBIP, respectively, using a difference score. We assessed whether gender, age, stage, BMI, or race influenced accuracy of adolescents' self-report using linear regression. Race was coded as ‘White’, ‘Black’ and ‘Other’. Structural equation modeling simultaneously examined how the physical exam was associated with steroid hormones, with separate models for boys and girls (since estradiol was measured in girls only). The physical exam was first modeled with basal hormones, removing non-significant coefficients. Poor model fit was indicated by significant χ2 values, CFI less than .95 or RMSEA greater than .10. Next, parallel models were fit substituting the respective self-report measures. To test whether models were parallel to the exam, we fixed coefficients to be identical to the physical exam and examined the reduction in model fit compared to when coefficients were unconstrained. If the indices of practical fit were too high/low or the χ2 was significant (indicating models were not parallel), we removed constraints on coefficients which resulted in the greatest model improvements.
Results and Discussion
How did self-report PDS map onto the physical exam?
Correlations between the physical exam and the PDS are presented in Table 1. The concordance between the physical exam and the PDS gonadal stage was modest, κ=.36, χ2(16)=93.0, p<.0001 (Table 2). Accuracy was defined as self-report of the same stage as the physical exam. Fifty-two percent of adolescents' gonadal scores were accurate (54% boys, 47% girls), while 18% overestimated (15% boys, 27% girls) and 30% underestimated stage (31% boys, 27% girls) compared to the exam. The concordance between the physical exam and the PDS adrenal stage was also modest, κ=.36, χ2(16)=90.6, p<.0001 (Table 3). Fifty percent of adolescents were accurate (60% boys, 44% girls), while 29% underestimated (26% boys, 34% girls) and 21% overestimated pubic hair (14% boys, 23% girls).
Table 1.
Intercorrelations of the Physical Exam, Picture-Based Interview about Puberty (PBIP), and Pubertal Development Scale (PDS) with girls above (in italics) and boys below (in bold).
| Physical Exam | PBIP | PDS | ||||
|---|---|---|---|---|---|---|
| Breast/Genital | Pubic Hair | Breast/Genital | Pubic Hair | Gonadal | Adrenal | |
| Physical Exam | ||||||
| Breast/Genital | .85 | .83 | .76 | .65 | .65 | |
| Pubic Hair | .93 | .75 | .88 | .69 | .71 | |
| PBIP | ||||||
| Breast/Genital | .60 | .60 | .79 | .77 | .72 | |
| Pubic Hair | .69 | .71 | .71 | .73 | .81 | |
| PDS | ||||||
| Gonadal | .65 | .63 | .59 | .68 | .72 | |
| Adrenal | .63 | .68 | .70 | .70 | .65 | |
| Mean (SD) Stage | ||||||
| Boys |
2.4 (1.3) |
2.3 (1.3) |
2.7 (1.2) |
2.3 (1.2) |
2.0 (1.1) |
2.3 (1.2) |
| Girls |
2.9 (1.5) |
2.8 (1.5) |
2.9 (1.2) |
2.7 (1.4) |
2.5 (1.3) |
2.9 (1.3) |
All intercorrelations have ps<.001. There are no mean differences in staging between the physical exam and PBIP, ps>.2, or PDS, ps>.06.
Table 2.
Concordance of the Physical Exam Breast/ Genital Stage, Pubertal Development Scale and the Picture-Based Interview about Puberty.
| A. Pubertal Development Scale (PDS) Gonadal Score | Physical Exam Breast/ Genital StageB | TotalA | ||||||
|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||||
| I. | 61.1 | 28.1 | 10.3 | 5.0 | 5.3 | 36 | ||
| II. | 19.4 | 53.1 | 20.7 | 15.0 | 5.3 | 34 | ||
| III. | 16.7 | 18.8 | 51.7 | 30.0 | 21.1 | 37 | ||
| IV. | 2.8 | 6.9 | 30.0 | 26.3 | 14 | |||
| V. | 10.3 | 20.0 | 42.1 | 15 | ||||
| Total (N) A | 36 | 32 | 29 | 20 | 19 | 136 | ||
| B. Picture-Based Interview About Puberty (PBIP) | Physical Exam Breast/ Genital StageB | |||||||
| I: No Development | 54.1 | 18.2 | 3.4 | 4.8 | 28 | |||
| II: Breast bud | 24.3 | 48.5 | 20.7 | 14.3 | 34 | |||
| II: Testes started to grow | ||||||||
| III: Breast tissue beyond areola | 18.9 | 30.3 | 48.3 | 19.0 | 4.8 | 36 | ||
| III: Penis growth in length | ||||||||
| IV: Areola second mound on breast | 2.7 | 3.0 | 17.2 | 61.9 | 61.9 | 33 | ||
| IV: Penis growth in width and length | ||||||||
| V: Adult-like development | 10.3 | 33.3 | 10 | |||||
| Total (N) A | 37 | 33 | 29 | 21 | 21 | 141 | ||
| C. Picture-Based Interview About Puberty | PDS Gonadal ScoreB | |||||||
| I. | 51.3 | 18.4 | 4.3 | 29 | ||||
| II. | 33.3 | 44.7 | 13.0 | 13.3 | 5.9 | 39 | ||
| III. | 12.8 | 28.9 | 41.3 | 26.7 | 11.8 | 41 | ||
| IV. | 2.6 | 7.9 | 32.6 | 53.3 | 52.9 | 36 | ||
| V. | 8.7 | 6.7 | 29.4 | 10 | ||||
| Total (N) A | 39 | 38 | 46 | 15 | 17 | 155 | ||
Number of Participants;
Column Percentages
Table 3.
Concordance of the Physical Exam Pubic Hair Stage, Pubertal Development Scale and the Picture-Based Interview about Puberty.
| A. Self-Report (PDS) | Physical Exam Pubic Hair StageB | |||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | TotalA | |
| I. | 77.8 | 25.8 | 18.2 | 3.8 | 48 | |
| II. | 17.8 | 51.6 | 27.3 | 23.1 | 16.7 | 38 |
| III. | 4.4 | 19.4 | 31.8 | 26.9 | 25 | 25 |
| IV. | 3.2 | 18.2 | 34.6 | 33.3 | 18 | |
| V. | 4.5 | 11.5 | 25 | 7 | ||
| Total (N)A | 45 | 31 | 22 | 26 | 12 | 136 |
| B. Picture-Based Interview About Puberty | Physical Exam Pubic Hair StageB | |||||
| I: No Development | 73.9 | 25 | 9.1 | 3.7 | 45 | |
| II: Sparse wispy strands | 19.6 | 50 | 22.7 | 3.7 | 31 | |
| III: Darker, courser hair | 6.5 | 21.9 | 36.4 | 14.8 | 14.3 | 24 |
| IV: Course hair along most of pubis | 3.1 | 31.8 | 55.6 | 35.7 | 28 | |
| V: Adult-like development, hair extends to upper thighs | 22.2 | 50.0 | 13 | |||
| Total (N)A | 46 | 32 | 22 | 27 | 14 | 141 |
| C. Picture-Based Interview About Puberty | PDS Adrenal ScoreB | |||||
| I. | 66.1 | 26.8 | 3.6 | 49 | ||
| II. | 26.8 | 36.6 | 14.3 | 9.1 | 36 | |
| III. | 7.1 | 24.4 | 32.1 | 18.2 | 12.5 | 28 |
| IV. | 12.2 | 39.3 | 63.6 | 12.5 | 31 | |
| V. | 10.7 | 9.1 | 75.0 | 11 | ||
| Total (N)A | 56 | 41 | 28 | 22 | 8 | 155 |
Number of Participants;
Column Percentages
How did the picture-based interview (PBIP) map onto the physical exam?
The concordance between the physical exam and PBIP breast/genital stage was modest, κ=.36, χ2(16)=120.9, p<.0001 (Tables 1 and 2). Forty-nine percent of adolescents reported the same breast/genital stage as the exam (41% boys, 57% girls), while 26% over-estimated (35% boys, 17% girls) and 25% underestimated stage (24% boys, 17% girls). The parallel concordance for pubic hair was good, κ=.43, χ2(16)=137.2, p<.0001 (Table 3). Fifty-six percent of adolescents reported the same pubic stage as the exam (54% boys, 58% girls), while 24% overestimated (26% boys, 21% girls) and 20% underestimated stage (19% boys, 21% girls).
How did self-report PDS map onto the PBIP?
Correlations between the two self-report measures are reported in Table 1. The concordance between the PDS gonadal stage and the breast/genital PBIP stage was low, κ=.29, χ2(16)=98.4, p<.0001 (Table 2). Forty-five percent of adolescents reported the same breast/genital PBIP stage as the PDS gonadal score (37% boys, 52% girls). The parallel concordance for pubic hair was moderate, κ=.37, χ2(16)=152.1, p<.0001 (Table 3). Fifty-two percent of adolescents reported the same pubic stage on the PBIP as the PDS (47% boys, 57% girls).
The physical exam is well-suited for a wide range of behavioral endocrinology-oriented questions or when an objective measure of physical development is desirable (Dorn et al., 2006). Though precise agreement was modest, the two self-report measures were correlated with the physical exam, suggesting they mutually capture underlying pubertal processes. If precision is not necessary, adolescents are relatively good observers. We should note that 13% of adolescents refused the physical exam but none refused the PBIP. Researchers conducting an exam might consider supplementation with a self-report measure to capture this subset.
Which adolescents were inaccurate informants?
Using linear regression, where the discrepancy between the physical exam and PDS was the outcome, we found that neither Sex nor BMI influenced accuracy, ps>.09. Stage (based on the exam) qualified an effect of age (β=.32, p<.003 for age; β=-.68 for stage, p<.001). In general, adolescents overestimated pubertal maturation when they were at lower stages of development relative to their peers and underestimated development when they were at higher stages than their peers. As expected, this distortion of staging was age-specific. For example, 11- and 12-year olds were accurate at stage 3 but overestimated stage 2 and underestimated stages 4+; 13- and 14-year olds were accurate at stage 4, but tended to overestimate stages 2 and 3 and underestimate stage 5. This may reflect the desirability of the adolescent to appear like the developmental stage that is most typical for their age. White adolescents overestimated stage more often than non-Caucasian adolescents, β=.21, p<.02.
Analyses of the discrepancies between the physical exam and PBIP yielded similar findings. Sex and BMI did not influence accuracy, ps>.15. Stage qualified the effect of age (β=.32, p<.003 for age; β=-.68, p<.001 for stage), such that adolescents sometimes overestimated development when at lower stages and underestimated development when at higher stages. Again, adolescents tended to report stages that were most typical of their age. White or Black adolescents overestimated stage more often than other adolescents, β=.19, p=.03.
Young adolescents may not be able to self-report an exact stage—particularly if they are maturing earlier or later than their peers. Measurement problems may primarily affect studies that employ a cut-score to describe adolescents as pre- or post-pubertal rather than as a continuous process; this may be especially problematic in research designed to isolate early and late maturing adolescents.
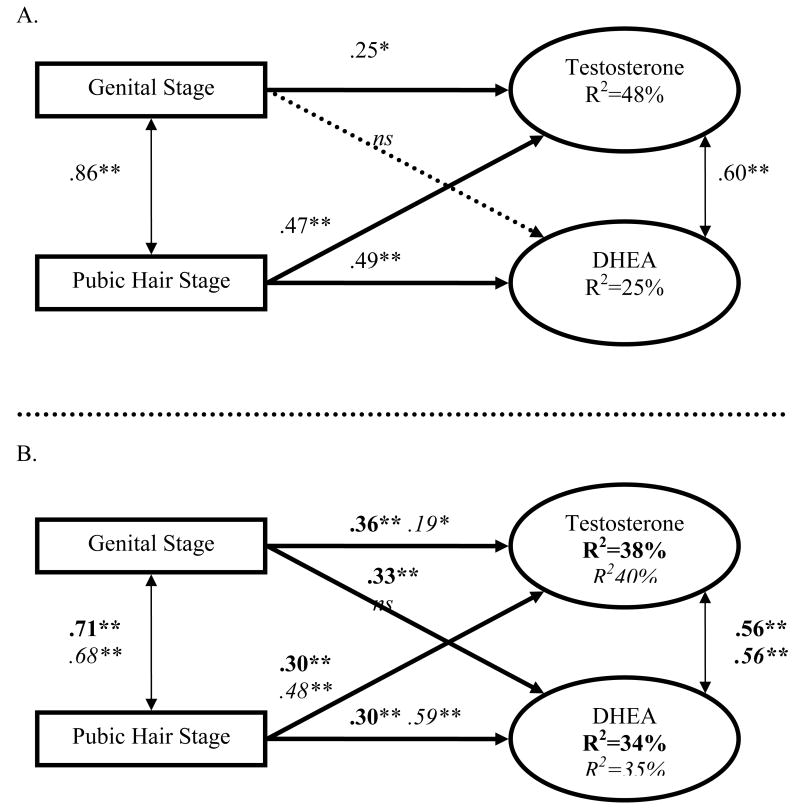
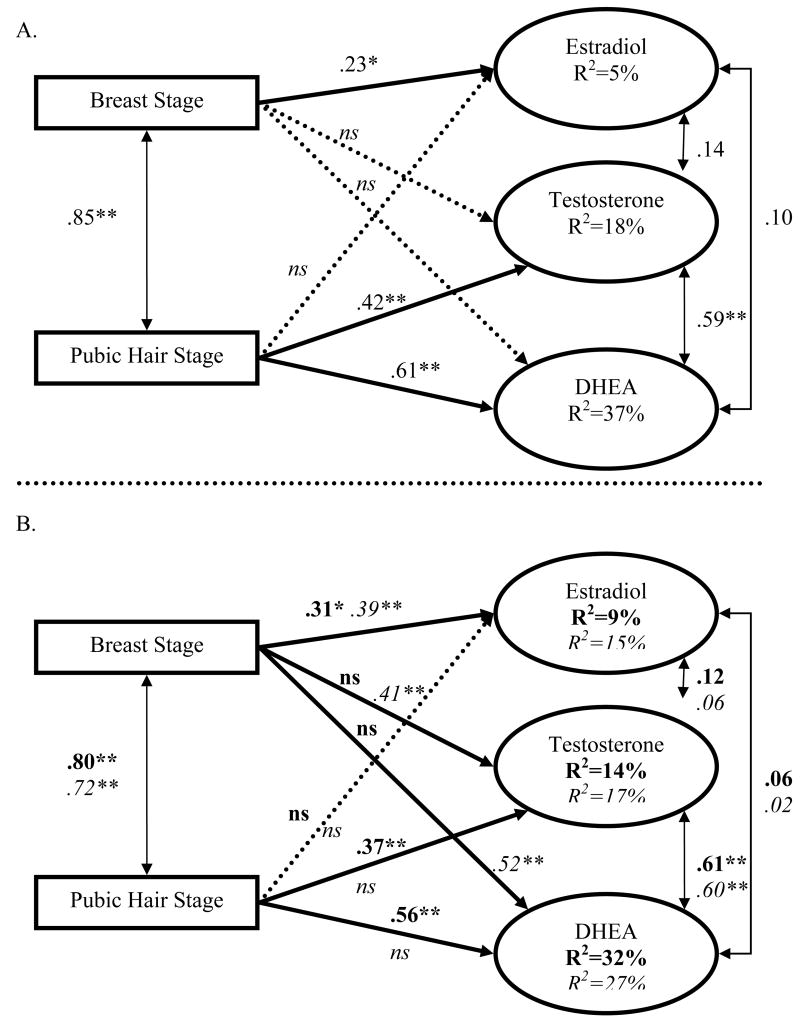
Was the physical exam stage associated with hormones? 1
Table 4 presents hormone values across Tanner stages for boys and girls. Boys' basal testosterone and DHEA were predicted by the physical exam, with one exception. Genital development was not associated with DHEA; dropping this coefficient did not change model fit, χ2(1)=.5, p=.46, CFI>.999, RMSEA<.0001 (Figure 1A). For girls, breast development was not associated with testosterone or DHEA, and pubic hair was not associated with estradiol (Figure 2A). These three coefficients were dropped without reducing the goodness of fit, χ2(3) 1.75, p=.63, CFI>.999, RMSEA<.0001. In sum, the physical exam captured basal testosterone and DHEA well in both sexes, though estradiol was modestly related to the exam.
Table 4.
Hormone Levels (and Standard Errors) in boys and girls across the physical exam stages.
| Boys | Girls | ||||
|---|---|---|---|---|---|
| Physical Exam Breast/Genital Stage | Testosterone | DHEA | Testosterone | DHEA | Basal EstradiolA |
| I | 15.90 (1.49) |
34.77 (4.93) |
18.66 (2.56) |
34.59 (6.05) |
4.36 (1.62) |
| II | 20.25 (2.90) |
42.42 (8.23) |
19.41 (2.01) |
47.09 (8.25) |
3.60 (0.44) |
| III | 29.87 (3.44) |
66.51 (13.99) |
26.93 (2.27) |
76.82 (11.97) |
3.81 (0.65) |
| IV | 54.57 (8.13) |
111.54 (22.07) |
29.85 (2.23) |
93.39 (13.97) |
5.96 (1.57) |
| VB | 50.87 (6.17) |
51.67 (9.09) |
32.12 (4.58) |
123.03 (19.95) |
3.81 (.57) |
| Physical Exam Pubic Hair Stage | |||||
| I | 16.25 (1.44) |
34.52 (5.06) |
16.86 (1.90) |
35.88 (8.48) |
4.61 (1.51) |
| II | 23.90 (3.57) |
46.77 (8.23) |
23.19 (2.21) |
48.94 (5.92) |
3.02 (0.34) |
| III | 29.38 (4.15) |
68.42 (15.42) |
27.06 (3.16) |
84.92 (13.56) |
4.10 (0.78) |
| IV | 53.23 (6.43) |
102.10 (20.25) |
29.41 (1.73) |
102.51 (14.58) |
5.58 (1.25) |
| VB | 59.80 (3.78) |
57.60 (15.52 |
34.26 (5.99) |
123.51 (22.18) |
3.85 (0.77) |
Estradiol was measured in girls only.
Five boys were genital stage V;
three were pubic stage V. Average Hormone Levels (not Basal) are presented to aid in comparison across studies.
Figure 1.
Structural Equation Model for Boys.
(A) Standardized β Coefficients when Testosterone and DHEA are Predicted by the Physical Exam Genital and Pubic Hair Stage.
(B) Parallel model to A with Testosterone and DHEA Predicted by the PDS stages (in italics) and PBIP stages (in bold). *p<.05, **p<.01; ns=non-significant.
Figure 2.
Structural Equation Model for Girls.
(A) Standardized β Coefficients when Testosterone, DHEA and Estradiol are Predicted by the Physical Exam Breast and Pubic Hair Stage.
(B) Parallel model to A with Basal Hormones Predicted by the PDS (in italics) and PBIP (in bold). *p<.05, **p<.01, ns=non-significant.
We were surprised that breast development explained such a small amount of variability in estradiol, and await replication in a different (and perhaps older) sample or in which more of the variability was basal. Pubic hair was particularly good at capturing basal hormones in both sexes. The endocrine signaling of pubic hair generally begins earlier (between ages 6-9) and may be more established than breast/genital development in this early age range. When interested in comparing boys and girls, pubic hair assessments may be emphasized as they performed well in both sexes.
Did self-report PDS lead to parallel relationships with hormones as did the physical exam?
An identical model substituting the PDS stages for boys demonstrated marginal model fit, χ2(4)=9.15, p=.06, CFI=.97, RMSEA=.13, but no single coefficient differed from the physical exam model, ps>.15. Like the exam, gonadal development measured using the PDS did not predict DHEA, p=.58, but all other coefficients were significant. The PDS basically led to parallel relationships with boys' basal hormones as did the physical exam (Figure 1B).
For girls, an identical model substituting PDS stages for the exam resulted in marginal model fit, χ2(6)=10.7, p=.10, CFI=.96, RMSEA=.10. Three coefficients were substantially different from the physical exam, accounting for most of the model misspecification, χ2(3)=8.4, p=.04, CFI=.96, RMSEA=.15. Unlike the physical exam, the PDS gonadal score was associated with testosterone and DHEA, and the PDS adrenal score was not as highly related to DHEA as in the physical exam model. Constraining the remaining coefficients to be parallel to the physical exam did not reduce model fit, χ2(3)=4.16, p=.25, CFI=.99, RMSEA=.07, indicating that the PDS gonadal score was more broadly related to basal hormones than the physical exam, while the adrenal score was less predictive of girls' basal DHEA (Figure 2B).
Given that the PDS is a common measure and is easily employed in a variety of settings (e.g., schools, screening mailers), it should be welcome news that this self-report measure captured basal hormones in parallel with the physical exam in boys; in girls, the gonadal score performed slightly better than the exam. That girls' adrenal score was not associated with basal hormones is perplexing because the adrenal score included items, such as body/pubic hair growth and skin changes, which are related to hormones like DHEA (Grumbach, 2002).
Did the picture-based interview (PBIP) lead to parallel relationships with hormones as did the physical exam?
An identical model which substituted PBIP stages for the physical exam for boys demonstrated poor model fit, χ2(4)=13.4, p<.01, CFI=.94, RMSEA=.17, indicating that PBIP was not parallel with the physical exam (Figure 1B). We allowed genital development to be related to DHEA and found it significantly predicted DHEA, p=.009. When the other three coefficients were constrained to be identical to the exam model, goodness of fit was excellent, χ2(3)=.87, p=.8, CFI>.999, RMSEA<.0001, suggesting that PBIP led to parallel relationships with basal hormones as did the physical exam, and additionally that the PBIP genital stage predicted DHEA better than the physical exam.
For girls, an identical model which substituted PBIP for the physical exam fit well, χ2(6)=3.96, p=.7, CFI>.999, RMSEA<.0001, indicating that PBIP captured basal hormones in a similar manner as the physical exam (Figure 2B). In sum, the PDS and PBIP were related to basal hormones in parallel or occasionally better than the physical exam.
That the PBIP mapped onto basal hormones in parallel to the physical exam (or slightly better for boys' prediction of basal DHEA) is an additional advantage of the PBIP for potentially addressing hormone-related research questions. Use of the PBIP is most viable (a) when high correlations with an objective measure like the physical exam are sought-after; (b) when the Tanner metric is desirable; (c) when basal hormones (particularly in boys) are outcomes of interest or are proximally associated with outcomes of interest. Nevertheless, even the best measure of external pubertal status captured less than half of the variability in basal hormones. Directly measuring hormones is often feasible.
While we were agnostic about which measure would be optimal, we were surprised that self-reported PDS and PBIP scores were occasionally better correlates with basal hormones than the exam. This may be due to the unique perspectives of clinicians and adolescents. While clinicians have a range of knowledge comparing one adolescent to another, rarely do they observe the same adolescent across time. In contrast, the adolescent has little experience with other individuals, yet they have daily insights into their own pubertal changes. The adolescent's perspective may be optimal for noticing changes in their body across months and years. Basal hormones likewise capture a gradual, continuous developmental process. Adolescents may generally be more attuned to the confluence of this internal process with external developmental changes. Choosing measures which encompass the subjective adolescent experiences may be suitable for many biopsychosocial research questions.
Limitations
Several limitations should be considered. First, while our study is ethnically and socioeconomically diverse, the sample size limited the extent to which we could explore individual differences such as mechanisms behind racial differences in accuracy of self-report. Second, other hormones involved in pubertal maturation (e.g., DHEA-sulfate, androstenedione and progesterone) could yield different relationships with exam and self-report measures. Third, estradiol varies across the menstrual cycle. This limitation is noticeable because estradiol was weakly related to pubertal development. Girls were not recruited to come to the laboratory during a particular phase of their menstrual cycle because: (a) estradiol begins to cycle years before girls' first menstruation (menarche) so this would reduce cycle effects in menarcheal but not premenarcheal girls; (b) after menarche, cycles are often irregular (48% of our girls), so it would be difficult to schedule by day-count; and (c) days in which girls were likely accurate (i.e., during menstruation) are when estradiol is at its nadir and least likely to differentiate early from late puberty (Dawes et al., 1999). More frequent or systematic estradiol measurement may reveal stronger associations with puberty than we found.
Conclusions
Here we reported different ways to measure pubertal development in early adolescence. Our broad goal was to understand the relationships between these measures so that researchers can be informed about which measure best addresses particular research questions. Because puberty encompasses a suite of changes and is not a single process, different measures may best capture different things. The answer about which measure(s) are best may depend on which aspects of puberty are of interest for a particular study or research question. Inclusion of pubertal measures provides essential information about developmental changes in adolescence.
Acknowledgments
This project was supported by grants from the National Institute of Mental Health to Elizabeth Shirtcliff (K01 MH077687), Ronald Dahl (R24 MH67346) and Seth Pollak (R01 MH61285, R01 MH068858). Infrastructure support was provided by P30-HD03352 (M. Seltzer, Center Director). Special thanks are due to the Pediatric Nurse Practitioners for their diligence and commitment to this research project: Marie Heilegenstein RN, Lois Hoornstra RN, and Kim Squires RN. We appreciate the research assistance of Patrick Bauer, Aaron Cohn, Johnna Dorshorst, Jamie Hanson, Chastity Jensen, and Abby Noack. Invaluable guidance was provided by Lorah D. Dorn and Elizabeth J. Susman throughout the study, including the use of the script for the interview measure. Finally, we thank the adolescents and their families who made this research possible.
Footnotes
We recalculated SEM analyses using a simple average instead of the HLM basal hormones. The fit of these models were similar. The RMSEA was on average .05 different and never exceeded .13. Similarly, the χ2 was on average 1.05 different and never exceeded 2.3. The percent of variance in each hormone explained by pubertal status was also very similar for the basal versus average models, differing on average by only 3.7% and never exceeding 9%.
References
- Angold A, Worthman CW. Puberty onset of gender differences in rates of depression: a developmental, epidemiologic and neuroendocrine perspective. J Affect Disord. 1993;29(23):145–158. doi: 10.1016/0165-0327(93)90029-j. [DOI] [PubMed] [Google Scholar]
- Biro FM, Lucky AW, Huster GA, Morrison JA. Pubertal staging in boys. J Pediatr. 1995;127(1):100–102. doi: 10.1016/s0022-3476(95)70265-2. [DOI] [PubMed] [Google Scholar]
- Boas SR, Falsetti D, Murphy TD, Orenstein DM. Validity of self-assessment of sexual maturation in adolescent male patients with cystic fibrosis. J Adolesc Health. 1995;17(1):42–45. doi: 10.1016/1054-139X(95)00042-Q. [DOI] [PubMed] [Google Scholar]
- Bond L, Clements J, Bertalli N, Evans-Whipp T, McMorris BJ, Patton GC, et al. A comparison of self-reported puberty using the Pubertal Development Scale and the Sexual Maturation Scale in a school-based epidemiologic survey. Journal of Adolescence. 2006;29(5):709–720. doi: 10.1016/j.adolescence.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Warren MP, Rosso J, Gargiulo J. Validity of self-report measures of girls' pubertal status. Child Dev. 1987;58(3):829–841. [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Jenni OG, Dahl RE, Spear LP. Adolescent brain development: Vulnerabilities and opportunities. New York Academy of Sciences; 2004. Regulation of adolescent sleep: Implications for behavior; pp. 276–291. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, Dement WC. Pubertal changes in daytime sleepiness. Sleep. 1980;2(4):453–460. doi: 10.1093/sleep/2.4.453. [DOI] [PubMed] [Google Scholar]
- Coleman L, Coleman J. The measurement of puberty: A review. Journal of Adolescence. 2002;25:535–550. doi: 10.1006/jado.2002.0494. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Arch Gen Psychiatry. 2000;57(1):21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- Dabbs JM., Jr Salivary testosterone measurements: Collecting, storing, and mailing saliva samples. Physiology and Behavior. 1991;49:815–817. doi: 10.1016/0031-9384(91)90323-g. [DOI] [PubMed] [Google Scholar]
- Dawes MA, Dorn LD, Moss HB, Yao JK, Kirisci L, Ammerman RT, et al. Hormonal and behavioral homeostasis in boys at risk for substance abuse. Drug Alcohol Depend. 1999;55(12):165–176. doi: 10.1016/s0376-8716(99)00003-4. [DOI] [PubMed] [Google Scholar]
- Desmangles JC, Lappe JM, Lipaczewski G, Haynatzki G. Accuracy of pubertal Tanner staging self-reporting. J Pediatr Endocrinol Metab. 2006;19(3):213–221. doi: 10.1515/jpem.2006.19.3.213. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, Biro F. Defining the boundaries of early adolescence: A user's guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science. 2006;10(1):30–56. [Google Scholar]
- Dorn LD, Susman EJ. Puberty Script: Assessment of Physical Development in Boys and Girls. Cincinnati, OH: Cincinnati Children's Hospital Medical Center; 2002. [Google Scholar]
- Fernandez-Garcia B, Lucia A, Hoyos J, Chicharro JL, Rodriguez-Alonso M, Bandres F, et al. The response of sexual and stress hormones of male pro-cyclists during continuous intense competition. Int J Sports Med. 2002;23(8):555–560. doi: 10.1055/s-2002-35532. [DOI] [PubMed] [Google Scholar]
- Finkelstein JW, D'Arcangelo MR, Susman EJ, Chinchilli VM, Kunselman SJ, Schwab J, et al. Self-assessment of physical sexual maturation in boys and girls with delayed puberty. J Adolesc Health. 1999;25(6):379–381. doi: 10.1016/s1054-139x(99)00014-2. [DOI] [PubMed] [Google Scholar]
- Frank GR. Role of estrogen and androgen in pubertal skeletal physiology. Med Pediatr Oncol. 2003;41(3):217–221. doi: 10.1002/mpo.10340. [DOI] [PubMed] [Google Scholar]
- Geller B, Rogol A, Knitter E. Preliminary data on the dexamethasone suppression test in children with major depressive disorder. American Journal of Psychiatry. 1983;140:620–622. doi: 10.1176/ajp.140.5.620. [DOI] [PubMed] [Google Scholar]
- Genentech. Assessment of pubertal stages 1997 [Google Scholar]
- Granger DA, Shirtcliff EA, Zahn-Waxler C, Usher B, Klimes-Dougan B, Hastings P. Salivary testosterone diurnal variation and psychopathology in adolescent males and females: individual differences and developmental effects. Dev Psychopathol. 2003;15(2):431–449. [PubMed] [Google Scholar]
- Grumbach MM. The neuroendocrinology of human puberty revisited. Horm Res. 2002;57 2:2–14. doi: 10.1159/000058094. [DOI] [PubMed] [Google Scholar]
- Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med. 2004;22(4):337–347. doi: 10.1055/s-2004-861550. [DOI] [PubMed] [Google Scholar]
- Hergenroeder AC, Hill RB, Wong WW, Sangi-Haghpeykar H, Taylor W. Validity of self-assessment of pubertal maturation in African American and European American adolescents. J Adolesc Health. 1999;24(3):201–205. doi: 10.1016/s1054-139x(98)00110-4. [DOI] [PubMed] [Google Scholar]
- Hiort O. Androgens and puberty. Best Pract Res Clin Endocrinol Metab. 2002;16(1):31–41. doi: 10.1053/beem.2002.0178. [DOI] [PubMed] [Google Scholar]
- Ikegami S, Moriwake T, Tanaka H, Inoue M, Kubo T, Suzuki S, et al. An ultrasensitive assay revealed age-related changes in serum oestradiol at low concentrations in both sexes from infancy to puberty. Clin Endocrinol (Oxf) 2001;55(6):789–795. doi: 10.1046/j.1365-2265.2001.01416.x. [DOI] [PubMed] [Google Scholar]
- Legro RS, Lin HM, Demers LM, Lloyd T. Rapid maturation of the reproductive axis during perimenarche independent of body composition. J Clin Endocrinol Metab. 2000;85(3):1021–1025. doi: 10.1210/jcem.85.3.6423. [DOI] [PubMed] [Google Scholar]
- Liben LS, Susman EJ, Finkelstein JW, Chinchilli VM, Kunselman S, Schwab J, et al. The effects of sex steroids on spatial performance: a review and an experimental clinical investigation. Dev Psychol. 2002;38(2):236–253. [PubMed] [Google Scholar]
- Lucky AW, Biro FM, Simbartl LA, Morrison JA, Sorg NW. Predictors of severity of acne vulgaris in young adolescent girls: results of a five-year longitudinal study. J Pediatr. 1997;130(1):30–39. doi: 10.1016/s0022-3476(97)70307-x. [DOI] [PubMed] [Google Scholar]
- MacGillivray MH, Morishima A, Conte F, Grumbach M, Smith EP. Pediatric endocrinology update: an overview. The essential roles of estrogens in pubertal growth, epiphyseal fusion and bone turnover: lessons from mutations in the genes for aromatase and the estrogen receptor. Horm Res. 1998;49 1:2–8. doi: 10.1159/000053061. [DOI] [PubMed] [Google Scholar]
- Maskarinec G, Morimoto Y, Novotny R, Nordt FJ, Stanczyk FZ, Franke AA. Urinary sex steroid excretion levels during a soy intervention among young girls: a pilot study. Nutr Cancer. 2005;52(1):22–28. doi: 10.1207/s15327914nc5201_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris NM, Udry RJ. Validation of a self-administered instrument to assess stage of adolescent development. Journal of Youth and Adolescence. 1980;9(3):271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Norris SA, Richter LM. Usefulness and reliability of tanner pubertal self-rating to urban Blac adolescents in South Africa. Journal of Research on Adolescence. 2005;15(4):609–624. [Google Scholar]
- Palmert MR, Hayden DL, Mansfield MJ, Crigler JF, Jr, Crowley WF, Jr, Chandler DW, et al. The longitudinal study of adrenal maturation during gonadal suppression: evidence that adrenarche is a gradual process. J Clin Endocrinol Metab. 2001;86(9):4536–4542. doi: 10.1210/jcem.86.9.7863. [DOI] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL, Devine N, Hunold JJ, Mauras N, Moshang T, Jr, Root AW. Salutary effects of combining early very low-dose systemic estradiol with growth hormone therapy in girls with Turner syndrome. J Clin Endocrinol Metab. 2005;90(12):6424–6430. doi: 10.1210/jc.2005-1081. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ. Androgens, brain, and behavior. Am J Psychiatry. 1996;153(8):974–984. doi: 10.1176/ajp.153.8.974. [DOI] [PubMed] [Google Scholar]
- Schmitz KE, Hovell MF, Nichols JF, Irvin VL, Keating K, Simon GM, et al. A validation study of early adolescents' pubertal self-assessments. Journal of Early Adolescence. 2004;24(4):357–384. [Google Scholar]
- Shirtcliff E, Zahn-Waxler C, Klimes-Dougan B, Slattery M. Salivary dehydroepiandrosterone responsiveness to social challenge in adolescents with internalizing problems. J Child Psychol Psychiatry. 2007;48(6):580–591. doi: 10.1111/j.1469-7610.2006.01723.x. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Schwartz E, Curran MJ. Use of salivary biomarkers in biobehavioral research: cotton-based sample collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology. 2001;26(2):165–173. doi: 10.1016/s0306-4530(00)00042-1. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Schwartz EB, Curran MJ, Booth A, Overman WH. Assessing estradiol in biobehavioral studies using saliva and blood spots: Simple radioimmunoassay protocols, reliability, and comparative validity. Horm Behav. 2000;38(2):137–147. doi: 10.1006/hbeh.2000.1614. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7(10):1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Gallagher lecture. The family at adolescence: transition and transformation. J Adolesc Health. 2000;27(3):170–178. doi: 10.1016/s1054-139x(99)00115-9. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9(2):69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Dahl R, Keating D, Kupfer D, Masten AS, Pine D. The study of developmental psychopathology in adolescence: Integrating affective neuroscience with the study of context. In: Cicchetti D, Cohen D, editors. Handbook of developmental psychopathology. 2nd. New York: Wiley; 2006. [Google Scholar]
- Tanner JM. Growth at adolescence. Springfield, IL: Thomas; 1962. [Google Scholar]