Abstract
Prior studies indicate that, in tests of recognition memory, ERPs elicited by correctly recognized test items differ according to whether the items were encoded in an emotionally arousing or an emotionally neutral study context. These prior studies employed only a relatively brief (ca. 10 min) retention interval, however. The present study contrasted the ERP correlates of incidental emotional retrieval as a function of study-test delay. Pictures of emotionally neutral objects were encoded in association with either emotionally negative or emotionally neutral scenes. In a repeated measures design (N=19), half of the objects were subjected to a recognition memory test 10 min after completion of the study phase, whereas the remainder were tested 24 hrs later. After the short delay, ERPs elicited by objects paired with emotional vs. neutral backgrounds differed from around 200 ms post-stimulus, the objects paired with the emotional scenes eliciting the more positive-going waveforms. After 24 hrs, differences between the ERPs elicited by the two classes of object were still apparent from around 200 ms post-stimulus. Strikingly, these effects differed from those obtained 10 min after study in both their polarity and scalp distribution. The early onset of these ERP effects suggests that they may reflect a form of memory independent of the conscious recollection of the associated study contexts. The qualitative differences in the effects at the two retention intervals raises the possibility that the encoded objects were subjected to consolidation processes that differed according to the emotional attributes of their study contexts.
Keywords: Memory, Retrieval, Emotion, Event-related potentials
Introduction
There is substantial evidence that memory for emotionally arousing information can be enhanced relative to non-arousing information (Christianson, 1992). The influence of emotion on memory has been attributed to the modulating effects of arousal on attention during encoding (Christianson & Loftus, 1991), as well as to post-encoding consolidation processes (McGaugh, 2000). The proposal that emotional arousal modulates consolidation is consistent with several reports that the beneficial effects of arousal increase with study-test delay (Kleinsmith & Kaplan, 1963; LaBar & Phelps, 1998; Sharot & Phelps, 2004). If encoded representations of emotionally arousing events are indeed modified over time, one might expect to see this reflected in differences in retrieval processing as a function of time. Thus, the question arises whether the processes supporting or contingent upon the retrieval of emotional information vary according to study-test delay.
In the present study we addressed this question by using event-related potentials (ERPs) to index the neural correlates of successful recognition memory. We employed an experimental procedure initially introduced by Maratos and Rugg (2001; see also Maratos et al. 2001 for a companion fMRI study) for the study of the incidental retrieval of emotionally arousing verbal information, and later modified by Smith et al. (2004a; see also Smith et al. 2004b, 2005 and 2006) for use with pictorial material. The procedure employs a study phase in which emotionally neutral pictures of objects are presented in the context of either emotionally arousing or emotionally neutral scenes. At test, the requirement is simply to discriminate between studied (old) and unstudied (new) objects. Differences in the neural activity elicited by old objects studied in an arousing versus a non-arousing context are assumed to reflect differences in activity associated with the incidental retrieval of emotionally arousing versus emotionally neutral information. Consistent with this assumption, fMRI findings from this procedure have demonstrated enhanced activity for old items studied in emotional contexts in brain regions engaged during the online processing of emotional stimuli, including amygdala, orbitofrontal cortex, and insula (Maratos et al, 2001; Smith et al, 2004b). In experiment 1 of their ERP study, Smith et al (2004a) also found evidence that the emotional characteristics of the encoding context modulated retrieval-related activity. Relative to pictures of new objects, ERPs elicited by correctly classified old objects demonstrated the parietally distributed ‘old/new’ effect held to be an ERP signature of successful episodic retrieval (see Rugg & Wilding, 2000 for a review). The contrast between the ERPs elicited by old objects according to their encoding context revealed two emotion effects. The first of these comprised a relatively early onsetting (ca. 300-500 ms), temporal-maximum positivity in the waveforms for objects encoded in emotional contexts. The second effect also took the form of a positive-going shift in the ERPs for emotionally encoded objects; in this case, the effect onset relatively late (ca. 700 ms), was frontally-distributed, and persisted for several hundred milliseconds. In view of its time-course and similarity with ERP effects elicited by emotionally arousing stimuli (Keil et al., 2002; Cuthbert et al., 2000; Schupp et al., 2000), Smith et al (2004a) interpreted the early effect as evidence that objects paired with emotional backgrounds had themselves acquired arousing properties through a process of associative learning. The late effect was interpreted as a reflection of frontally-mediated monitoring operations engaged by incidental retrieval of emotionally arousing contextual information (see also Maratos & Rugg, 2001).
In the present study, we adopted the same procedure as Smith et al. (2004a; experiment 1). Instead of testing memory and obtaining ERP data once only, after a study-test delay of a few minutes, we did so on two occasions separated by 24 hours. This interval was chosen because of the evidence that the ‘window’ for the post-encoding consolidation of emotional information extends over a period of no more than a few hours (McGaugh, 2006), and findings indicating that the behavioral consequences of consolidation are clearly evident after a 24 hr delay (e.g. Sharot & Phelps, 2004). By testing memory at these two different study-test delays we were thus able to address the question whether, as has been reported for behavioral measures of memory (see above), the ERP correlates of incidental retrieval of emotional information are exaggerated at the long relative to the short delay. Such a finding would add further weight to the notion that memories for emotionally arousing events are strengthened by post-encoding consolidation to a greater extent than memories for non-arousing events.
Results
Behavioral data
Mean accuracy and reaction time (RT) of items paired with emotionally negative backgrounds (emotional hits), emotionally neutral backgrounds (neutral hits) and correctly rejected items (New) are shown in table 1. ANOVA of the accuracy data [factors of item type (emotional, neutral, new) and delay (short, long)] gave rise to significant effects for item type, F(1.6,28.5) = 32.96, p < 0.0001, delay, F(1,18) = 91.24, p < .0001, and for the item type X delay interaction, F(1.5,26.3) = 16.12, p < .0001 (here and in all subsequent ANOVAs, the degrees of freedom associated with effects involving factors with more than two levels were corrected for nonsphericity by the Greenhouse-Geisser procedure). These results indicate that accuracy was greater at the short than at the long delay. They further indicate that subjects were more accurate responding to new than to old items, and that this difference was more prominent at the long delay. Subsidiary ANOVAs revealed that there were no significant differences between emotional and neutral hit rates at either delay (F < 1).
Table 1.
Mean (SD) accuracies and RTs (ms)
| Delay 1 | Delay 2 | |||||
|---|---|---|---|---|---|---|
| Emotional | Neutral | New | Emotional | Neutral | New | |
| Recognition accuracy | 0.82 (0.1) | 0.81 (0.08) | 0.89 (0.06) | 0.61 (0.13) | 0.61 (0.10) | 0.86 (0.09) |
| RTs | 872 (179) | 856 (166) | 916 (177) | 988 (186) | 989 (197) | 1007 (188) |
ANOVA of RTs revealed main effects for item type, delay, and for the interaction between these two factors [item type: F(1.4,25.5) = 4.90, p < .025; delay: F(1,18) = 12.12, p < .003; item type X delay: F(1.4,25.2) = 4.41, p < .034]. These results indicate that the subjects responded more quickly for hits than correct rejections at both delays, although this difference between response types was greater for the short delay. The results further showed that responses were faster for all item types after the short than the long delay. Subsidiary ANOVAs revealed that emotional and neutral hit RTs did not differ significantly after either delay (F < 2.1).
ERP data
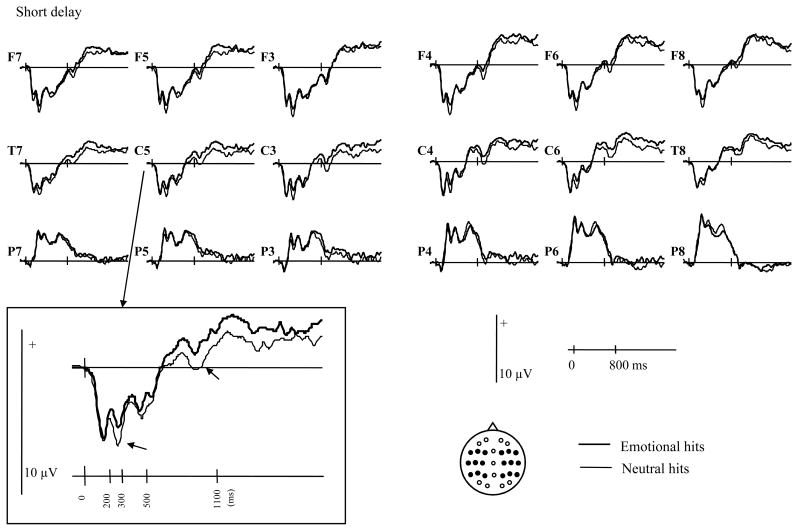
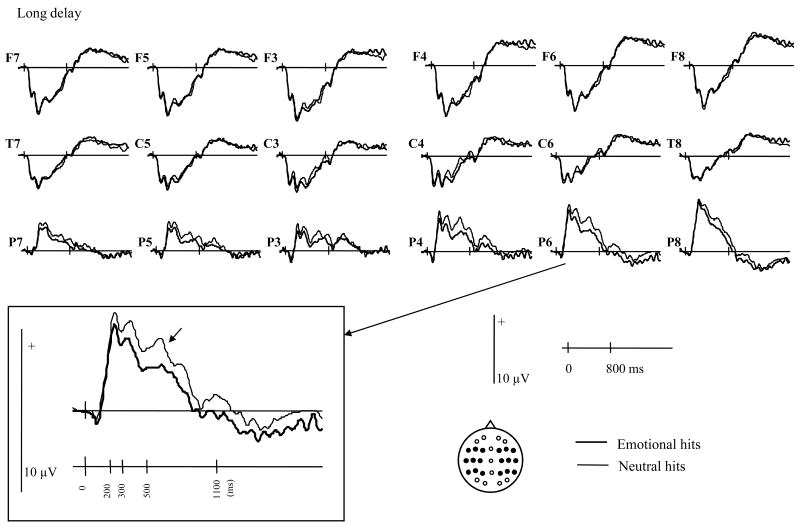
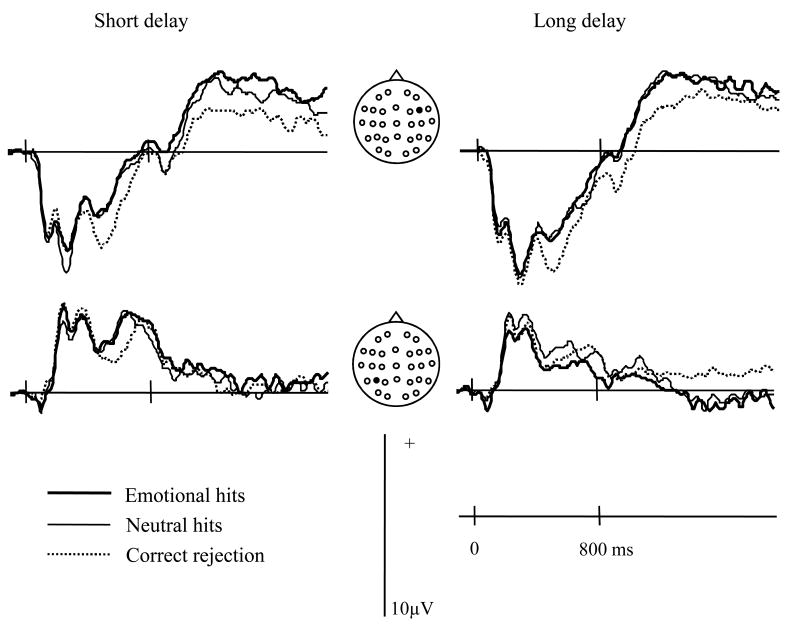
Analysis of ERPs was conducted in two parts. First, the ERPs elicited by correctly classified old items (hits) were directly contrasted in order to investigate the effects of encoding context (emotional vs. neutral) as a function of delay. Second, the old/new effects associated with the emotional and neutral hits were analyzed. Grand average ERPs elicited by emotional and neutral hits are shown in figure 1. For each item type, the mean numbers (and range) of trials that contributed to the waveforms were: 29 (16-43), 30 (17-42), 32 (17-44) for the short delay emotional hits, neutral hits and correct rejections respectively, and 26 (17-43), 25 (18-41), and 36 (19-45) for the equivalent items after the long delay.
Figure 1.
Grand average waveforms elicited by test items associated at study with emotional vs. neutral contexts when tested after the short and long study-test delays. Locations of the electrode sites are depicted on the insert.
Emotional vs. Neutral Hits
Magnitude analyses
As can be seen in figure 1, ERPs elicited by emotional and neutral hits begin to differ from approximately 200 ms post stimulus. However, these differences appear to differ qualitatively across the two delays. At the short delay, emotional hits appear to elicit a more positive-going waveform than the neutral hits between approximately 200 and 1100 ms. At the long delay, by contrast, emotional hits elicit a sustained, posteriorly distributed waveform that is less positive-going than the waveform elicited by neutral hits.
Quantification of these data was accomplished by measuring the mean amplitude (relative to the mean of the 102 ms pre-stimulus baseline) of the following epochs: 200-300, 300-500, 500-1100, and 1100-1900 ms. Selection of these epochs was based on the time-courses of two emotional effects obtained in a previous study (Smith et al. 2004a), as well as on visual inspection of the waveforms elicited in the present experiment.
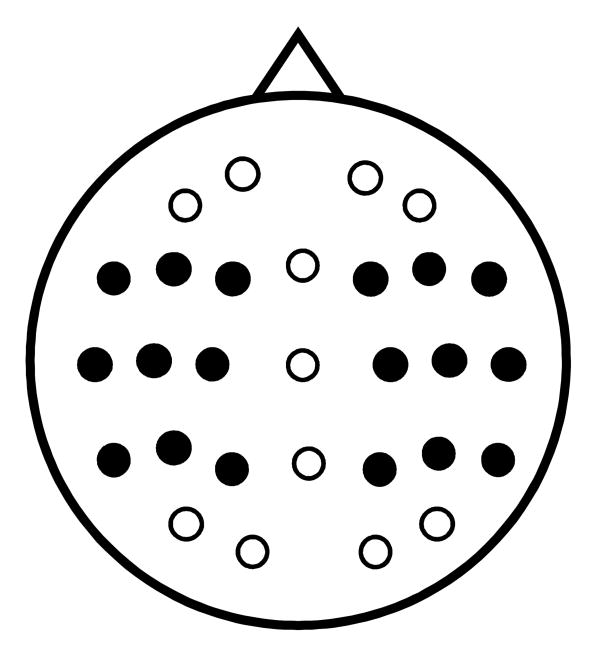
Statistical analyses were conducted on the data from the electrode sites depicted in figure 2. The analyses involved three stages. In the first stage, an overall ANOVA was performed, factored according to item type (emotional hits, neutral hits), delay (short, long), hemisphere, chain (frontal, central, parietal), and site (inferior, middle, superior). For each epoch in which the initial ANOVA revealed a reliable interaction between item type and delay, subsidiary ANOVAs were performed on the data from each delay separately. Further ANOVAs were conducted as necessary to identify electrode clusters where any emotion effect was most in evidence. The outcomes of the initial and subsidiary ANOVAs for each epoch are shown in table 2 (see figure 3 for graphical depiction of the key findings).
Figure 2.
Electrode montage and sites employed for analyses of ERP magnitudes (black in-fills).
Table 2.
Significant ANOVA results for ERPs elicited by the contrast between emotional and neutral hits for each epoch
| Epochs | 200-300 | 300-500 | 500-1100 |
|---|---|---|---|
| Delay interaction | |||
| Delay |
F(1,18) = 9.8 p < 0.006 |
F(1,18) = 6.86 p < 0.017 |
|
| Item type × delay |
F(1,18) = 8.36 p < 0.01 |
||
| Item type × delay × site |
F(1.1,19.6) = 9.62 p < 0.005 |
F(1.1,19.5) = 4.41 p < 0.046 |
|
| Item type × delay × site × chain |
F(3.0,53.6) = 3.63 p < 0.019 |
||
| Short Delay | |||
| Item type |
F(1,18) = 8.3 p < 0.01 |
||
| Item type × site |
F(1.1,19.2) = 4.64 p < 0.042 |
||
| Item type × site × hemisphere |
F(1.3,22.8) = 4.59 p < 0.035 |
F(1.4,25.4) = 4.01 p < 0.043 |
|
| Long Delay | |||
| Item type × chain |
F(1.1,20) = 7.16 p < 0.013 |
||
| Item type × site |
F(1.1,19) = 4.43 p < 0.047 |
F(1.1,19.1) = 5.73 p < 0.025 |
|
Item type (emotional hits/neutral hits), delay (short delay/long delay), hemisphere (left/right), site (inferior/middle/superior), chain (anterior/central/posterior)
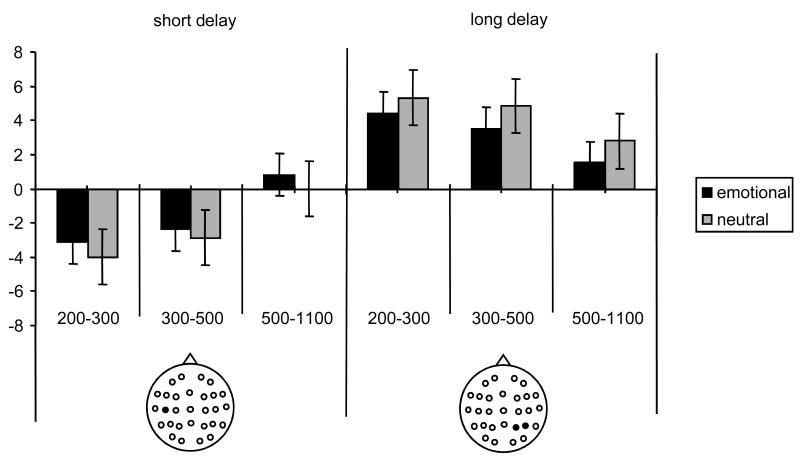
Figure 3.
Mean amplitudes (and standard errors) of the emotional and neutral effects for the 200-300, 300-500, and 500-1100 ms epochs elicited in the short delay in the C5 electrode and in the long delay in the P6 and P4 electrodes. Location of the electrode sites is depicted on the insert.
As can be seen from the table 2, there were interactions between the factors of item and delay, in association with various topographic factors, in the 200-300, 300-500, and 500-1100 ms epochs. For the short delay, effects involving item type were evident in each epoch. In the earliest epoch, the effect interacted with the factor of site, reflecting the fact that the difference between the two classes of hit was maximal over superior sites, where emotional hits elicited a more positive-going waveform than neutral hits. In the subsequent epochs, reliable interactions involving the factors of site and hemisphere revealed a positive shift elicited by emotional hits over the inferior and middle sites of the left hemisphere. Subsidiary analyses revealed that the item type effect was independently reliable only for the 200-300 ms epoch over the central electrode chain (F1,18 = 9.20, p < .01). The item effects in the subsequent epochs were not reliable when tested at each chain separately.
As shown in table 2, subsidiary ANOVAs for the long-delay condition also revealed item effects in each epoch, the effects interacting either with the variables of site or chain. Follow-up analyses revealed that, in all three epochs, effects were reliable for the parietal electrode chain (F1,18 of 8.61 to 18.77, p < .01 to p < .0001), a finding consistent with the impression from figure 1 that the difference between emotional and neutral hits was maximal over parietal locations.
Topographic analyses
The scalp distributions of the foregoing emotional effects (emotional hits – neutral hits) are illustrated in figure 4 for the three epochs in which there were reliable item type × delay interactions in the magnitude analyses. For each delay, the scalp distributions were contrasted according to epoch to determine whether the distributions varied over time. The analyses were performed on difference scores from all 29 electrode locations after range normalization to remove the confounding effects of global amplitude differences (McCarthy & Wood, 1985). There were no significant effects involving the factor of epoch for either of the delays, and hence no evidence that the scalp distribution of the ERP emotion effects varied over time.
Figure 4.
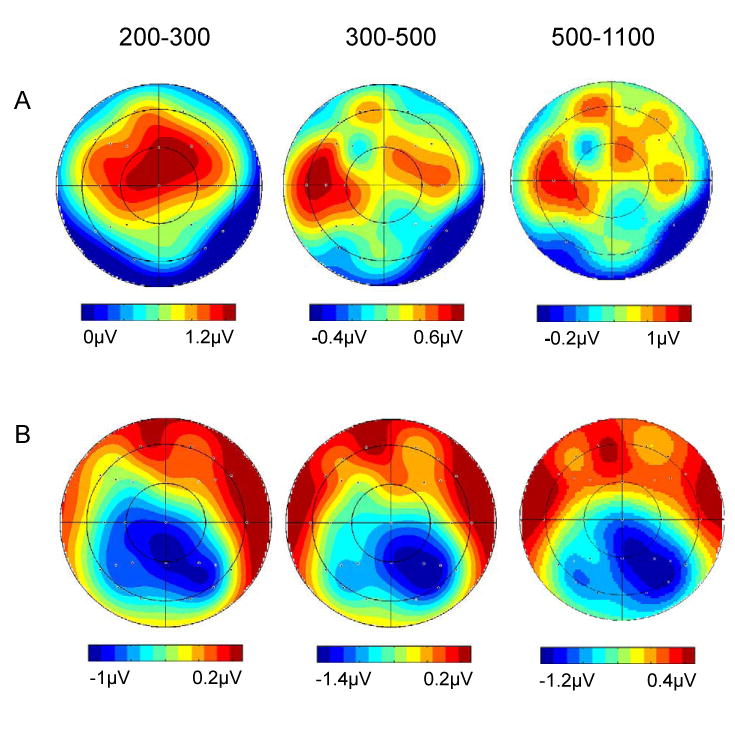
Scalp topographies of the differences in mean amplitude between the ERPs elicited by emotional hits versus neutral hits after the short (A) and long (B) study-test delays. The epoch depicted in each plot is given at the top, and the range in microvolts is indicated underneath. Increasing redness indicates greater positivity.
Old/New Effects
Inspection of figure 5 suggests that, relative to correct rejections, emotional and neutral hits from the short delay condition both elicited more positive-going waveforms between approximately 400 and 700 ms post-stimulus at frontal and parietal locations. We interpret these effects as examples of the ‘frontal’ and ‘parietal’ old/new effects described in numerous prior studies of recognition memory (Rugg & Curran, 2007). At the longer delay, the frontal effects are preserved, whereas the parietal effects appear to be attenuated.
Figure 5.
Grand average waveforms elicited by emotional hits, neutral hits and correct rejection at the short and long study-test delays. Locations of the electrode sites are depicted on the insert. The emotional effects for these electrode sites are also depicted in figure 1.
Old/new effects were quantified as the mean amplitude in the 400-700 ms epoch. An initial ANOVA, with factors of item type (emotional hit vs. neutral hit vs. correct rejection), delay, hemisphere, chain and site revealed a main effect of item type (F 1.8, 31.9 = 8.26, p < .002) and a three-way interaction between item type, hemisphere and chain (F2.3, 41.5 = 3.43, p < .05). The four-way interaction between item type, delay, hemisphere, and chain approached significance (F4.7, 84.3 = 2.22, p < .07). A subsidiary ANOVA on the data for the early delay revealed a main effect for item type (F1.7, 30.5 = 5.54, p < .025) and no other effects. The effects reflected the fact that, collapsed across electrode locations, ERPs elicited by old items were more positive-going than those to new items (emotional hits vs. correct rejections: F1,18 = 8.49, p < .025; neutral hits vs. correct rejections: F1,18 = 5.84, p < .05).
ANOVA of the data for the long delay revealed significant effects for the interactions between item type and chain (F2.2,40.4 = 7.31, p < .001), item type and site (F2.1,37.3 = 3.83, p < .05), and item type, hemisphere and chain (F3.0,54.8 = 3.32, p < .05). Collapsed across the two classes of hit, there was a significant interaction between item type and chain (F1,18 = 8.99, p < .01), reflecting the fact that old/new effects were larger in magnitude over the frontal than the parietal scalp (2.13 μV vs. 0.15 μV). Follow-up ANOVAs revealed significant old/new effects for both emotional and neutral hits at frontal locations (emotional: F1,18 = 4.94, p < .05; neutral: F1,18 = 6.64, p < .025). Effects at parietal locations were reliable (in the form of an item type × site interaction) for the neutral hits only (F1.1,20.3 = 5.62, p < .025).
In summary, the foregoing analyses indicate that whereas old/new effects did not differ significantly according to scalp location for the early delay, the effects were focused over the frontal scalp after the long delay, particularly in the case of the emotional hits.
Discussion
Behavioral data
There was no accuracy difference in the recognition of objects associated with emotionally arousing and emotionally neutral contexts at either delay. These findings are consistent with the data reported by Smith et al. (2004a), who also reported no difference in recognition accuracy for items encoded in the context of negatively valenced versus neutral backgrounds. Recognition accuracy did however decline markedly as a function of delay. Thus, there was no evidence that memory for items encoded in association with an arousing background sustained less forgetting over time than did items encoded against neutral backgrounds (cf. Kleinsmith & Kaplan, 1963; LaBar & Phelps, 1998; Sharot & Phelps, 2004).
ERP data
Replicating prior studies (Maratos et al, 2001; Smith et al, 2004a), ERPs elicited by recognized objects differed according to whether the objects had been paired at study with an emotionally arousing or a neutral context. Contrary to our prediction, which was that the effects of emotional context on the object-elicited ERPs would increase in magnitude with increasing study-test delay, ERP ‘emotion effects’ differed qualitatively rather than quantitatively across the two delays: after the short delay objects studied with emotional contexts elicited waveforms that were more positive than those elicited by objects studied with neutral contexts, but this effect reversed after the long delay. Thus, although there was no sign of a delay-dependent emotion effect on behavior, the associated ERP data suggest that the emotionally arousing contexts modulated the processing of the objects with which they were paired in a delay-dependent manner.
The ERP emotion effects evident at the short delay comprised a sustained positive-going modulation in the ERPs elicited by items paired with emotional relative to non-emotional contexts. Although the topography of this effect did not differ significantly over time, these results resemble those reported by Smith et al. (2004a). These authors described two effects of emotional context in their data, a small, transient effect onsetting around 300 ms post-stimulus, and a larger, more diffusely distributed and sustained effect that onset around 700 ms. As was noted in the Introduction, Smith et al. proposed that the earlier of these effects indicated that objects paired at study with the emotionally arousing contexts had acquired arousing properties, and did not depend upon the conscious recollection of the associated contexts (indexed by the later-onsetting ‘parietal old/new effect’). By contrast, they proposed that the later effect reflected monitoring or evaluative processes triggered by recollection of the contexts. Given the similar time courses of the effects due to emotion in the present and prior studies, these proposals appear to provide a good account of the present findings, even though the late effect in the current experiment was more left-lateralized than, and not as large as, the effect found by Smith et al. (2004a). These differences between the studies may reflect the absence of a positively-valenced condition in the present study.
At the longer of the two study-test delays the ERPs elicited by emotional and neutral hits diverged in the same three epochs where effects were observed after the short delay. These effects were however qualitatively different from those observed in the preceding test phase, in that they demonstrated a reversal in their magnitude differences, a sharply focused posterior scalp distribution, and a more sustained modulation of the waveform. These dramatic differences as a function of delay may indicate that the influence of emotional context on retrieval-related neural activity is modulated by emotionally-specific consolidation mechanisms. As was mentioned previously, these mechanisms are assumed to be responsible for the relative strengthening of emotional relative to non-emotional memories with time (McGaugh, 2000). Previous studies suggest that, as is the case in experimental animals, the amygdala plays a crucial role in this consolidation process in humans (see Phelps, 2006, for review). Indeed, functional neuroimaging studies employing the same pictorial material and experimental design as in the present experiment reported greater amygdala activation during the encoding of the emotionally arousing relative to the neutral contexts (Smith et al, 2006). Amygdala activation was also demonstrated during retrieval of items encoded in association with emotional contexts (Smith et al, 2005; 2006; see Buchanan, 2007, for review). Therefore, although in the present experiment we cannot infer the specific brain regions responsible for the pattern of ERP effects found at each delay, it is possible that the effects reflect the differential influence of the amygdala on the consolidation of item memories encoded in association with emotionally arousing versus neutral contexts.
The present findings of delay-dependent differences in the ERPs elicited by negative and neutral hits are however also consistent with an alternative account. Parietally-distributed ERP old/new effects, which are commonly held to be a neural correlate of episodic retrieval (or ‘recollection’), were markedly attenuated at the longer delay, to the extent they were undetectable in the ERPs elicited by emotional hits. This finding implies that whereas recognition of test items after the short delay was associated with recovery of details of the study episode (such as the nature of the study context), recognition after the longer delay was likely mediated primarily by an acontextual sense of familiarity in the absence of recollection. This proposal receives additional support from the finding that, in contrast to the parietal old/new effect, a frontal effect – often interpreted as a neural correlate of familiarity-driven recognition (Rugg & Curran, 2007) – did not vary with delay (see figure 5). Thus, it could be argued that the delay-dependent ERP emotion effects are a consequence not of delay per se, but of a shift in the basis of the associated recognition judgments. By this argument, ERPs elicited by recognition test items are differentially modulated depending on whether or not the items are associated with explicit recollection of their study contexts (cf. Smith et al., 2005).
Resolution of the above two opposing accounts of the present findings will depend on studies in which delay and probability of recollection are independently manipulated. Although it remains unclear whether or not the pattern we found would be elicited in association with recollection after a 24hr study-test delay, the present findings nonetheless highlight the relatively long-lasting effects that emotionally arousing contexts can have on the later processing of associated, ostensibly neutral, stimulus events, even when these events fail to elicit recollection of the study episode (see also Smith et al, 2005). Finally, it is noteworthy that there was little evidence to suggest that the onset latency of these emotion effects was affected by delay; in both cases, reliable effects could be detected no later than 300 ms post-stimulus. As was first proposed by Smith et al. (2004a), the early onset of such effects suggests that study items paired with the emotional contexts acquired some of the affective or arousing properties of those contexts.
Experimental procedures
Subjects
Twenty six individuals (12 females) aged between 18 and 26 years (mean = 21) participated in return for payment of $15/h. The subjects were recruited from the UCI undergraduate and graduate communities, were right-handed, and were native English speakers with normal or corrected-to-normal vision. Informed consent was obtained in accordance with UCI Institutional Review Board guidelines. Of these 26 subjects, 7 were rejected from all analyses. Three subjects were rejected due to insufficient trials in critical conditions, 3 due to excessive eye movement artifact, and one because they failed to appear for the second test session.
Stimuli
The stimuli were similar to those employed by Smith et al (2004a). They consisted of a pool of 300 pictures of emotionally neutral objects and a pool of 200 background contexts that varied in their emotional arousal and valence. Context backgrounds subtended maximum vertical and horizontal angles of 12.2° and 19.2° respectively at the 1 m viewing distance. The objects (maximum visual angle 5.7° × 6.8°) were presented within a white box (subtending 7.5° × 7.5°) which demarcated their separation from the contexts during study and from the monitor background at test. Approximately 50 % of the contexts were taken from the International Affective Picture System (IAPS; Lang et al., 1997), which consists of a series of pictures standardized for arousal and emotional valence. The remaining contexts were obtained from a variety of internet sources. All contexts were rated for arousal and valence by 10 North-American subjects (5 females, mean age 22 years) who did not participate in the ERP experiment. The ratings were acquired with two 5 point Likert scales that assessed arousal (1 = very calming, 5 = very arousing) and valence (1 = very negative, 3 = neutral, 5 = very positive). The contexts selected as experimental materials consisted of 100 highly arousing emotionally negative pictures (mean arousal 4.46, SD 0.66; mean valence 1.44, SD 0.62) and 100 moderately arousing neutral pictures (mean arousal 2.98, SD 0.79; mean valence 3.36, SD 0.73). No positively valenced pictures were included. Most of the selected contexts were scenes that contained people (82% of the emotionally negative, and 74% of the emotionally neutral contexts). The remaining contexts depicted a mixture of animals, landscapes and objects. None of the contexts depicted sexual material. The set of critical objects belonged to a variety of different semantic categories and had been confirmed as neutrally valenced in a pilot study conducted by Smith et al. Allocation of stimuli to experimental conditions was randomized on a subject-specific basis. For each participant, the 200 backgrounds were randomly paired with 200 of the objects at encoding; and the remaining 100 objects served as “new” items at test. Fifty exemplars of each item type (that is, objects paired with emotionally arousing or emotionally neutral contexts, and new objects) were assigned as test items for each delay, giving a total of 150 items per test phase. To allow rest breaks, the study phase was presented in four blocks and each test session was divided into three blocks. The first two context/item pairs of each study block, and the first two items of each test block, were filler items drawn from additional pools of objects and backgrounds. These pools of additional items were also employed to construct the study and test practice lists.
Study procedure
On each study trial a white fixation cross was initially presented against a black background for 750 ms, serving as a warning for the upcoming presentation of a context. Each context was presented for 3 sec, during which the subjects verbally rated its emotional valence [from -2 (very negative) to +2 (very positive)]. The study object was then superimposed across the center of the context and the stimulus pairing presented for 5 sec. During this period subjects were required to visualize a connection between the two items. The white fixation cross was then re-presented, indicating the onset of a new trial. The stimulus onset asynchrony (SOA) was thus 8,750 ms. The presentation of the study contexts was pseudo-randomized such that no more than three similarly valenced backgrounds were presented consecutively. A practice study session, administered prior to the study session proper, consisted of six trials in which the subjects were required to describe verbally the connection they had been made between the objects and the backgrounds. Rest breaks were given after each 52 trials in the study session proper.
Test procedure
The short and the long delay test phases followed the encoding phase after 10 min and 24 hours respectively, and were procedurally identical. On each trial a red fixation cross was initially presented against a black background for 500 ms, serving as a warning for the upcoming presentation of the test object. Test objects were presented for 750 ms followed by a white fixation cross against a black background for 1750 ms, giving an SOA of 3 sec. Subjects kept their left and right forefingers resting on a response-pad. They were instructed to respond by pressing one button when the test object had been presented in the study phase, and by pressing the other button for new objects. The response button assignment (left versus right) was counterbalanced across subjects. Each practice test phase consisted of the presentation of 3 items from the practice study phase and 2 completely new items. Rest breaks were given after every 52 trials in the test phase proper.
ERP recording and analysis
Electroencephalographic activity (EEG) was recorded continuously from 31 silver/silver-chloride electrodes. Twenty-nine of these electrodes were embedded in an elastic cap and one was placed on each mastoid process. The locations of the cap electrodes were based on the International 10–20 system (American Electroencephalographic Society, 1994) and corresponded to midline sites (Fz, Cz, Pz) as well as homotopic (left/right) pairs of sites (Fp1/Fp2, AF7/AF8, F3/F4, F5/F6, F7/F8, C3/C4, C5/C6, T7/T8, P3/P4, P5/P6, P7/P8, PO7/PO8, O1/O2). Vertical and horizontal electro-oculograms (EOG) were recorded from bipolar electrode pairs situated above and below the left eye and on each outer canthus. Data were acquired with Contact Precision amplifiers (London, UK) at a 256-Hz sampling rate and an amplifier bandwidth of 0.01-40 Hz (-3dB). Electrode impedances were adjusted to below 5 kΩ. Offline, the continuous EEG data were epoched to 2048 ms duration with a 102 ms pre-stimulus baseline. The resulting epochs were downsampled to a 125-Hz sampling rate and algebraically re-referenced to linked mastoids. Trials containing movement artifact, EOG artifact other than blinks, or excessive baseline drift were rejected. The averaged ERPs were smoothed with a 5-point moving-window filter at a cutoff of 19.4 Hz (-3dB). A previously described linear regression method (e.g., Henson et al., 2004) was used to correct blink artifacts for all subjects.
Acknowledgments
Antonio Jaeger was supported by CAPES/PDEE. The research was supported by the National Institute of Mental Health, grant number R01-MH072966. The authors thank two anonymous reviewers for helpful comments on an earlier version of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Electroencephalographic Society. Guidelines for standard electrode position nomenclature. J Clin Neurophysiol. 1994;11:111–113. [PubMed] [Google Scholar]
- Buchanan TW. Retrieval of emotional memories. Psychol Bull. 2007;133:761–799. doi: 10.1037/0033-2909.133.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson SA. The handbook of emotion and memory. Hillsdale, NJ: Erlbaum; 1992. [Google Scholar]
- Christianson SA, Loftus EF. Remembering emotional events: the fate of detailed information. Cogn Emot. 1991;5:81–108. [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biol Psychol. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rylands A, Ross E, Vuilleumeir P, Rugg MD. The effect of repetition lag on electrophysiological and haemodynamic correlates of visual object priming. Neuroimage. 2004;21:1674–1689. doi: 10.1016/j.neuroimage.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang PJ. Large-scale neural correlates of affective picture processing. Psychophysiology. 2002;39:641–649. doi: 10.1017.S0048577202394162. [DOI] [PubMed] [Google Scholar]
- Kleinsmith L, Kaplan S. Paired-associated learning as a function of arousal and interpolated interval. J Exp Psychol. 1963;65:190–193. doi: 10.1037/h0040288. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. The international affective picture system (IAPS): Photographic slides. Gainesville, FL: University of Florida; 1997. [Google Scholar]
- LaBar KS, Phelps EA. Arousal-mediated memory consolidation: Role of the medial temporal lobe in humans. Psychol Sci. 1998;9:490–493. [Google Scholar]
- Maratos EJ, Dolan RJ, Morris JS, Henson RNA, Rugg MD. Neural activity associated with episodic memory for emotional context. Neuropsychologia. 2001;39:910–920. doi: 10.1016/s0028-3932(01)00025-2. [DOI] [PubMed] [Google Scholar]
- Maratos EJ, Rugg MD. Electrophysiological correlates of the retrieval of emotional and non-emotional context. J Cogn Neurosci. 2001;13:877–891. doi: 10.1162/089892901753165809. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Wood CC. Scalp distribution of event-related potentials: An ambiguity associated with analysis of variance models. Electroencephalogr Clin Neurophysiol. 1985;62:203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Make mild moments memorable: add a little arousal. Trends Cogn Sci. 2006;10:345–347. doi: 10.1016/j.tics.2006.06.001. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory-A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Ann Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends Cogn Sci. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Wilding EL. Retrieval processing and episodic memory. Trends Cogn Sci. 2000;4:108–115. doi: 10.1016/s1364-6613(00)01445-5. [DOI] [PubMed] [Google Scholar]
- Sharot T, Phelps EA. How arousal modulates memory: disentangling the processes of attention and retention. Cogn Affect Behav Neurosci. 2004;4:294–306. doi: 10.3758/cabn.4.3.294. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Smith APR, Dolan RJ, Rugg MD. Event-related potentials correlates of the retrieval of emotional and nonemotional context. J Cogn Neurosci. 2004a;16:760–775. doi: 10.1162/089892904970816. [DOI] [PubMed] [Google Scholar]
- Smith APR, Henson RN, Dolan RJ, Rugg MD. fMRI correlates of the episodic retrieval of emotional contexts. Neuroimage. 2004b;22:868–878. doi: 10.1016/j.neuroimage.2004.01.049. [DOI] [PubMed] [Google Scholar]
- Smith APR, Henson RN, Rugg MD, Dolan RJ. Modulation of retrieval processing reflects accuracy of emotional source memory. Learn Mem. 2005;12:472–479. doi: 10.1101/lm.84305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith APR, Stephan KE, Rugg MD, Dolan RJ. Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron. 2006;49:631–638. doi: 10.1016/j.neuron.2005.12.025. [DOI] [PubMed] [Google Scholar]