Abstract
By describing patterns of disease distribution within populations, identifying risk factors, and finding associations, epidemiological studies have contributed to our current understanding of schizophrenia. Advanced paternal age and the association with auto-immune diseases are some of the newly described epidemiological finding in schizophrenia epidemiology, shaping our current definition of schizophrenia. Though early intervention strategies have gained momentum, primary prevention of schizophrenia still seems a very distant aspiration. In this article we review the major epidemiological features of schizophrenia, with particular attention to the recent advances using population-based data. We also discuss some pervasive myths in schizophrenia epidemiology, such as the universal distribution and the gender equality myths. Review of the available evidence shows that schizophrenia does not distribute itself equally across cultures and countries, and the disease is more prevalent among males.
Introduction
The epidemiology of schizophrenia has progressed from descriptive accounts to a surge in analytic epidemiologic findings over the last two decades. This article reviews the epidemiology of schizophrenia, concentrating on results which are most credible methodologically and consistent across studies, focusing particularly on the most recent developments. We also provide comments about some misconceptions regarding schizophrenia epidemiology, specifically pointing to widespread misinterpretation of evidence regarding the incidence of schizophrenia and the gender ratio of the disease.
Descriptive Epidemiology: Prevalence and Incidence of schizophrenia
Prevalence
The point prevalence of schizophrenia is the proportion of the population at a point in time that has the disorder. The point prevalence of schizophrenia is about five per thousand in the population. The estimate depends on the age distribution of the population– if persons too young to be at risk are included in the denominator, for example, the estimates will be lower. Table 1 presents findings from areas in which credible estimates of both prevalence and incidence are available. The range in prevalence in Table 1 is from 2.7/1000 to 8.3/1000, and this range would not be much affected if several dozen other studies, available from prior reviews, were included 1. Lifetime prevalence has been estimated by surveys with examinations by medically trained persons, with resulting estimates not too different from those shown in Table 1 1.
TABLE 1.
Prevalence and incidence of Schizophrenia per 1000 population.
| Area | Date | Author | Age | Prevalence | Incidence | |
|---|---|---|---|---|---|---|
| Type | Rate | |||||
| Denmark | 1977 | Nielsen | 15 + | Lifetime | 2.7 | |
| 1972 | Munk-Jorgensen | All | Annual | 0.12 | ||
| Baltimore, Maryland, USA | 1963 | Wing | All | One year | 7 | |
| 1963 | Warthen | All | Annual | 0.7 | ||
| Camberwell, England | 1963 | Wing | 15+ | One year | 4.4 | |
| 1971 | Hailey | All | Annual | 0.11 | ||
| Ireland | 1973 | Walsh | 15+ | Point | 8.3 | |
| 1986 | WHO | 15–54 | Annual | 0.22 | ||
| Portogruaro, Italy | 1982–9 | de Salvia et al. | 2.7 | |||
| 1989 | de Salvia et al. | Annual | 0.19 | |||
| Hampstead, England | 1991–5 | Jeffreys et al. | 5.1 | |||
| 1991–5 | McNaught et al. | Annual | 0.21 | |||
Selected from reviews by Eaton (1985, 1991), with additions of Jeffreys et al. (1997), McNaught et al. (1997), and de Salvia et al. (2000).
Incidence
The incidence of schizophrenia is about 0.20/1000/year. The incidences presented are all estimated for one year, making the comparison somewhat tighter. The range in annual incidence in Table 1 is from 0.11/1000/year to 0.70/1000/year, and this range would not be much affected if several dozens of other studies, reviewed elsewhere, were included 2, 3. The presentation of prevalence and incidence figures from the same areas in juxtaposition shows that the point prevalence is usually more than ten times the annual incidence, indicating the chronic nature of the disorder.
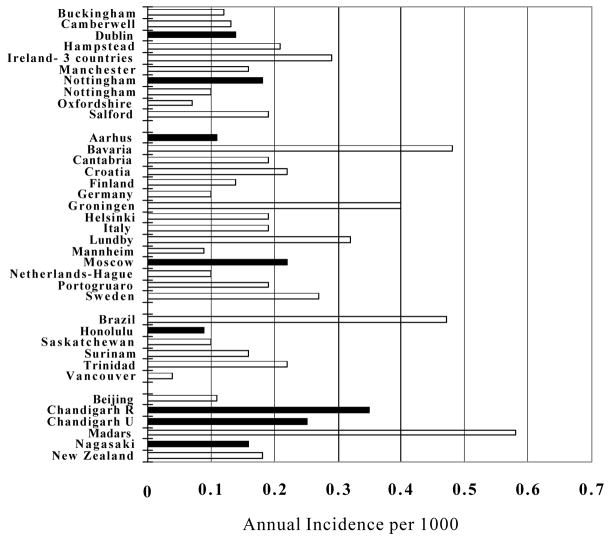
There is considerable variation in incidence rates around the world, as shown in Figure 1. The dark bars represent the WHO study of incidence, which reveal a smaller variation, presumably due to the standardization of method 4. The conclusion of that study suggested to some that there was little or no variation in schizophrenia around the world, which would make schizophrenia a very unusual disease indeed. Figure 1 shows variation greater than one order of magnitude, from a low estimate in Vancouver of .04/1000/year to a high estimate in Madras of 0.58/1000/year. Both the Vancouver 5 and Madras 6 studies were carefully done and their estimates are credible.
FIGURE 1. Incidence of Schizophrenia in Selected Studies published after 1985.
Criteria: study focus is the general population of a defined geographic area; diagnosis is made by a psychiatrist; case finding includes inpatient and outpatient services; greater than 25000 person years of risk in age group studies.
The force of morbidity for schizophrenia peaks in young adulthood. The age of onset varies between men and women, where males tend to have a younger onset 7. The peak incidence for males and females is in the decade 15–24. The peak for young adults is more marked for males, and the females have a second peak in the years 55–64. Evidence suggests that males have higher lifetime risk of schizophrenia, which is born out in two meta-analysis addressing that issue, showing that males have about 30%–40% higher lifetime risk of developing schizophrenia 8, 9.
Analytical epidemiology: Natural History and Risk Factors
Natural History
Onset
The onset of schizophrenia is varied. In the classic long-term follow-up study of Ciompi, about 50% had an acute onset, and 50% a long prodrome. The intensive study of prodrome by Hafner and colleagues 10 suggests onset of negative symptoms tends to occur about five years before the initial psychotic episode, with onset of positive symptoms much closer to the first hospitalization.
Childhood developmental abnormalities
Many long term follow-up studies, both retrospective and prospective, suggest a variety of signs, symptoms, conditions, and behaviors are associated with raised risk for schizophrenia, but none with such strength or uniqueness as to be useful in prediction. Earlier work on high risk (HR) groups has shown that offspring of schizophrenic parents were more likely to have a lower IQ, poor attention skills, thought disorder-like symptoms, poor social adjustment, and psychiatric symptoms as compared to the offspring of controls 10–12. Although several concerns have been raised regarding the generalizability of HR findings to non- familial forms of schizophrenia, recent longitudinal studies conducted in the United Kingdom, Sweden, Finland, and New Zealand have provided evidence that individuals with schizophrenia differ from their peers even in early childhood, in a variety of developmental markers, such as the age of attaining developmental milestones 13–15, levels of cognitive functioning 16, 17, educational achievement 13, 18–20, neurological and motor development 21–23, social competence 19, 24, and psychological disturbances 24.:More recent evidence also suggest the association between low IQ is specific to schizophrenia as it was not found in bipolar disorder 25. It is noteworthy that there seems to be no common causal paths which link these developmental markers with schizophrenia 26. Indeed, individuals who later develop schizophrenia or related disorders may have already experienced a general or pan-developmental impairment early as in their childhood. Prospectively collected data from the 1972–73 birth cohort in New Zealand, showed that 20 schizophrenic subjects may have suffered a significant deficit in neuromotor, language, and cognitive development in the first decade of their lives. The compelling evidence linking an array of childhood developmental abnormalities and schizophrenia is echoing with the hypothesis that schizophrenia is a neurodevelopmental disorder, for which causes may be traced to a defect in the early brain development 27–30.
Course
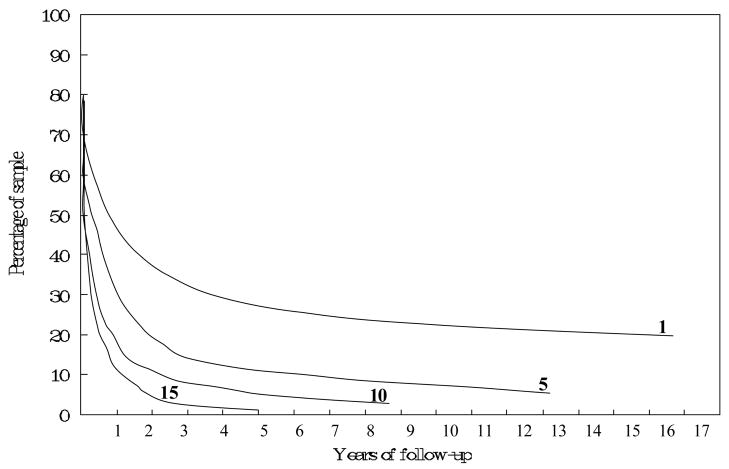
The symptomatic course of schizophrenia is varied. In Ciompi’s classic study, about half had an undulating course, with partial or full remissions followed by recurrences, in an unpredictable pattern. About one-third had relatively chronic, unremitting course with poor outcome. A small minority in that study had a steady pattern of recovery with good outcome. Follow-up studies which are not strictly prospective, such as the study by Ciompi, can be deceptive, because there is a tendency to focus on a residue of chronic cases, making the disorder appear more chronic than it actually is. Figure 2 shows data on time to rehospitalization for a cohort of patients with schizophrenia in Denmark. The proportion remaining in the community without rehospitalization is shown on the vertical axis, and time is on the horizontal axis. After the initial hospitalization, about 25% are not rehospitalized even after 15 years. For that subgroup of the cohort with ten hospitalizations, more than 90% are rehospitalized within three years following the tenth episode. While it could be that the occurrence of episodes are reinforcing the illness, (so-called “schubweis (stepwise)” process), or that hospitalization itself is damaging 31, it seems more likely the cohort is sorting itself into those with tendency for more versus less chronicity of disorder. This process may lead clinicians and others to overestimate the chronicity of the disorder, since they see individuals in the bottom curve of Figure 2 about fifteen times as often as individuals in the top curve (Cohen and Cohen 1984). For this reason, the natural history of schizophrenia is best studied with cohorts of first onsets 32.
FIGURE 2. Community survival in schizophrenia (1, 5, 10, and, 15 represent the number of hospital discharges).
Source: Mortensen and Eaton (1994).
Outcome
Predictors of outcome for schizophrenia remain elusive, for the most part. In a review of thirteen prospective studies of course in first onset cohorts, negative symptoms predicted poor outcome in four studies, and gradual onset, typical of negative symptoms as noted above, predicted poor outcome in several studies 32. There is variation in the course of schizophrenia around the world, with better prognosis in so-called “developing” countries. Table 2 shows a summary of data from the WHO study on this issue (1979), with the right most columns extracted by us from the publication of Leff and colleagues 33. Those in developing countries are less likely to have been chronically psychotic over the period of follow-up, and more likely to have no residual symptoms after five years, than those in the developed countries. This result remains to be explained. It could be that individuals meeting criteria for schizophrenia in developing countries include a subset destined for better prognosis because of the risk factor structure in those countries—more deaths of compromised fetuses, for example; or a cause connected to good prognosis, such as a parasite which is rare in developed countries. Another interpretation is that the environment of recovery in the developed world is more pernicious, involving harsher economic competition, a greater degree of stigma, and smaller family networks who can share the burden of care for persons with schizophrenia.
TABLE 2.
WHO Followup of Schizophrenia.
| Developed Countries | Sample size | Percent with no symptoms | Percent with chronic psychosis |
|---|---|---|---|
| London, England | 50 | 6 | 40 |
| Aarhus, Denmark | 64 | 5 | 14 |
| Moscow, Russia | 66 | 17 | 21 |
| Washington, D.C., USA | 65 | 6 | 23 |
| 51 | 3 | 23 | |
| Developing countries | |||
| Agra, India | 73 | 42 | 10 |
| Cali, Colombia | 91 | 11 | 21 |
| Ibadan, Nigeria | 68 | 34 | 10 |
Source: Leff et al. (1992).
The course of schizophrenia, from early prodrome through to later outcome, is influenced by social variables, including socioeconomic position and marital status 34. The individual who eventually is diagnosed with schizophrenia is more likely to be single than others, even as many as 20 years prior to diagnosis, where the relative odds is about 4. The relative odds of being single, as compared to those never diagnosed with schizophrenia, peak at the time of admission, at more than 15, and remain high for decades afterward. The effect is greater for males, possibly because their earlier onset occurs during the years of formation of marriages. Likewise, the individual who eventually is diagnosed with schizophrenia is more likely to be unemployed than others, many years earlier than the first diagnosis of schizophrenia, and many years afterward. Although there is a long literature on the relationship of low socioeconomic position to risk for schizophrenia 35, 36, it seems likely that the association is due to the effects of insidious onset on the ability of the individual to compete in the job market. Recent studies from Scandinavia suggest that, if anything, the parents of persons with schizophrenia are likely to come from a higher, not lower, social position 37.
Risk Factors
The genetics of schizophrenia, including family history as a risk factor, are beyond the scope of a general review on the epidemiology of schizophrenia. Below are presented risk factors which have found at least in several credible studies and in which it is fairly clear that the risk factor has been present prior to the onset of schizophrenia.
Season of Birth
For a long time it has been known that individuals with schizophrenia are more likely to be born in the winter, and the results have been reported from the samples in the Northern and Southern Hemispheres. The relative risk is small, on the order of a 10% increase for those born in the winter versus summer, but it has been replicated many times 38, 39. One possible explanation is that the mother is passing through the second trimester of her pregnancy in the height of the flu season, and that infections during that period raise risk for schizophrenia in the offspring. Another explanation offered by a recent study suggests that the seasonal effects may increase one’s risk of schizophrenia via the interaction with genetic vulnerability 40.
Birth complications
The finding regarding season of birth suggests that something about pregnancy and birth might be awry in individuals who later develop schizophrenia. There have been case-control studies available for decades on this issue, but the generally positive findings were clouded by the possibility that the mother’s recall was biased. In the last fifteen years there have been many studies reporting a relative odds of about two for those with one or another sort of birth complication, and several meta-analyses on this topic exist 41–43. Later analyses have begun to specify the individual type of birth complication, with the hope of elucidating the causal mechanism. A recent meta-analytic review of this literature categorizes the types of birth complications as 1) complications of pregnancy (bleeding, diabetes, rhesus incompatibility, preeclampsia); 2) abnormal fetal growth and development: (low birthweight, congenital malformations, reduced head circumference), and 3) complications of delivery (uterine atony, asphyxia, emergency Cesarean section) 22. The review concludes that the investigations into specific mechanisms need to move now from the epidemiological perspective to include a combination of disciplines and approaches. The complications variously suggest as a possible cause malnutrition 44, extreme prematurity, and hypoxia or ischemia 30, 45–47.
Parental age
The role of advanced parental age in relation to a higher risk of schizophrenia was first proposed in the mid-20th century, and has gained extensive scientific attention in recent years. Based upon the family background data of 1000 patients in the Ontario hospital, Canada, Gregory 48 reported that parents of patients with schizophrenia were, on average, 2–3 years older than those of the general population. However, subsequent investigations showed inconsistent findings 49, 50, and it was argued that observed maternal age-associated higher risk in schizophrenia might be largely confounded by raised paternal age 49, 51. Recently, several population-based epidemiological studies in Demark, Israel, Sweden, and the United States have provided stronger evidence as to the role of paternal age in schizophrenia 9, 52–56. A population-based birth cohort study from in Israel found that the relative risk of schizophrenia rose monotonically in each 5-year group of paternal age, with a maximum relative risk of 2.96 (95% CI: 1.60–5.47) in the group aged 55 or above in comparison with the age of 20–24. Additionally, once paternal age is statistically adjusted, maternal age no longer is a significant predictor of schizophrenia. The evidence from one nested case-control study indicates that the paternal age-related excess in the risk of schizophrenia is generally greater in females 55. In addition, current population-based cohort research tends to support that advancing paternal age-related increased risk of schizophrenia only appears significant among those without family history, indicating the possibility of accumulation of de novo mutations in paternal sperm 57]
Infections and the immune system
A series of ecologic studies suggest that persons whose mothers were in their second trimester of pregnancy during a flu epidemic have higher risk for schizophrenia {Munk-Jorgensen, 2001 #72; Mednick, 1988 #69; Brown, 2002 #9; Brown, 2004 #153}. Infection during pregnancy as a risk factor is consistent with the neurodevelopmental theory of schizophrenia 28, 58. Later studies, which are more convincing, include individual assessment of infection, either via comparison of antibodies in adults with schizophrenia versus normal individuals 59, or, even more convincing, prospective studies in which the infection can be determined to have occurred during the pregnancy. There is consistent evidence that individuals with antibodies to Toxoplasmosis Gondii have higher prevalence of schizophrenia 60. One study suggests a relative risk of 5.2 for individuals with documented infection by the rubella virus during fetal development 61. Another prospective study found higher risk for psychosis in individuals whose mothers had higher levels of antibodies to herpes simplex virus 62. A study in Brazil compared individuals who had meningitis during the 1971–1974 epidemic, with their sibs who did not have meningitis, and found that that the prevalence of psychosis, and schizophrenia specifically, was five times higher in those who had meningitis. The finding is intriguing because the average age of infection with meningitis was 26 months, i.e., much later than prenatal infection 63. If this finding is replicated it will have important implications for the neurodevelopmental theory of schizophrenia.
Autoimmune diseases
A relatively small but consistent literature indicates that persons with schizophrenia have unusual resistance or susceptibility to autoimmune diseases. Studies have consistently shown that individuals with schizophrenia are somehow less likely to have rheumatoid arthritis 64. While it could be that medications for schizophrenia are protective for rheumatoid arthritis in some unknown way, some of the studies were conducted prior to the era in which neuroleptic medications were available. It could be that other physiologic consequences of schizophrenia are protective, or it could be that a single gene raises risk for the one disorder and protects for the other. A single small study suggests that mothers of individuals with schizophrenia have lower risk for rheumatoid arthritis, but it’s size and quality are not convincing 65. It is intriguing, in this regard, that case control studies have shown that persons taking non-steroidal anti-inflammatory medications, which primarily treat arthritis, may be protected from dementia 66, 67.
Other autoimmune disorders have been linked to schizophrenia, including thyroid disorders 68, type 1 diabetes 69, and celiac disease 70. Currently the evidence is strongest for thyroid disorders and celiac disease. In a study from the Danish population registers, persons whose parents had celiac disease were three times as likely to later be diagnosed with schizophrenia. Celiac disease is an immune reaction to wheat gluten. One possible explanation is that the increased permeability of the intestine brought about by celiac disease increases the level of antigen exposure increasing risk of autoimmune response. It is also possible that gluten proteins are broken down into psychoactive peptides 71.
The results linking schizophrenia to autoimmune disease are paralleled by the clinical and laboratory study of autoimmune processes in schizophrenia. There are apparently abnormalities of the immune system in schizophrenia, but it is not clear whether these are causal or a consequence of schizophrenia or its treatment 72. It is possible that a single weakness in the immune system in those with schizophrenia explains both the data on infections and the results on autoimmune disorders, but this remains to be proven 73. Meanwhile, there are ongoing clinical trials of anti-inflammatory 74 and antibiotic 75 agents for schizophrenia.
Ethnicity
Ethnic status is a relatively easy to identify characteristic of an individual which indicates a shared history with others. Markers of ethnic status include race, country of origin, and religion. Country of origin has proven to be a consistent risk factor for schizophrenia in the United Kingdom and the Netherlands. In the United Kingdom, those immigrating from Africa or the Caribbean, and their second generation offspring, have rates of schizophrenia up to ten times higher than those in the general population 76. Since immigrant groups who do not have black skin do not have higher rates, and since the second generation is affected, it is unlikely to be the stresses of immigration. Since rates in the countries of origin are not elevated it is unlikely to be a genetic difference between races. The cause appears to be the psychological conditions associated with being Black in England, or being from Surinam in Holland. It could be discrimination, or a more subtle form of difficulty associated with planning one’s life when the future is as uncertain as it is for racial groups at the structural bottom of society 77, 78.
Cannabis Use
There are numerous case control studies showing that persons with schizophrenia are more likely to have taken, or be using, cannabis 79. Recently there have been prospective studies in Sweden, the Netherlands, New Zealand, and Israel, showing higher risk ranging from 2 to as high as 25 80–83. It could be that individuals in the premorbid phase of schizophrenia are responding to initial, mild symptoms of schizophrenia by using drugs, even though these studies have attempted to control for premorbid conditions. On the other hand, it could be that cannabis precipitates, or even causes, an episode of schizophrenia 84–89.
Urban residence
In the 1930’s Faris and Dunham showed that, while the addresses of first admissions for manic depressive illness were distributed more or less randomly throughout Chicago, admissions for schizophrenia tended to come from the center of the city (1939), with decreasing rates as one moves outward into zones of transition, working class, and family. This finding, and other similar findings 90, were interpreted as due to the selection into the city of individuals who would develop schizophrenia. But later studies from Europe were strictly prospective, with the cohort defined in late adolescence, well prior to onset 91, or even at birth 92. The relative risk is about 2–4 times higher for those born in urban areas. The difficulty is identifying the plausible biological process associated with urban residence. It could include differences in the physical environment, such as the higher concentration of lead in the soil and air in cities; differences in the cultural environment, such as the expectation to leave the family of origin and define a new life plan 77, differences in birth practices, such as breastfeeding 93, crowding which might permit spread of infections 94, discussed below, differences in the manner in which animals are, or are not, brought into the household 95 and a host of other factors 96.
Myths in schizophrenia epidemiology
In recent years reviews have yield a reconsideration of some widely cited, but poorly supported by evidence, aspects of schizophrenia epidemiology 97. The first is the notion that schizophrenia has universal incidence across cultures and countries. The second is the belief that schizophrenia distributes itself equality in males and females. Taken together these beliefs could be conceptualized as (1) schizophrenia is an equalitarian disorder, and (2) schizophrenia is an exceptional disorder 97. It is puzzling that these two interrelated beliefs are usually cited as evidence for a biological origin of the disease, when most diseases in medicine do vary across cultures, countries, and gender.
As seen in Table 1 and Figure 1 this paper, the incidence of schizophrenia varies significantly across countries. Another study found the incidence of schizophrenia varying significantly, with a median value of 15.2 per 100 000, with a range of 7.7 to 43 8. Regarding the male to female rate ratio, a review of available data from 31 studies, estimate the median rate to be 1.4:1 97. Regarding the gender ratio in schizophrenia, two independent meta-analyses, with some overlap in study sampling, have shown increased risk for men in schizophrenia 8, 9.
Discussion
What has been accomplished over the last several decades, and what are prospects for future progress? Even as late as one-quarter century ago, the epidemiology of schizophrenia was nearly a blank page. There was even argumentation about the value of the concept itself. The only risk factors which seemed strong and consistent were the conditions of lower social class life, and the family history of schizophrenia. Since that time, there has been considerable progress delineating a more or less consistent picture of the descriptive epidemiology and the natural history of schizophrenia. Research in analytic epidemiology has generated a series of heretofore unsuspected risk factors, as described above. In general, the risk factors have been considered in the context of theories of how schizophrenia might actually be developing in the psychological and physiological life of the individual— even if the linkage is sometimes speculative. These developments are healthy.
In the future there will be concerted efforts to study risk factors in combination. This process has begun already. For example, Mortensen et al have studied the combined effects of season of birth, urbanization of birthplace, and family history of schizophrenia. The combination is informative in evaluating the importance of the risk factors. Although the relative risk for urban birth is much smaller than the risk associated with having a parent who has schizophrenia, the importance of urban birth is greater, because a much larger proportion of the population are born in urban areas than the proportion with parents who have schizophrenia—the situation of relative versus population-attributable risk (Mortensen et al. 1999). If the causal path connected to urban birth could be identified, the prospects for prevention would be much stronger.
The combination of risk factors will facilitate prospective studies of high risk individuals, in which the high risk is not simply due to family history, as in earlier high risk studies. Furthermore, combination of risk factors will raise the positive predictive value of the risk formulation, to the point where it may be ethically feasible to approach the individual, identify the risk, and begin efforts to protect them from the catastrophic effects of the first episode of schizophrenia. Studies such as these have begun, albeit very cautiously (McGorry et al. 1996; Tsuang et al. 1999; Woods et al. 2003). In general, epidemiologic research has built a strong knowledge base over the past quarter century, and this knowledge base will continue to contribute to public health efforts at prevention of schizophrenia in the coming decades.
Acknowledgments
Supported by NIMH Grant #53188
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eaton WW. Epidemiology of schizophrenia. Epidemiologic reviews. 1985;7:105–126. doi: 10.1093/oxfordjournals.epirev.a036278. [DOI] [PubMed] [Google Scholar]
- 2.Eaton WW. Update on the epidemiology of schizophrenia. Epidemiologic reviews. 1991;13:320–328. doi: 10.1093/oxfordjournals.epirev.a036075. [DOI] [PubMed] [Google Scholar]
- 3.Eaton W. Evidence for universality and uniformity of schizophrenia around the world: assessment and implications. Darmstadt: Steinkopf; 1999. [Google Scholar]
- 4.Sartorius N, Jablensky A, Korten A, Ernberg G, Anker M, Cooper JE, Day R. Early manifestations and first-contact incidence of schizophrenia in different cultures. A preliminary report on the initial evaluation phase of the WHO Collaborative Study on determinants of outcome of severe mental disorders. Psychological medicine. 1986;16(4):909–928. doi: 10.1017/s0033291700011910. [DOI] [PubMed] [Google Scholar]
- 5.Beiser M, Erickson D, Fleming JA, Iacono WG. Establishing the onset of psychotic illness. The American journal of psychiatry. 1993;150(9):1349–1354. doi: 10.1176/ajp.150.9.1349. [DOI] [PubMed] [Google Scholar]
- 6.Rajkummar S. Incidence of Schizophrenia in an Urban Community in Madras. Indian J Psychiat. 1993;35:18–21. [PMC free article] [PubMed] [Google Scholar]
- 7.Munk-Jorgensen P. First-admission rates and marital status of schizophrenics. Acta Psychiatr Scand. 1987;76:210–216. doi: 10.1111/j.1600-0447.1987.tb02886.x. [DOI] [PubMed] [Google Scholar]
- 8.McGrath J, Saha S, Welham J, El Saadi O, MacCauley C, Chant D. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC medicine. 2004;2:13. doi: 10.1186/1741-7015-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Archives of general psychiatry. 2003;60(6):565–571. doi: 10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- 10.Hafner H, Maurer K, Loffler W. Onset and prodromal phase as determinants of the course. In: Gattaz WF, Hafner H, editors. Search for the Causes of Schizophrenia: Vol IV-- Balance of the Century. Darmstadt: Steinkopf Springer; 1999. pp. 35–58. [Google Scholar]
- 11.Tarrant CJ, Jones PB. Precursors to schizophrenia: do biological markers have specificity? Canadian journal of psychiatry. 1999;44(4):335–349. doi: 10.1177/070674379904400403. [DOI] [PubMed] [Google Scholar]
- 12.Niemi LT, Suvisaari JM, Tuulio-Henriksson A, Lonnqvist JK. Childhood developmental abnormalities in schizophrenia: evidence from high-risk studies. Schizophrenia research. 2003;60(2–3):239–258. doi: 10.1016/s0920-9964(02)00234-7. [DOI] [PubMed] [Google Scholar]
- 13.Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344(8934):1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- 14.Jones P. The early origins of schizophrenia. British medical bulletin. 1997;53(1):135–155. doi: 10.1093/oxfordjournals.bmb.a011596. [DOI] [PubMed] [Google Scholar]
- 15.Isohanni M, Jones PB, Moilanen K, Rantakallio P, Veijola J, Oja H, Koiranen M, Jokelainen J, Croudace T, Jarvelin M. Early developmental milestones in adult schizophrenia and other psychoses. A 31-year follow-up of the Northern Finland 1966 Birth Cohort. Schizophrenia research. 2001;52(1–2):1–19. doi: 10.1016/s0920-9964(00)00179-1. [DOI] [PubMed] [Google Scholar]
- 16.Gunnell D, Harrison G, Rasmussen F, Fouskakis D, Tynelius P. Associations between premorbid intellectual performance, early-life exposures and early-onset schizophrenia. Cohort study. Br J Psychiatry. 2002;181:298–305. doi: 10.1192/bjp.181.4.298. [DOI] [PubMed] [Google Scholar]
- 17.David AS, Malmberg A, Brandt L, Allebeck P, Lewis G. IQ and risk for schizophrenia: a population-based cohort study. Psychological medicine. 1997;27(6):1311–1323. doi: 10.1017/s0033291797005680. [DOI] [PubMed] [Google Scholar]
- 18.Isohanni I, Jarvelin MR, Nieminen P, Jones P, Rantakallio P, Jokelainen J, Isohanni M. School performance as a predictor of psychiatric hospitalization in adult life. A 28-year follow-up in the Northern Finland 1966 Birth Cohort. Psychological medicine. 1998;28(4):967–974. doi: 10.1017/s0033291798006928. [DOI] [PubMed] [Google Scholar]
- 19.Done DJ, Crow TJ, Johnstone EC, Sacker A. Childhood antecedents of schizophrenia and affective illness: social adjustment at ages 7 and 11. BMJ (Clinical research ed. 1994;309(6956):699–703. doi: 10.1136/bmj.309.6956.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cannon TD, Rosso IM, Bearden CE, Sanchez LE, Hadley T. A prospective cohort study of neurodevelopmental processes in the genesis and epigenesis of schizophrenia. Development and psychopathology. 1999;11(3):467–485. doi: 10.1017/s0954579499002163. [DOI] [PubMed] [Google Scholar]
- 21.Leask SJ, Done DJ, Crow TJ. Adult psychosis, common childhood infections and neurological soft signs in a national birth cohort. Br J Psychiatry. 2002;181:387–392. doi: 10.1192/bjp.181.5.387. [DOI] [PubMed] [Google Scholar]
- 22.Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. The American journal of psychiatry. 2002;159(7):1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- 23.Cannon M, Jones P, Huttunen MO, Tanskanen A, Huttunen T, Rabe-Hesketh S, Murray RM. School performance in Finnish children and later development of schizophrenia: a population-based longitudinal study. Archives of general psychiatry. 1999;56(5):457–463. doi: 10.1001/archpsyc.56.5.457. [DOI] [PubMed] [Google Scholar]
- 24.Malmberg A, Lewis G, David A, Allebeck P. Premorbid adjustment and personality in people with schizophrenia. Br J Psychiatry. 1998;172:308–313. doi: 10.1192/bjp.172.4.308. discussion 314–305. [DOI] [PubMed] [Google Scholar]
- 25.Zammit S, Allebeck P, David AS, Dalman C, Hemmingsson T, Lundberg I, Lewis G. A longitudinal study of premorbid IQ Score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Archives of general psychiatry. 2004;61(4):354–360. doi: 10.1001/archpsyc.61.4.354. [DOI] [PubMed] [Google Scholar]
- 26.Jones PB, Tarrant CJ. Specificity of developmental precursors to schizophrenia and affective disorders. Schizophrenia research. 1999;39(2):121–125. doi: 10.1016/s0920-9964(99)00110-3. discussion 161. [DOI] [PubMed] [Google Scholar]
- 27.Weinberger DR. From neuropathology to neurodevelopment. Lancet. 1995;346(8974):552–557. doi: 10.1016/s0140-6736(95)91386-6. [DOI] [PubMed] [Google Scholar]
- 28.Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? British medical journal (Clinical research ed. 1987;295(6600):681–682. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isohanni M, Murray GK, Jokelainen J, Croudace T, Jones PB. The persistence of developmental markers in childhood and adolescence and risk for schizophrenic psychoses in adult life. A 34-year follow-up of the Northern Finland 1966 birth cohort. Schizophrenia research. 2004;71(2–3):213–225. doi: 10.1016/j.schres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Isohanni M, Lauronen E, Moilanen K, Isohanni I, Kemppainen L, Koponen H, Miettunen J, Maki P, Rasanen S, Veijola J, Tienari P, Wahlberg KE, Murray GK. Predictors of schizophrenia: evidence from the Northern Finland 1966 Birth Cohort and other sources. The British journal of psychiatry. 2005;48:s4–7. doi: 10.1192/bjp.187.48.s4. [DOI] [PubMed] [Google Scholar]
- 31.Eaton WW., Jr Mental hospitalization as a reinforcement process. American sociological review. 1974;39(2):252–260. [PubMed] [Google Scholar]
- 32.Ram R, Bromet EJ, Eaton WW, Pato C, Schwartz JE. The natural course of schizophrenia: a review of first-admission studies. Schizophrenia bulletin. 1992;18(2):185–207. doi: 10.1093/schbul/18.2.185. [DOI] [PubMed] [Google Scholar]
- 33.Leff J, Sartorius N, Jablensky A, Korten A, Ernberg G. The International Pilot Study of Schizophrenia: five-year follow-up findings. Psychological medicine. 1992;22(1):131–145. doi: 10.1017/s0033291700032797. [DOI] [PubMed] [Google Scholar]
- 34.Agerbo E, Byrne M, Eaton WW, Mortensen PB. Marital and labor market status in the long run in schizophrenia. Archives of general psychiatry. 2004;61(1):28–33. doi: 10.1001/archpsyc.61.1.28. [DOI] [PubMed] [Google Scholar]
- 35.Dohrenwend BP, Levav I, Shrout PE, Schwartz S, Naveh G, Link BG, Skodol AE, Stueve A. Socioeconomic status and psychiatric disorders: the causation-selection issue. Science. 1992;255(5047):946–952. doi: 10.1126/science.1546291. [DOI] [PubMed] [Google Scholar]
- 36.1854 Comission on Lunacy. Report on Insanity and Idiocy in Massachusetts. Boston: Harvard University Press; 1971. [Google Scholar]
- 37.Byrne M, Agerbo E, Eaton WW, Mortensen PB. Parental socio-economic status and risk of first admission with schizophrenia- a Danish national register based study. Social psychiatry and psychiatric epidemiology. 2004;39(2):87–96. doi: 10.1007/s00127-004-0715-y. [DOI] [PubMed] [Google Scholar]
- 38.Davies G, Welham J, Chant D, Torrey EF, McGrath J. A systematic review and meta-analysis of Northern Hemisphere season of birth studies in schizophrenia. Schizophrenia bulletin. 2003;29(3):587–593. doi: 10.1093/oxfordjournals.schbul.a007030. [DOI] [PubMed] [Google Scholar]
- 39.Torrey EF, Miller J, Rawlings R, Yolken RH. Seasonality of births in schizophrenia and bipolar disorder: a review of the literature. Schizophrenia research. 1997;28(1):1–38. doi: 10.1016/s0920-9964(97)00092-3. [DOI] [PubMed] [Google Scholar]
- 40.Carrion-Baralt JR, Smith CJ, Rossy-Fullana E, Lewis-Fernandez R, Davis KL, Silverman JM. Seasonality effects on schizophrenic births in multiplex families in a tropical island. Psychiatry research. 2006;142(1):93–97. doi: 10.1016/j.psychres.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Verdoux H, Geddes JR, Takei N, Lawrie SM, Bovet P, Eagles JM, Heun R, McCreadie RG, McNeil TF, O’Callaghan E, Stober G, Willinger MU, Wright P, Murray RM. Obstetric complications and age at onset in schizophrenia: an international collaborative meta-analysis of individual patient data. The American journal of psychiatry. 1997;154(9):1220–1227. doi: 10.1176/ajp.154.9.1220. [DOI] [PubMed] [Google Scholar]
- 42.Geddes JR, Verdoux H, Takei N, Lawrie SM, Bovet P, Eagles JM, Heun R, McCreadie RG, McNeil TF, O’Callaghan E, Stober G, Willinger U, Murray RM. Schizophrenia and complications of pregnancy and labor: an individual patient data meta-analysis. Schizophrenia bulletin. 1999;25(3):413–423. doi: 10.1093/oxfordjournals.schbul.a033389. [DOI] [PubMed] [Google Scholar]
- 43.Geddes JR, Lawrie SM. Obstetric complications and schizophrenia: a meta-analysis. Br J Psychiatry. 1995;167(6):786–793. doi: 10.1192/bjp.167.6.786. [DOI] [PubMed] [Google Scholar]
- 44.Susser ES, Lin SP. Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944–1945. Archives of general psychiatry. 1992;49(12):983–988. doi: 10.1001/archpsyc.1992.01820120071010. [DOI] [PubMed] [Google Scholar]
- 45.Zornberg GL, Buka SL, Tsuang MT. Hypoxic-ischemia-related fetal/neonatal complications and risk of schizophrenia and other nonaffective psychoses: a 19-year longitudinal study. The American journal of psychiatry. 2000;157(2):196–202. doi: 10.1176/appi.ajp.157.2.196. [DOI] [PubMed] [Google Scholar]
- 46.Rosso IM, Cannon TD, Huttunen T, Huttunen MO, Lonnqvist J, Gasperoni TL. Obstetric risk factors for early-onset schizophrenia in a Finnish birth cohort. The American journal of psychiatry. 2000;157(5):801–807. doi: 10.1176/appi.ajp.157.5.801. [DOI] [PubMed] [Google Scholar]
- 47.Dalman C, Allebeck P, Cullberg J, Grunewald C, Koster M. Obstetric complications and the risk of schizophrenia: a longitudinal study of a national birth cohort. Archives of general psychiatry. 1999;56(3):234–240. doi: 10.1001/archpsyc.56.3.234. [DOI] [PubMed] [Google Scholar]
- 48.Gregory I. Factors influencing first admission rates to Canadian mental hospitals: III; an analysis by education, marital status, country of birth, religion, and rural-urban residence, 1950–1952. Canadian journal of psychiatry. 1959;4:133–151. doi: 10.1177/070674375900400209. [DOI] [PubMed] [Google Scholar]
- 49.Hare EH, Moran PA. Raised parental age in psychiatric patients: evidence for the constitutional hypothesis. Br J Psychiatry. 1979;134:169–177. doi: 10.1192/bjp.134.2.169. [DOI] [PubMed] [Google Scholar]
- 50.Granville-Grossman KL. Parental age and schizophrenia. Br J Psychiatry. 1966;112(490):899–905. doi: 10.1192/bjp.112.490.899. [DOI] [PubMed] [Google Scholar]
- 51.Kinnell HG. Parental age in schizophrenia. Br J Psychiatry. 1983;142:204. doi: 10.1192/bjp.142.2.204a. [DOI] [PubMed] [Google Scholar]
- 52.Zammit S, Allebeck P, Dalman C, Lundberg I, Hemmingson T, Owen MJ, Lewis G. Paternal age and risk for schizophrenia. Br J Psychiatry. 2003;183:405–408. doi: 10.1192/bjp.183.5.405. [DOI] [PubMed] [Google Scholar]
- 53.Malaspina D, Harlap S, Fennig S, Heiman D, Nahon D, Feldman D, Susser ES. Advancing paternal age and the risk of schizophrenia. Archives of general psychiatry. 2001;58(4):361–367. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- 54.Dalman C, Allebeck P. Paternal age and schizophrenia: further support for an association. The American journal of psychiatry. 2002;159(9):1591–1592. doi: 10.1176/appi.ajp.159.9.1591. [DOI] [PubMed] [Google Scholar]
- 55.Byrne M, Agerbo E, Ewald H, Eaton WW, Mortensen PB. Parental age and risk of schizophrenia: a case-control study. Archives of general psychiatry. 2003;60(7):673–678. doi: 10.1001/archpsyc.60.7.673. [DOI] [PubMed] [Google Scholar]
- 56.Brown AS, Schaefer CA, Wyatt RJ, Begg MD, Goetz R, Bresnahan MA, Harkavy-Friedman J, Gorman JM, Malaspina D, Susser ES. Paternal age and risk of schizophrenia in adult offspring. The American journal of psychiatry. 2002;159(9):1528–1533. doi: 10.1176/appi.ajp.159.9.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sipos A, Rasmussen F, Harrison G, Tynelius P, Lewis G, Leon DA, Gunnell D. Paternal age and schizophrenia: a population based cohort study. BMJ (Clinical research ed. 2004;329(7474):1070. doi: 10.1136/bmj.38243.672396.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Archives of general psychiatry. 1987;44(7):660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 59.Yolken RH, Torrey EF. Viruses, schizophrenia, and bipolar disorder. Clinical microbiology reviews. 1995;8(1):131–145. doi: 10.1128/cmr.8.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torrey EF, Yolken RH. Toxoplasma gondii and schizophrenia. Emerging infectious diseases. 2003;9(11):1375–1380. doi: 10.3201/eid0911.030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown AS, Cohen P, Greenwald S, Susser E. Nonaffective psychosis after prenatal exposure to rubella. The American journal of psychiatry. 2000;157(3):438–443. doi: 10.1176/appi.ajp.157.3.438. [DOI] [PubMed] [Google Scholar]
- 62.Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Archives of general psychiatry. 2001;58(11):1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- 63.Gattaz WF, Abrahao AL, Foccacia R. Childhood meningitis, brain maturation and the risk of psychosis. European archives of psychiatry and clinical neuroscience. 2004;254(1):23–26. doi: 10.1007/s00406-004-0431-3. [DOI] [PubMed] [Google Scholar]
- 64.Eaton WW, Hayward C, Ram R. Schizophrenia and rheumatoid arthritis: a review. Schizophrenia research. 1992;6(3):181–192. doi: 10.1016/0920-9964(92)90001-l. [DOI] [PubMed] [Google Scholar]
- 65.McLaughin D. Racial and Sex differences in Length of Hospitalization of Schizophrenics. Honolulu: 1977. [Google Scholar]
- 66.in ’t Veld BA, Launer LJ, Breteler MM, Hofman A, Stricker BH. Pharmacologic agents associated with a preventive effect on Alzheimer’s disease: a review of the epidemiologic evidence. Epidemiologic reviews. 2002;24(2):248–268. doi: 10.1093/epirev/mxf001. [DOI] [PubMed] [Google Scholar]
- 67.Etminan M, Gill S, Samii A. Effect of non-steroidal anti-inflammatory drugs on risk of Alzheimer’s disease: systematic review and meta-analysis of observational studies. BMJ (Clinical research ed. 2003;327(7407):128. doi: 10.1136/bmj.327.7407.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.DeLisi LE, Boccio AM, Riordan H, Hoff AL, Dorfman A, McClelland J, Kushner M, Van Eyl O, Oden N. Familial thyroid disease and delayed language development in first admission patients with schizophrenia. Psychiatry research. 1991;38(1):39–50. doi: 10.1016/0165-1781(91)90051-p. [DOI] [PubMed] [Google Scholar]
- 69.Wright P, Sham PC, Gilvarry CM, Jones PB, Cannon M, Sharma T, Murray RM. Autoimmune diseases in the pedigrees of schizophrenic and control subjects. Schizophrenia research. 1996;20(3):261–267. doi: 10.1016/0920-9964(96)82950-1. [DOI] [PubMed] [Google Scholar]
- 70.Eaton W, Mortensen PB, Agerbo E, Byrne M, Mors O, Ewald H. Coeliac disease and schizophrenia: population based case control study with linkage of Danish national registers. BMJ (Clinical research ed. 2004;328(7437):438–439. doi: 10.1136/bmj.328.7437.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dohan FC. Hypothesis: genes and neuroactive peptides from food as cause of schizophrenia. Advances in biochemical psychopharmacology. 1980;22:535–548. [PubMed] [Google Scholar]
- 72.Ganguli R, Brar JS, Rabin BS. Immune abnormalities in schizophrenia: evidence for the autoimmune hypothesis. Harvard review of psychiatry. 1994;2(2):70–83. doi: 10.3109/10673229409017120. [DOI] [PubMed] [Google Scholar]
- 73.Rothermundt M, Arolt V, Bayer TA. Review of immunological and immunopathological findings in schizophrenia. Brain, behavior, and immunity. 2001;15(4):319–339. doi: 10.1006/brbi.2001.0648. [DOI] [PubMed] [Google Scholar]
- 74.Muller N, Riedel M, Scheppach C, Brandstatter B, Sokullu S, Krampe K, Ulmschneider M, Engel RR, Moller HJ, Schwarz MJ. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. The American journal of psychiatry. 2002;159(6):1029–1034. doi: 10.1176/appi.ajp.159.6.1029. [DOI] [PubMed] [Google Scholar]
- 75.Dickerson FB, Boronow JJ, Stallings CR, Origoni AE, Yolken RH. Reduction of symptoms by valacyclovir in cytomegalovirus-seropositive individuals with schizophrenia. The American journal of psychiatry. 2003;160(12):2234–2236. doi: 10.1176/appi.ajp.160.12.2234. [DOI] [PubMed] [Google Scholar]
- 76.Eaton W, Harrison G. Ethnic disadvantage and schizophrenia. Acta psychiatrica Scandinavica. 2000;(407):38–43. doi: 10.1034/j.1600-0447.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- 77.Eaton W, Harrison G. Life chances, life planning, and schizophrenia: a review and interpretation of research on social deprivation. International Journal of Mental Health. 2001;30:58–81. [Google Scholar]
- 78.Leao TS, Sundquist J, Frank G, Johansson LM, Johansson SE, Sundquist K. Incidence of schizophrenia or other psychoses in first- and second-generation immigrants: a national cohort study. The Journal of nervous and mental disease. 2006;194(1):27–33. doi: 10.1097/01.nmd.0000195312.81334.81. [DOI] [PubMed] [Google Scholar]
- 79.Hall W, Degenhardt L. Cannabis use and psychosis: a review of clinical and epidemiological evidence. The Australian and New Zealand journal of psychiatry. 2000;34(1):26–34. doi: 10.1046/j.1440-1614.2000.00685.x. [DOI] [PubMed] [Google Scholar]
- 80.Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ (Clinical research ed. 2002;325(7374):1199. doi: 10.1136/bmj.325.7374.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weiser M, Reichenberg A, Rabinowitz J, Kaplan Z, Caspi A, Yasvizky R, Mark M, Knobler HY, Nahon D, Davidson M. Self-reported drug abuse in male adolescents with behavioral disturbances, and follow-up for future schizophrenia. Biological psychiatry. 2003;54(6):655–660. doi: 10.1016/s0006-3223(03)00110-0. [DOI] [PubMed] [Google Scholar]
- 82.van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. Cannabis use and psychosis: a longitudinal population-based study. American journal of epidemiology. 2002;156(4):319–327. doi: 10.1093/aje/kwf043. [DOI] [PubMed] [Google Scholar]
- 83.Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ (Clinical research ed. 2002;325(7374):1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Veen ND, Selten JP, van der Tweel I, Feller WG, Hoek HW, Kahn RS. Cannabis use and age at onset of schizophrenia. The American journal of psychiatry. 2004;161(3):501–506. doi: 10.1176/appi.ajp.161.3.501. [DOI] [PubMed] [Google Scholar]
- 85.Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophrenia bulletin. 2005;31(3):608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- 86.Degenhardt L, Hall W. Is cannabis use a contributory cause of psychosis? Canadian journal of psychiatry. 2006;51(9):556–565. doi: 10.1177/070674370605100903. [DOI] [PubMed] [Google Scholar]
- 87.Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, Taylor A, Arseneault L, Williams B, Braithwaite A, Poulton R, Craig IW. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biological psychiatry. 2005;57(10):1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 88.Barnes TR, Mutsatsa SH, Hutton SB, Watt HC, Joyce EM. Comorbid substance use and age at onset of schizophrenia. Br J Psychiatry. 2006;188:237–242. doi: 10.1192/bjp.bp.104.007237. [DOI] [PubMed] [Google Scholar]
- 89.Arendt M, Rosenberg R, Foldager L, Perto G, Munk-Jorgensen P. Cannabis-induced psychosis and subsequent schizophrenia-spectrum disorders: follow-up study of 535 incident cases. Br J Psychiatry. 2005;187:510–515. doi: 10.1192/bjp.187.6.510. [DOI] [PubMed] [Google Scholar]
- 90.Eaton WW. Residence, social class, and schizophrenia. Journal of health and social behavior. 1974;15(4):289–299. [PubMed] [Google Scholar]
- 91.Lewis G, David A, Andreasson S, Allebeck P. Schizophrenia and city life. Lancet. 1992;340(8812):137–140. doi: 10.1016/0140-6736(92)93213-7. [DOI] [PubMed] [Google Scholar]
- 92.Marcelis M, Navarro-Mateu F, Murray R, Selten JP, Van Os J. Urbanization and psychosis: a study of 1942–1978 birth cohorts in The Netherlands. Psychological medicine. 1998;28(4):871–879. doi: 10.1017/s0033291798006898. [DOI] [PubMed] [Google Scholar]
- 93.McCreadie RG. The Nithsdale Schizophrenia Surveys. 16. Breast-feeding and schizophrenia: preliminary results and hypotheses. Br J Psychiatry. 1997;170:334–337. doi: 10.1192/bjp.170.4.334. [DOI] [PubMed] [Google Scholar]
- 94.Torrey EF, Yolken RH. At issue: is household crowding a risk factor for schizophrenia and bipolar disorder? Schizophrenia bulletin. 1998;24(3):321–324. doi: 10.1093/oxfordjournals.schbul.a033329. [DOI] [PubMed] [Google Scholar]
- 95.Torrey EF, Yolken RH. Could schizophrenia be a viral zoonosis transmitted from house cats? Schizophrenia bulletin. 1995;21(2):167–171. doi: 10.1093/schbul/21.2.167. [DOI] [PubMed] [Google Scholar]
- 96.van Os J, Krabbendam L, Myin-Germeys I, Delespaul P. The schizophrenia envirome. Current opinion in psychiatry. 2005;18(2):141–145. doi: 10.1097/00001504-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 97.McGrath JJ. Myths and plain truths about schizophrenia epidemiology--the NAPE lecture 2004. Acta Psychiatr Scand. 2005;111(1):4–11. doi: 10.1111/j.1600-0447.2004.00467.x. [DOI] [PubMed] [Google Scholar]