Abstract
Background
The cathelicidin family of anti-microbial peptides is an integral component of the innate immune response that exhibits activity against bacterial, fungal and viral pathogens. Eczema herpeticum (ADEH) develops in a subset of AD patients due to disseminated infection with herpes simplex virus (HSV).
Objective
This study investigated the potential role of cathelicidins in host susceptibility to HSV infection.
Methods
Glycoprotein D was measured by real-time RT-PCR as a marker of HSV replication in skin biopsies and human keratinocyte cultures. Cathelicidin expression was evaluated in skin biopsies from AD (n=10) without history of HSV skin infection and ADEH (n=10) patients.
Results
The cathelicidin peptide LL-37 exhibited activity against HSV in an anti-viral assay with significant killing (p<0.001) within the physiologic range. The importance of cathelicidins in anti-viral skin host defense was confirmed by the observation of higher levels of HSV-2 replication in cathelicidin deficient (Cnlp-/-) mouse skin (2.6 ± 0.5 pg HSV/pg GAPDH; p<0.05) compared to their wild type counterparts (0.9 ± 0.3). Skin from ADEH patients exhibited significantly (p<0.05) lower levels of cathelicidin protein expression than AD. We also found a significant inverse correlation between cathelicidin expression and serum IgE levels (r2=0.46, p<0.05) in AD and ADEH patients.
Conclusion
This study demonstrates that the cathelicidin peptide LL-37 possesses anti-viral activity against HSV and the importance of variable skin expression of cathelicidins in controlling susceptibility to ADEH. Additionally, serum IgE may be a surrogate marker for innate immune function and serve as a biomarker for which AD patients are susceptible to ADEH.
Clinical Implication
A deficiency of LL-37 may render AD patients susceptible to eczema herpeticum. Therefore increasing production of skin LL-37 may prevent herpes infection in AD.
Keywords: antimicrobial peptides, herpes simplex virus, atopic dermatitis, eczema herpeticum
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease that affects approximately 17% of children and is associated with recurrent skin infections.1,2 Recent studies have shown that the innate immune response, and more specifically anti-microbial peptides (AMPs), are decreased in the skin of AD patients. It has been postulated that this may explain susceptibility of these individuals to recurrent bacterial skin infections.3,4
Eczema herpeticum (ADEH) is a disseminated herpes simplex virus (HSV)-1 or -2 infection that occurs in a subset of patients with AD.5,6 Left un-treated, ADEH may be fatal due to systemic viremia.7 However, only a small subset of AD patients develop problems with recurrent viral infections, suggesting they may have an AMP phenotype distinct from most AD patients. Therefore this study was conducted to investigate the role of human cathelicidin in controlling HSV infection and to examine differences in cathelicidin expression between uncomplicated AD and ADEH patients.
Methods
Patients
Study participants included 10 AD patients without a history of EH (mean age: 29.3 ± 6.9 years) and 10 AD patients with a history of ADEH (mean age: 39.8 ± 4.2 years). Patients were classified as ADEH based on clinical signs of ADEH, as diagnosed by a dermatologist, and a confirmation of HSV infection by either PCR or serology. Total serum IgE was measured using the UniCAP system (Pharmacia, Uppsala, Sweden). Patients in these studies were never on oral steroids or systemic calcineurin inhibitors and were taken off of topical calcineurin inhibitors for a minimum of 1 week prior to enrollment. These studies were conducted according to the Declaration of the Helsinki Guidelines and approved by the institutional review board at National Jewish Medical and Research Center in Denver and Ludwig Maximilian's University in Munich. All patients gave written informed consent prior to participation in these studies.
Skin biopsies were collected from the lesional eczematoid skin rash of AD and ADEH patients. Following collection, skin biopsies were fixed in formalin and archived.
Mice
BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Cnlp-/- (Cnlp knockout) mice were obtained from R. L. Gallo (Veterans Affairs Medical Center and the University of San Diego, CA) and backcrossed onto the BALB/c background. All protocols with these animals were approved by the Institutional Animal Care and Use Committee at National Jewish Medical and Research Center. This institution has an animal welfare assurance number (A3026-1) on file with the Office of Protection and Research Risks.
Preparation of Virus
Human Herpes Virus type 2 (HSV-2; gift from Dr. Adriana Weinberg, UCHSC) was grown and passaged in human embryonic lung fibroblasts in Earle's minimal essential medium (MEM) with 2.5% fetal calf serum (FCS) and antibiotics. Freshly trypsinized lung fibroblasts were grown three days to confluence and inoculated with approximately 1 plaque-forming units (pfu) per cell in culture medium. Cells were checked daily for cytopathic effects. The culture supernatant was harvested after 48-72 hours of incubation at 37° in 5% CO2, freeze thawed five times and centrifuged 15 minutes at 1000 RPM. For virus titration, ten-fold dilutions of stock were made and 0.1 ml of each dilution was added to the fibroblast cell sheets in 24 well tissue culture plates. Adsorption was allowed to take place for one hour at 37° in 5% CO2 and was followed by the addition of E-MEM with 2.5% FCS. Forty-eight hours following infection, media was removed and cells fixed with formalin-crystal violet. Plaques were visualized on an Inverted Nikon Microscope under 1.3 × 10 magnification. Virus stocks were stored at -70°.
Peptide Preparations
Human cathelicidin (LL-37) and an irrelevant control peptide 8044 (GLNGPDIYKGUYQFKSVEFD) were synthesized via solid-phase t-BOC chemistry using standard methodology and purified to homogeneity via high purified liquid chromatography by the Molecular Resource Center at National Jewish. Peptide 8044 was chosen from a library of existing peptides for use as a control having no sequence identity with the test peptide. The identity of LL-37 was confirmed by mass spectroscopy. Concentrations of LL-37 and control peptides used in these experiments ranged from 0 – 100 μM.
Viral Killing Assay
BS-C-1 African green monkey kidney cells were seeded at 2 × 105 cells/well in 24 well plates (Becton Dickinson, Torreyana, CA) and allowed to grow to confluence overnight at 37°C, 5% CO2 in Earle's MEM with 10% FCS and antibiotics. To examine the effects of LL-37 and control peptide, 0 – 100 μM was incubated with 2 × 103 pfu HSV-2 for 24 hours, 37°C in a volume not to exceed 0.1 ml. Growth media was removed from the cell sheet and rinsed once using Earle's MEM with 2.5% FCS. The virus:protein complex was added to the cells and adsorbed for one hour at 37°C, 5% CO2. Growth media was added to 0.5 ml and incubated for 24 hours for RNA analysis of HSV gene expression and 48 hours for plaque development. For the plaque assay, the medium was removed and wells were overlaid with 0.5 ml 4% buffered formalin, allowed to fix for 10 minutes at room temperature. The formalin was removed and 0.5 ml 0.1% crystal violet in PBS was added to the wells for 5 minutes at room temperature. Wells were then aspirated and the air-dried for visualization of plaques. We found the most accurate results with the virus alone forming 50-80 plaques per well.
Keratinocyte Cell Culture
HaCaT cells, a human keratinocyte cell line, were cultured in Dulbecco's modified eagle's media (Cellgro) supplemented with 10% FCS (Gemini Bio Products) and 1% of the following: penicillin/streptomycin, L-glutamine, MEM with non-essential amino acids (GIBCO), MEM vitamins solution (GIBCO) until confluent.
To evaluate the anti-viral activity of LL-37, HaCaT cells were infected with 0.05 pfu/cell of HSV-2 for six hours. Following the incubation, HSV-2 was removed and cells were washed with media to remove remaining HSV-2. LL-37 (0 – 100 μM) was added to the cells and allowed to incubate for an additional 18 hours. RNA was isolated from the cells for analysis of HSV-2 gene expression.
Murine Skin Explant Cultures
The dorsal thorax of all mice was clipped and treated with the depilatory agent Nair® to remove hair. Seventy-two hours following hair removal, mice were euthanized via carbon dioxide asphyxiation. Six-mm punch biopsies were collected from the dorsal thorax and immediately placed in a 96 well plate and RPMI (Cellgro) supplemented with 10% FCS (Gemini Bio Products). Murine skin biopsies were cultured in the presence of media alone or 2 × 104 pfu HSV-2 for 24 hours. Following the exposure period, media was removed and biopsies submerged in Tri-Reagent (Molecular Research Center Inc., Cincinnati, OH) for RNA isolation. Three independent experiments were conducted with a total of 15 mice in each exposure group. Data from one representative experiment are shown.
Real-Time RT-PCR
Total RNA was isolated from skin biopsies by chloroform:phenol extraction and isopropanol precipitation according to manufacturer's guidelines (Molecular Research Center Inc.). RNeasy Mini Kits (Qiagen, Valencia, CA) were used according to the manufacturer's protocol to isolate RNA from cell cultures and to further purify RNA from skin biopsies. Real-time RT-PCR was performed using an ABI 7000 Sequence Detection system (Applied Biosystems, Foster City, CA) as previously described.4 Human GAPDH and rodent GAPDH were purchased from Applied Biosystems. Primer and probe sequences for cathelicidin were designed as previously described.3 Primer and probe sequences used to assay HSV-2 gene transcripts were: Forward, 5′- CGC TCT CGT AAA TGC TTC CCT -3′, Reverse, 5′-TCT ACC CAC AAC AGA CCC ACG -3′. This region of the genome encodes the glycoprotein D (gD) of HSV-2.8 Relative expression levels were calculated by the relative standard curve method as outlined in the manufacturer's technical bulletin. To allow for comparisons between samples and groups, quantities of all targets in test samples were normalized to the corresponding GAPDH or total RNA levels and expressed as Target Gene normalized to GAPDH or Target Gene normalized to Total RNA. A standard curve was generated using cDNA from purified herpes virus.
Cathelicidin Protein Expression
Paraffin-embedded tissues were cut into 5 μm sections, de-paraffinized, re-hydrated, and then stained with rabbit anti-LL-37 (5μg/ml) as previously described.3 All slides were coded to ensure patient anonymity and readings were done blinded so that the slide reader was unaware of the identity of the slides. Images were collected at 40× magnification and the intensity of the immunostaining was scored on a scale from 0 to 5, with 0 indicating no staining and 5 the most intense staining.
Statistical Analyses
All statistical analysis was conducted using Graph Pad Prism, version 3.01 (San Diego, CA). Statistical differences in gene expression or protein staining between multiple groups was determined using a one-way analysis of variance (ANOVA) and significant differences determined by a Tukey-Kramer test.9 Statistical differences in total serum IgE were determined using a student's t-test.
Results
Anti-HSV Activity of LL-37
In our initial experiments, we examined whether LL-37 (0 – 100 μM) could directly kill HSV. As shown in Figure 1A, we observed a concentration-dependent inhibition of viral replication measured by real-time RT-PCR. Significant reduction in viral replication by LL-37 was observed with concentrations as low as 5 μM (mean: 2444 ± 1223 ng HSV/ng total RNA; p<0.001) as compared to HSV alone (mean: 32620 ± 4061). This was confirmed using a standard viral plaque assay in which pre-incubation of HSV with 5 μM of LL-37 significantly (p<0.001) reduced the number of plaques from 78.3 ± 1.5 (HSV alone) to 40.3 ± 1.9 (Figure 1B). The control peptide 8044 possessed no anti-viral activity against HSV.
Figure 1.

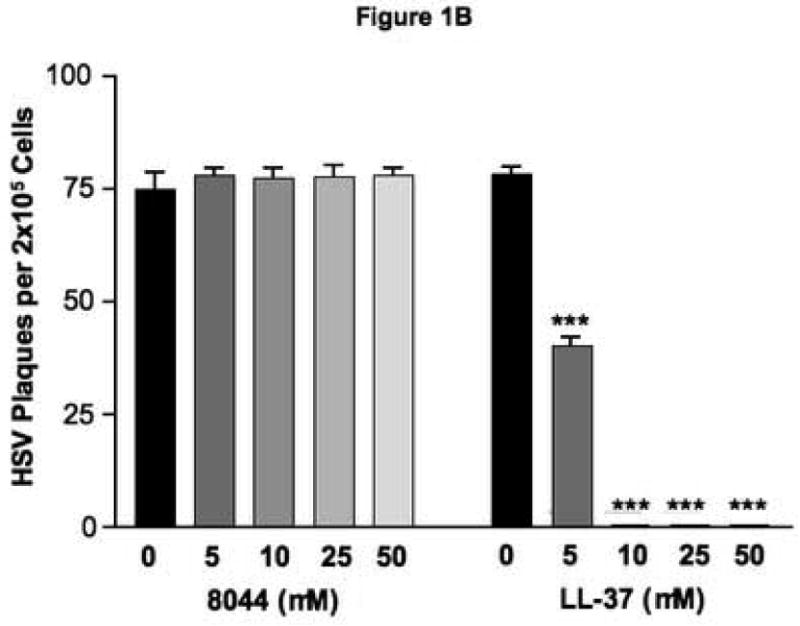
LL-37 exhibits anti-viral activity against HSV. Physiologic concentrations of LL-37 were pre-incubated with HSV-2 for 24 hours and then added to BS-C-1 for an additional 24 hours to evaluate HSV-2 gene expression by real-time RT-PCR (A) or 48 hours to investigate functionally active virus using a standard plaque assay (B). *** indicates a significant difference of p<0.001 as compared to 0 μM.
Role of Cathelicidins in Controlling HSV Replication in the Skin
To examine a more physiologic condition, human keratinocyte cultures were pre-infected with HSV for six hours and then treated with exogenous LL-37 to determine whether intracellular viral replication could be halted with physiologic concentrations of LL-37. Figure 2 demonstrates that concentrations of LL-37 as low as 25 μM (mean: 563 ± 71 ng HSV/ng GAPDH) were able to significantly (p<0.01) reduce the levels of HSV gene expression in previously infected keratinocytes (HSV mean: 1614 ± 158).
Figure 2.
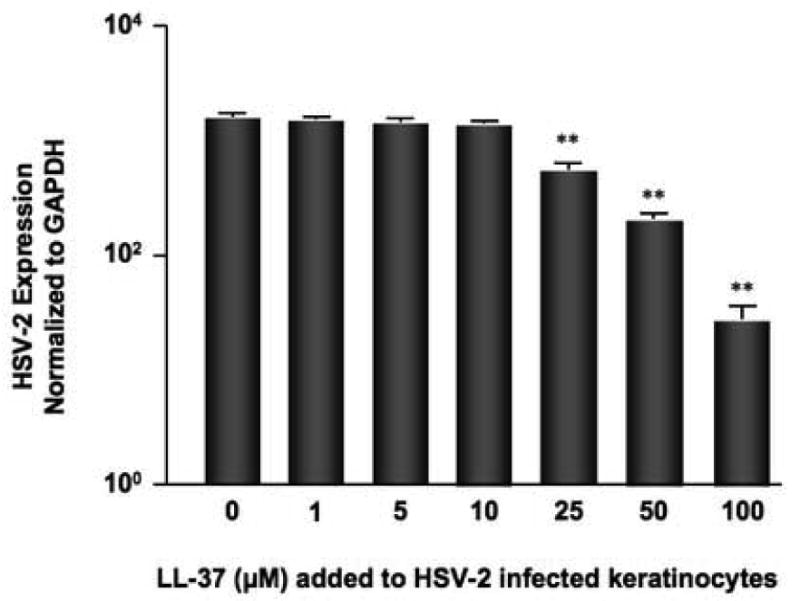
Exogenous LL-37 rescues HSV infected keratinocytes. Human keratinocytes were infected with 0.05 pfu/cell HSV-2 for six hours and then treated with physiologic concentrations of LL-37 for an additional 18 hours. RNA was isolated from the cells and the levels of HSV-2 gene expression evaluated by real-time RT-PCR. **indicates a significant difference of p<0.01 as compared to HSV alone.
To demonstrate the clinical relevance of LL-37 as compared to other potential arms of the innate immune response in limiting HSV infection, we used mice deficient in Cnlp, the murine cathelicidin. Significantly higher levels of HSV replication were observed in skin biopsies from Cnlp KO mice (BALB/c background) (2.6 ± 0.5 pg HSV/pg GAPDH; p<0.05) as compared to skin biopsies from wild type BALB/c mice (0.9 ± 0.3) (Figure 3) suggesting that cathelicidins play an important role in controlling HSV skin infection. These data are representative of three independent experiments with a total of 15 mice.
Figure 3.

Essential role of cathelicidins in controlling HSV replication in the skin. Skin biopsies from BALB/c (n=5) and Cnlp KO (n=5) mice were stimulated with HSV-2 for 24 hours and evaluated for HSV-2 gene expression. RNA was collected from the tissue and the levels of HSV-2 evaluated by real-time RT-PCR. *indicates a significant difference of p<0.05.
Deficiency of LL-37 in Skin of ADEH Patients
Skin biopsies were collected from the skin lesions of adult patients with AD and ADEH patients. To investigate cathelicidin expression, biopsies were stained with a polyclonal antibody specific for LL-37. All slides were coded prior to analysis and readings were done blinded so that the slide reader was unaware of the identity of the slides. Figure 4A illustrates that skin sections from AD patients exhibited more staining for cathelicidin than skin lesions from ADEH patients. The composite data for cathelicidin immunostaining in all samples is shown in Figure 4B. The intensity of cathelicidin staining in ADEH skin lesions was significantly (p<0.05) lower than skin lesions from AD patients.
Figure 4.
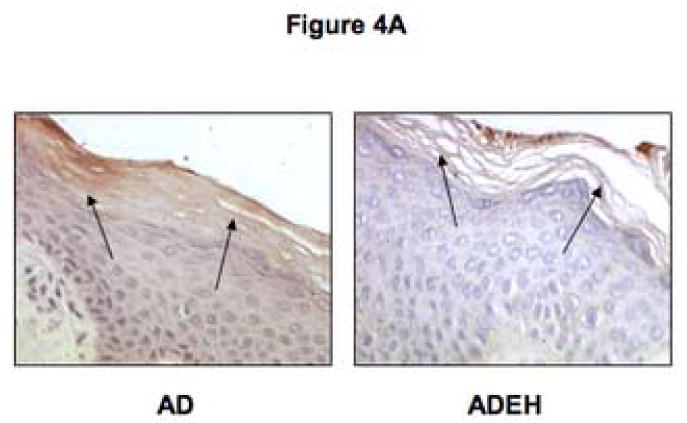

Expression of cathelicidin elevated in AD as compared to ADEH skin. A. Paraffin embedded skin explants from AD (n=10) and ADEH (n=10) patients were cut into 5 μm sections and stained for human cathelicidin. B. The intensity of the immunostaining was visually scored on a scale from 0 to 5, with 0 indicating no staining and 5 the most intense staining. *indicates statistical significance of p<0.05.
Correlation between Serum IgE and Cathelicidin Expression
Previous studies have suggested that Th2 cytokines could inhibit cathelicidin production.10 Due to the limited amount of archived tissue available, we were unable to investigate potential differences in IL-4 and IL-13 expression between AD and ADEH patients. However, IL-4 and IL-13 are Th2 cytokines essential in the production of IgE.10 Therefore, we examined whether elevations in serum IgE levels might correlate with cathelicidin expression in ADEH and AD patients. Since antibody generation is exponential, we log transformed the serum IgE values for further statistical analysis. Using linear regression analysis, we demonstrate a significant correlation (r2=0.46; p<0.05) between total serum IgE levels and cathelicidin protein expression in AD and ADEH patients (Figure 5).
Figure 5.
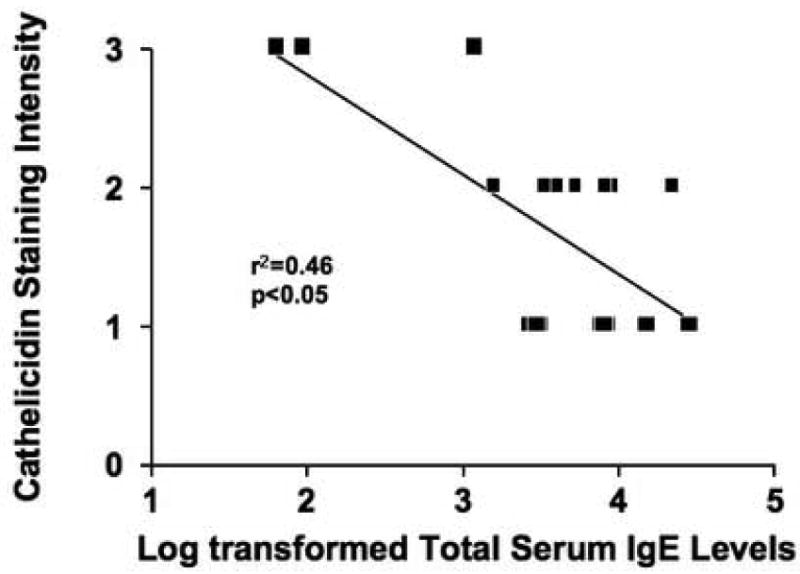
Correlation between serum IgE levels and cathelicidin expression in AD and ADEH patients. Serum IgE levels were determined from AD (n=9) and ADEH (n=9) patients. Regression analysis was performed on log transformed serum IgE values and LL-37 protein expression.
Discussion
AMPs are an integral part of the innate immune response as they have been shown to be effective in killing bacterial3 and viral11 pathogens. Cathelicidin is produced by several cells in the skin including keratinocytes where it is induced in response to inflammatory stimuli.12,13 Upon release, the cathelicidin precursor protein is processed into the biologically active antimicrobial peptide LL-37. In this study we demonstrate that LL-37 exhibits anti-viral activity against HSV. Our previous studies have demonstrated that AD patients, in general, have a reduced ability to generate cathelicidin in their skin, as compared to patients with psoriasis or allergic contact dermatitis, and this may predispose them to microbial skin infection.3,14 However, the propensity of AD patients to serious skin infection such as ADEH has not previously been explored. Results from the current study indicate that a more exaggerated reduction in cathelicidin expression may predispose a subset of AD patients to develop ADEH. Thus, there is heterogeneity in the expression of cathelicidin within AD such that the individuals with the lowest levels are most prone to disseminated viral infection.
It was previously reported that LL-37 exhibits little activity against HSV-1 or -2.15 In contrast, we demonstrate in this study that LL-37 exhibits potent anti-viral activity against HSV. In the previous study, Yasin et al. demonstrated that 44.5 μM of LL-37 provided 28 and 46% protection against HSV-1 and HSV-2, respectively, using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cytotoxicity assay. In the MTT assay, anti-viral activity of LL-37 is determined by increases in cellular proliferation compared to cells treated with virus alone. In our current study, we measured HSV replication by evaluating glycoprotein D gene expression using real-time RT-PCR. By directly measuring the virus activity rather than cellular proliferation, it provides a more direct and appropriate measurement of the anti-viral activity of LL-37 against HSV accounting for differences between our results and those of Yasin et al.15 Our observation is further supported by Gordon et al.16 who recently demonstrated that LL-37 exhibits anti-viral activity against HSV-1 in corneal and conjunctival epithelia.
In this study, we demonstrate that concentrations as low as 10 μM of LL-37 reduce HSV levels by over 10,000-fold. Since psoriatic skin can contain up to 1605 micromolar LL-37,3 this demonstrates that physiologic concentrations of LL-37 are effective at controlling HSV replication. This was further supported using a more physiological approach in which keratinocytes were pre-infected with HSV for six hours and then incubated with LL-37 for an additional 18 hours. Again, we saw greater than a 60% reduction in the levels of HSV when as little as 25 μM of LL-37 was added to previously infected keratinocytes. The importance of cathelicidins in skin innate immune responses to HSV is also strongly supported by our current finding that skin explants from mice deficient in the cathelicidin gene Cnlp, and it's AMP product CRAMP, sustain higher levels of HSV replication following inoculation as compared to their wild type counterparts. Mouse CRAMP is very similar to human LL-37 in structure, tissue distribution and antimicrobial activity and is therefore a reasonable model of the human cathelicidin. The observation that CRAMP deficient mice support a higher level of HSV replication reinforces the important impact that a cathelicidin deficiency would have on HSV skin infection in humans. These data support a conclusion that decreased cathelicidin expression will significantly increase the potential for disseminated skin infection to occur.
Since not all AD patients develop ADEH, we investigated the abundance of cathelicidin in the skin of AD patients and ADEH patients to determine whether the development of ADEH corresponded with decreased cathelicidin expression. Skin biopsies were obtained from naturally induced inflammatory skin rashes of AD and ADEH. This allowed for comparisons between similarly stimulated skin samples. Lesional skin from ADEH patients exhibited significantly lower levels of cathelicidin protein than skin lesions of uncomplicated AD patients. Our results suggest that AD patients with the lowest levels of cathelicidin are most susceptible to developing ADEH and that lack of this molecule may serve as a biomarker for patients at risk for disseminated viral skin infection.
AD skin is characterized by the over-expression of the Th2 cytokines IL-4 and IL-13.16 Previously we demonstrated that Th2 cytokines down-regulate cathelicidin.10 However, due to the difficulty in identifying ADEH patients and insufficient amounts of archived tissue, we were unable to investigate the levels of IL-4 and IL-13 in AD and ADEH patients for potential differences. IL-4 and IL-13 are Th2 cytokines essential in the production of IgE.10 Therefore, the measurement of serum IgE may serve as a biomarker for levels of Th2 responses. Wollenberg et al.6 and Lagace-Simard et al.18 have previously demonstrated that ADEH patients exhibited higher total serum IgE levels than AD patients. We confirmed this observation in the current study by demonstrating significantly higher serum IgE levels in AD patients as compared to ADEH patients. We further determined that there is a strong correlation between the levels of serum IgE and cathelicidin protein expression in AD and ADEH patients. Therefore serum IgE levels may serve as a surrogate marker for the expression of cathelicidin in the skin of AD and ADEH patients and may separate those who are more susceptible to disseminated viral infection.
The current study therefore demonstrates that AD is a heterogeneous population of patients expressing different levels of cathelicidin in the skin. This explains, in part, why a subgroup of AD patients is susceptible to developing ADEH following HSV infection. Additionally this study demonstrates the importance of the cathelicidin in controlling the replication of HSV in the skin. This is supported by significantly higher levels of HSV replication in the skin of Cnlp knockout mice and the significant reduction of HSV gene expression in keratinocytes treated with LL-37. Overall these data suggest further clinical studies are warranted to examine whether augmentation of LL-37 expression in AD skin may be useful in prevention of ADEH and the perplexing challenge of controlling microbial infection in this common skin problem.
Acknowledgments
The authors are indebted to Dr. Adriana Weinberg at the University of Colorado Health Science Center for the contribution of the human embryonic lung fibroblasts and herpes simplex virus. The authors thank Maureen Sandoval for her assistance in the preparation of this manuscript and the nursing staff in the General Clinical Research Center for their help in patient recruitment and sample collection.
This work was supported in part by the NIH National Research Service Award (T32 AI 07365) and AAAAI Fujisawa Skin Diseases Award (M.D.H.); NIH grants AI052453, AR45676, and a VA Merit Award (R.L.G.); NIH grants AR41256, 5R21AR051634, NIH/NIAID contracts N01 AI40029 and N01 AI40030, General Clinical Research Center grant MO1 RR00051 from the Division of Research Resources, the Edelstein Family Chair in Pediatric Allergy and Immunology, and the University of Colorado Cancer Center (D.Y.M.L.)
Abbreviations
- AD
Atopic dermatitis
- ADEH
Eczema herpeticum
- AMP
Anti-microbial peptide
- CRAMP
Mouse cathelicidin
- FCS
Fetal calf serum
- HSV
Herpes simplex virus
- IL
Interleukin
- LL-37
Human cathelicidin
- MEM
minimal essential media
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PFU
Plaque forming unit
- Th
T helper
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leung DY, Bieber T. Atopic dermatitis. Lancet. 2003;361:151–60. doi: 10.1016/S0140-6736(03)12193-9. [DOI] [PubMed] [Google Scholar]
- 2.Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–7. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 4.Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–9. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 5.Wollenberg A, Wetzel S, Burgdorf WHC, Haas J. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol. 2003;112:667–74. doi: 10.1016/j.jaci.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Wollenberg A, Zoch C, Wetzel S, Plewig G, Przybilla B. Predisposing factors and clinical features of eczema herpeticum: a retrospective analysis of 100 cases. J Am Acad Dermatol. 2003;49:198–205. doi: 10.1067/s0190-9622(03)00896-x. [DOI] [PubMed] [Google Scholar]
- 7.Sanderson IR, Brueton LA, Savage MO, Harper JI. Eczema herpeticum: a potentially fatal disease. Br Med J (Clin Res Ed) 1987;294:693–4. doi: 10.1136/bmj.294.6573.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weidmann M, Meyer-Konig U, Hufert FT. Rapid detection of herpes simplex virus and varicella-zoster virus infections by real-time PCR. J Clin Microbiol. 2003;41:1565–8. doi: 10.1128/JCM.41.4.1565-1568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tukey J. Exploratory Data Analysis. Reading, NY: Addison Wesley; 1977. [Google Scholar]
- 10.Bacharier LB, Geha RS. Molecular mechanisms of IgE regulation. J Allergy Clin Immunol. 2000;105:S547–58. doi: 10.1016/s0091-6749(00)90059-9. [DOI] [PubMed] [Google Scholar]
- 11.Howell MD, Jones JF, Kisich KO, Streib JE, Gallo RL, Leung DY. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J Immunol. 2004;172:1763–7. doi: 10.4049/jimmunol.172.3.1763. [DOI] [PubMed] [Google Scholar]
- 12.Erdag G, Morgan JR. Interleukin-1alpha and interleukin-6 enhance the antibacterial properties of cultured composite keratinocyte grafts. Ann Surg. 2002;235:113–24. doi: 10.1097/00000658-200201000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–63. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 14.Howell MD, Novak N, Bieber T, Pastore S, Girolomoni G, Boguniewicz M, et al. Interleukin-10 downregulates anti-microbial peptide expression in atopic dermatitis. J Invest Dermatol. 2005;125:738–45. doi: 10.1111/j.0022-202X.2005.23776.x. [DOI] [PubMed] [Google Scholar]
- 15.Yasin B, Pang M, Turner JS, Cho Y, Dinh NN, Waring AJ, et al. Evaluation of the inactivation of infectious Herpes simplex virus by host-defense peptides. Eur J Clin Microbiol Infect Dis. 2000;19:187–94. doi: 10.1007/s100960050457. [DOI] [PubMed] [Google Scholar]
- 16.Gordon YJ, Huang LC, Romanowski EG, Yates KA, PRoske RJ, McDermott AM. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr Eye Res. 2005;30:385–94. doi: 10.1080/02713680590934111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994;94:870–6. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagace-Simard J, Portnoy JD, Wainberg MA. High levels of IgE in patients suffering from frequent recurrent herpes simplex lesions. J Allergy Clin Immunol. 1986;77:585–5. doi: 10.1016/0091-6749(86)90349-0. [DOI] [PubMed] [Google Scholar]


