Abstract
Background
Elevated body-mass index (BMI, weight in kilograms/height in meters2), in the obese range (≥30) is associated with an excess risk of heart failure (HF). However, impact of overweight or preobese (BMI 25 to 29.9) status and physical activity to HF risk is unclear.
Methods and Results
In a prospective cohort of 21,094 men (mean age, 53 years) without known coronary heart disease at baseline in the Physicians’ Health Study, we examined the individual and combined effects of BMI and vigorous physical activity (exercise to the point of breaking a sweat) to HF incidence from 1982 to 2007. We evaluated BMI as both a continuous (per 1 kg/m2 increment) and a categorical (lean, <25, overweight, 25 to 29.9, and obese, ≥30) variable, and vigorous physical activity primarily as a dichotomous variable (inactive [rarely/never] vs. active [≥1 to 3 times a month]). During follow-up (mean 20.5 years), 1109 participants developed new-onset HF. In multivariable analyses, every 1 kg/m2 increase in BMI was associated with an 11% (95%CI 9 to 13%) increase in HF risk. Compared with lean participants, overweight participants had a 49% (95%CI 32 to 69%) and obese participants had a 180% (95%CI 124 to 250%) increase in HF risk. Vigorous physical activity conferred an 18% (95%CI 4 to 30%) decrease in HF risk. No interaction was found between BMI and vigorous physical activity to HF risk (p=0.96). Lean-active men had the lowest and obese-inactive men had the highest risk of HF. Compared with lean-active men, the hazard ratios (95%CI) were 1.19 (0.94 to 1.51), 1.49 (1.30 to 1.71), 1.78 (1.43 to 2.23), 2.68 (2.08 to 3.45), and 3.93 (2.60 to 5.96) in lean-inactive, overweight-active, overweight-inactive, obese-active, and obese-inactive men respectively.
Conclusions
In this cohort of men, elevated BMI, even in preobese range, was associated with an increased risk of HF and vigorous physical activity was associated with a decreased risk. Public health measures to curtail excess weight, maintain optimal weight, and promote physical activity may limit the scourge of HF.
Keywords: Exercise, Heart Failure, Obesity, Overweight, Physical Activity
INTRODUCTION
Excess body weight, sedentary lifestyle, and heart failure (HF) are major public health problems in the United States and worldwide.1–6 In adult men and women living in the community, body-mass index (BMI, calculated as weight in kilograms divided by the square of height in meters) is associated with the risk of HF7–13 in a positive,7–13 linear,10–12 and dose-response manner.11 Although BMI in the obese range (≥30 kg/m2) is associated with an increased risk of HF,9,11,13 the risk conferred in the overweight or preobese (25 to 29.9 kg/m2) individuals is unclear.9,11 Consequently, the Heart Failure Society of America recommends a BMI of <30 kg/m2 as the target to prevent the development of HF.14
Physical activity is a key determinant of body weight and an important component for weight reduction and weight maintenance.1,2,15 Numerous health benefits of physical activity have been reported,15 but its influence on the risk of HF, especially in men, remains uncertain.8 Furthermore, the interaction of BMI and physical activity with the risk of HF is not known. Therefore, we investigated the relation of individual and combined effects of BMI and physical activity with the risk of HF in a large prospective cohort of men in the Physicians’ Health Study (PHS).
METHODS
Study sample
The study design and methods of the PHS have been published previously.16,17 Briefly, the PHS was a randomized, double-blind, placebo-controlled trial that began in 1982 to evaluate the efficacy of low-dose aspirin and beta carotene according to a two-by-two factorial design among 22,071 US male physicians, 40 to 84 years of age, in the primary prevention of cardiovascular disease and cancer. At baseline, physicians with personal history of myocardial infarction, stroke, transient ischemic attack, cancer (except nonmelanoma skin cancer), liver or renal disease, peptic ulcer, gout, contraindications to aspirin consumption, and current use of aspirin, or other platelet active drugs, or nonsteroidal anti-inflammatory agents, or a vitamin A supplement were excluded. For the present investigation, we excluded 977 participants (4.4%) with missing baseline information on height, weight, or physical activity (248 participants), missing information on other covariates (712 participants), and HF prior to baseline examination (17 participants). After these exclusions, 21,094 participants comprised our baseline population. All participants provided written informed consent for enrollment in the Physicians’ Health Study. The institutional review board at the Brigham and Women’s Hospital (Boston, Mass), approved the research protocol for the present investigation.
Data collection
Baseline information on self-reported demographic, medical history, and lifestyle variables was collected using a mailed questionnaire in 1982. Every 6 months for the first year and annually thereafter, participants provided information on compliance with randomized treatment assignments, various risk factors for chronic diseases, and newly diagnosed conditions in follow-up questionnaires. After the termination of the randomized aspirin16 and beta-carotene17 components of the trial, participants continued to provide information on risk factors and relevant outcomes in yearly mailed questionnaires.
Exposures and covariates
On the baseline questionnaire, each physician reported his weight (in pounds) and height (in inches). The weight in kilograms was divided by the square of the height in meters to calculate BMI.1,2 The level of physical activity was ascertained at baseline using the single question: “How often do you exercise vigorously enough to work up a sweat?” Response options were rarely/never, 1 to 3 times a month, 1 time a week, 2 to 4 times a week, 5 to 6 times a week, and daily. Each physician also reported his age, parental history of myocardial infarction, cigarette smoking, frequency of alcohol consumption, and history of hypertension, diabetes mellitus, and hypercholesterolemia. On follow-up questionnaires, physicians reported the occurrence of myocardial infarction, which was confirmed on review of medical records by the PHS Endpoints Committee using the World Health Organization Criteria.18
Outcome ascertainment
A diagnosis of HF was self-reported by physician participants on the yearly follow-up questionnaires. To validate the diagnosis of HF using established epidemiologic criteria, we randomly selected 100 participants with a recent self-reported diagnosis of HF. Of these 100 participants, 8 had previously indicated that they not be contacted for additional information, and 4 had died. The remaining 88 participants were mailed a questionnaire to collect information on symptoms, signs, and laboratory investigations at the time of first diagnosis of HF and list of current medications for the treatment of HF. After two mailings followed by telephone contact, we collected and reviewed data from 76 of 88 (86%) to verify the diagnosis of HF. Among these 76 responders, 68 (89%) were on current treatment for HF and/or met the Framingham Heart Study criteria for HF at the time of first diagnosis.19 This extent of confirmation paralleled that reported by other investigators applying epidemiologic criteria to the validation of the physician-determined diagnosis of HF.20,21
Statistical analysis
We performed all analyses using SAS software version 9.1.3 (SAS Institute, Cary, NC). We computed means (±standard deviations [SD]) for continuous variables and proportions (expressed as percentage) for categorical variables according to various categories of BMI and levels of vigorous physical activity. We constructed cumulative incidence curves for HF according to exposure categories using the Kaplan-Meier estimation method.
We used Cox-proportional hazards regression models to examine the effects of BMI and vigorous physical activity on the risk of HF. We expressed these results as hazard ratios, 95% confidence intervals (CIs), and p-values. We considered a two-sided p-value of <0.05 as statistically significant. To examine the individual effects of exposure variables on the risk of HF, we considered BMI as both a continuous variable (per increment of 1 kg/m2) and a categorical variable (lean <25.0 [referent], overweight 25.0 to 29.9, and obese ≥30 kg/m2);1,2 and categorized vigorous physical activity as both a dichotomous variable (inactive [rarely or never active, referent], and active [any vigorous activity ≥1 to 3 times a month]) and a 4-category variable (rarely/never [referent], 1–3 times a month, 1–4 times a week, and 5–7 times a week). To evaluate the combined effects of BMI and vigorous physical activity on the risk of HF, we used the aforementioned three categories of BMI (lean, overweight, and obese) and two levels of vigorous physical activity (inactive and active) and constructed six groups: lean and active [referent], lean and inactive, overweight and active, overweight and inactive, obese and active, and obese and inactive. Of note, because there were very few participants in the underweight category (n=46, BMI <18.5 kg/m2),1,2 we combined this category with normal group (n=12,087; body mass index 18.5 to <25.0 kg/m2) 1,2 and classified them as lean group.
In multivariable analyses evaluating BMI and the risk of HF, we adjusted for (a) age only, (b) age, cigarette smoking (never [referent], past only, and current), alcohol consumption (rarely/never, monthly, weekly, and daily [referent]), parental history of myocardial infarction and random assignment to aspirin or beta-carotene (baseline variables not likely in the causal pathway), (c) the aforementioned baseline covariates and vigorous physical activity (rarely/never [referent] vs. ≥1 to 3 times a month), (d) all above covariates and presence or absence of history of hypertension, diabetes mellitus, and hypercholesterolemia (baseline variables likely on the causal pathway), and (e) all of the above baseline variables plus myocardial infarction (likely on the causal pathway) during follow-up as a time-dependent variable. We considered variables as likely or not likely in the causal pathway on the basis of prior epidemiologic evidence and biological plausibility.22 We used the same model-building approach in the evaluation of physical activity and the risk of HF (except that BMI was considered as a variable possibly in the causal pathway between vigorous physical activity and the risk of HF). We constructed trend models to assess for a gradient of risk across multicategory exposure variables for the risk of HF.
In multivariable models including all baseline covariates, we examined for effect modification between baseline covariates and BMI (per 1 kg/m2 increment), and between baseline covariates and vigorous physical activity (inactive vs. any vigorous activity) on the risk of HF by introducing appropriate interaction terms. In the presence of statistically significant interactions (defined as p-value <0.05), we conducted stratified analyses according to various levels of the baseline covariate.
Of note, in statistical models adjusting for history of hypercholesterolemia, we introduced a dummy variable for missing information on this variable that made up about 12% of study participants. Finally, in secondary analyses, to account for the competing risk of death that may alter the probability of experiencing HF (the event of interest), we evaluated the individual effects of BMI and vigorous physical activity on the risk of composite of death or HF.
Statement of Responsibility
Dr. Kenchaiah had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and agree to the manuscript as written.
RESULTS
Baseline characteristics
About 40% of physicians in our sample were overweight and approximately 5% were obese at baseline. As anticipated, a greater proportion of obese individuals exercised less and had a history of hypertension and diabetes mellitus (Table 1). However, a marginally greater proportion of obese were current smokers. Physicians who rarely or never exercised vigorously were older, had higher BMI, smoked cigarettes more often, and had a greater prevalence of hypertension and diabetes mellitus. By design, randomized aspirin and beta-carotene treatment was similar across all categories of BMI and physical activity.
Table 1.
Baseline Characteristics According to Body Mass Index and Vigorous Physical Activity Categories in the Physicians’ Health Study*
| Body-mass index categories | Vigorous physical activity categories | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Lean (n=12133) | Overweight (n=8032) | Obese (n=929) | p-value | Inactive (n=2841) | Active (n=18253) | p-value |
| Age (years) | 52.9 ± 9.7 | 53.6 ± 9.1 | 52.8 ± 8.8 | <0.001 | 55.7 ± 10.0 | 52.7 ± 9.3 | <0.0001 |
| Body mass index (kg/m2) | 23.0 ± 1.3 | 26.6 ± 1.3 | 32.4 ± 2.8 | <0.0001 | 25.2 ± 3.2 | 24.7 ± 2.7 | <0.0001 |
| Lean (%) | 100 | – | – | 51.6 | 58.4 | ||
| Overweight (%) | – | 100 | – | 42.1 | 37.4 | ||
| Obese (%) | – | – | 100 | 6.3 | 4.1 | ||
| Vigorous physical activity (%) | <0.0001 | ||||||
| Inactive (rarely/never) | 12.1 | 14.9 | 19.3 | 100 | – | ||
| Low active (1-3/month) | 12.6 | 16.1 | 18.3 | – | 16.4 | ||
| Medium active (1-4/week) | 56.0 | 56.3 | 53.0 | – | 64.7 | ||
| High active (5-7/week) | 19.3 | 12.7 | 9.5 | – | 18.9 | ||
| Parental history of myocardial infarction | 9.0 | 9.6 | 10.5 | 0.12 | 8.2 | 9.4 | 0.025 |
| Cigarette smokers (%) | <0.0001 | <0.0001 | |||||
| No | 51.7 | 47.1 | 42.8 | 45.1 | 50.2 | ||
| Past | 38.1 | 41.0 | 43.5 | 39.7 | 39.4 | ||
| Current | 10.2 | 11.9 | 13.7 | 15.1 | 10.4 | ||
| Alcohol consumption (%) | <0.0001 | <0.0001 | |||||
| Rarely | 14.4 | 15.2 | 19.5 | 19.8 | 14.1 | ||
| Monthly | 10.7 | 11.2 | 15.8 | 14.1 | 10.7 | ||
| Weekly | 49.1 | 49.5 | 46.6 | 40.1 | 50.5 | ||
| Daily | 25.8 | 24.1 | 18.1 | 26.1 | 24.6 | ||
| History of hypertension (%) | 18.9 | 28.8 | 42.4 | <0.0001 | 29.8 | 22.8 | <0.0001 |
| Blood pressure (mm Hg) | |||||||
| Systolic | 124.3 ± 11.4 | 128.1 ± 11.7 | 132.4 ± 12.8 | <0.0001 | 127.8 ± 12.5 | 125.8 ± 11.7 | <0.0001 |
| Diastolic | 77.7 ± 7.4 | 80.0 ± 7.3 | 82.7 ± 7.9 | <0.0001 | 79.7 ± 7.6 | 78.7 ± 7.5 | <0.0001 |
| History of diabetes mellitus (%) | 2.2 | 3.2 | 6.7 | <0.0001 | 5.8 | 2.3 | <0.0001 |
| History of hypercholesterolemia (%) | |||||||
| Present | 9.8 | 11.6 | 10.9 | <0.001 | 11.5 | 10.4 | 0.066 |
| Missing | 11.4 | 12.2 | 14.7 | 11.0 | 12.0 | ||
| Study medications | |||||||
| Aspirin (%) | 50.3 | 49.5 | 49.7 | 0.52 | 49.5 | 50.0 | 0.57 |
| Beta-carotene | 49.9 | 50.0 | 51.9 | 0.50 | 49.2 | 50.2 | 0.39 |
Body mass index is weight in kilograms (kg) divided by the square of the height in meters (m2). The body mass index was <25 kg/m2 in lean participants, 25 to 29.9 kg/m2 overweight participants, and ≥30 kg/m2 in obese participants. Data on blood pressure were available for 10755 participants in the lean group, 7124 participants in the overweight group, 818 participants in the obese group, 2457 participants in the inactive group, and 16240 participants in the active group. Plus – minus values are means ± standard deviation.
BMI and the risk of heart failure
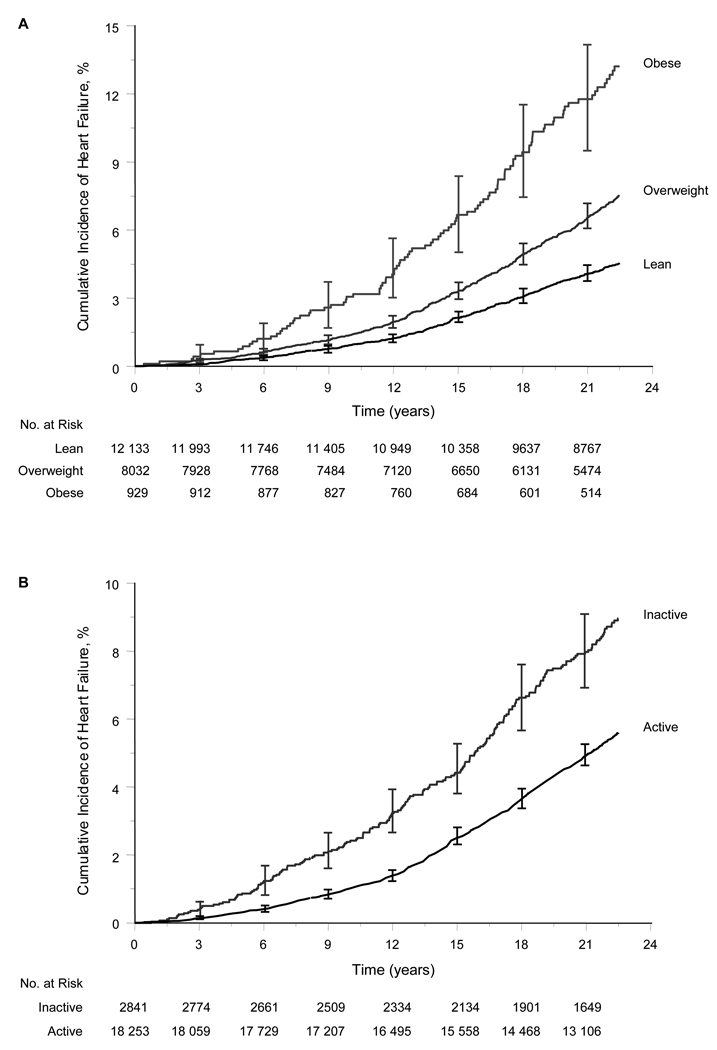
During a mean ± SD follow-up of 20.5 ± 5.4 years (maximum, 24.8 years; 431,654 total person-years), 1109 men developed new-onset HF. The cumulative incidence of HF increased with increasing categories of BMI (Figure 1A).
Figure 1. Cumulative Incidence of Heart Failure According to Categories of Body-mass Index (Panel A) and Vigorous Physical Activity (Panel B) at the Base-line Examination.
Body mass index is weight in kilograms (kg) divided by the square of the height in meters (m2). The body mass index was <25 kg/m2 in lean participants, 25 to 29.9 kg/m2 in overweight participants, and ≥30 kg/m2 in obese participants. Active participants vigorously exercised for at least 1 to 3 times a month and inactive participants rarely/never exercised vigorously. Vertical bars represent 95% confidence intervals for the cumulative incidence.
In models adjusting for variables not likely in the causal pathway between BMI and HF (age, smoking, alcohol consumption, parental history of myocardial infarction), each 1 kg/m2 increase in BMI was associated with a 13% increase in the risk of HF (Table 2). Compared with lean men, overweight men showed a 62%-increased risk of HF and obese men a 240%-increased risk. Increasing categories of BMI were associated with a stepwise increase in the risk of HF (p for trend <0.0001). Including vigorous physical activity as a covariate in multivariable analyses did not materially alter the excess risk of HF associated with elevated BMI. Additional adjustment for baseline variables possibly in the causal pathway between elevated BMI and HF (hypertension, diabetes mellitus, and hypercholesterolemia) resulted in decline of the hazard ratio from 1.62 to 1.49 in overweight men and from 3.38 to 2.80 in obese men, thus explaining approximately 21.0% and 24.4% of the excess risk of HF in overweight and obese men respectively.
Table 2.
Results of Cox Proportional Hazards Regression Models Evaluating the Association of Body Mass Index and Vigorous Physical Activity to the Risk of Heart Failure
| No. of events/No. at risk (%) | Follow-Up, Person-y (Rate per 10 000 Person-y) | Hazard ratio (95% confidence interval) |
||||
|---|---|---|---|---|---|---|
| Age-adjusted | Multivariable-adjusted* | Multivariable-adjusted† | Multivariable-adjusted‡ | |||
| I. Body mass index (kg/m2) | ||||||
| A. As continuous variable | ||||||
| Per unit increment | 1109/21094 (5.3) | 431,654 (25.7) | 1.14 (1.12–1.16) | 1.13 (1.11–1.15) | 1.13 (1.11–1.15) | 1.11 (1.09–1.13) |
| B. As categorical variable | ||||||
| Lean (<25) | 484/12133 (4.0) | 251,471 (19.2) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Overweight (25 to 29.9) | 528/8032 (6.6) | 162,773 (32.4) | 1.65 (1.46–1.87) | 1.62 (1.43–1.83) | 1.61 (1.42–1.82) | 1.49 (1.32–1.69) |
| Obese (≥30) | 97/929 (10.4) | 17,411 (55.7) | 3.54 (2.85–4.41) | 3.38 (2.71–4.21) | 3.35 (2.69–4.17) | 2.80 (2.24–3.50) |
| P for trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| II. Vigorous Physical Activity | ||||||
| A. As dichotomous variable | ||||||
| Inactive (rarely/never) | 206/2841 (7.3) | 53,713 (38.4) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Active (≥1 to 3 times a month) | 903/18253 (4.9) | 377,941 (23.9) | 0.74 (0.63–0.86) | 0.76 (0.66–0.89) | 0.79 (0.67–0.92) | 0.82 (0.70–0.96) |
| B. As 4-category variable | ||||||
| Rarely/never | 206/2841 (7.3) | 53,713 (38.4) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| 1 to 3 times a month | 145/2991 (4.8) | 62,190 (23.3) | 0.76 (0.61–0.94) | 0.77 (0.62–0.96) | 0.76 (0.62–0.94) | 0.78 (0.63–0.97) |
| 1 to 4 times a week | 610/11813 (5.2) | 245,208 (24.9) | 0.78 (0.66–0.91) | 0.80 (0.68–0.94) | 0.82 (0.70–0.96) | 0.86 (0.73–1.01) |
| 5 to 7 times a week | 148/3449 (4.3) | 70,543 (21.0) | 0.60 (0.49–0.74) | 0.64 (0.52–0.79) | 0.69 (0.56–0.85) | 0.73 (0.59–0.90) |
| P for trend | <0.0001 | <0.001 | 0.003 | 0.016 | ||
Adjusted for age, cigarette smoking (never [referent], past only, and current), alcohol consumption (rarely/never, monthly, weekly, and daily [referent]), parental history of myocardial infarction, and random assignment to aspirin or beta-carotene in all models (baseline covariates not likely in the causal pathway).
Adjusted for aforementioned baseline covariates and vigorous physical activity (rarely/never [referent], and ≥1-3 times a month) in models IA and IB and body-mass index (lean [referent], overweight, and obese) in models IIA and IIB.
Adjusted for all above baseline covariates and presence or absence of history of hypertension, diabetes mellitus, and hypercholesterolemia (baseline covariates likely in the causal pathway)
During follow-up, 764 (6.3%) in the lean, 702 (8.7%) in the overweight, and 117 (12.6%) in the obese group developed myocardial infarction. Interim myocardial infarction preceded the onset of HF in 77 (15.9%), 87 (16.5%), and 14 (14.4%) of lean, overweight, and obese participants with HF, respectively. The association between BMI and HF remained robust after adjustment for all baseline covariates and interim myocardial infarction as a time-dependent variable. In these models, every 1kg/m2 increment in BMI was associated with a hazard ratio of 1.11 (95% CI 1.09 to 1.12). Compared with lean participants, the hazard ratios were 1.46 (95% CI 1.29 to 1.66) and 2.65 (95% CI 2.12 to 3.31) in overweight and obese participants, respectively.
Vigorous physical activity and the risk of heart failure
Compared with participants in the inactive group, those in the active group had a lower cumulative incidence of HF (Figure 1, Panel B). The divergence of these curves was apparent within 5 years of follow-up.
In models adjusting for lifestyle and other variables not likely in the causal pathway, vigorous physical activity of at least 1 to 3 times a month was associated with a significant 26% reduction in the risk of HF (Table 2). Compared with men who rarely or never vigorously exercised, there was a 23%, 20%, and 36% reduction in the risk of HF among men who vigorously exercised for 1 to 3 times a month, 1 to 4 times a week, and 5 to 7 times a week respectively (P for trend across categories, <0.001). Inclusion of BMI as an additional covariate in multivariable analyses resulted in a minor change in the effect of vigorous physical activity on the risk of HF. After adjustment for known baseline variables presumably in the causal pathway (BMI, hypertension, diabetes mellitus, and hypercholesterolemia), the risk reduction of HF conferred by vigorous physical activity changed from 24% to 18%, thereby accounting for about 25% of the decreased risk. In these analyses, a statistically significant trend toward decreasing HF risk was found with increasing levels of vigorous physical activity (p for trend 0.016).
During follow-up, interim myocardial infarction occurred in a greater proportion of inactive (278 events, 9.8%) compared with active (1305 events, 7.2%) participants. Myocardial infarction preceded the onset of HF among similar proportion of inactive (47 events, 22.8%) and active (206 events, 22.8%) participants who developed HF. In models adjusting for all covariates at baseline and myocardial infarction during follow-up as a time-dependent variable, the association between vigorous physical activity and HF remained significant. As compared with inactive participants, the hazard ratio for incident HF among active participants was 0.85 (95% CI 0.73 to 0.99).
Combined effect of BMI and vigorous physical activity on the risk of heart failure
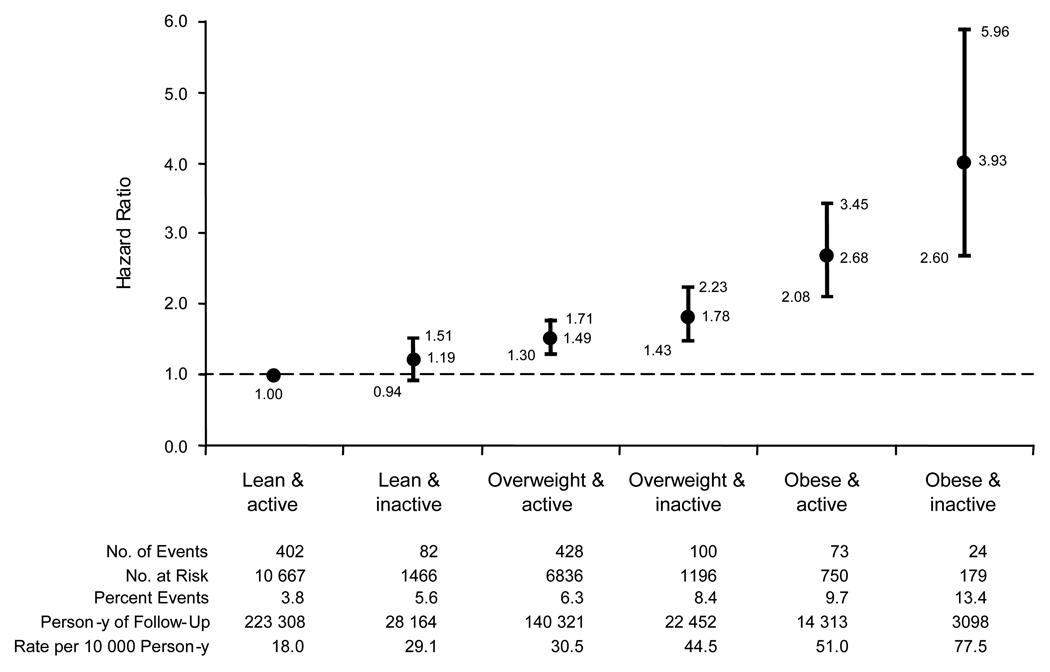
In models evaluating BMI as a continuous variable (per increment of 1 kg/m2) and vigorous physical activity as a dichotomous variable (inactive vs. active), no significant interaction (p=0.96) between BMI and vigorous physical activity with the risk of HF. After adjustment for all baseline covariates, compared with participants who were lean and active, the risk of HF was found increased progressively by 19% (albeit statistical nonsignificant) in the lean and inactive, 49% in the overweight and active, 78% in the overweight and inactive, 168% in the obese and active, and 293% in the obese and inactive groups (Figure 2).
Figure 2. Risk of Heart Failure According to Combined Categories of Body-mass Index and Vigorous Physical Activity at the Base-Line Examination.
Vertical bars represent the 95 percent confidence intervals for the hazard ratios. The referent group includes men with a BMI lower than 25 and who vigorously exercised at least 1 to 3 times a month. Hazard ratios were adjusted for age, cigarette smoking (never [referent], past only, current), alcohol consumption (rarely/never, monthly, weekly, daily [referent]), and presence or absence of parental history of myocardial infarction, history of hypertension, diabetes mellitus, and hypercholesterolemia, and random assignment to aspirin or beta-carotene at base-line.
Effect modification by other baseline covariates
Elevated BMI was associated with a relatively greater risk of HF among younger compared with older participants (p for interaction =0.020) and among nondiabetic as compared with diabetic participants (p for interaction <0.0001) (Table 3). Other baseline covariates inclusive of parental history of myocardial infarction, cigarette smoking, alcohol consumption, history of hypertension, history of hypercholesterolemia, or aspirin intake did not alter the effect of BMI to the risk of HF (all p for interaction >0.40).
Table 3.
Results of Cox Proportional Hazards Regression Models Evaluating the Association of Body Mass Index and Vigorous Physical Activity to the Risk of Heart Failure Stratified According to Various Levels of Baseline Covariates
| No. of events/No. at risk (%) | Follow-Up, Person-y (Rate per 10000 Person-y) | Multivariable Hazardratio (95% CI)* | P for Interaction | |
|---|---|---|---|---|
| I. Body mass index as a continuous variable (per 1 kg/m2 increment) | ||||
| A. Age | ||||
| <50 years | 126/8704 (1.4) | 194,427 (6.5) | 1.15 (1.10–1.20) | 0.020 |
| 50–59.9 years | 405/7147 (5.7) | 150,136 (27.0) | 1.09 (1.06–1.12) | |
| ≥60 years | 578/5243 (11.0) | 87,271 (66.2) | 1.09 (1.06–1.12) | |
| B. Diabetes mellitus | <0.0001 | |||
| No | 1012/20,505 (4.9) | 422,477 (24.0) | 1.11 (1.09–1.13) | |
| Yes | 97/589 (16.5) | 9178 (105.7) | 1.07 (1.02–1.12) | |
| II. Vigorous physical activity as a dichotomous variable | ||||
| A. Diabetes Mellitus | <0.001 | |||
| No | ||||
| Inactive (rarely/never) | 183/2675 (6.8) | 51,334 (35.6) | 1.00 (referent) | |
| Active (≥1 to 3 times a month) | 829/17,830 (4.6) | 371,143 (22.3) | 0.80 (0.68–0.94) | |
| Yes | ||||
| Inactive (rarely/never) | 23/166 (13.9) | 2380 (96.6) | 1.00 (referent) | |
| Active (≥1 to 3 times a month) | 74/423 (17.5) | 6798 (108.9) | 1.02 (0.63–1.64) | |
Adjusted for cigarette smoking (never [referent], past only, and current), alcohol consumption (rarely/never, monthly, weekly, and daily [referent]), parental history of myocardial infarction, random assignment to aspirin or beta-carotene, and presence or absence of history of hypertension, and hypercholesterolemia in all models, and additionally for diabetes mellitus and vigorous physical activity (rarely/never [referent], and ≥1-3 times a month) in model IA, age (per year increment) and vigorous physical activity in model IB, and age and body-mass index (per 1 kg/m2) in model IIA.
Vigorous physical activity conferred a reduction in the risk of HF among non-diabetic but not among diabetic participants (p for interaction <0.001) (Table 3). None of the other baseline covariates influenced the relation between vigorous physical activity and incident HF (all p for interaction ≥0.10).
Secondary analyses
During follow-up, 3159 (26.0%) in the lean group, 2473 (30.8%) in the overweight group, and 399 (42.9%) in the obese group died or developed HF. In analyses adjusting for all baseline covariates, each increment of 1 kg/m2 in BMI was associated with a hazard ratio of 1.06 (95% CI 1.05 to 1.06) to the risk of death or HF. Compared with participants in the lean group, hazard ratios were 1.12 (95% CI 1.06 to 1.18) for overweight participants and 1.97 (95% CI 1.77 to 2.19) for obese participants (p for trend <0.0001).
The composite outcome of death or HF occurred in a lesser proportion of active men (4897 events, 26.8%) as compared with inactive men (1134 events, 39.9%). In multivariable analyses adjusting for all baseline covariates, vigorous physical activity was associated with a lower risk of death or HF (hazard ratio 0.81, 95% CI 0.76 to 0.87). In analyses of vigorous physical activity as a 4-level categorical variable, the hazard ratios were 0.80 (95% CI 0.73–0.88) for 1 to 3 times a month, 0.81 (95% CI 0.76 to 0.87) for 1 to 4 times a week, and 0.84 (95% CI 0.77 to 0.91) for 5 to 7 times a week of vigorous physical activity (p for trend <0.0001).
DISCUSSION
Principal findings
In men, we found that higher BMI was associated with a greater risk of HF. This increased risk occurred in a linear fashion without evidence of a threshold and was evident not only in obese (BMI ≥30 kg/m2) but also in overweight or preobese (BMI 25 to 29.9 kg/m2) men. Vigorous physical activity was associated with a reduced risk of HF, and increasing levels of vigorous physical activity were associated with a graded reduction in the risk of HF. BMI and vigorous physical activity did not modify each other's individual effect on HF risk. Lean and active individuals had the lowest risk of HF; obese and inactive individuals had the highest risk. The statistically significant association of elevated BMI with an increased risk of the composite of death or HF and vigorous physical activity with a decreased risk of this combined outcome suggests that death as a competing event is an unlikely reason for the observed association between BMI and vigorous physical activity with the risk of HF.
Elevated BMI was associated with a greater risk of HF in all subgroups, although its effect was stronger in younger as compared with older participants and in non-diabetic as compared with diabetic participants. The beneficial impact of vigorous physical activity was evident in all categories of baseline covariates except among diabetics, where no association was noted, likely because of the small sample size of this subgroup.
Comparison with previous studies
The increased risk of HF among obese individuals noted in our investigation is consistent with that found in previously published studies.7–11,13 However, statistically non-significant association between overweight but not obese (preobese) status and the risk of HF has been reported among men in a nested case-control analyses from United Kingdom-based General Practice Research Database (589 cases vs. about 2500 age-matched controls, relative risk 0.9, and 95% CI 0.7 to 1.3)9 and a prospective cohort analyses from the Framingham Heart Study (2704 men, 252 events, mean follow-up 14 years, hazard ratio 1.20, and 95% CI 0.87 to 1.64).11 The observed non-significant association may have been due to the smaller number of men and HF endpoints evaluated in these studies. In the present investigation, we noted a statistically significant 49% increase in the risk of HF among overweight men, suggesting a continuous gradient of HF risk across increasing categories of BMI (lean to overweight to obese).
In multivariable analyses of 5545 men in the First National Health and Nutrition Examination Survey (NHANES I) Epidemiologic Follow-up Study, compared with low levels of recreational physical activity (determined using the question: “Do you get much exercise in things you do for recreation [sports, or hiking, or anything like that], or hardly any exercise, or in between?”), medium to high levels of recreational physical activity were associated with a statistically nonsignificant 12% (95% CI −6 to 28%) decline in the risk of HF.8 In contrast, in our cohort of 21,094 men, vigorous physical activity (defined as exercise to the point of breaking a sweat) for at least 1 to 3 times a month conferred a significant 18% reduction in the risk of HF. The larger sample size of men investigated in our study and difference in the definition of physical activity may partially explain the reason for demonstrating a significant association between vigorous physical activity and the reduced risk of HF in our investigation.
Mechanisms
Several mechanisms by which elevated BMI increases the risk of HF have been proposed. These include promotion of atherogenic risk factors such as hypertension,23,24 insulin resistance,25,26 diabetes mellitus,27–29 and dyslipidemia,1 that enhance the risk of myocardial infarction.30,31 These factors may mediate or independently increase the risk of HF.7,8,10,12,30,32 Excess weight may alter cardiac structure and function,33–35 activate neuroendocrine pathways,36–38 predispose to sleep-disordered breathing,39–41 and promote chronic kidney disease42–45 that may subsequently manifest as overt HF.32 Increased BMI is an anthropometric surrogate of surplus total body fat,46 and evidence from animal models suggest direct myocardial lipotoxicity in the pathogenesis of cardiomyopathy.47–49
Greater levels of physical activity promote weight loss,50 improve lipoprotein profile,51 and reduce the risk of hypertension,52 diabetes mellitus,53,54 and coronary artery disease.55,56 These favorable influences on cardiovascular risk profile may, in turn, reduce the likelihood of HF.
Study strengths and limitations
The large sample size, prospective nature of analyses, long duration of follow-up, adequate adjustment for confounding variables (including interim myocardial infarction), and evaluation of composite of HF and all-cause death as an endpoint to account for competing risk of death are particular strengths of our study. Several limitations are also relevant. First, although the mean age of PHS participants were similar to other published cohorts from the general population,7–11 the incidence rate of HF was lower in the PHS. This is likely explained by the fact that the PHS is made up of physicians who were generally healthier in terms of coronary risk factors, presumably had better access to healthcare, and as a group belonged to a higher socioeconomic status. Second, measures of weight and height and frequency of vigorous physical activity were self-reported. This is likely to result in modest nondifferential misclassification that may underestimate the true hazard ratios of HF. Third, the small sample size (n=46) precluded evaluation of the impact of underweight status (BMI <18.5 kg/m2) on the risk of HF. Fourth, our study assessed the impact of vigorous physical activity, and its frequency was ascertained at one point in time. The intensity and frequency of activity may change over time, which conceivably underestimates the magnitude of the true association between vigorous physical activity and HF. Fifth, data on left ventricular function at the time of onset of HF were not available to assess the influence of excess weight and vigorous physical activity to the risk of HF with preserved versus impaired left ventricular ejection fraction. Finally, our study population consisted of men, and the results of our investigation cannot be generalized to women.
Future Implications
It is intriguing to note that exercise to the point of breaking a sweat even at frequency as low as 1 to 3 times a month was associated with a reduced risk of HF. This finding may imply that any vigorous physical activity is an indicator of healthier lifestyle in men at risk of developing HF. Whether specific domains (at work, for transport, in domestic duties, or in leisure time),3 modes (aerobic vs. muscle-strengthening, and intentional vs. usual activities), types (e.g., walking, jogging, swimming, bicycling, or stair-climbing), duration (short vs. long bouts of activity), or amount (total energy expenditure)15 of physical activity have different degrees of beneficial impact on the risk of HF needs further evaluation.
Our findings indicate that both overweight (preobese) and obese men have an increased risk of HF, and men with higher levels of vigorous physical activity have a reduced risk. Additional research is warranted to ascertain whether intentional weight reduction to optimal levels in overweight and obese individuals, together with improved physical activity would lessen the probability of HF. Meanwhile, on the basis of published evidence7–11 inclusive of prospective cohort data from the Framingham Heart study11 and the present investigation from the PHS that demonstrate significantly increased risk of HF in overweight (preobese) women and men respectively, perhaps a BMI of <25 kg/m2 represents an optimal goal for the primary prevention of HF. Clinical trials targeting improvements in BMI and physical activity levels will be critically important in determining whether our observational data bear clinical relevance.
In the United States, approximately 37% are overweight (preobese), 25% are obese, 38% do not achieve the recommended amount of physical activity, and 14% are inactive.4 Recent global estimates also reveal comparable high prevalence of excess weight and physical inactivity.5 Concurrently, HF continues to impose substantial morbidity, mortality, and financial costs.6 Hence, public health approaches to curtail excess weight, to maintain optimal weight, and to promote regular physical activity have the potential to limit the scourge of HF.
Acknowledgements
We thank the staff at the Physicians’ Health Study for data management.
Funding/Support:
The study was supported in part by grants HL-26490 and HL-34595 from the National Heart, Lung and Blood Institute, Bethesda, Md; and grants CA-34944, CA-40360, and CA-097193 from the National Cancer Institute, Bethesda, Maryland.
Role of Sponsor:
The sponsoring organizations played no role in the design and conduct of the study; in the collection, management, analyses, and interpretation of data; or in the preparation, review, and approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is an un-copyedited author manuscript that was accepted for publication in Circulation, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the "Fair Use of Copyrighted Materials" (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at http://circ.ahajournals.org. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
Conflict of Interest Disclosures:
None
REFERENCES
- 1.National Institutes of Health; Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. 1998 Sep; Report No.: NIH Publication No. 98–4083. [PubMed]
- 2.Obesity: Preventing and Managing the Global Epidemic. Geneva, Switzerland: World Health Organization; WHO Technical Report. 2000 [PubMed]
- 3.Geneva, Switzerland: The World Health Report 2002: Reducing risks, promoting healthy life. 2002 doi: 10.1080/1357628031000116808. [DOI] [PubMed]
- 4. [Accessed October 15, 2008];Department of Health and Human Services, Behavioral Risk Factor Surveillance System, Centers for Disease Control and Prevention; Atlanta, Ga: United States overweight and obesity prevalence estimates for 2006 and physical activity prevalence estimates for 2007. Available at http://apps.nccd.cdc.gov/brfss/list.asp?cat=OB&yr=2006&qkey=4409&state=All and http://apps.nccd.cdc.gov/PASurveillance/StateSumV.asp.
- 5. [Accessed October 15, 2008];World Health Organization; Geneva, Switzerland: Global overweight and obesity estimates for 2005 and physical inactivity: a global public health problem. Available at http://www.who.int/mediacentre/factsheets/fs311/en/print.html and http://www.who.int/dietphysicalactivity/factsheet_inactivity/en/print.html.
- 6.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 7.Chen YT, Vaccarino V, Williams CS, Butler J, Berkman LF, Krumholz HM. Risk factors for heart failure in the elderly: a prospective community- based study. Am J Med. 1999;106:605–612. doi: 10.1016/s0002-9343(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 8.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 9.Johansson S, Wallander MA, Ruigomez A, Garcia Rodriguez LA. Incidence of newly diagnosed heart failure in UK general practice. Eur J Heart Fail. 2001;3:225–231. doi: 10.1016/s1388-9842(00)00131-8. [DOI] [PubMed] [Google Scholar]
- 10.Wilhelmsen L, Rosengren A, Eriksson H, Lappas G. Heart failure in the general population of men--morbidity, risk factors and prognosis. J Intern Med. 2001;249:253–261. doi: 10.1046/j.1365-2796.2001.00801.x. [DOI] [PubMed] [Google Scholar]
- 11.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 12.Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294:334–341. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 13.Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E, Szklo M, Lima JA. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51:1775–1783. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 14.Heart Failure Society Of America. HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006;12:e1–e2. doi: 10.1016/j.cardfail.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 16.Steering Committee of the Physicians' Health Study Research Group. Final report on the aspirin component of the ongoing Physicians' Health Study. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 17.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W, Peto R. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 18.Copenhagen, Denmark: World Health Organization; World Health Organization. IHD registers: report of the fifth working group. 1971
- 19.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 20.Mosterd A, Deckers JW, Hoes AW, Nederpel A, Smeets A, Linker DT, Grobbee DE. Classification of heart failure in population based research: an assessment of six heart failure scores. Eur J Epidemiol. 1997;13:491–502. doi: 10.1023/a:1007383914444. [DOI] [PubMed] [Google Scholar]
- 21.Einhorn PT, Davis BR, Massie BM, Cushman WC, Piller LB, Simpson LM, Levy D, Nwachuku CE, Black HR. The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Heart Failure Validation Study: diagnosis and prognosis. Am Heart J. 2007;153:42–53. doi: 10.1016/j.ahj.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 22.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stamler R, Stamler J, Riedlinger WF, Algera G, Roberts RH. Weight and blood pressure. Findings in hypertension screening of 1 million Americans. JAMA. 1978;240:1607–1610. doi: 10.1001/jama.240.15.1607. [DOI] [PubMed] [Google Scholar]
- 24.Stamler J. Epidemiologic findings on body mass and blood pressure in adults. Ann Epidemiol. 1991;1:347–362. doi: 10.1016/1047-2797(91)90045-e. [DOI] [PubMed] [Google Scholar]
- 25.Abbasi F, Brown J, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. Journal of the American College of Cardiology. 2002;40:937–943. doi: 10.1016/s0735-1097(02)02051-x. [DOI] [PubMed] [Google Scholar]
- 26.Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. Journal of Clinical Investigation. 1997;100:1166–1173. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colditz GA, Willett WC, Stampfer MJ, Manson JE, Hennekens CH, Arky RA, Speizer FE. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol. 1990;132:501–513. doi: 10.1093/oxfordjournals.aje.a115686. [DOI] [PubMed] [Google Scholar]
- 28.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–969. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- 29.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 30.Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care. 1979;2:120–126. doi: 10.2337/diacare.2.2.120. [DOI] [PubMed] [Google Scholar]
- 31.Manson JE, Colditz GA, Stampfer MJ, Willett WC, Rosner B, Monson RR, Speizer FE, Hennekens CH. A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med. 1990;322:882–889. doi: 10.1056/NEJM199003293221303. [DOI] [PubMed] [Google Scholar]
- 32.Kenchaiah S, Gaziano JM, Vasan RS. Impact of obesity on the risk of heart failure, and its influence on survival after the onset of heart failure. Med Clin North Am. 2004;88:1273–1294. doi: 10.1016/j.mcna.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA. 1991;266:231–236. [PubMed] [Google Scholar]
- 34.Lauer MS, Anderson KM, Levy D. Separate and joint influences of obesity and mild hypertension on left ventricular mass and geometry: the Framingham Heart Study. J Am Coll Cardiol. 1992;19:130–134. doi: 10.1016/0735-1097(92)90063-s. [DOI] [PubMed] [Google Scholar]
- 35.de Simone G, Devereux RB, Roman MJ, Alderman MH, Laragh JH. Relation of obesity and gender to left ventricular hypertrophy in normotensive and hypertensive adults. Hypertension. 1994;23:600–606. doi: 10.1161/01.hyp.23.5.600. [DOI] [PubMed] [Google Scholar]
- 36.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 37.Lyon CJ, Law RE, Hsueh WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology. 2003;144:2195–2200. doi: 10.1210/en.2003-0285. [DOI] [PubMed] [Google Scholar]
- 38.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PW, D'Agostino RB. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 39.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 40.Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A. Sleep apnea and sleep disruption in obese patients. Arch Intern Med. 1994;154:1705–1711. [PubMed] [Google Scholar]
- 41.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier NF, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 42.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 43.Chae CU, Albert CM, Glynn RJ, Guralnik JM, Curhan GC. Mild renal insufficiency and risk of congestive heart failure in men and women > or =70 years of age. Am J Cardiol. 2003;92:682–686. doi: 10.1016/s0002-9149(03)00822-1. [DOI] [PubMed] [Google Scholar]
- 44.Fried LF, Shlipak MG, Crump C, Bleyer AJ, Gottdiener JS, Kronmal RA, Kuller LH, Newman AB. Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol. 2003;41:1364–1372. doi: 10.1016/s0735-1097(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 45.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 46.Bouchard C. BMI, fat mass, abdominal adiposity and visceral fat: where is the 'beef'? Int J Obes (Lond) 2007;31:1552–1553. doi: 10.1038/sj.ijo.0803653. [DOI] [PubMed] [Google Scholar]
- 47.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–822. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Philip-Couderc P, Smih F, Pelat M, Vidal C, Verwaerde P, Pathak A, Buys S, Galinier M, Senard JM, Rouet P. Cardiac transcriptome analysis in obesity-related hypertension. Hypertension. 2003;41:414–421. doi: 10.1161/01.HYP.0000057573.32425.95. [DOI] [PubMed] [Google Scholar]
- 50.Slentz CA, Duscha BD, Johnson JL, Ketchum K, Aiken LB, Samsa GP, Houmard JA, Bales CW, Kraus WE. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE--a randomized controlled study. Arch Intern Med. 2004;164:31–39. doi: 10.1001/archinte.164.1.31. [DOI] [PubMed] [Google Scholar]
- 51.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 52.Kokkinos PF, Narayan P, Colleran JA, Pittaras A, Notargiacomo A, Reda D, Papademetriou V. Effects of regular exercise on blood pressure and left ventricular hypertrophy in African-American men with severe hypertension. N Engl J Med. 1995;333:1462–1467. doi: 10.1056/NEJM199511303332204. [DOI] [PubMed] [Google Scholar]
- 53.Hu FB, Sigal RJ, Rich-Edwards JW, Colditz GA, Solomon CG, Willett WC, Speizer FE, Manson JE. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA. 1999;282:1433–1439. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- 54.Weinstein AR, Sesso HD, Lee IM, Cook NR, Manson JE, Buring JE, Gaziano JM. Relationship of physical activity vs body mass index with type 2 diabetes in women. JAMA. 2004;292:1188–1194. doi: 10.1001/jama.292.10.1188. [DOI] [PubMed] [Google Scholar]
- 55.Wannamethee SG, Shaper AG, Walker M. Changes in physical activity, mortality, and incidence of coronary heart disease in older men. Lancet. 1998;351:1603–1608. doi: 10.1016/S0140-6736(97)12355-8. [DOI] [PubMed] [Google Scholar]
- 56.Manson JE, Hu FB, Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Speizer FE, Hennekens CH. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med. 1999;341:650–658. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]