Abstract
A juvenile hormone acid methyltransferase (JHAMT) was isolated as an abundant EST in a library of the corpora allata of the adult female mosquito Aedes aegypti. Its full-length cDNA encodes a 278-aa protein that has 43 % amino acid identity with BmJHAMT, a juvenile hormone acid methyltransferase previously cloned from Bombyx mori. Heterologous expression produced a recombinant protein that metabolizes farnesoic acid (FA) into methyl farnesoate, as well as juvenile hormone acid into juvenile hormone III (JH III) with exquisite stereo specificity. Real time PCR experiments showed that JHAMT mRNA levels are not an unequivocal indicator of JH III synthesis rates; the A. aegypti JHAMT gene, silent in female pupae, was transcriptionally activated just 4–6 hours before adult eclosion. Radiochemical methyltransferase assays using active and inactive corpora allata glands (CA) dissected from sugar and blood-fed females respectively, clearly indicated that significant levels of JHAMT enzymatic activity are present when the CA shows very low spontaneous rates of JH III synthesis. Having the last enzymes of the JH synthetic pathway readily available all the time might be critical for the adult female mosquito to sustain rapid dynamic changes in JH III synthesis in response to nutritional changes or peripheral influences, such as mating or feeding. These results suggest that this gene has different roles in the regulation of JH synthesis in pupal and adult female mosquitoes, and support the hypothesis that the rate-limiting steps in JH III synthesis in adult female mosquitoes are located before entrance of FA into the synthetic pathway.
Keywords: Aedes aegypti, mosquito, juvenile hormone, farnesoic acid, methyltransferase
INTRODUCTION
The addition of a methyl group to biologically active molecules such as hormones, neurotransmitters, lipids, proteins and nucleic acids causes a change in the physicochemical properties of these molecules. The biological functions of methylation are wide ranging and include biosynthesis, metabolism, detoxification, signal transduction, protein sorting and repair, and nucleic acid processing (Martin and McMillan, 2002). The diversity in the roles of methylation is matched by the vast number of methyltransferase (MT) enzymes (EC 2.1.1). Although several classes of MT enzymes are known, the great majority of methylation reactions are catalyzed by the S-adenosylmethionine-dependent MTs (SAM-MTs). This class of enzyme utilizes the methyl donor S-adenosylmethionine (SAM or AdoMet) to methylate substrates. Around 120 members of the SAM-MT family have been classified (EC 2.1.1.X) based on their substrate (Martin and McMillan, 2002).
Juvenile hormone III (JH) is the key hormone regulating metamorphosis and previtellogenic ovarian development in mosquitoes (Klowden, 1997). JH is synthesized and released from the corpora allata (CA), a pair of endocrine glands with nervous connections to the brain (Li et al., 2003a).
The biosynthetic pathway of JHs is divided conventionally into two steps, the early steps and the late steps (Goodman and Granger, 2005). The early steps of JH III biosynthesis follow the mevalonate pathway, with the formation of five-carbon (5C) isoprenoid units from acetate via mevalonic acid, with the sequential head to tail condensation of three 5C units to form farnesyl pyrophosphate (FPP) (Bellés et al., 2005). In the late steps FPP is hydrolyzed by a pyrophosphatase to farnesol, then oxidized successively to farnesal and farnesoic acid (FA) by an alcohol dehydrogenase and an aldehyde dehydrogenase, respectively (Goodman and Granger, 2005). The last two steps diverge depending on the insect order. In Lepidoptera, a C-10, 11 epoxidation by a P450 monooxygenase converts the FA to the epoxy acid (JH acid or JHA), that is afterward methylated by an O-(SAM) dependent methyltransferase (JHAMT) to form the methyl ester. In Orthoptera and Dictyoptera epoxidation follows methylation (Schooley and Baker, 1985; Goodman and Granger, 2005); this is also believed to be the case in mosquitoes (Li et al., 2003a, 2003b).
Because the late-step enzymes are highly specific to insects, they could be excellent targets for selective insect growth regulators; thus, characterization of these molecules is of great importance. Here we describe the molecular and functional characterization of an A. aegypti juvenile hormone acid methyltransferase (or AaJHAMT) that is highly expressed in the CA. It is a member of the same protein family involved in the methylation of JHA in the lepidopteran Bombyx mori (Shinoda and Itoyama, 2003) and in Drosophila melanogaster (Niwa et al., 2008). In addition, we describe the developmental stage-dependent expression of this gene in correlation to methyl farneosate and JH synthesis in the CA, as well as the expression in normal and nutritional deficient female mosquitoes. These studies suggest that AaJHAMT plays different roles in JH synthesis regulation during metamorphosis and reproduction.
MATERIALS AND METHODS
Chemicals
(E,E)-farnesoic acid and (E,E)-methyl farneosate (MF) were purchased from Echelon (Salt Lake City, UT) JH III (79% purity) was purchased from Scitech (Prague, Czech Republic). Methyllaurate, methylpalmitate, lauric acid and palmitatic acid were purchased from DOOSAN Serdary Research Laboratories (Toronto, Canada). Linolenic acid, linoleic acid and tridecanoic acid were purchased from Acros Organics (Geel, Belgium).
Insects
Aedes aegypti of the Rockefeller strain were reared at 28°C and 80 % relative humidity under a photoperiod of 16 h light: 8 h dark. Mated adults were offered a cotton pad soaked in 3% sucrose solution. We will refer to the cotton pad sucrose-fed adults as sugar fed. Three-day-old female mosquitoes were fed pig blood equilibrated to 37 °C, and ATP was added to the blood meal to a final concentration of 1mM immediately before use as previously described (Noriega et al., 1999).
Nutrient-deficient mosquitoes
small nutrient-deficient mosquitoes were produced as previously described (Caroci et al., 2004); briefly, larvae were reared in pans (23 cm x 35 cm x 13 cm) containing 1 liter of distilled water, with the following amounts of a 10 % solution of bovine liver powder (ICN, Aurora, OH) diet: 0.75 ml on day 1, 3, 5 and 7. Under these rearing conditions, most larvae pupate at day 8. Only those females with wing length under 2.5 mm were used.
AaJHAMT cloning
Eleven full length AaJHAMT sequences were obtained from an A. aegypti corpora-allata + corpora cardiaca library, constructed and sequenced as previously described (Noriega et al., 2006). The AaJHAMT EST sequence was queried against the A. aegypti database at VectorBase (http://www.vectorbase.org/index.php); it revealed an identical sequence with the accession number AAEL006280-RA.
Secondary structure analysis
Related sequences from other insect JHAMTs were identified by using BLAST (Altschul et al., 1990). Sequence identities with A. aegypti are as follows: Culex pipiens 68% (accession number: XM_001853694), Anopheles gambiae 60% (accession number: AGAP005256-PA), Drosophila melanogaster 48% (accession number: AB113579) and Bombyx mori 43% (accession number: NP_001036901). Multiple sequence alignment was performed with the T-COFFEE program (Notredame et al., 2000). Secondary structure analysis was performed using the PSIPRED (Jones, 1999) and Jnet programs (Cuff and Barton, 2000). DISOPRED, a disorder prediction program, was utilized to confirm loop regions (Ward et al., 2004). A consensus within the different programs was employed to select the secondary structure elements.
Expression of recombinant protein
The coding region of the AaJHAMT cDNA was cloned into the expression vector pET28a(+) (Novagen). E. coli BL21(DE3) strain cells were transformed with the construct and expressed as previously described (Shinoda and Itoyama, 2003).
Recombinant His-tagged protein was purified from the supernatant by using a HiTrap chelating column (Amersham Pharmacia). Elution buffer containing 300 mM imidazole was exchanged to Tris-Cl (50 mM, pH 7.5) with a PD-10 desalting column (Amersham Pharmacia). Glycerol was added to the enzyme solution (final concentration 50%), and the sample was stored at −80°C until use. Quantification and SDS-PAGE analysis of the purified protein were performed as described (Shinoda and Itoyama, 2003).
Enzyme assays
Recombinant AaJHAMT crude extracts were used to test the enzymatic properties against several juvenoid acids and fatty acids following the protocol previously described by Shinoda and Itoyama (2003) with small modifications. Farnesoic acid (FA; 85 µM), racemic JH III acid (JHA III; 700 µM), linoleic acid (288 µM), linolenic acid (285 µM), palmitic acid (PA; 78 µM), lauric acid (LA; 100 µM), and tridecanoic acid (93 µM) were assayed in 500 µl of Tris-Cl buffer (50 mM, pH 7.4) containing 200 µM SAM and 250 µl of crude extracts for 2 hours at 25°C. For negative control, bacterial crude extracts without the expression vector were added to the same reaction mixtures. The reaction mixtures were extracted and the production of methyl esters were analyzed by RP-HPLC and GC-MS as previously described (Shinoda and Itoyama, 2003). In an other series of experiments, purified recombinant AaJHAMT was incubated in 500 µl of Tris-Cl (50 mM, pH 7.5) buffer containing SAM (500 µM) and either one of the following substrate: FA (50 µM), racemic JHA III (50 µM), LA (100 µM), or PA (100 µM). Amount of AaJHAMT and incubation times at 25°C were adjusted to consume less than 15% of the initial substrates during the assay: 2.6 µg and 10 min for FA and JHA III; 10 µg and 60 min for LA and PA. Reactions were stopped by adding 500 µl of CH3CN and vortexing. To analyze the methylated products of FA (MF) and racemic JHA III (JH III), the samples were cleared by centrifugation and the supernatants were analyzed directly by reversed-phase HPLC (Niwa et al., 2008). To analyze the methylated LA (methyllaurate) and PA (methylpalmitate), the supernatants were extracted with hexane containing 5 µg/ml methyltridecanoate as an internal standard and analyzed by GC-MS. Enzyme activities were measured by at least three independent reactions for each substrate. To analyze the stereo specificity of the JH III produced, purified AaJHAMT (2.6 ug) was incubated in 500 µl of Tris-Cl (50 mM, pH 7.5) buffer containing SAM (500 µM) and racemic JHA III (5 µg) for 60 min at 25°C, and the reaction was stopped with 500 µl of CH3CN as described above. After centrifugation the supernatant was extracted with hexane and analyzed by chiral-HPLC as previously described (Ichikawa et al., 2007).
Quantitative real-time PCR (qPCR)
Total RNA was isolated using RNA-binding glass powder as previously described (Noriega and Wells, 1993). Contaminating genomic DNA was removed using the DNA-freeTM kit (Ambion, Austin, TX). Reverse transcription was carried out using the Reverse-iTTM First Strand Synthesis Kit (ABgene, Epsom, UK) by an oligo dT priming method according to the manufacturer’s recommendations, using 300 ng of total RNA in 20 µl reactions. Real-time PCR was performed with the 7300 Real Time PCR System using TaqMan® Gene Expression Assays together with TaqMan® Universal PCR Master Mix (Applied Biosystems, Foster City, CA). The primer probes for house keeping gene 60S ribosomal protein L32 (rpL32; AAEL003396 from VectorBase) and for juvenile hormone acid methyltransferase (AaJHAMT) genes were as follows:
-
rpL32 Forward: 5’ CCATCAGTCCGATCGCTATGA 3’;
rpL32 Reverse: 5’GTTGTCAATACCTTTCGGCTTACG 3’;
rpL32 Probe: 5’CAAGCTTGCCCCCAACTG 3’;
AaJHAMT Forward: 5’ CCAAGCCAGATTCCTAACATTCAAA 3’;
AaJHAMT Reverse: 5’ TGCCCTGTCCGCAACC 3’;
AaJHAMT Probe: 5’ CTTGCAATCATTACCACAGCAC3’.
Primer/probes were synthesized by Applied Biosystems and reactions were carried in a 20 µl volume according to manufacturer recommendations for Custom TaqMan® Gene Expression Assays. Reactions were run in duplicate using 1µl of cDNA per reaction. Standard curves to quantify relative gene copy number were made from serial dilutions of plasmids containing rpL32 or AaJHAMT gene (300,000; 30,000; 3,000; 300; 30 copies of a plasmid per reaction). Real-time data were collected by 7300 System SDS Software and analyzed in Microsoft Excel. AaJHAMT transcript levels were normalized with rpL32 transcript levels in the same sample. Relative AaJHAMT transcript levels are expressed as a number of copies of AaJHAMT transcript per 10,000 copies of rpL32 transcript. Each RT-PCR data point is average of three independent biological replicates.
Corpora allata in vitro radiochemical assay for AaJHAMT activity
The CA complexes were isolated as previously described (Li et al., 2003a). Rates of MF and JH III biosynthesis were estimated using an in vitro radiochemical assay (Feyereisen and Tobe, 1981; Feyereisen, 1985a), as previously modified (Li et al., 2003a; 2003b). Briefly, CA complexes were incubated for 4h in fresh medium containing 3H-labeled methionine. After extraction and separation by thin-layer chromatography, the MF and JH III bands were removed, placed into scintillation cocktail and assayed for 3H. The quantity of MF and JH III produced was calculated from the specific activity of the 3H-labeled methionine in the medium. The effect of FA on MF and JH III synthesis was tested by adding the precursor directly into the CA incubation medium.
Statistical analysis
Statistical analysis of the data was performed by t-test using GraphPad Prism version 3.00 for Windows, GraphPad Software (San Diego, CA). The results were expressed as mean ± SEM, and considered significantly different at P <0.05.
RESULTS
A juvenile hormone acid methyltransferase sequence from a corpora allata EST collection
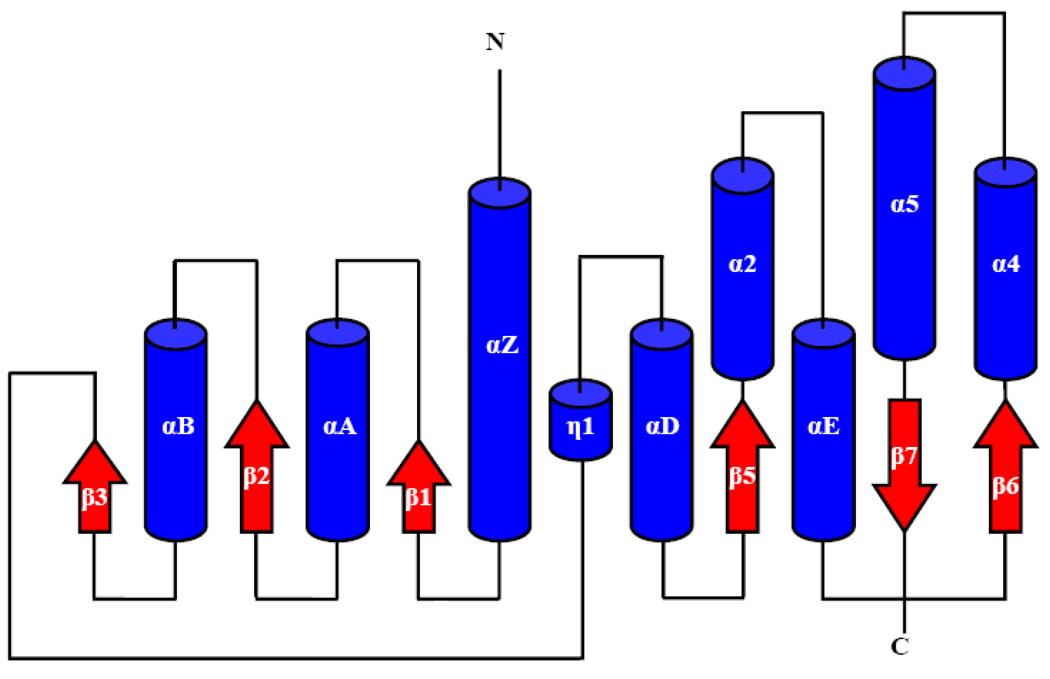
A cDNA library was prepared using corpora-allata + corpora cardiaca from two-and three-day-old A. aegypti adult females (80 pair) as previously described (Noriega et al., 2006). Sequencing revealed 11 ESTs encoding full length ORFs coding for a protein of 278 amino acids (Fig. 1A), that had 43 % amino acid identity with BmJHAMT, a juvenile hormone acid methyltransferase previously cloned from B. mori (Shinoda and Itoyama, 2003). Analysis of the protein sequence showed the presence of a typical SAM-MT fold composed of alternating 6 stranded β sheets with 9 α helices (Fig. 1A). The SAM-binding region, localized close to the N-terminal, was very well conserved among B. mori, D. melanogaster and 3 different species of mosquitoes (Fig. 1A). The expected topology of the fold of AaJHAMT was based on comparisons with previous studies (Martin and McMillan, 2002) (Fig. 1B).
Fig. 1. Juvenile hormone acid methyltransferase sequences and secondary structure comparison.
(A) Multiple alignments of several insect juvenile hormone acid methyltransferases. Secondary structure elements are named according to the literature, α -helices and β-sheets are blue and red colored respectively. The conserved SAM-binding region is highlighted by a brown box. (B). Schematic showing the expected topology of the fold of the A. aegypti juvenile hormone acid methyltransferase. Helices are shown as blue cylinders and β-sheets as red arrows.
Functional characterization of AaJHAMT
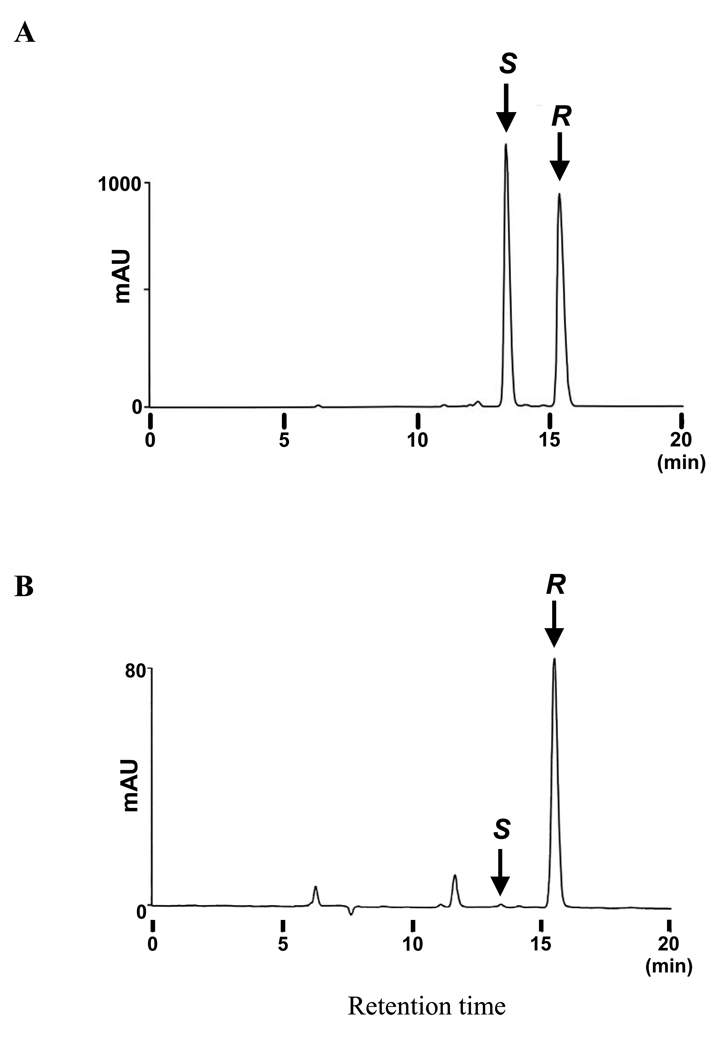
Using crude E. coli extracts expressing recombinant AaJHAMT, we detected high catalytic activity against FA and JHA III in the presence of SAM (data not shown). However, such activity was not detected in the reaction lacking SAM or with crude extract of E. coli transformed with an empty vector in the presence of SAM. No significant activity was detected against linoleic acid, linolenic acid, palmitic acid, lauric acid and tridecanoid acid (data not shown). We used purified rAaJHAMT to further characterize the enzyme specificity; rAaJHAMT showed high activity against racemic JHA III (kcat (min-1) = 1.033) and FA (kcat (min-1) = 0.260) (Table 1). In contrast, rAaJHAMT showed trace activity against lauric acid and palmitic acid even at a higher concentration (100 µM). When the JH III enzymatically produced from racemic JHA III by rAaJHAMT was analyzed by chiral-HPLC, it contained almost only (10R)-JH III (>99.5%), showing that this enzyme has high stereo specificity against (10R)-JHA III (Fig. 2). These results revealed that AaJHAMT is a member of the previously described juvenile hormone acid O-methyltransferase family (Shinoda and Itoyama, 2003); because AaJHAMT has the ability to efficiently use two juvenoid acid substrates (FA and JHA), it could functionally be defined as a juvenoid acid methyltransferase.
Table 1.
Enzymatic activity of purified recombinant AaJHAMT on juvenile hormone acid III, farnesoic acid and two fatty acids
| Substrate | Activity (mol /mol enzyme−1/ min−1) |
|---|---|
| JHA III | 1.033 ± 0.008 |
| FA | 0.260 ± 0.009 |
| Palmitic acid | 0.00089 ± 0.00012 |
| Lauric acid | 0.00010 ± 0.00017 |
Fig. 2. Enantioselectivity of rAaJHAMT.
Chiral-HPLC analysis was performed with (A) racemic JH III, and (B) metabolites from racemic JHA III incubated with the purified rAaJHAMT protein. Arrows indicate (10S)-JH III (S: retention time 13.4 min) and (10R)-JH III (R: retention time 15.5 min), respectively. The S:R ratio of the racemic JHA III used as the substrate should have been ∼50:50 (data not shown), as the JHA III was synthesized by alkaline hydrolysis from the racemic JH III showing 50:50 S:R ratio in (A). The JH III (0.83 µg) produced from racemic JHA III (5 µg) contained almost only (10R)-JH III (>99.5%) and trace amount of (10S)-JH III (B).
Tissue-specific and developmental expression of AaJHAMT
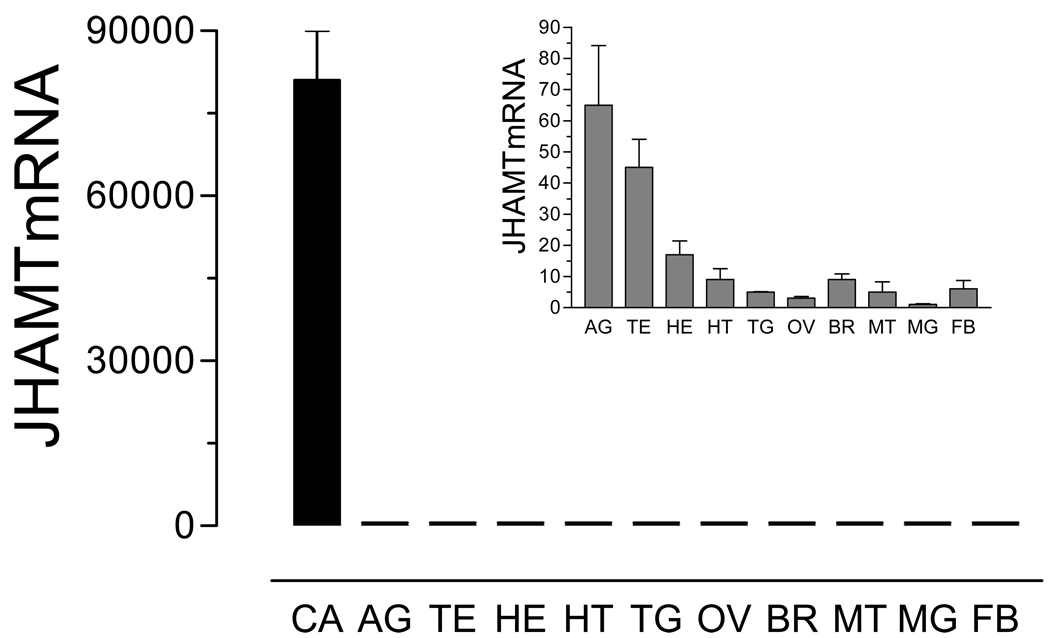
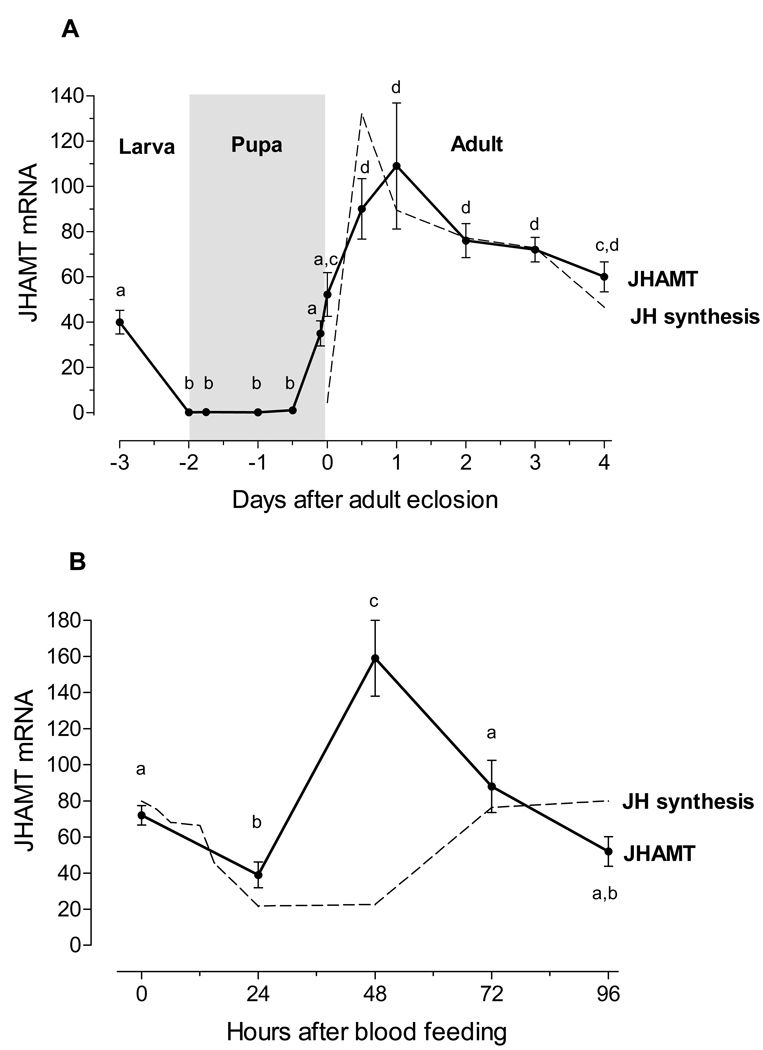
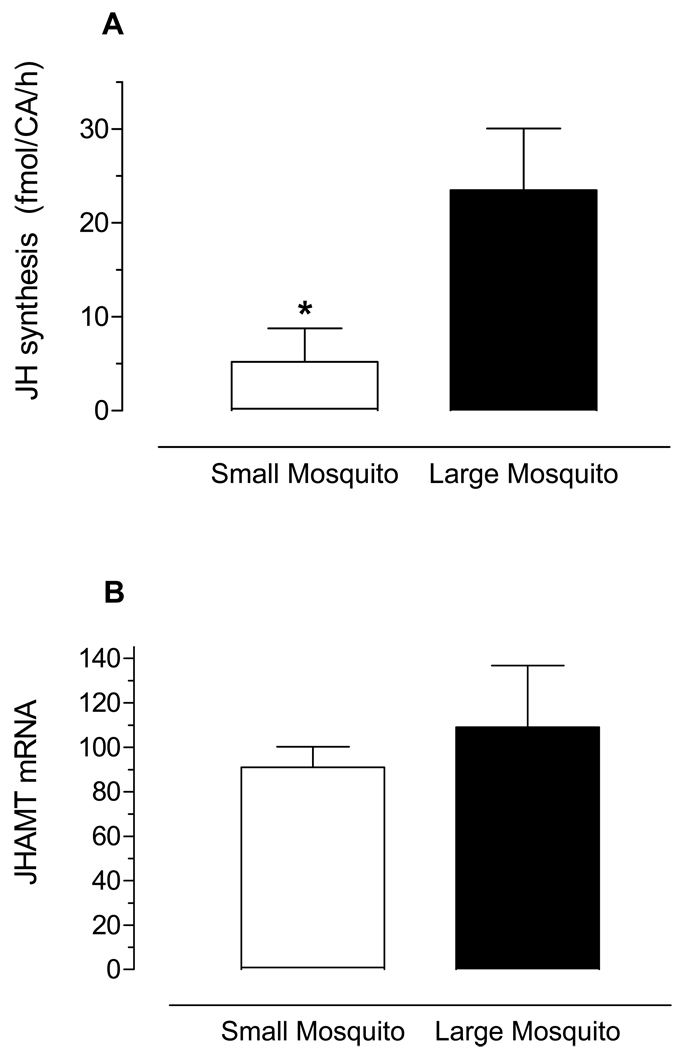
Real time PCR was used to analyze the transcript tissue specificity. AaJHAMT mRNA expression was very high in the CA, with trace amounts present in the thoracic ganglia, head, heart, and other female tissues, as well as in the testis and accessory glands of the male (Fig. 3). Transcripts for AaJHAMT in thorax are most likely produced in the corpora allata; there was a 500 fold difference in AaJHAMT mRNA expression between the first thoracic segment containing the CA and the rest of the thorax (results not shown). Therefore, the whole thorax was used to assess changes of AaJHAMT mRNA during larvae, female pupal and adult development. Transcript levels were high in early 4th instar larvae, undetectable in early and one-day old pupae, and rapidly increased 44–46 h after pupation to relatively high levels at adult eclosion (approximately 48–50 h after pupation). With sugar-fed females, the highest transcript level was observed on day 1 after adult eclosion (Fig. 4A), at the time of maximum biosynthetic activity of the CA (Li et al., 2003a). On the other hand, the messenger level significantly decreased 24 h after blood feeding, when JH synthesis is low, to increase again 48 h after a blood meal, just before the reactivation of JH synthesis observed 3 days after blood feeding (Fig. 4B). Expression of AaJHAMT transcript was also studied in nutrient-deficient mosquitoes; at 24 h after eclosion, the relative levels of AaJHAMT mRNA were similar in large and small mosquitoes (Fig. 5).
Fig. 3. Tissue specific expression of AaJHAMT.
All tissues were dissected from three-day old sugar-fed females, except for testis (TE) and accessory glands (AG) dissected from three-day old sugar-fed males. CA: corpora allata; HE: head; HT: heart; TG: thoracic ganglia; OV: ovaries; BR: brain; MT: Malpighian tubules; MG: midgut, and FB: fat body. The insert shows a detail of values of tissues with less expression. AaJHAMT mRNA is expressed as copy number of AaJHAMT mRNA/ 10,000 copies of rpL32 mRNA. Each RT-PCR data point is average of three independent biological replicates of 10–20 tissue samples.
Fig. 4. Developmental expression of AaJHAMT.
A) Expression of AaJHAMT mRNA in thorax of newly-molted last instar larvae, pupa and sugar-fed females. B) Expression of AaJHAMT mRNA in thorax of blood-fed females. AaJHAMT mRNA (solid lane) is expressed as copy number of AaJHAMT mRNA/ 10,000 copies of rpL32 mRNA. Each RT-PCR data point is average of three independent biological replicates of three insects. Values labeled with the different letters denote significant differences (unpaired t-test P≤0.05). JH biosynthesis values (dotted lane) are based on Li et al., 2003a, and are expressed as relative levels.
Fig. 5. Expression of AaJHAMT mRNA and JH synthesis in nutrient-deficient mosquitoes.
A) JH biosynthesis was evaluated in vitro in CA dissected 24h after emergence, and it is expressed as femtomols per pair of glands per hour. Each bar represents the means ± SEM of 5–10 independent determinations of individual CA (unpaired t-test; *P≤ 0.05). B)AaJHAMT mRNA levels are expressed as copy number of AaJHAMT mRNA/ 10,000 copies of rpL32 mRNA. Each RT-PCR data point is average of three independent biological replicates of three insects. Large are control mosquitoes (dark bar) and small are nutrient-deficient mosquitoes (white bar).
Farnesoic acid methyltransferase activity in isolated corpora allata
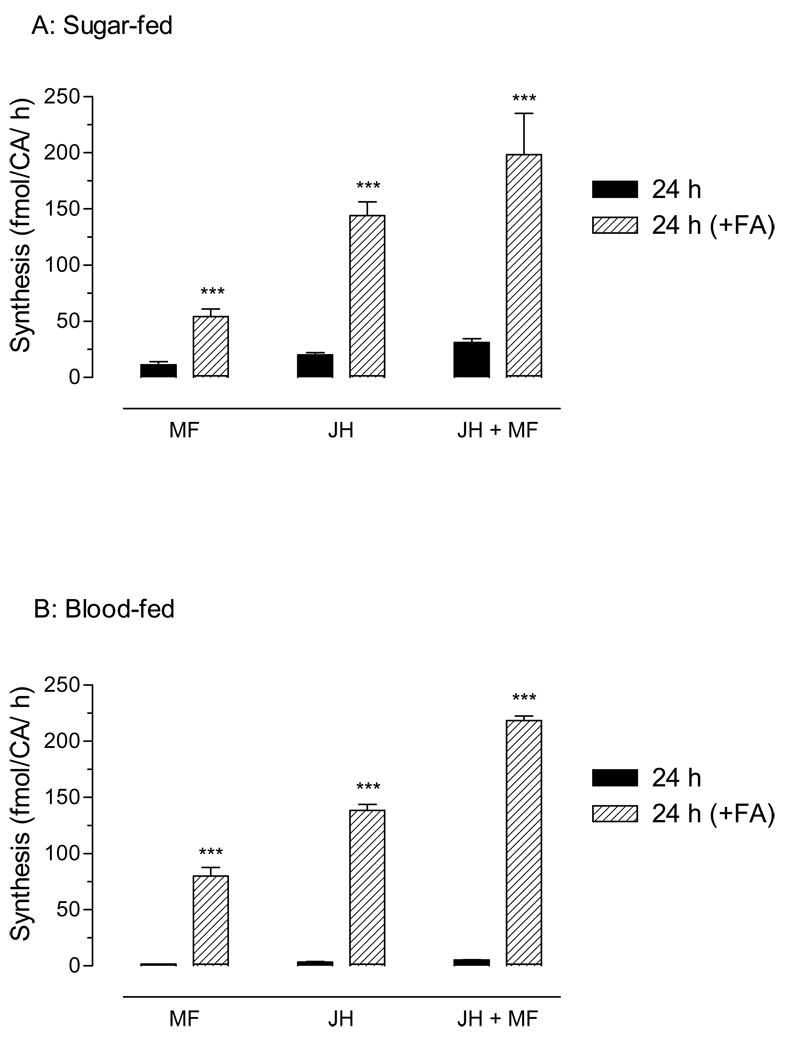
Changes in the activity of AaJHAMT in mosquito corpora allata were analyzed using an in vitro radiochemical assay. Methylation of FA results in the formation of radioactive MF, which is further transformed by a P450 epoxidase into radioactive JH III; therefore the sum of the two juvenoids (MF and JH III) represents the endogenous catalytic activity of AaJHAMT. The addition of exogenous FA dramatically enhanced the production of MF and JH III in the active glands of the 24 h old sugar-fed females, as well as in the inactive gland of the 24 h blood-fed female (Fig. 6).
Fig. 6. Farnesoic acid methyltransferase activity in isolated corpora allata.
CA were incubated in vitro in culture medium alone (solid bars) or with 40 µM FA (stripped bars). A) Sugar-fed: CA from 3-day-old sugar-fed females. B) Blood-fed: CA from females 24h after blood feeding. MF: methyl farneosate, JH: Juvenile hormone III. Bars represent the means ± S.E.M. of 5–10 independent replicates of individual CA. (unpaired t-test; ***P≤0.001).
DISCUSSION
AaJHAMT is structurally and functionally a juvenile hormone acid methyltransferase
We have structurally and functionally characterized a novel A. aegypti juvenile hormone acid methyltransferase involved in the synthesis of JH in the mosquito corpora allata. AaJHAMT was isolated as a fairly abundant EST from the CA of female A. aegypti (Noriega et al., 2006). Analysis of the secondary structure of AaJHAMT revealed the presence of a typical SAM-MT fold, composed by a 6-stranded β sheet with 9 α helices. In all SAM-MT studied, the SAM-binding region is located close to the N-terminal part of the protein (Martin and McMillan, 2002). The substrate-binding region is located in the C-terminal part of the polypeptide, and varies entirely in structure and topology among different groups of SAM-MTs (Martin and McMillan, 2002; Boissier et al., 2006). When lipids are methyl acceptor substrates, they are expected to bind internal cavities of a mostly non-polar character, as observed in Mycobacterium tuberculosis mycolic acid cyclopropane synthase (Boissier et al., 2006; Huang et al., 2002) and Mycobacterium leprae lipid methyltransferase (Graña et al., 2007). These lipid binding pockets structurally are hydrophobic tunnels extending from the surface to the SAM binding site; the entrance of the hydrophobic tunnel is often defined by the α2 and α3 domains (Graña et al., 2007). Common additional features of lipid MTs are: a poorly formed αC, a short helix inserted between β4 and αD, 1 or 2α helices inserted between β5 and αE and 2 or 3 α helices inserted between β6 and β7. With the exception of the absence of β4 and αC these structural features can be recognized in the predicted AaJHAMT secondary structure (Fig. 1A), suggesting that it is a member of the lipid transfer SAM-MT family. This fact was confirmed studying the activity of the recombinant AaJHAMT. Our enzymatic assays showed that rAaJHAMT efficiently recognized the 15-carbon isoprenoid chain of FA or JHA. It had very weak activity against palmitic acid (16C), and did not methylate shorter fatty acids such as lauric (12C) or tridecanoic acid (13C) or the longer linoleic or linolenic acids (18C). It is remarkable that when the JH III generated from the JHA III racemate was analyzed by chiral HPLC it contained almost only (R)-JH III (R:S=99.5:0.5). Whether JHA is present in CA of mosquitoes remains to be seen. However, if it plays any role on JH synthesis, the chirality of (10R)-JH III would be ensured by the stereospecificity of this enzyme.
Transcription of AaJHAMT is suppressed during metamorphosis
Metamorphosis is a developmentally regulated process that is tightly controlled by hormones. Once the insect larva has reached its species-specific size, JH concentrations decrease, allowing pupation and metamorphosis to proceed (Riddiford, 2008). There are no reports on JH titers or synthesis in mosquito pupae; presumably the CA is inactive before adult eclosion. Supporting this statement is the observation that JH-stimulated adult-specific genes are not expressed in pupae, but can be induced by raising them in the presence of JH analogues (Noriega and Wells, 1999). To guarantee a successful completion of metamorphosis, it is reasonable that transcription of genes coding for JH synthetic enzymes is suppressed during pupa development; similar low levels of JHAMT mRNA were described in pupa of D. melanogaster (Niwa et al., 2008) and B. mori (Kinjoh et al., 2007), suppression of JHAMT transcription was recently described as critical for induction of larval-pupal metamorphosis in Tribolium castaneum (Minakuchi et al., 2008). Transcripts levels were high in early 4th instar A. aegypti larvae. Conversely, AaJHAMT mRNA was undetectable in CA of newly pupated mosquitoes or 1 day-old pupae; this is the only time in which we did not detect AaJHAMT transcripts. AaJHAMT mRNA steady state levels sharply increased during the last 4–6 hours before adult eclosion, and newly eclosed females had significantly higher levels of AaJHAMT mRNA. At the same time, JH titers (Shapiro et al., 1986) and spontaneous CA synthetic activity (Li et al., 2003a) are very low in newly eclosed females. Remarkably, the synthesis of juvenoids can be rapidly activated if the CA of the newly eclosed females is stimulated with allatotropin and FA (Li et al., 2003b), suggesting that JHAMT activity is already present.
AaJHAMT plays a different role in JH synthesis during reproduction
Sexual maturation and reproduction in adult mosquitoes are nutrient-dependent processes (Briegel, 1990). JH is part of the signal transduction pathway that connects changes in the nutritional status with activation of specific physiological events during reproduction (Noriega, 2004). The JH level increase during the first day after adult emergence, remains high in sugar-fed females, and rapidly falls after a blood meal (Shapiro et al., 1986). The initial rise in JH after eclosion is essential for female’s reproductive maturation, it signals that ecdysis of the adult has finished and reproductive processes should begin (Klowden, 1997). Teneral reserves are utilized to initiate previtellogenic ovarian development in mosquitoes. Only when nutrients are appropriate can factors released from the brain induce the CA to synthesize enough JH to activate reproductive maturation (Caroci et al., 2004). Allocation of these nutritional reserves for ovarian development is life-threatening if the resources are not sufficient (Martinez-Hernandez et al., 2007). Nutritional deficient females having emerged with low teneral reserves have reduced JH synthesis and previtellogenic development (Caroci et al., 2004), however, they show JHAMT mRNA levels equivalent to those of normal mosquitoes with high JH synthesis. For the female mosquito a tight regulation of JH titers is vital. Having the last enzymes of the JH synthetic pathway readily available all the time might be critical for rapid dynamic changes in JH synthesis in response to nutritional changes or peripheral influences, such as mating, feeding, ovarian development, etc. Evidence of the dynamic regulation comes from the observation that spontaneous and FA stimulated rates of AaJHAMT activity are not correlated. For example, after blood meal, the spontaneous rates are very low, but the stimulated rates are among the highest detected. The rate of spontaneous juvenoid biosynthesis is a direct estimate of the overall activity of the CA and is clearly linked with JH titers (Li et al., 2003a). The FA stimulated rates indicate the relative importance of rate-limitation at early steps in JH biosynthesis; exogenous FA is efficiently utilized by the CA to nullify the effect of rate-limiting factors at an early step(s) in JH synthesis (Feyereisen, 1985b; Li et al., 2003b). The spontaneous synthesis of juvenoids in CA dissected from both sugar-fed and blood-fed females is always markedly stimulated by the addition of FA to the medium, demonstrating that the supply of this precursor is a rate limiting factor in these glands, and not the activity of AaJHAMT.
Conclusions
In summary, these studies demonstrate that the AaJHAMT isolated from a mosquito CA EST library is structurally and functionally a juvenile hormone acid methyltransferase that catalyzes the methylation of farnesoic acid into methyl farnesoate, as well as juvenile hormone acid into juvenile hormone with exquisite stereo specificity. The combination of transcriptional studies and biosynthetic assays showed that the gene has different roles in the regulation of JH synthesis in pupa and adult mosquitoes. Our studies using inactive CA glands from nutritional deficient mosquitoes as well as CA dissected from blood-fed females, clearly suggest that significant levels of the enzyme are always available after adult eclosion, and the rate-limiting factors in JH synthesis, as well as the targets for allatoregulatory peptides (Li et al., 2006), are located before the entrance of FA into the synthetic pathway.
ACKNOWLEDGEMENTS
This work was supported by NIH grant AI 45545 to FGN, and the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN) to TS. AER thanks the UF High Performance Computing Center and the Large Resource Allocations Committee through grant TG-MCA05S010 for supercomputing time. AGT thanks the Pew foundation for generous support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Altschul SF, Gish W, Miller W, Myerst EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bellés X, Martin D, Piulachs M-D. The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu. Rev. Entomol. 2005;50:181–190. doi: 10.1146/annurev.ento.50.071803.130356. [DOI] [PubMed] [Google Scholar]
- Boissier F, Bardou F, Guillet V, Uttenweiler-Joseph S, Daffe M, Quemard A, Mourey L. Further insight into S-adenosylmethionine-dependent methyltransferases Structural characterization of Hma, an enzyme essential for the biosynthesis of oxygenated mycolic acids in Mycobaterium tuberculosis. J. Biol. Chemistry. 2006;281:4434–4445. doi: 10.1074/jbc.M510250200. [DOI] [PubMed] [Google Scholar]
- Briegel H. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J. Insect Physiol. 1990;36:165–172. [Google Scholar]
- Caroci A, Li Y, Noriega FG. Reduced juvenile hormone synthesis in mosquitoes with low teneral reserves prevents ovarian previtellogenic development in Aedes aegypti. J. Exp. Biol. 2004;207:2685–2690. doi: 10.1242/jeb.01093. [DOI] [PubMed] [Google Scholar]
- Cuff JA, Barton GJ. Application of multiple sequence alignment profiles to improve protein secondary structure prediction. Proteins. 2000;40:502–511. doi: 10.1002/1097-0134(20000815)40:3<502::aid-prot170>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Feyereisen R. Radiochemical assay for juvenile hormone III biosynthesis in vitro. In: Law JH, Rilling HC, editors. Methods in Enzymology. vol. III. Orlando, FL: Academic Press; 1985a. pp. 530–539. [Google Scholar]
- Feyereisen R. Regulation of juvenile hormone titer: synthesis. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Vol 7. Oxford: Pergamon Press; 1985b. pp. 391–429. [Google Scholar]
- Feyereisen R, Tobe SS. A rapid partition assay for routine analysis of JH release by insect CA. Anal. Biochem. 1981;111:372–375. doi: 10.1016/0003-2697(81)90575-3. [DOI] [PubMed] [Google Scholar]
- Goodman WG, Granger NA. In: The juvenile Hormones. Gilbert LI, Gil SS, editors. Vol 6. Elsevier: Comprehensive Molecular Insect Science; 2005. pp. 55–115. [Google Scholar]
- Graña M, Haouz A, Buschiazzo A, Miras I, Wehenkel A, Bondet V, Shepard W, Schaeffer F, Cole ST, Alzar PM. The crystal structure of M lepraeML2640c defines a large family of putative S-adenosylmethionine-dependent methyltransferase in mycobacteria. Protein Science. 2007;16:1896–1904. doi: 10.1110/ps.072982707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Smith CV, Glickman MS, Jacobs WR, Sacchettini JC. Crystal structure of mycolic acid cyclopropane synthases from Mycobaterium tuberculosis. J. Biol. Chemistry. 2002;277:11559–11569. doi: 10.1074/jbc.M111698200. [DOI] [PubMed] [Google Scholar]
- Ichikawa A, Ono H, Furuta K, Shiotsuki T, Shinoda T. Enantioselective separation of racemic juvenile hormone III by normal-phase high-performance liquid chromatography and preparation of [2H3]juvenile hormone III as an internal standard for liquid chromatography-mass spectrometry quantification. J. Chromatogr. A. 2007;1161:252–260. doi: 10.1016/j.chroma.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Jones DT. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 1999;292:195–202. doi: 10.1006/jmbi.1999.3091. [DOI] [PubMed] [Google Scholar]
- Kinjoh T, Kaneko Y, Itoyama K, Mita K, Hiruma K, Shinoda T. Control of juvenile hormone biosynthesis in Bombyx mori: cloning of the enzymes in the mevalonate pathway and assessment of their developmental expression in the corpora allata. Insect Biochem. Mol. Biol. 2007;37:808–818. doi: 10.1016/j.ibmb.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Klowden MJ. Endocrine aspects of mosquito reproduction. Arch. Insect Biochem. Physiol. 1997;35:491–512. [Google Scholar]
- Li YP, Hernandez-Martinez S, Unnithan GC, Feyereisen R, Noriega FG. Activity of the corpora allata of adult female Aedes aegypti: effects of mating and feeding. Insect Biochem. Mol. Biol. 2003a;33:1307–1315. doi: 10.1016/j.ibmb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Li Y, Unnithan GC, Veenstra JA, Feyereisen R, Noriega FG. Stimulation of JH biosynthesis by the corpora allata of adult female Aedes aegypti in vitro: effect of farnesoic acid and Aedes allatotropin. J. Exp. Biol. 2003b;206:1825–1832. doi: 10.1242/jeb.00371. [DOI] [PubMed] [Google Scholar]
- Li Y, Hernandez-Martinez S, Fernandez F, Mayoral JG, Topalis P, Priestap H, Perez M, Navare A, Noriega FG. Biochemical, Molecular, and functional characterization of PISCF-allatostatin a regulator of juvenile biosynthesis in the mosquito Aedes aegypti. J. Biol. Chem. 2006;281:34048–34055. doi: 10.1074/jbc.M606341200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, McMillan FM. SAM (dependent) I AM: the S-adenosylmethionine-dependent methyltransferase fold. Curr. Op. Struct. Biol. 2002;12:783–793. doi: 10.1016/s0959-440x(02)00391-3. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez S, Mayoral JG, Li Y, Noriega FG. Role of juvenile hormone and allatotropin on nutrient allocation, ovarian development and survivorship in mosquitoes. J. Insect Physiol. 2007;53:230–234. doi: 10.1016/j.jinsphys.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakuchi C, Namiki T, Yoshiyama M, Shinoda T. RNAi-mediated knockdown of juvenile hormone acid O-methyltransferase gene causes precocious metamorphosis in the red flour beetle Tribolium castaneum. Febs J. 2008;275:2919–2931. doi: 10.1111/j.1742-4658.2008.06428.x. [DOI] [PubMed] [Google Scholar]
- Niwa R, Niimi T, Honda N, Yoshiyama M, Itoyama K, Kataoka H, Shinoda T. Juvenile hormone acid O-methyltransferase in Drosophila melanogaster. Insect. Biochem. Molec. Biol. 2008;38:714–720. doi: 10.1016/j.ibmb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Noriega FG. Nutritional regulation of JH synthesis: a mechanism to control reproductive maturation in mosquitoes? Insect Biochem Molec Biol. 2004;34:687–693. doi: 10.1016/j.ibmb.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Wells MA. A comparison of three methods to isolate RNA from mosquitoes. Insect Mol. Biol. 1993;2:21–24. doi: 10.1111/j.1365-2583.1993.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Wells MA. A molecular view of trypsin synthesis in the midgut of Aedes aegypti. J. Insect Physiol. 1999;45:613–620. doi: 10.1016/s0022-1910(99)00052-9. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Colonna AE, Wells MA. Increase in the size of the amino acid pool is sufficient to activate translation of early trypsin mRNA in Aedes aegypti midgut. Insect Biochem. Mol. Biol. 1999;29:243–247. doi: 10.1016/s0965-1748(98)00132-5. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Ribeiro JMC, Koener JF, Valenzuela JG, Hernandez-Martinez S, Pham VM, Feyereisen R. Genomic endocrinology of insect juvenile hormone biosynthesis. Insect Biochem. Mol. Biol. 2006;36:366–374. doi: 10.1016/j.ibmb.2006.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notredame C, Higgins DG, Heringa J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000;302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- Riddiford LM. Juvenile hormone action: A 2007 perspective. J Insect Physiol. 2008;54:895–901. doi: 10.1016/j.jinsphys.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Schooley DA, Baker FC. Juvenile hormone biosynthesis. In: Kerkut GA, Gilbert LI, editors. Comprehensive Ins. Physiol. Biochem. Pharm. vol 7. Oxford: Pergamon Press; 1985. pp. 363–389. [Google Scholar]
- Shapiro AB, Wheelock GD, Hagedorn HH, Baker FC, Tsai LW, Schooley DA. Juvenile hormone and juvenile hormone esterase in adult females of the mosquito Aedes aegypti. J. Insect Physiol. 1986;32:867–877. [Google Scholar]
- Shinoda T, Itoyama K. Juvenile hormone acid methyltransferase: a key regulatory enzyme for insect metamorphosis. Proc. Natl. Acad. Sci. USA. 2003;100:11986–11991. doi: 10.1073/pnas.2134232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JJ, McGuffin LJ, Bryson K, Buxton BF, Jones DT. The DISOPRED server for the prediction of protein disorder. Bioinformatics. 2004;20:2138–2139. doi: 10.1093/bioinformatics/bth195. [DOI] [PubMed] [Google Scholar]