Abstract
Of the 4 million neonatal deaths and 500,000 maternal deaths that occur annually worldwide, almost 99% are in developing countries and one-third are associated with infections. Implementation of proven interventions and targeted research on a select number of promising high-impact preventative and curative interventions are essential to achieve Millennium Development Goals for reduction of child and maternal mortality. Feasible, simple, low-cost interventions have the potential to significantly reduce the mortality and severe morbidity associated with infection in these settings. Studies of chlorhexidine in developing countries have focused on three primary uses: 1) intrapartum vaginal and neonatal wiping, 2) neonatal wiping alone, and 3) umbilical cord cleansing. A study of vaginal wiping and neonatal skin cleansing with chlorhexidine, conducted in Malawi in the 1990s suggested that chlorhexidine has potential to reduce neonatal infectious morbidity and mortality. A recent trial of cord cleansing conducted in Nepal also demonstrated benefit. Although studies have shown promise, widespread acceptance and implementation of chlorhexidine use has not yet occurred. This paper is derived in part from data presented at a conference on the use of chlorhexidine in developing countries and reviews the available evidence related to chlorhexidine use to reduce mortality and severe morbidity due to infections in mothers and neonates in low-resource settings. It also summarizes issues related to programmatic implementation.
Background
One-third of the 4 million neonatal deaths (< 28 days) and 500,000 maternal deaths that occur annually worldwide are associated with infections.[1,2] In areas with 28-day neonatal mortality rates (NMR) > 45 per 1000 births, up to 50% of these deaths are due to infection.[2] The effectiveness of essential maternal and newborn care (EMNC) interventions such as clean delivery, hygienic cord care, thermal care and breastfeeding has been estimated to decrease neonatal deaths due to serious infections by 20–50%.[3,4] Chlorhexidine antisepsis also has shown promise in preliminary studies; however, the additive value of this intervention to reduce maternal and neonatal mortality and morbidity, in the context of EMNC, should be considered. In October 2006, a conference on chlorhexidine in developing countries was organized by staff from USAID and NICHD. The data presented below is derived in part from data presented at that conference.
Chlorhexidine pharmacology
Chlorhexidine is a bisbiguanide compound that acts by binding to the bacterial cell wall and disrupting its membrane, leading to increased permeability and cell content leakage. It has a shelf life of 20 to 24 months, is stable at room temperature when stored in an opaque container, and has no known drug interactions.[5] hlorhexidine is widely used in hospital settings as a topical skin antiseptic to reduce infection.
Preparations of chlorhexidine acetate, diacetate, gluconate and digluconate - primarily in an aqueous or saline solution - have been used as a vaginal disinfectant to reduce the number of organisms and associated maternal and newborn infections. Concentrations have ranged from 0.05% to 4%, and the timing of doses has ranged from a single application to use with each vaginal examination during labor.[6] Concentrations applied to neonatal skin have generally been < 1% and a concentration of 4% has been popular for cord applications.[7] Chlorhexidine is produced by a number manufacturers throughout the world. Most commonly the digluconate form is prepared in 20% solutions and then diluted with water or saline to achieve the final concentration.[6]
Safety data
Chlorhexidine is generally safe to use. Despite extensive use in medical settings since the 1950s, only isolated adverse events such as contact sensitivity, dermatitis, urticaria and photosensitivity have been reported.[8,9] However, there have been isolated reports of anaphylactic reactions.[10–12] In obstetric use, desquamation of the vaginal mucosa has been reported.[13, 14] Wilson et al used a vaginal wipe with concentrations ranging from 0.25% to 2% every 4 hours and found no significant adverse reactions at concentrations < 1%.[15] At 1% concentration, 13% of the women reported burning or itching sensations.
There have been extensive studies of the safety of chlorhexidine in newborns. Reports have documented percutaneous absorption of chlorhexidine with both body wash[16] and cord care wipe,[17] but no adverse clinical consequences have been reported. Hypothermia has been identified as a potential neonatal risk associated with chlorhexidine skin cleansing following delivery. However, a Nepal study examined the effect of chlorhexidine wipe on skin temperature and found minimal increased risk of hypothermia.[18]
Chlorhexidine to prevent infection
Studies of chlorhexidine to prevent neonatal and/or maternal infection and mortality have been conducted in both developed and developing countries and have focused on three classes of intervention: intrapartum vaginal wipe in conjunction with newborn wipe, neonatal wipe alone and umbilical cord care.
Vaginal/neonatal chlorhexidine research
In developing countries, sepsis, meningitis, tetanus, acute respiratory infection and diarrhea all contribute to neonatal mortality.[19] In comparison to developed countries, where group B streptococcus (GBS) is often the most important pathogen, the causal organisms are frequently gram-negative bacteria such as Klebsiella pneumoniae and Escherichia coli, that likely originate in the maternal genital tract. These are most likely acquired during labor, either from the fetus aspirating infected amniotic, or being exposed to the organisms as they pass through the birth canal.[20–22] Researchers have speculated that prevention of peripartal infection of the mother and fetus-neonate might be possible by intrapartum cleansing of the vagina and cervix with an anti-bacterial agent such as chlorhexidine.[6]
Two studies of neonatal outcomes using a 0.25% chlorhexidine vaginal/neonatal treatment in developing countries have been published (Table 1).[23–25] The first, a hospital-based study in Malawi, involved a two-month control period with no treatment, followed by a three-month period where everyone in labor received treatment, followed by a second one-month control period.[23] With treatment, there were significant reductions in overall newborn admissions, sepsis admissions, early neonatal mortality and neonatal mortality due to sepsis, as well as significant reductions in maternal hospital admissions, length of stay, and admissions due to sepsis during the intervention period. Investigators in Egypt conducted a similar study. In their trial of 4400 women, chlorhexidine treatment resulted in significant reductions in newborn hospital admissions and admissions due to sepsis, newborn deaths due to infections, as well as maternal hospital admissions.[25] Both studies were criticized because the patients were not randomized. At least two hospital-based trials of an intrapartum vaginal chlorhexidine wash are ongoing in developing countries, one in Pakistan and one in South Africa (http://www.clinicaltrials.gov). In addition, a pilot study of intrapartum vaginal 1% chlorhexidine wash was recently completed in Zimbabwe. These trials should provide additional data regarding the efficacy of chlorhexidine to reduce neonatal and maternal morbidity.
Table 1.
Summary of selected vaginal/neonatal chlorhexidine studies aimed at improving pregnancy outcomes in low-resource settings
| Author, Year | Location | Study design, sample size | Study Groups | Outcome |
|---|---|---|---|---|
| Bakr 2005 | Egypt | Pre-post comparison, N = 4415 | Manual wiping of birth canal with 0.25% CHX solution; babies wiped | Neonatal admissions were comparable, the nonintervention group showed a significantly higher rate of admissions due to sepsis (p=0.0002) and deaths caused by sepsis (0.004). |
| Taha 1997 | Malawi | Pre-post comparison, N = 6965 | Vaginal wipe with0.25% CHX; babies wiped. | Intervention phase, overall neonatal admissions were reduced (634/3743 (16.9%) v 661/3417(19.3%), P<0.01), as were admissions for neonatal sepsis (7.8 v 17.9 per 1000 live births, P<0.0002), overall neonatal mortality (28.6 v 36.9 per 1000 live births, P<0.06), and mortality due to infectious causes (2.4 v 7.3 per 1000 live births, P<0.005). |
| Mullany 2006 | Nepal | Cluster randomized, placebo controlled trial, N = 15,855 | 4.0% CHX, soap and water on days 1,2,3,4,6,8,10 or dry cord care | 75% reduction in severe cord infection (95% CI0.12,0.53) |
| Tielsch 2005 | Nepal | Cluster randomized, placebo controlled trial, N = 15,855 | Neonatal wipe with normal wipes or wipes with 0.25% CHX solution after delivery. | Overall, no significant effect, but in a secondary analysis, a 28% reduction in mortality among low birth weight infants (RR=0.72, CI: 0.54, 0.95) and no impact on infants 2500 g or above (RR=1.14, CI: 0.75, 1.73). |
| Studies in the field or not yet reported | ||||
| Goldenberg | Pakistan | Randomized, placebo controlled trial, Planned N = 5000 | Intrapartum vaginal wipe with 0.6% CHX, babies wiped | 28-day neonatal mortality, infection-related morbidity |
| Schrag | South Africa | Randomized trial, Planned N = 8000 | Intrapartum vaginal wash with 0.5% CHX, babies wiped | Infection-related hospitalization |
| Tolosa, Chipato | Zimbabwe | Pilot study, N = 500 | Intrapartum vaginal wash with 1% CHX, babies wiped | Safety and feasibility of 1% CHX washing of the vaginal canal and neonate. Change in vaginal flora related to CHX wash. |
In developed countries, where the NMR is much lower, GBS is a common neonatal pathogen and causes the majority of neonatal sepsis.[26] Most developed country studies of chlorhexidine vaginal wipes have focused on reducing neonatal GBS infections.[14,26–30] Several have shown a statistically significant reductions in maternal-to-newborn GBS transmission following the use of vaginal/newborn chlorhexidine.[15,25] Since only one in several hundred GBS transmissions results in neonatal sepsis, a more important question is whether vaginal use reduces neonatal sepsis. Two studies suggest this may occur.[14,30] In the largest study (n=4483), in both GBS positive and negative women, significant reductions were seen in all newborn infections from 0.3% to <0.1%, respiratory disorders/infections from 1.6% to 0.9%, and hospital admissions from 2.9% to 2.0%.[30] In a second study, while reductions in a number of infectious outcomes were seen in the chlorhexidine compared to controls, only a composite diagnosis of adverse outcomes was significantly different (7.9% vs. 4.9%, p<0.05).[14] Another study compared vaginal chlorhexidine use to intrapartum antibiotics. Both treatments displayed the same efficacy in preventing vertical transmission of GBS, but the rate of Escherichia coli colonization was less in the chlorhexidine treated group.[31] These studies suggest that in high-resource countries, the neonatal benefits of chlorhexidine, although potentially significant, are likely to be small. In two U.S. studies, investigators attempted to reduce the maternal infectious outcomes of chorioamnionitis and postpartum endometritis using a 0.2% chlorhexidine 200 ml douche, compared to a saline douche, applied once in the first study and every 6 hrs during labor in the second. In over 2000 women studied, there were no significant differences in any maternal or neonatal outcomes. High rates of intrapartum antibiotics use may have reduced the impact of chlorhexidine.[28,29]
Neonatal wipe research
In addition to studies of vaginal/neonatal chlorhexidine applications, studies of the efficacy of a chlorhexidine neonatal wipe alone to reduce neonatal infectious morbidity have been conducted in developed countries. Outcomes examined included superficial infections, but have not been adequately powered to assess impact on neonatal mortality.[32–33] Only one randomized study of neonatal cleansing alone has been conducted in a developing country.[35] In this Nepal trial, at delivery, local workers provided a full-body skin cleansing intervention. There was a non-significant reduction in overall neonatal mortality; however, a sub-analysis of outcome among low birth weight infants showed a significant reduction in mortality in the chlorhexidine compared to the control group.
Umbilical cord cleansing research
In many developing countries, umbilical cord infection constitutes an important cause of neonatal morbidity and poses an increased risk for mortality. Umbilical infection rates from 2 to 54 per 1000 live births have been reported from hospital studies.[7, 36] A WHO report suggested that clean cord care should usually be sufficient to reduce infections except where harmful practices such as putting cow dung on the stump are prevalent.[37] In the latter case, WHO recommends use of a topical antiseptic such as chlorhexidine to replace the harmful practice. A review of umbilical cord care regimens based on data from developed countries concluded that although bacterial colonization could be reduced with antimicrobial applications, evidence was lacking to recommend use of antimicrobials in place of dry cord care to reduce infections.[38] The only trial of chlorhexidine of umbilical cord care in a low-resource setting was completed in Nepal. This nested, cluster-randomized, controlled trial of umbilical cord cleansing examined the impact of care using 4% chlorhexidine compared to usual care or cord care with soap and water to reduce neonatal infections.[18] Daily cleansing of the umbilical cord and stump with chlorhexidine reduced cord infections by 32% to 75%, and reduced neonatal mortality by 24%.
Translating research into products for global health
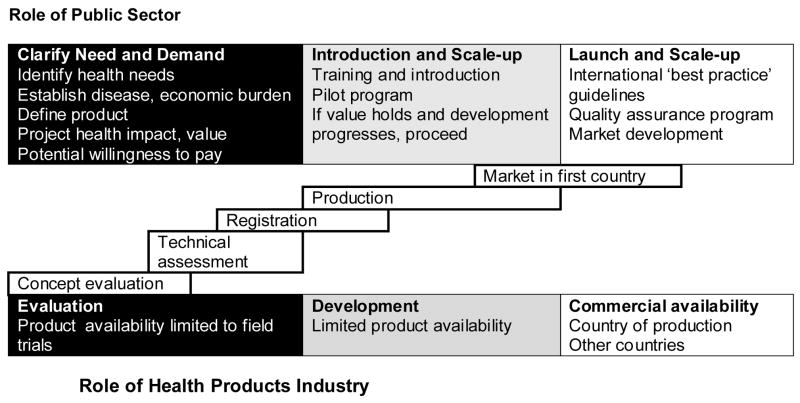
When a product such as chlorhexidine is considered for widespread introduction, product development includes establishing underlying disease and economic burdens, defining the key product performance characteristics, projecting the health impact and considering whether there is a willingness to pay for the intervention (Figure 1). After these initial issues have been addressed, the next steps involve introduction of the product in pilot programs to refine understanding of value. If the value holds - that is, the challenges are manageable and initial studies confirm expected findings - the next step is widespread implementation. Steps toward scale-up have included development of the international ‘best practice’ policy statement and guidelines, a quality assurance program and market development.
Figure 1.
Steps to Scale up Chlorhexidine Implementation
Chlorhexidine: programmatic considerations
Developing a chlorhexidine product for low-resource countries requires defining user complexity, including compliance and dose delivery and control; and manufacturing complexity including cost, packaging, and quality assurance. During the research phase, evaluating the effectiveness in the field and conceptualizing the product development are important considerations for implementation. Coordination of research activities with program development can increase the efficiency of implementation (Panel 1). However, until there is a conclusive level of evidence, manufacturers may be reluctant to invest in a product, especially one with low potential for financial yield. Positive results of at least two independent randomized trials have been considered the standard for adoption of study interventions as standard practice by WHO and national drug authorities. To date, none of the potential uses for chlorhexidine have met this standard.
Discussion
Chlorhexidine is an inexpensive, safe and simple intervention with the potential to reduce a major cause of perinatal morbidity and mortality in low-resource settings. Promising results are available for chlorhexidine used as a vaginal or neonatal wipe and for cord care. Nevertheless, despite positive results from studies using each type of application in developing countries, chlorhexidine use to prevent perinatal morbidity and mortality has not been widely adopted.[39] Based on this review, it appears that this failure is due in part to a lack of sufficient randomized trial evidence of efficacy. However, the lack of consistency in the studies in terms of application site evaluated, and type, concentration, and dosing schedule of the chlorhexidine preparation used have also contributed to the failure to adopt the chlorhexidine into routine clinical care.
Considerations for further research and evaluation that may enhance adoption, if ongoing study results are positive, are summarized in Panel 2. To facilitate comparability across studies that evaluate chlorhexidine as a tool for perinatal infection prevention, the product should be described in a consistent way. An aqueous-based solution (without alcohol) of chlorhexidine digluconate appears to be the most common preparation used in studies and probably should be the preparation used in future studies. For clinical use of chlorhexidine for vaginal or neonatal wipes, a 1% concentration appears to be the upper limit, while concentrations as low as 0.25% appear to be effective. To achieve maximum efficacy while staying below the 1% concentration where side effects begin to appear, the two ongoing studies have chosen concentrations of 0.5 to 0.6%. For cord care regimens, a 4% chlorhexidine concentration has been used without side effects, but whether lower doses would be equally effective is unknown. Since providing a single concentration for multiple uses would be most efficient, whether a 0.6% – 1% solution would be effective in umbilical cord care as well as tolerable for the vaginal and/or neonatal wipe should be addressed.
Based on the above discussions, further research related to chlorhexidine should focus on confirmatory randomized controlled trials of vaginal/neonatal wipes to reduce neonatal and maternal morbidity and mortality and a clinical trial of a neonatal chlorhexidine wipe alone with the power to perform sub-group analyses by birth weight strata. Trials of cord care with chlorhexidine that address issues such as concentration and dosing schedules are also important. The use of chlorhexidine in community compared to hospital settings should also be evaluated.
In summary, vaginal and neonatal chlorhexidine applications have demonstrated potential in reducing neonatal and maternal mortality and morbidity in low-resource/high mortality settings. As the research continues, collaboration between investigators, funding agencies as well as product development experts could facilitate prioritization of research activities and simultaneous product development, all crucial to ensure the adoption and the sustainability of this intervention.
Panel 1. Key milestones in the transition from chlorhexidine research to implementation by consortium of producers, funders and researchers
Evaluate whether further evidence is needed to demonstrate safety and effectiveness, and determine how evidence should be obtained
Select product(s) and screen product presentations for acceptability and cost
Conduct market study (who buys and distributes, affordability of product)
Describe commercial investment potential of chlorhexidine scale-up
Prepare supply strategy (local, multinational or combination)
Develop a regulatory strategy, if required
Implement a multi-site pilot introduction (product from “alpha” supplier and conduct evaluation (phase IV post market studies)
Determine whether value would convert to willingness to pay
Build supply and distribution network (‘sell’ the ‘value proposition’ to local suppliers, Ministries of Health, NGOs, and multilaterals)
Develop appropriate training and promotional materials and guidelines
Develop quality assurance strategy for ongoing monitoring, including testing program and certification of suppliers
Source: PATH/HealthTech; adapted from Free (2005)
Panel 2. Recommended steps for parallel progress in chlorhexidine research and product development
CHX effectiveness and efficacy
Determine the optimal chlorhexidine concentration, preparation, volume, dosing, cost
Assess the additive benefit over chlorhexidine over full implementation of known EMNC interventions
Evaluate the effectiveness and acceptability in high risk geographically and culturally diverse communities including both institutional and non-institutional birth settings
Identify and characterize factors which may influence efficacy, such as gestational age and rupture of membranes, concomitant antibiotics
Monitor program effects where the intervention is introduced
Product and program implementation
Express the product across studies in a standard way that includes the concentration of total salt.
Identify the steps for scaling up and integration with other programs, such as with the use of clean delivery kits and essential newborn care practices
Define the product, including target applications (vaginal, skin, and umbilical cord), use scenarios (hospital, health center, and home) and format (swabs, solutions, creams, aerosols)
Identify the potential cultural barriers
Management of potential side effects
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. World Health Report 2005: Making every mother and child count. Geneva: WHO; 2005. [Google Scholar]
- 2.Lawn J, Cousens S, Zupan J. 4 million neonatal deaths: When? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 3.Darmstadt G, Bhutta ZA, Cousens S, Adam T, Walker N. Evidence-based cost-effective intervention: how many newborn babies can we save? Lancet. 2005;365:977–88. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- 4.Bhutta ZA, Darmstadt GL, Hasan B, Haws R. Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: review of the evidence. Pediatrics. 2005;115:519–617. doi: 10.1542/peds.2004-1441. [DOI] [PubMed] [Google Scholar]
- 5.Goodman LS, editor. The pharmacological basis of therapeutics. New York: Macmillan Publishing Co; 1985. [Google Scholar]
- 6.Goldenberg RL, McClure EM, Saleem S, Rouse DJ, Vermund S. Use of vaginally administered chlorhexidine during labor to improve pregnancy outcomes. Obstet Gynecol. 2006;107(5):1139–46. doi: 10.1097/01.AOG.0000215000.65665.dd. [DOI] [PubMed] [Google Scholar]
- 7.Mullany L, Darmstadt GL, Tielsch JM. Safety and impact of chlorhexidine antisepsis interventions for improving neonatal health in developing countries. Pediatr Infect Dis J. 2006;25(8):665–75. doi: 10.1097/01.inf.0000223489.02791.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bechgaard E, Ploug E, Hjorth N. Contact sensitivity to chlorhexidine? Contact Dermatitis. 1985;13(2):53–5. doi: 10.1111/j.1600-0536.1985.tb02504.x. [DOI] [PubMed] [Google Scholar]
- 9.Goon A, White IR, Rycroft RJ, McFadden JP. Allergic contact dermatitis from chlorhexidine. Dermatitis. 2004;15(1):45–7. doi: 10.2310/6620.2004.20438. [DOI] [PubMed] [Google Scholar]
- 10.Beaudouin E, Kanny G, Morisset M, Renaudin JM, Mertes M, Laxenaire MC, Mouton C, Jacson F, Moneret-Vautrin DA. Immediate hypersensitivity to chlorhexidine: literature review. Allerg Immunol. 2004;36(4):123–6. [PubMed] [Google Scholar]
- 11.Okano M, Nomura M, Hata S, Okada N, Sato N, Kitano Y, Tashiro M, Yoshimoto Y, Hama R, Aoki T. Anaphylactic symptoms due to chlorhexidine gluconate. Arch Dermatol. 1989;125(1):50–2. [PubMed] [Google Scholar]
- 12.Ohtoshi T, Yamauchi N, Tadokoro K. IgE antibody-mediated shock reaction caused by topical application of chlorhexidine. Clin Allergy. 1986;16:155–61. doi: 10.1111/j.1365-2222.1986.tb00759.x. [DOI] [PubMed] [Google Scholar]
- 13.Shippey S, Malan TK. Desquamating vaginal mucosa from chlorhexidine gluconate. Obstet Gynecol. 2004;103(5):1048–50. doi: 10.1097/01.AOG.0000121834.67077.03. [DOI] [PubMed] [Google Scholar]
- 14.Stray-Pedersen B, Burgan T, Hafstad A, Normann E, Grogaard J, Vangdaal M. Vaginal disinfection with chlorhexidine during childbirth. Int J Antimicrob Agents. 1999;12:245–51. doi: 10.1016/s0924-8579(99)00068-0. [DOI] [PubMed] [Google Scholar]
- 15.Wilson C, Gray G, Read JS, Mwatha A, Lala S, Johnson S, et al. Safety and tolerance of different concentrations of chlorhexidine for peripartum vaginal and infant washes: HIVNET 025. J Acquir Immune Defic Syndr. 2004;35:138–43. doi: 10.1097/00126334-200402010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vorheer H, Ulrich JA, Messer RH, et al. Antimicrobial effect of chlorhexidine and povidone-iodine on bacteria of groin, perineum and vagina. J Reprod Med. 1980;24:153–7. [PubMed] [Google Scholar]
- 17.Mullany LC, Darmstadt GL, Khatry SK, LeClerq SC, Tielsch JM. Safety of neonatal skin cleansing in rural Nepal. Indian Pediatr. 2006;43(2):117–24. [PMC free article] [PubMed] [Google Scholar]
- 18.Mullany LC, Darmstadt G, Khatry SK, Katz J, LeClerq SC, Shrestha S, Adhikari R, Tielsch JM. Topical applications of chlorhexidine to the umbilical cord for prevention of omphalitis and neonatal mortality in southern Nepal: a community-based, cluster-randomised trial. Lancet. 2006;367(9514):910–8. doi: 10.1016/S0140-6736(06)68381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoll BJ. The global impact of neonatal infection. Clinics in Perinat. 1997;24:1–21. [PubMed] [Google Scholar]
- 20.Goldenberg RL, Jobe AH. Prospects for research in reproductive health and birth outcomes. JAMA. 2001;285(5):633–9. doi: 10.1001/jama.285.5.633. [DOI] [PubMed] [Google Scholar]
- 21.Goldenberg RL, Thornton C. The infectious origins of stillbirth. Am J Obstet Gynecol. 2003;189:861–73. doi: 10.1067/s0002-9378(03)00470-8. [DOI] [PubMed] [Google Scholar]
- 22.Goldenberg RL. Factors influencing perinatal outcomes. Ann NY Acad Sci. 2004;1038:227–34. doi: 10.1196/annals.1315.032. [DOI] [PubMed] [Google Scholar]
- 23.Taha TE, Biggar R, Broadhead RL, Mtimavalye LA, Justesen LGAB, et al. Effect of cleansing the birth canal with antiseptic solution on maternal and newborn morbidity and mortality in Malawi: clinical trial. BMJ. 1997;315:216–9. doi: 10.1136/bmj.315.7102.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biggar RJ, Miotti PG, Taha TE, Mtimavalye L, Broadhead R, Justesen A, et al. Perinatal intervention trial in Africa: effect of a birth canal cleansing intervention to prevent HIV transmission. Lancet. 1996;347:1647–50. doi: 10.1016/s0140-6736(96)91486-5. [DOI] [PubMed] [Google Scholar]
- 25.Bakr AF, Karkour T. Effect of predelivery vaginal antisepsis on maternal and neonatal morbidity and mortality in Egypt. J Womens Health. 2005;14:496–501. doi: 10.1089/jwh.2005.14.496. [DOI] [PubMed] [Google Scholar]
- 26.Stade B, Shah V, Ohlssen A. Vaginal chlorhexidine during labour to prevent early-onset neonatal group B streptococcal infection. Cochrane Database Syst Rev. 2004;3:CD003520. doi: 10.1002/14651858.CD003520.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Lumbiganon P, Thinkhamrop J, Thinkhamrop B, Tolosa JE. Vaginal chlorhexidine during labour for preventing maternal and neonatal infections (excluding Group B Streptococcal and HIV) Cochrane Database Syst Rev. 2004 Oct 18;4:CD004070. doi: 10.1002/14651858.CD004070.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Rouse DJ, Cliver S, Lincoln TL, Andrews WW, Hauth JC. Clinical trial of chlorhexidine vaginal irrigation to prevent peripartal infection in nulliparous women. Am J Obstet Gynecol. 2003;189:166–70. doi: 10.1067/mob.2003.322. [DOI] [PubMed] [Google Scholar]
- 29.Rouse DJ, Hauth J, Andrews WW, Mills BB, Maher JE. Chlorhexidine vaginal irrigation for the prevention of peripartal infection: a placebo-controlled randomized clinical trial. Am J Obstet Gynecol. 1997;176:617–22. doi: 10.1016/s0002-9378(97)70557-x. [DOI] [PubMed] [Google Scholar]
- 30.Burman LG, Christensen P, Christensen K, Fryklund B, Helgesson, Svenningsen NW, Tullus K. Prevention of excess neonatal morbidity associated with group B streptococci by vaginal chlorhexidine disinfection during labour. The Swedish Chlorhexidine Study Group. Lancet. 1992;340:65–9. doi: 10.1016/0140-6736(92)90393-h. [DOI] [PubMed] [Google Scholar]
- 31.Facchinetti F, Piccinini F, Mordini B, Volpe A. Chlorhexidine vaginal flushings versus systemic ampicillin in the prevention of vertical transmission of neonatal group B streptococcus, at term. J Matern Fetal Neonatal Med. 2002;11(2):84–8. doi: 10.1080/jmf.11.2.84.88. [DOI] [PubMed] [Google Scholar]
- 32.Tuke W. Hibiscrub in the control of staphyoccal in neonates. Nurs Times. 1975;71(37):20. [PubMed] [Google Scholar]
- 33.Maloney M. Chlorhexidine: a hexachlorophane substitute in the nursery. Nurs Times. 1975;71(37):21. [PubMed] [Google Scholar]
- 34.Meberg A, Schoyen K. Bacterial colonization and neonatal infections. Effects of skin and umbilical disinfection in the nursery. Acta Paediatr Scand. 1985;74(3):366–71. doi: 10.1111/j.1651-2227.1985.tb10985.x. [DOI] [PubMed] [Google Scholar]
- 35.Tielsch JM, Darmstadt GL, Mullany LC, Khatry SK, Katz J, Leclerq SC, et al. Impact of Newborn Skin-Cleansing With Chlorhexidine on Neonatal Mortality in Southern Nepal: A Community-based Cluster-Randomized Trial. Pediatrics. 2007 Jan 8; doi: 10.1542/peds.2006-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullany LC, Darmstadt GL, Tielsch JM. Role of antimicrobial applications to the umbilical cord in neonates to prevent bacterial colonization and infection: a review of the evidence. Pediatr Infect Dis J. 2003;22(11):996–1002. doi: 10.1097/01.inf.0000095429.97172.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. Care of the Umbilical Cord: A review of the evidence. Geneva: WHO; 1998. [Google Scholar]
- 38.Zupan J, Garnder P, Omari AA. Topical umbilical cord care at birth. Cochrane Database Syst Rev. 2004;3:CD001057. doi: 10.1002/14651858.CD001057.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biggar RJ. Vaginal cleansing and the gold standard. J Womens Health. 2005;14(6):531–3. doi: 10.1089/jwh.2005.14.531. [DOI] [PubMed] [Google Scholar]