Abstract
Background
We and others have reported mutations in the cardiac predominant sodium channel gene SCN5A in patients with AF. We also have reported that SCN1B is associated with Brugada syndrome and isolated cardiac conduction disease. Here we tested the hypothesis that mutations in the 4 sodium channel β-subunit genes SCN1B – SCN4B contribute to atrial fibrillation (AF) susceptibility.
Methods and Results
Screening for mutations in the 4 β-subunit genes was performed in 480 patients with AF (118 patients with lone AF and 362 patients with AF and cardiovascular disease) and 548 controls (188 ethnically-defined anonymized subjects and 360 subjects without AF). The effects of mutant β-subunits on SCN5A mediated currents were studied using electrophysiological studies. We identified 2 non-synonymous variants in SCN1B (resulting in R85H, D153N) and 2 in SCN2B (R28Q, R28W) in patients with AF. These occur at residues highly conserved across mammals, and were absent in controls. In 3 of 4 mutation carriers, the electrocardiograms showed saddle back type ST-segment elevation in the right precordial leads. Transcripts encoding both SCN1B and SCN2B were detected in human atrium and ventricle. In heterologous expression studies using Chinese hamster ovary cells, the mutant β1- or β2-subunits reduced SCN5A mediated current and altered channel gating compared to coexpression of wild-type subunits.
Conclusions
Loss of function mutations in sodium channel β-subunits were identified in patients with AF, and were associated with a distinctive ECG phenotype. These findings further support the hypothesis that decreased sodium current enhances AF susceptibility.
Keywords: arrhythmia, sodium channel, electrophysiology, genetics, mutations
Introduction
Risk factors for the development of AF include male gender, increasing age, hypertension, type II diabetes, metabolic syndrome, and obesity as well as underlying heart disease.1–3 In addition to these risk factors, multiple studies now support a genetic contribution to AF susceptibility. In isolated patients and families, mutations in multiple ion channel genes including KCNQ1, KCNE2, KCNJ2, KCNA5, SUR2A, and SCN5A as well as the gap junction gene GJA5 and the nuclear protein NUP155 have been associated with AF.4–6 In addition, linkage of AF to 4 further loci has been reported, although the disease genes in the loci have not yet been identified.4 In population studies, increased AF susceptibility has been associated with common polymorphisms in ion channels (KCNE1, KCNE5, SCN5A), a G-protein subunit (GNB3), angiotensinogen (AGT), and a locus near the atrial transcription factor PITX2.4, 5, 7
Sodium channels are multi-subunit protein complexes composed of pore-forming α-subunits, auxiliary function-modifying β-subunits,8 and multiple other proteins.9 In humans, 4 sodium channel β-subunits (β1 to β4, encoded by SCN1B to SCN4B) have been identified. They share a common predicted protein topology with an extracellular immunoglobulin-like domain, a single transmembrane spanning segment, and an intracellular C-terminal domain.8 Functions attributed to β-subunits include an increase in sodium channel expression at the cell surface, modulation of channel gating and voltage dependence, and a role in cell adhesion and recruitment of cytosolic proteins such as ankyrin G.8
Mutations in SCN5A, encoding the predominant cardiac sodium channel α-subunit, cause a range of inherited arrhythmia diseases including the long QT syndrome, the Brugada syndrome, progressive cardiac conduction disease, and sick sinus syndrome.10 Moreover, mutations in SCN1B and SCN4B have also been implicated .in the Brugada syndrome and/or conduction disease, and long QT syndrome, respectively.11, 12 In addition, SCN5A mutations and polymorphisms have been associated with AF, and we recently reported SCN5A mutations in 5.9% of patients with AF.4, 5, 13 Taken together, these data suggest β-subunits as candidates for AF pathogenesis. Therefore, we have tested this hypothesis by screening sodium channel β-subunit genes for variants in patients with AF and controls.
Methods
Study subjects
The study protocol was approved by the Institutional Review Board of Vanderbilt University and all subjects gave informed consent. This study included two sets of patients with AF: 1) 375 patients including 118 patients with lone AF and 257 patients with AF and cardiovascular disease from the Vanderbilt AF Registry (356 Caucasians [94%], 19 African Americans [5%], 3 Hispanics [8%], 1 Asian [3%]), and 2) 105 patients from the Vanderbilt Cardiac Surgery Registry (101 Caucasians [96%], 3 African Americans [3%], 1 Hispanics [1%]) who had not had AF prior to or during surgery and in whom AF was documented in the postoperative period.13, 14
Control populations
There were three sets of controls in this study. 1) 188 ethnically-identified but otherwise anonymized subjects (Caucasian, African-American, Hispanic, Asian, n=47 for each group) from the Coriell Cell Repositories (Camden, NJ). 2) For the lone AF controls, we used 94 subjects (51 Caucasians [54%], 43 African-Americans [46%]) who on screening had no significant medical history, normal physical examinations, and no personal or family history of AF.15 The subjects were matched on the basis of age, gender and ethnicity to the lone AF cohort. 3) For the group with AF in association with heart disease or other risk factors, we included 266 patients (211 Caucasians [81%], 51 African-Americans [19%]) from the Cardiac Surgery Registry who had no personal or family history of AF, and no post cardiac operative AF.13, 14 The patients were matched for age, gender, ethnicity and ejection fraction (±5%) to the cohort with AF and heart disease.
Resequencing and follow-up genotyping
The coding regions and flanking intronic sequences of all 4 β-subunit genes, including exon 3A of β1B,16 were resequenced in all of the AF (N = 488 patients; 118 patients with lone AF, 257 patients with AF and cardiovascular disease, 105 patients with AF in the postoperative period) and the Coriell control (N = 188 subjects) cohorts in the Vanderbilt DNA Sequencing Facility or the NHLBI-supported Resequencing and Genotyping Service at the J. Craig Venter Institute. The patient controls (94 healthy subjects and 266 controls from the Cardiac Surgery Registry) were genotyped at the variant sites identified in the AF cohort using the MassArray SNP genotyping system (Sequenom Inc., San Diego, CA) at the Vanderbilt DNA Resource Core. Proband samples were included in triplicate as positive controls.
Frequency of ST-segment elevation
A high prevalence of right precordial ST-segment elevation has recently been reported in a group of patients with lone AF.17, 18 We therefore studied the frequency of the ST-segment elevation in lone AF patients and lone AF controls (healthy subjects).
Quantitative Real-Time PCR
Poly A+ RNA pooled separately from atria and ventricles of healthy hearts from ≥15 Caucasians (Clontech, Mountain View, CA) was analyzed. cDNA was synthesized from 2 µg of the RNA and used as template. Genes of interest subcloned into the pEGFP-IRES vector (SCN1B, SCN2B, SCN5A; Clontech) or the pRC-CMV vector (β-actin; Invitrogen, Carlsbad, CA) were used for absolute quantification. Real-time polymerase chain reaction (PCR) was performed with pre-designed TaqMan assays (SCN1B, Hs00168897_m1; SCN2B, Hs00394952_m1; SCN5A, Hs00965681_m1; β-actin, Hs99999903_m1) using the 7900HT Real-Time Instrument (Applied Biosystems, Foster City, CA).
Functional analysis
Full length human SCN1B cDNA (Gen Bank accession No. NM_001037) and SCN2B cDNA (NM_004588) subcloned into a bicistronic vector (pEGFP-IRES, Clontech) also carrying GFP were supplied by Dr. Alfred George, Jr. Mutations were prepared using the QuickChange II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA) and were verified by resequencing. The SCN5A cDNA (NM_198056) was subcloned into the pBK-CMV vector (Stratagene). SCN1B or SCN2B constructs (1 µg) were cotransfected with the plasmid encoding SCN5A (1 µg) in Chinese hamster ovary (CHO) cells. When SCN5A DNA was transfected without β-subunits, the pEGFP-IRES vector was cotransfected to identify fluorescent cells for voltage-clamp.
Whole-cell voltage-clamp was performed at room temperature using an Axopatch 200B amplifier and pClamp9.2 software (Molecular Devices, Union City, CA) as described previously.12 Transfected cells were clamped with ∼1.0-MΩ glass microelectrodes and were held at a resting potential of −120 mV. Data for voltage-dependence were fitted with the Boltzmann equation: y = (1+exp((V‒V1/2)/k))−1, where V1/2 is the voltage required to achieve half-maximal conductance or channel availability and k is the slope factor. Pulse protocols are shown as insets in the Figures.
Statistical Analysis
Data are presented as mean ± SEM. Student’s unpaired t-test, one-way ANOVA, or Fisher’s exact test were used to test for significant differences. A value of P <0.05 was considered statistically significant. The authors had full access to the data and take responsibility for its integrity. All authors have read and agreed to the manuscript as written.
Results
Resequencing the AF population identified two non-synonymous variants in the reference SCN1B sequence and two in SCN2B in three Caucasian and one African-American. These variants were absent in the Coriell controls and in the AF population controls, including a total of 309 Caucasians and 141 African-Americans. Resequencing SCN5A in these 4 patients carrying β-subunit mutations did not identify any coding region or splice junction variant. No AF-unique non-synonymous variant was identified in SCN3B or SCN4B.
Clinical features (Table 1)
Table 1.
Clinical Characteristics of patients carrying β-subunit mutation
| Patient No. |
Sex | Age at onset (years) |
Type of AF | PR interval (ms) |
QRS interval (ms) |
QTc (ms) |
ST segment elevation* |
LVDD (mm) |
LVEF (%) |
LA (mm) |
Nucleotide substitution |
Amino acid substitution |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 58 | Paroxysmal | 140 | 83 | 385 | Yes | 50 | 55 | 47 | SCN1B 254G→A | β1 R85→H |
| 2 | F | 35 | Paroxysmal | 132 | 84 | 400 | No | 46 | 65 | 48 | SCN1B 457G→A | β1 D153→N |
| 3 | M | 55 | Paroxysmal | 200 | 80 | 354 | Yes | 50 | 55 | 40 | SCN2B 82C→T | β2 R28→W |
| 4 | M | 57 | Paroxysmal | 180 | 88 | 398 | Yes | 49 | 60 | 42 | SCN2B 83G→A | β2 R28→Q |
ST-segment elevation in the right precordial leads. AF = atrial fibrillation; LA = left atrium; LVDD = left ventricular diastolic diameter; LVEF = left ventricular ejection fraction; QTc = corrected QT interval by Bazett’s formula.
Patient 1
A heterozygous missense mutation in exon 3 of SCN1B (c.254G→A) resulting in p.R85H was identified in a 68 year-old Caucasian female with paroxysmal AF and moderate aortic stenosis (pressure gradient, 31 mmHg; aortic valve area, 0.82 cm2) (Figure 1). There was no history of hypertension. AF was diagnosed when she was 58 years old. The 12-lead electrocardiogram (ECG) showed saddle-back type ST-segment elevation in leads V1 to V3 (Figure 1). The ST-segment elevation was evident both during AF and sinus rhythm with beat-to-beat and day-to-day variability. She did not have ischemic heart disease, congestive heart failure, electrolyte abnormality, or antiarrhythmic drug therapy to explain the ST-segment elevation. Amiodarone failed to maintain sinus rhythm and did not exacerbate ST-segment elevation. Echocardiography revealed left atrial enlargement. No family member had documented AF, although her grandmother and daughter had a history of stroke. Her father had a history of myocardial infarction. Mutations in SCN1B have been previously reported in the generalized epilepsy with febrile seizures plus (GEFS+ ) syndrome and R85H was initially found in a patient with GEFS+.19, 20 However, there was no personal or family history of seizures in this or any of the other 3 patients having β-subunit mutations described here.
Figure 1.
SCN1B mutations in patients with atrial fibrillation. A. The 12-lead ECG in patient 1 showed ST-segment elevation in leads V1 to V3. B. Heterozygous single-nucleotide change in SCN1B (c.254G→A) resulting in p.R85H in patient 1. Left and right panels indicate sequences in a control subject and the patient, respectively. C. Heterozygous single-nucleotide change in SCN1B (c.457G→A) resulting in p.D153N in patient 2. The arrows in panels B and C indicate heterozygous mutations. D. Alignment of β1 amino acid sequences in human, mouse, rat, and dog. The sites of the mutations are indicated by the boxes. E. Locations of mutations in the predicted topology of the β1-subunit (circles).
Patient 2
A heterozygous missense mutation in exon 4 of SCN1B (c.457G→A) resulting in p.D153N was identified in a 57 year-old African-American female with paroxysmal lone AF (Figure 1). AF was initially diagnosed when she was 35 years old. Her ECG was normal and did not show ST-segment elevation in the right precordial leads or any conduction abnormality. Echocardiography revealed left atrial enlargement. When she was 54 years old, she developed episodes of paroxysmal AF with rapid ventricular responses, unresponsive to sotalol, propafenone, and amiodarone; there was no ST segment elevation during therapy with antiarrhythmic drugs. She underwent atrioventricular nodal ablation followed by implantation of dual-chamber pacemaker. There was no family history of AF, although her mother had hypertension and a pacemaker.
Patient 3
A heterozygous missense mutation in exon 2 of SCN2B (c.82C→T) resulting in p.R28W was identified in a 61 year-old Caucasian male with paroxysmal AF and hypertension (Figure 2). AF was initially diagnosed when he was 55 years old. The ECG showed saddle-back type ST-segment elevation in the right precordial leads during sinus rhythm with a prolonged PR interval of 220 ms. The magnitude of ST-segment elevation showed day-to-day variability. Echocardiography was normal. Holter recording during sinus rhythm did not reveal atrial tachycardia. Sotalol failed to maintain sinus rhythm and did not exacerbate ST-segment elevation. There was no family history of AF.
Figure 2.
SCN2B mutations in patients with atrial fibrillation. A. The 12-lead ECG in patient 3 showed ST-segment elevation in leads V1 to V3. B. Heterozygous single-nucleotide change in SCN2B (c.82C→T) resulting in p.R28W in patient 3. C. c.83G→A resulting in p.R28Q in patient 4. The arrows in panels B and C indicate heterozygous mutations. D. Alignment of β2 amino acid sequences in human, mouse, rat, and dog. R28 is indicated by the box. E. Location of mutations in the predicted topology of the β2-subunit (circle).
Patient 4
A heterozygous missense mutation in exon 2 of SCN2B (c.83G→A) resulting in p.R28Q was identified in a 57 year-old Caucasian male patient with paroxysmal lone AF (Figure 2). AF was initially diagnosed when he was 57 years old. There was saddle back type ST-segment elevation in the right precordial leads. Echocardiography revealed slight left atrial enlargement. He did not receive any antiarrhythmic drugs to restore AF. His father and mother had AF and coronary heart disease.
There was no history of ventricular tachyarrhythmias or syncope in any of the 4 patients. Electrophysiologic study has not been performed in any of the patients. DNA was not available in any family members of the 4 probands.
ST-segment elevation in lone AF
Right precordial ST-segment elevation during sinus rhythm was identified more frequently in lone AF patients (8/118, 6.8%) than in control subjects (1/94, 1.1%, P <0.05). The 8 patients in the lone AF group included one with the SCN2B mutation described above and one with a H445D SCN5A mutation 13. None of the 8 patients except for the SCN2B mutation carrier with a long PR interval (described above) showed a conduction abnormality.
Conservation of mutated amino acids
The sites of the mutations identified here, R85 and D153 in β1, and R28 in β2 (Figure 1 and Figure 2) are completely conserved across human, dog, rat, and mouse sequences, suggesting that these amino acids are functionally important.
Real-Time PCR in human heart
As a first step to establishing the functional significance of SCN1B and SCN2B in the genesis of AF, we studied their expression in atrial tissue. Figure 3 shows that the transcripts were readily detected in both atrium and ventricle. The abundance of SCN1B and SCN5A transcripts was greater in ventricle than atrium (68% and 35% of ventricle, respectively), but SCN2B transcript levels were similar in the two chambers.
Figure 3.

Expression profile of SCN1B, SCN2B, and SCN5A in non-diseased human heart tissues using Real-Time PCR. The graph represents the relative expression levels normalized to those of β actin in atrium (filled bars) and ventricle (open bars). Data are expressed as mean ± SEM.
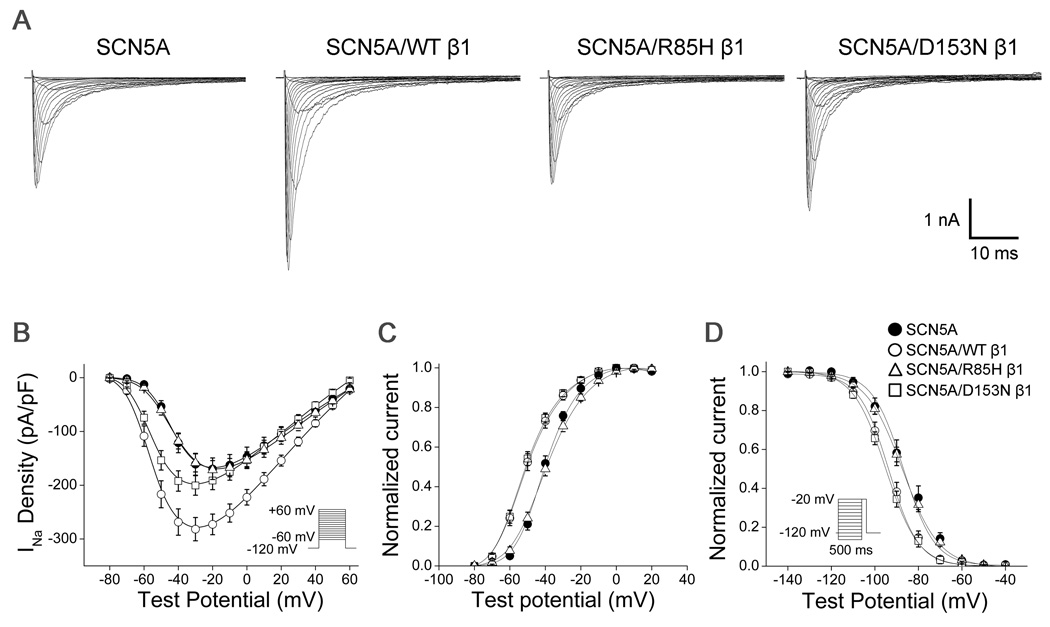
Electrophysiology
Each of the 4 mutant subunits generated a loss of function phenotype. Peak sodium current amplitude was increased by 75% at a test pulse of −30 mV when wild-type β1 was co-expressed with SCN5A (Table 2, Figure 4, P <0.001). This effect was markedly blunted with the D153N mutation (24% increase versus SCN5A alone, P <0.05), and absent with the R85H mutation, resulting in smaller sodium current amplitude for the mutants than wild-type β1 (P <0.001 for each). D153N did not affect the voltage dependence of sodium channel activation or inactivation compared to wild-type β1. However, R85H resulted in a positive shift of both voltage dependence of activation (+10.6 mV, P <0.001) and of inactivation (+6.2 mV, P <0.001) compared to wild-type β1. There was no difference in persistent sodium current among wild-type (1.0±0.1%), D153N (0.9±0.1%), and R85H β1 (1.1±0.2%).
Table 2.
Biophysical Parameters for β1 Variants Associated with Atrial Fibrillation
| Peak Current Density at −30 mV |
Voltage Dependence of Activation |
Voltage Dependence of Inactivation |
||||||
|---|---|---|---|---|---|---|---|---|
| (pA/pF) | N | V 1/2 (mV) | k (mV) | N | V 1/2 (mV) | k (mV) | N | |
| SCN5A | −161.0±20.4 | 18 | −38.7±0.5 | 7.7±0.3 | 18 | −86.9±1.5 | 7.1±0.7 | 18 |
| SCN5A/WT β1 | −281.7±21.7* | 37 | −48.9±0.8* | 8.5±0.5 | 37 | −93.6±0.8* | 6.8±0.4 | 36 |
| SCN5A/R85H β1 | −158.5±16.8† | 35 | −38.3±0.7† | 8.1±0.5 | 35 | −87.4±0.6† | 8.5±0.3 | 34 |
| SCN5A/D153N β1 | −200.4±18.2*, † | 27 | −50.8±0.9* | 8.7±0.6 | 27 | −95.0±0.7* | 7.5±0.5 | 26 |
P<0.05 vs. SCN5A
P<0.05 vs. SCN5A/WT β1.
Figure 4.
Electrophysiologic characteristics of β1-subunit variants in CHO cells expressing SCN5A and β1-subunits. A. Representative current traces. B. Current-voltage relationships of SCN5A alone (filled circles), SCN5A coexpressed with wild-type (WT) β1 (open circles), SCN5A coexpressed with R85H β1 (open triangles), and SCN5A coexpressed with D153N β1 (open squares). The voltage dependence of activation (C) and inactivation (D).
In contrast to β1, wild-type β2 did not increase peak sodium current amplitude compared to SCN5A alone (Table 3, Figure 5, P = NS). However, coexpression of R28W or of R28Q reduced peak current amplitude by 30% (P <0.05) and by 36% (P <0.01) at −30 mV, respectively, compared to wild-type β2. In addition, R28W produced a positive shift in the voltage dependence of activation (+5.1 mV, P <0.001) compared to wild-type, but did not affect the voltage dependence of inactivation (P = NS). R28Q produced a positive shift in both of the voltage dependence of activation (+7.4 mV, P <0.001) and of inactivation (+2.8 mV, P <0.01) compared to wild-type β2. There was no difference in persistent sodium current among wild-type (1.2±0.3%), R28W (1.1±0.3%), and R28Q β2 (1.2±0.2%).
Table 3.
Biophysical Parameters for β2 Variants Associated with Atrial Fibrillation
| Peak Current Density at −30 mV |
Voltage Dependence of Activation |
Voltage Dependence of Inactivation |
||||||
|---|---|---|---|---|---|---|---|---|
| (pA/pF) | N | V 1/2 (mV) | k (mV) | N | V 1/2 (mV) | k (mV) | N | |
| SCN5A | −157±13.6 | 25 | −39.6±0.5 | 7.6±0.2 | 25 | −84.4±0.7 | 6.9±0.3 | 24 |
| SCN5A/β2 WT | −161.6±13.3 | 27 | −38.0±0.6 | 6.2±0.2 | 27 | −80.2±0.6* | 6.8±0.3 | 26 |
| SCN5A/R28W β2 | −112.6±12.2*† | 31 | −32.9±0.8*† | 8.3±0.4 | 31 | −79.4±0.7 | 7.5±0.4 | 29 |
| SCN5A/R28Q β2 | −103.4±11.8*† | 29 | −30.6±0.6*† | 6.3±0.2 | 29 | −77.4±0.7*† | 7.2±0.3 | 26 |
P<0.05 vs. SCN5A
P<0.05 vs. SCN5A/WT β2.
Figure 5.
Electrophysiologic characteristics of β2-subunit variants in CHO cells expressing SCN5A and β2-subunits. A. Representative current traces. B. Current-voltage relationships of SCN5A alone (filled circles), SCN5A coexpressed with wild-type (WT) β2 (open circles), SCN5A coexpressed with R28W β2 (open triangles), and SCN5A coexpressed with R28Q β2 (open squares). The voltage dependence of activation (C) and inactivation (D).
Discussion
We report here rare non-synonymous variants in SCN1B and SCN2B in patients with AF. These variants affect highly conserved residues and were not present in large control populations. Thus, SCN1B and SCN2B are candidate genes for increasing AF susceptibility. The findings that SCN1B and SCN2B are expressed in atrium, and that mutant β1 and β2 produced loss-of function effects on SCN5A-mediated currents further support the association of the variants with AF.
The reported effects of coexpressing β1 on SCN5A channels are controversial.8 Some groups have reported that β1 increases SCN5A currents without affecting the voltage dependence of gating or kinetics, while others have reported β1-mediated changes in channel gating and/or kinetics.8 In some reports, β1 has no effect on SCN5A-mediated current.8 In β1 null mice, an increase in sodium current amplitude without a change in channel gating or kinetics has been reported.21 In our experiments, wild-type β1 increased SCN5A currents and modulated channel gating, and the p.R85H and p.D153N mutants showed loss of β1 function with significantly decreased current amplitudes.
The effects of β2 on SCN5A currents have been less extensively studied. While one group reported that β2 has no effect on SCN5A currents using heterologous expression,22 another group reported a negative shift of the voltage dependence of activation.23 Sinus node dysfunction has been reported in β2 null mice.24 In the present study, while β2 had no effects on SCN5A currents except for a minor positive shift of the voltage dependence of inactivation, both the p.R28W and p.R28Q mutants strikingly decreased peak sodium current amplitude. The patients had no evidence of sinus node dysfunction.
All of 4 mutations identified in SCN1B and SCN2B were located in the extracellular domain, which has a critical role in modulation of cell surface expression and gating of sodium channel.25 In previous studies of skeletal muscle and neuronal sodium channel α-subunits, deletion of the intracellular domain of the β1-subunit had no effect on its modulation of α-subunit function whereas deletions within the extracellular domain block modulation.26–28 Our recent study, which describes loss-of-function mutations in SCN1B in the extracellular domain supports functional importance of the extracellular domain.12 However, it is also possible that specific residues may not be as important as preservation of overall structural motifs, since β-subunit modulates sodium channel via the membrane anchor plus additional intracellular or extracellular regions.29
Variation in SCN5A is associated with AF.4, 5 Loss-of-function mutations in SCN5A has been associated with AF as well as with dilated cardiomyopathy, sinus node dysfunction, and/or conduction disease.30 Screening for SCN5A variants in a large AF cohort, which was also used for this study, found SCN5A mutations in 5.9% of those with AF.13 A common polymorphism in SCN5A (H558R) has also been associated with AF susceptibility,4 although this was not reproduced in another study.31
Sodium channels play a critical role not only in the initiation of the action potential, but also in the maintenance of the action potential dome,10 and loss of sodium channel function can cause shortening of refractoriness and slowing of conduction.32 Shortening of refractory period by a reduction in inward current and/or an increase in outward current has been proposed as creating a substrate for reentry,32 and this concept has been supported by evidence that loss of function mutations in SCN5A or gain of function mutations in potassium channel genes that shorten action potential duration contribute to AF susceptibility.31, 33–35 Slow conduction, which is also promoted by decreased sodium current, is another important substrate for reentry.32 Thus, mutations in SCN1B and SCN2B that reduce sodium current can generate an AF-prone substrate through multiple mechanisms even in the presence of other susceptibility modifiers.
The clinical features of the AF cases we identified here seem to share molecular and pathophysiologic characteristics with the Brugada syndrome, characterized by ST-segment elevation in the right precordial leads, episodes of ventricular fibrillation, and occasionally AF.10, 36 Moreover, loss-of-function mutations in SCN5A have been reported in the Brugada syndrome as well as AF,4, 5, 10 and we have recently reported a loss-of-function mutation in SCN1B in the Brugada syndrome.12 Brugada-type ST-segment elevation, similar to this study, has been reported in patients with lone AF, and a genetic etiology is suggested by a high frequency of a family history of AF, although no molecular mechanism was identified in previous studies.17, 18 Taken together, these data implicate loss of sodium channel function due to β-subunit mutations as a further mechanism underlying the Brugada-type ECG and AF susceptibility. We also identified ST-segment elevation in other subjects with AF (more commonly than in controls), but mutations in SCN5A, SCN1B, or SCN2B were only identified in a minority; thus other genetic mechanisms likely play a role.10
Sodium channel blocking drugs are widely used to restore and maintain sinus rhythm in paroxysmal AF. They are also used to exaggerate or unmask ST-segment elevation in Brugada syndrome, where they can increase ventricular arrhythmia susceptibility. Therefore, these drugs may be pro-arrhythmic (or at least ineffectual) in cases of AF – such as those we describe here – in which decreased sodium current plays a role in pathogenesis of the arrhythmia. ST-segment elevation in the right precordial leads may be useful to identify such patients.
Mutations in SCN1B were originally identified in familial epilepsy, GEFS+.19 However, there was no history of epilepsy in our patients carrying mutations including R85H, previously reported as an epilepsy mutation.20 In addition, there was no history of seizure disorder in patients with SCN1B mutations in conduction disease and Brugada syndrome that we have recently described.12 Conversely, to our knowledge, defects in cardiac function have not been investigated in SCN1B mutation carriers presenting with epilepsy, and AF has not been described in the family with R85H and seizures.19, 20 The mechanism underlying this difference between the brain and heart phenotypes is not known, but gender, age, and genetic modifiers (e.g. common polymorphisms) are commonly invoked as modulators of such clinical phenotypes. One possibility is that the Sudden Unexpected Death in Epilepsy (SUDEP) syndrome is a cardiac arrhythmia manifestation of β-subunit or other mutations contributing to epilepsy.37
Limitations
Screening for β-subunit genes was performed in large cohorts including ethnically-defined and population-matched controls, and mutations were identified only in patients with AF. However, it is difficult to have controls definitely free from AF. We believe a cohort of patients with heart disease undergoing cardiac surgery but without AF is a very robust control set. Linkage or segregation analysis was not conducted because DNA was not available in family members of affected patients. The variants are rare and thus genetic variants in β-subunit genes may not be responsible in a large number of patients with AF. Evidence supporting a critical role of β-subunits in AF includes expression of SCN1B and SCN2B in atrium and loss of sodium channel function in the heterologous expression studies. The functional analyses used a conventional heterologous expression system, where the environment is different from that in the native cardiomyocyte, and other proteins associated with the sodium channel complex (including other β-subunits) are absent. Nevertheless, the in vitro characteristics of the mutations were consistent with the phenotype in the patients, further supporting the disease causality of the mutations. The alterations in gating observed indicate that the mutant subunits are expressed and likely coassemble with SCN5A to form dysfunctional channels.
Conclusions
In summary, we have identified mutations in sodium channel β1 and β2 subunit genes in patients with AF, and have shown that sodium currents were reduced and channel gating was altered when the mutant β1 or β2 was coexpressed with SCN5A, compared to coexpression with wild-type β-subunits. Three out of four mutation carriers showed ST-segment elevation in the right precordial leads, further implicating loss of sodium current as a disease mechanism for AF. We speculate that sodium channel blockers may have pro-arrhythmic effects in cases of AF in which decreased sodium current plays a role in pathogenesis of the arrhythmia.
Clinical Perspective
There is a positive family history in many patients with AF, especially lone AF. Recent genetic studies have identified both rare and common genetic variants that appear to predispose to the arrhythmia, and this includes variants in SCN5A, encoding the cardiac sodium channel pore-forming α-subunit. Sodium channels are multi-protein complexes, and so in this study, 4 function-modifying sodium channel β-subunit genes (SCN1B to SCN4B) were screened for mutations in a large number of patients with lone AF and AF associated with cardiovascular disease. This screening effort identified 4 subjects with mutations resulting in changes in amino acids highly conserved across species in SCN1B and SCN2B. All 4 mutations showed decreased sodium current, a change similar to that seen with loss-of-function mutations in SCN5A and SCN1B in Brugada syndrome. AF is relatively common in the Brugada Syndrome, and 3 of the 4 AF patients carrying a mutation in a β-subunit gene showed Brugada Syndrome-like ST-segment elevation, further reinforcing the idea that loss of sodium channel function increases AF susceptibility. Indeed, in some series, saddleback or other ST segment deformities are reported in up to 10% of patients with lone AF, suggesting these patients represent a distinct subgroup of AF due to reduced sodium current through mutations in SCN5A, SCN1B, SCN2B, or other sodium channel-associated protein genes. Exposure to sodium channel blockers could be used to identify this subgroup, although long-term therapy with these drugs would be undesirable because they can increase ventricular arrhythmia susceptibility in Brugada syndrome.
Acknowledgment
We thank Alfred George Jr, Cara Sutcliffe, Christiana Ingram, Gayle Kucera, Tanya Stubblefield, Tao Yang, Dao Wang, Justine Stassun, and Lynn Hall at Vanderbilt University for their assistance in performing and/or analyzing this work. The Vanderbilt DNA Resources Core provided technical assistance for this work.
Funding Sources
Supported by grants from the United States Public Health Service, Bethesda, MD (DR, HL65962, HL49989; DD, HL HL075266), from the Fondation Leducq, Paris, France (DR, Trans-Atlantic Network of Excellence: Preventing Sudden Cardiac Death, 05-CVD-01), and from University Leipzig, Leipzig, Germany (DH, HU 1679/1-1, DFG and NBL Formel.1–109). Also supported by the Resequencing and Genotyping Program of the NHLBI. The Vanderbilt DNA Core Resource is supported in part by Vanderbilt CTSA grant 1 UL1 RR024975 from NCRR/NIH.
Footnotes
Conflict of Interest Disclosures
None.
References
- 1.Wang TJ, Parise H, Levy D, D'Agostino RB, Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 2.Movahed MR, Hashemzadeh M, Jamal MM. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int J Cardiol. 2005;105:315–318. doi: 10.1016/j.ijcard.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe H, Tanabe N, Watanabe T, Darbar D, Roden DM, Sasaki S, Aizawa Y. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation. 2008;117:1255–1260. doi: 10.1161/CIRCULATIONAHA.107.744466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fatkin D, Otway R, Vandenberg JI. Genes and atrial fibrillation: a new look at an old problem. Circulation. 2007;116:782–792. doi: 10.1161/CIRCULATIONAHA.106.688889. [DOI] [PubMed] [Google Scholar]
- 5.Darbar D. Genetics of atrial fibrillation: rare mutations, common polymorphisms, and clinical relevance. Heart Rhythm. 2008;5:483–486. doi: 10.1016/j.hrthm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Chen S, Yoo S, Chakrabarti S, Zhang T, Ke T, Oberti C, Yong SL, Fang F, Li L, de la Fuente R, Wang L, Chen Q, Wang QK. Mutation in nuclear pore component NUP155 leads to atrial fibrillation and early sudden cardiac death. Cell. 2008;135:1017–1027. doi: 10.1016/j.cell.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, Jonasdottir A, Baker A, Thorleifsson G, Kristjansson K, Palsson A, Blondal T, Sulem P, Backman VM, Hardarson GA, Palsdottir E, Helgason A, Sigurjonsdottir R, Sverrisson JT, Kostulas K, Ng MC, Baum L, So WY, Wong KS, Chan JC, Furie KL, Greenberg SM, Sale M, Kelly P, MacRae CA, Smith EE, Rosand J, Hillert J, Ma RC, Ellinor PT, Thorgeirsson G, Gulcher JR, Kong A, Thorsteinsdottir U, Stefansson K. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448:353–357. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- 8.Meadows LS, Isom LL. Sodium channels as macromolecular complexes: implications for inherited arrhythmia syndromes. Cardiovasc Res. 2005;67:448–458. doi: 10.1016/j.cardiores.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Abriel H, Kass RS. Regulation of the voltage-gated cardiac sodium channel Nav1.5 by interacting proteins. Trends Cardiovasc Med. 2005;15:35–40. doi: 10.1016/j.tcm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Terrenoire C, Simhaee D, Kass RS. Role of sodium channels in propagation in heart muscle: how subtle genetic alterations result in major arrhythmic disorders. J Cardiovasc Electrophysiol. 2007;18:900–905. doi: 10.1111/j.1540-8167.2007.00838.x. [DOI] [PubMed] [Google Scholar]
- 11.Medeiros-Domingo A, Kaku T, Tester DJ, Iturralde-Torres P, Itty A, Ye B, Valdivia C, Ueda K, Canizales-Quinteros S, Tusie-Luna MT, Makielski JC, Ackerman MJ. SCN4B-encoded sodium channel beta4 subunit in congenital long-QT syndrome. Circulation. 2007;116:134–142. doi: 10.1161/CIRCULATIONAHA.106.659086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe H, Koopmann TT, Le Scouarnec S, Yang T, Ingram CR, Schott JJ, Demolombe S, Probst V, Anselme F, Escande D, Wiesfeld AC, Pfeufer A, Kaab S, Wichmann HE, Hasdemir C, Aizawa Y, Wilde AA, Roden DM, Bezzina CR. Sodium channel beta1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J Clin Invest. 2008;118:2260–2268. doi: 10.1172/JCI33891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darbar D, Kucera G, Stubblefield T, Wang J, George AL, Roden DM. Cardiac Sodium Channel (SCN5A) Variants Associated with Atrial Fibrillation. Circulation. 2008;117:1927–1935. doi: 10.1161/CIRCULATIONAHA.107.757955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pretorius M, Donahue BS, Yu C, Greelish JP, Roden DM, Brown NJ. Plasminogen activator inhibitor-1 as a predictor of postoperative atrial fibrillation after cardiopulmonary bypass. Circulation. 2007;116:I1–I7. doi: 10.1161/CIRCULATIONAHA.106.677906. [DOI] [PubMed] [Google Scholar]
- 15.Warsy I, Norris K, Roden D, Kannankeril P. Ibutilide-induced QT Prolongation Varies Markedly Among Individuals in a Gender and Exercise-independent Fashion. Circulation. 2004;110:623. [Google Scholar]
- 16.Qin N, D'Andrea MR, Lubin ML, Shafaee N, Codd EE, Correa AM. Molecular cloning and functional expression of the human sodium channel beta1B subunit, a novel splicing variant of the beta1 subunit. Eur J Biochem. 2003;270:4762–4770. doi: 10.1046/j.1432-1033.2003.03878.x. [DOI] [PubMed] [Google Scholar]
- 17.Beldner S, Lin D, Marchlinski FE. Flecainide and propafenone induced ST-segment elevation in patients with atrial fibrillation: clue to specificity of Brugada-type electrocardiographic changes. Am J Cardiol. 2004;94:1184–1185. doi: 10.1016/j.amjcard.2004.07.091. [DOI] [PubMed] [Google Scholar]
- 18.Junttila MJ, Raatikainen MJ, Perkiomaki JS, Hong K, Brugada R, Huikuri HV. Familial clustering of lone atrial fibrillation in patients with saddleback-type ST-segment elevation in right precordial leads. Eur Heart J. 2007;28:463–468. doi: 10.1093/eurheartj/ehl474. [DOI] [PubMed] [Google Scholar]
- 19.Wallace RH, Wang DW, Singh R, Scheffer IE, George AL, Jr, Phillips HA, Saar K, Reis A, Johnson EW, Sutherland GR, Berkovic SF, Mulley JC. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta1 subunit gene SCN1B. Nat Genet. 1998;19:366–370. doi: 10.1038/1252. [DOI] [PubMed] [Google Scholar]
- 20.Scheffer IE, Harkin LA, Grinton BE, Dibbens LM, Turner SJ, Zielinski MA, Xu R, Jackson G, Adams J, Connellan M, Petrou S, Wellard RM, Briellmann RS, Wallace RH, Mulley JC, Berkovic SF. Temporal lobe epilepsy and GEFS+ phenotypes associated with SCN1B mutations. Brain. 2007;130:100–109. doi: 10.1093/brain/awl272. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Santiago LF, Meadows LS, Ernst SJ, Chen C, Malhotra JD, McEwen DP, Speelman A, Noebels JL, Maier SK, Lopatin AN, Isom LL. Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals. J Mol Cell Cardiol. 2007;43:636–647. doi: 10.1016/j.yjmcc.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhar Malhotra J, Chen C, Rivolta I, Abriel H, Malhotra R, Mattei LN, Brosius FC, Kass RS, Isom LL. Characterization of sodium channel alpha- and beta-subunits in rat and mouse cardiac myocytes. Circulation. 2001;103:1303–1310. doi: 10.1161/01.cir.103.9.1303. [DOI] [PubMed] [Google Scholar]
- 23.Johnson D, Bennett ES. Isoform-specific effects of the beta2 subunit on voltage-gated sodium channel gating. J Biol Chem. 2006;281:25875–25881. doi: 10.1074/jbc.M605060200. [DOI] [PubMed] [Google Scholar]
- 24.Maier SK, Westenbroek RE, Chen C, Isom LL, Maass AH, Catteral lWA, Scheuer T. Knock out of the · -subunit of voltage-gated sodium channels causes arrhythmias and disarrangement of sodium channel · -subunits in the heart. Heart Rhythm. 2006;3:S33–S34. [Google Scholar]
- 25.Isom LL. Sodium channel beta subunits: anything but auxiliary. Neuroscientist. 2001;7:42–54. doi: 10.1177/107385840100700108. [DOI] [PubMed] [Google Scholar]
- 26.Chen C, Cannon SC. Modulation of Na+ channel inactivation by the beta 1 subunit: a deletion analysis. Pflugers Arch. 1995;431:186–195. doi: 10.1007/BF00410190. [DOI] [PubMed] [Google Scholar]
- 27.McCormick KA, Isom LL, Ragsdale D, Smith D, Scheuer T, Catterall WA. Molecular determinants of Na+ channel function in the extracellular domain of the beta1 subunit. J Biol Chem. 1998;273:3954–3962. doi: 10.1074/jbc.273.7.3954. [DOI] [PubMed] [Google Scholar]
- 28.McCormick KA, Srinivasan J, White K, Scheuer T, Catterall WA. The extracellular domain of the beta1 subunit is both necessary and sufficient for beta1-like modulation of sodium channel gating. J Biol Chem. 1999;274:32638–32646. doi: 10.1074/jbc.274.46.32638. [DOI] [PubMed] [Google Scholar]
- 29.Zimmer T, Benndorf K. The human heart and rat brain IIA Na+ channels interact with different molecular regions of the beta1 subunit. J Gen Physiol. 2002;120:887–895. doi: 10.1085/jgp.20028703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNair WP, Ku L, Taylor MR, Fain PR, Dao D, Wolfel E, Mestroni L. SCN5A mutation associated with dilated cardiomyopathy, conduction disorder, and arrhythmia. Circulation. 2004;110:2163–2167. doi: 10.1161/01.CIR.0000144458.58660.BB. [DOI] [PubMed] [Google Scholar]
- 31.Ellinor PT, Nam EG, Shea MA, Milan DJ, Ruskin JN, Macrae CA. Cardiac sodium channel mutation in atrial fibrillation. Heart Rhythm. 2007;19:19. doi: 10.1016/j.hrthm.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 33.Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu WY, Jin HW, Sun H, Su XY, Zhuang QN, Yang YQ, Li YB, Liu Y, Xu HJ, Li XF, Ma N, Mou CP, Chen Z, Barhanin J, Huang W. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y, Xia M, Jin Q, Bendahhou S, Shi J, Chen Y, Liang B, Lin J, Liu Y, Liu B, Zhou Q, Zhang D, Wang R, Ma N, Su X, Niu K, Pei Y, Xu W, Chen Z, Wan H, Cui J, Barhanin J. Identification of a KCNE2 gain-of-function mutation in patients with familial atrial fibrillation. Am J Hum Genet. 2004;75:899–905. doi: 10.1086/425342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia M, Jin Q, Bendahhou S, He Y, Larroque MM, Chen Y, Zhou Q, Yang Y, Liu Y, Liu B, Zhu Q, Zhou Y, Lin J, Liang B, Li L, Dong X, Pan Z, Wang R, Wan H, Qiu W, Xu W, Eurlings P, Barhanin J. A Kir2.1 gain-of-function mutation underlies familial atrial fibrillation. Biochem Biophys Res Commun. 2005;332:1012–1019. doi: 10.1016/j.bbrc.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 36.Eckardt L, Kirchhof P, Loh P, Schulze-Bahr E, Johna R, Wichter T, Breithardt G, Haverkamp W, Borggrefe M. Brugada syndrome and supraventricular tachyarrhythmias: a novel association? J Cardiovasc Electrophysiol. 2001;12:680–685. doi: 10.1046/j.1540-8167.2001.00680.x. [DOI] [PubMed] [Google Scholar]
- 37.Monte CP, Arends JB, Tan IY, Aldenkamp AP, Limburg M, de Krom MC. Sudden unexpected death in epilepsy patients: Risk factors. A systematic review. Seizure. 2007;16:1–7. doi: 10.1016/j.seizure.2006.10.002. [DOI] [PubMed] [Google Scholar]