Abstract
Serine/threonine protein phosphatases (PPs) are important mediators of general cellular function as well as neurodegenerative processes. We have previously shown inhibition of PPs to be as neurotoxic as glutamate-induced neuronal death but resistant to neuroprotection by estrogens. In this study, the mechanism by which phosphatase inhibition via okadaic acid (OA) induce neurotoxicity is explored. Neurons were exposed to OA or glutamate in the presence or absence of various protein kinases inhibitors, and/or one of four estrogens. Both OA and glutamate induced cell death via increased reactive oxygen species (ROS), protein carbonylation, lipid peroxidation, caspase-3 activity, and mitochondrial dysfunction. All estrogens attenuated glutamate-mediated responses, but not OA-induced responses. In addition, inhibition of PKC and MAPK pathway was neuroprotective against glutamate but not OA toxicity. Interestingly, inhibition of MAPK pathway with PD98096 or U0126 caused a decrease in ROS production suggesting that activation of ERK1/2 could further exacerbate the oxidative stress caused by glutamate-induced toxicity; however, these inhibitors had no effect on OA-induced toxicity. Collectively, these results indicate that both glutamate and OA neurotoxicities are mediated by persistent activation of ERK1/2 and/or PKC and a resulting oxidative stress, and that protein phosphatase activity is an important and necessary aspect of estrogen-mediated neuroprotection.
Keywords: estradiol, estrogen analogues, okadaic acid, phosphatases, protein kinases, oxidative stress
Oxidative stress has been shown to cause protein oxidation, lipid peroxidation, DNA oxidation, and accumulation of reactive oxygen species (ROS), which are increased in a diverse group of neuropathological conditions such as Alzheimer's disease, stroke, and Parkinson's disease (Butterfield, 2006), suggesting a role for oxidative stress in the pathogenesis of these neurodegenerative diseases (Coyle and Puttfarcken, 1993; Mattson et al., 2001; Sultana et al., 2006). It has been demonstrated by various laboratories that dysfunction of protein phosphatases are evident during oxidative stress. For example, calcineurin activity was recently shown to be reduced in lymphocytes of amyotrophic lateral sclerosis patients via an oxidation-mediated mechanism (Ferri et al., 2004). Indeed, it has been known for some time that PP2A activity is reduced in the cortices of Alzheimer's patients as compared with control patients (Gong et al., 1995), and ERK has been found to be activated in these tissues (Pei et al., 2002). Tau is a well-described ERK1/2 target (Pei et al., 2003), and the hyperphosphorylation of tau protein and the development of neurofibrillary tangles in Alzheimer's disease pathology could reflect aberrant ERK1/2 activity (Pei et al., 2003). Interestingly, mice expressing a dominant negative form of PP2A in neurons display features of Alzheimer's pathology (Kins et al., 2003). Inactivation of PP1/PP2A via oxidative stress has been shown in vitro and in vivo to be involved in hyperphosphorylation of tau and prolonged phosphorylation of ERK 1/2 (Rahman et al., 2005; Poppek et al., 2006; Ho et al., 2007). Thus, it is intriguing to postulate that oxidative stress mediated PP1 and PP2A inhibition in Alzheimer's disease may account for enhanced ERK1/2 activity and subsequent tau hyperphosphorylation and neurofibrillary tangle formation.
Okadaic acid, a potent and non-selective inhibitor of serine/threonine phosphatases, has been shown to be cytotoxic in a variety of cell lines. Okadaic acid increases phosphorylation of microtubule associated protein and tau, which are concomitant with early changes in neuronal cytoskeleton that ultimately leads to cell death in primary cortical neurons and in neuroblastoma cell lines (Arias et al., 1993). In cerebellar granule cells, okadaic acid induces disintegration of neurites and swelling of cell bodies (Fernandez et al., 1991). Okadaic acid has also been shown to produce condensation of chromatin, reorganization of cytoskeleton, and DNA fragmentation characteristic of apoptosis (Boe et al., 1991; Fernandez-Sanchez et al., 1996). We have previously shown okadaic acid to induce neuronal death, and estrogens, which are known potent neuroprotectants, could not rescue these neurons (Yi et al., 2005). In the present study, we compared the mechanisms by which okadaic acid and glutamate induce neuronal cell death and the effects of estrogens against these neurotoxicities.
Materials and Methods
Chemicals
17β-estradiol and 17α-estradiol was purchased from Steraloids, Inc. (Wilton, NH). The enantiomer of 17β-estradiol (ENT E2) and ZYC3 were prepared as described previously (Green et al., 2001; Liu et al., 2002). All steroids were dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 mM and diluted to appropriate concentration in culture media. Calcein AM and 2,7-dichlorofluorescin diacetate (DCFH-DA) was purchased from Molecular Probes, Inc. (Eugene, OR). Okadaic acid, L-glutamate, trichloroacetic acid, 2-thiobarbituric acid (TBA), 1,1,3,3-tetramethoxypropane, HCl and DMSO were purchased from Sigma-Aldrich (St Louise, MO). PD 98059, U0126, bis-indolylmaleimide (BIM), H-89, LY294002, and Akt inhibitor were purchased from Calbiochem (Gibbstown, NJ).
Culture of primary cortical neurons
Cerebral cortices of rat embryos (18-day) were dissected and harvested in preparation medium (DMEM, glucose 4.5g/L, Penicillin 100 U/ml, Streptomycin 100μg/ml). The cortical tissue was treated with trypsin. The tissue was washed three times using washing medium (Hank's medium, glucose 4.5g/L, Penicillin 100 U/ml, Streptomycin 100μg/ml) and individual cells were isolated by mechanical trituration using three different sizes of fire polished Pasteur pipettes. The cells were harvested in seeding medium (DMEM, glucose 4.5g/L, Penicillin 100 U/ml, Streptomycin 100μg/ml, Glutamine 2mM, 19% horse serum) and filtered through 40μm filter. The cerebral cortical cells were seeded in poly-L-lysine treated dishes and plates at variety of cell densities. The cells were incubated in neurobasal medium (DMEM, glucose 4.5g/L, Penicillin 100 U/ml, Streptomycin 100μg/ml, glutamine 2mM) supplemented with B-27 with antioxidants in normal cell culture condition of 37°C in a humid atmosphere of 5% CO2. The cells were allowed to mature for 14 days before initiation of experiments. Two hours before treatment with inhibitors and/or estrogens, the media was replaced with neurobasal medium supplemented with B-27 without antioxidants.
Dose and Sampling time
17β-estradiol, 17α-estradiol, and enantiomer of 17β-estradiol were used at a concentration of 100 nM, which has been shown to be potently neuroprotective (Perez et al., 2005; Yi et al., 2008) and to preserve protein phosphatase activity against glutamate toxicity (Yi and Simpkins, 2008). ZYC3 was used at 10 nM given its greater neuroprotective potency (Perez et al., 2005; Yi et al., 2008) and enhanced ability to preserve protein phosphatase activity against glutamate toxicity (Yi and Simpkins, 2008). Glutamate (50 μM) and OA (50 nM) concentrations were utilized to produce 50% cell death in primary cortical cultures (Yi et al., 2005). Doses of MEK inhibitors PD 98059 (50 μM) and U0126 (10 μM), PKC inhibitor BIM (2.5 μM), PKA inhibitor H-89 (1 μM), PI3K inhibitor LY294002 (50 μM) and Akt inhibitor (100 μM) were at least 50 times the IC50 for each compound. Glutamate, OA, and/or estrogens were added simultaneously; while, the inhibitors of various kinases were incubated one hour prior to addition of the other three compounds.
All samples were collected 24 hr after treatment with glutamate, OA, estrogens, and/or kinase inhibitors because we have shown in our previous studies that OA abolishes the estrogen mediated neuroprotection against glutamate when sampled at 24 hr (Yi et al., 2005; Yi et al., 2008).
MTT Assay
Mitochondrial function was indirectly determined by MTT (Sigma Chemical Co., St. Louis, MO) reduction by mitochondrial dehydrogenases. Primary cortical neurons were plated in 96-well plates at a density of 25,000 cells/well. MTT was added to each well 22 h after the beginning of the insult at a final concentration of 250 μM. After 2-hr incubation at 37°C, the media was removed, and cells dissolved in DMSO. The assay of the formation of formazan was performed by measuring the amount of reaction product by absorbance change (570 nM) using a microplate reader (BioTek, Highland Park, VT).
Measurement of Cytosolic ROS
The extent of cytosolic cellular oxidative stress was estimated by monitoring the amount of ROS by the fluorescent dye DCFH-DA (Molecular Probes, Eugene, OR). Primary cortical neurons were plated in 96-well plates at a density of 25,000 cells/well. Following seven days in vitro, the neurons were loaded with DCFH-DA at a final concentration of 50 μM for 45 min at 37°C. After incubation, DCFH-DA was removed, and cells were washed twice with PBS, pH 7.4. Following treatment with various compounds for 24 hr, DCF2,7-dichlorofluorescin fluorescence was determined at an excitation of 485 nm and an emission of 538 nm using an FL600 microplate-reader (BioTek, Highland Park, VT). Values were normalized to percentage of untreated control groups.
Lipid Peroxidation Measurement
Lipid peroxidation was monitored by measuring malondiadehyde (MDA), a stable end product of lipid peroxidation cascades using the thiobarbituric acid reactive substances (TBARS) assay. As one of the main compounds among TBARS, MDA reacts with TBA under acidic and high heat conditions and the product of this reaction can be detected spectrophotometrically or fluorometrically. Primary neuronal cells were plated in 100-mm dishes at the density of 500,000 cells/ml. Cells were exposed to 50 μM glutamate for 24 hr in the presence of estrogens or vehicle. Cells were washed twice with ice-cold PBS and harvested with 0.6 ml/dish ice-cold PBS using rubber policeman. Then, cells were homogenized by sonication. To prevent sample oxidation during homogenization, 0.5 M BHT (10 μl/ml cell suspension) was added before sonication. Cell homogenates were centrifuged at 3000 × g at 4°C for 10 min. The clear supernatant was used for TBARS assay and protein determination. For MDA measurement, 100 μl of sample was added into 48-well plate followed by addition of a solution containing 1% TBA, 12.5% trichloroacetic acid, and 0.8 N HCl. Reaction mixtures were incubated at 50°C for 60 min, and then precipitated proteins were removed by centrifuging at 12,000 rpm for 2 min. Supernatants were transferred to 96-well plates, and relative fluorescence values were determined using a BioTek FL600 plate reader (BioTek, Highland Park, VT) at an excitation wavelength of 530 ± 25 nm, emission wavelength of 590 ± 20 nm, and sensitivity of 100. External standards used in the TBARS assay were made from 1,1,3,3-tetramethoxypropane in reagent grade ethanol and diluted in 0.9% normal saline to give concentrations ranging from 0 to 20 μM. Values were normalized to percentage of untreated control groups.
Protein Carbonyl Assay
The accumulation of oxidized proteins was evaluated by the carbonyl group content via reaction with DNPH (2,4-dinitrophenylhydrazine) at 360 nm (Fagan et al., 1999). Neurons were treated with different paradigms and cells were collected and centrifuged at 1500 × g for 10 min at 4°C. The pellet was solubilized in 1 ml of ice-cold buffer (trichloroacetic acid (TCA)) and centrifuged (1500 × g, 10 min). The sediments were incubated with 1 ml of 10 mM DNPH (freshly prepared in 2 M HCl, in the dark) for 1 h at room temperature, with vortex agitation every 10 min. Following this incubation, 1 ml of 20% TCA was added and samples were centrifuged at 20,000 × g, for 3 min. The supernatant was discarded and the pellet mixed with 1 ml of a 1:1 ethanol:ethyl acetate solution. The pellet was then incubated with 1 ml of 6 M guanidine (prepared in PBS, pH 6.5), for 15 min at 37 °C and centrifuged at 1500 × g for 10 min. The supernatant was collected and protein oxidation was estimated spectrophotometrically at 360 nm in 96-well plates. For all samples a blank was prepared, which was incubated with 2 M HCl instead of DNPH. The carbonyl content was calculated using a molar extinction coefficient of 22 mM−1 cm−1 for DNPH and was expressed as nmoles DNPH/mg protein. Values were normalized to percentage of untreated control groups.
Caspase-3/7 Activity Assay
Caspase-3/7 enzyme activity was measured by proteolytic cleavage of the fluorogenic substrate Ac-DEVD-AFC by counting on a microplate fluorometer (Bio-Tek FL600 Winooski, VT). After incubation with 50 μM glutamate, 100 nM 17β-estradiol, and/or 50 nM OA for 24 hr, cells was harvested and washed once with cold PBS. The cells were lysed using lysis buffer containing 10mM EDTA, 0.5% Triton X-100, and 10mM Tris–HCl (pH 8.0) at room temperature for 10 min. Then, assay buffer (100mM HEPES; pH 7.5, 10mM dithiothreitol, 10% sucrose, 0.1% CHAPS, 0.1% BSA) and substrate (50 μM) were added on ice. Fluorescence at 400 nm (excitation) and 505 nm (emission) was measured using a BioTek FL600 plate reader (BioTek, Highland Park, VT) after incubation at 37 °C for 1-3 hr. Values were normalized to percentage of untreated control groups.
Statistical analysis
Statistical significance was determined by one-way analysis of variance (ANOVA) followed by a Tukey's multiple comparison test. p < 0.05 was considered significant for all experiments. Each set of data represents six or more individual assays performed separately, with each containing at least two replicate wells. The values are reported as the mean ± SEM.
Results
Cellular markers of oxidative stress in glutamate or okadaic acid mediated cell death in primary cortical neurons
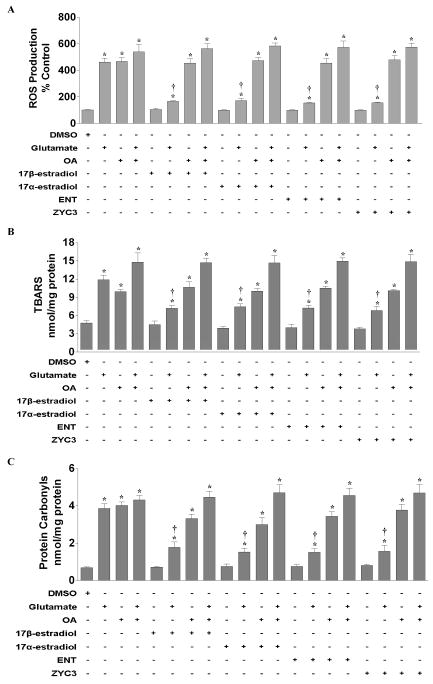
In order to determine if OA caused production of reactive oxygen species (ROS) in primary cortical neurons, we simultaneously treated cortical cultures with 17β-estradiol, 17α-estradiol, the enantiomer of 17β-estradiol (ENT E2), or ZYC3 with glutamate and/or OA. Treatment with glutamate or OA induced an approximately 4.5-fold increase in hydrogen peroxide (H2O2) production as measure by DCFH-DA florescent dye (Fig 1A). Co-treatment of glutamate and OA exacerbated the ROS production seen with one or the other compound. None of the estrogens had an effect on basal ROS production. All estrogens attenuated the glutamate-induced generation of hydrogen peroxide; however, they were ineffective against the OA-induced ROS production. In addition, the presence of OA abolished the estrogen-mediated down-regulation of glutamate-induced ROS generation. Similar effects were seen with lipid peroxidation production (Fig 1B) and protein carbonylation (Fig 1C).
Figure 1. Effects of glutamate, okadaic acid, 17β-estradiol, 17α-estradiol, ENT E2, and/or ZYC3 on oxidative stress markers in primary cortical neurons.
Primary cortical neurons were seeded into 96-well plates at a density of 25,000 cells/well for the ROS assays and 100 mm dishes at a density of 500,000 cells/well for the lipid peroxidation and protein carbonyl assays. The various assays were performed following 24 hr treatment. A) ROS production was measured in neurons treated simultaneously with 50 nM okadaic acid, 50 μM glutamate, and/or 100 nM 17β-estradiol, 100 nM 17α-estradiol, 100 nM ENT E2, and/or 10 nM ZYC3. B) Lipid peroxidation was measured in cortical neurons 24 hr following simultaneously treatment with 50 nM okadaic acid, 50 μM glutamate, and/or 100 nM 17β-estradiol, 100 nM 17α-estradiol, 100 nM ENT E2, and/or 10 nM ZYC3. C) Protein carbonylation was measured in cortical neurons 24 hr following simultaneously treatment with 50 nM okadaic acid, 50 μM glutamate, and/or 100 nM 17β-estradiol, 100 nM 17α-estradiol, 100 nM ENT E2, and/or 10 nM ZYC3. All data were normalized to % non-treated control. Depicted are mean ± SEM for six independent experiments with two replicates per experiment for ROS production and six independent experiments each for lipid peroxidation and protein carbonylation experiments. * P <0.05 vs. vehicle control.
MTT formazen reduction and caspase 3/7 activity in glutamate or okadaic acid mediated cell death in primary cortical neurons
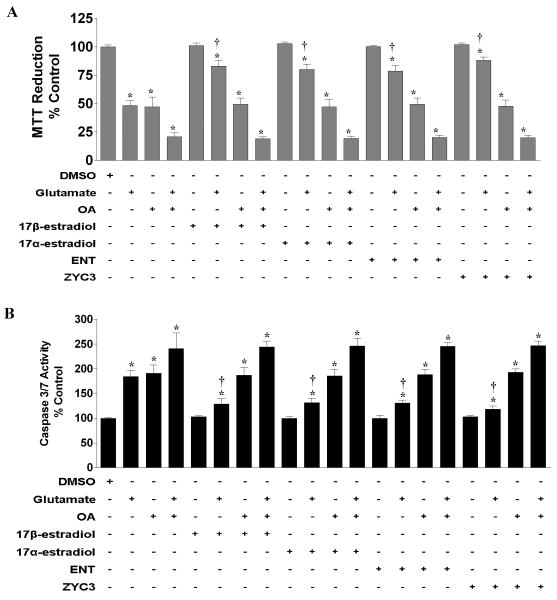
We examined mitochondrial function and caspase activity in response to treatment with glutamate, OA, and/or various forms of estrogen in primary cortical neurons. Estrogen treatment alone did not affect MTT formazen reduction (Fig 2A) or caspase 3/7 activity (Fig 2B); however, the various estrogens effectively attenuated MTT reduction (Fig 2A), an indirect indication of mitochondrial function, as well as caspase activation (Fig 2B) caused by glutamate-induced oxidative/excitotoxic stress. Treatment of primary cortical neurons with OA induced MTT reduction and the activation of caspase 3/7 to a level similar to that caused by glutamate treatment. All estrogens were effective against glutamate-mediated MTT reduction and caspase activation but did not significantly alter the OA induced effects. In addition, presence of OA blocked the estrogen mediated protective effects against glutamate induced dysfunctions.
Figure 2. Effects of glutamate, okadaic acid, 17β-estradiol, 17α-estradiol, ENT E2, and/or ZYC3 on MTT reduction and caspase activity in primary cortical neurons.
Primary cortical neurons were seeded into 96-well plates at a density of 25,000 cells/well for the MTT assays and 100 mm dishes at a density of 500,000 cells/well for the caspase activity assays. The various assays were performed following 24 hr treatment. A) MTT reduction was measured in neurons treated simultaneously with 50 nM okadaic acid, 50 μM glutamate, and/or 100 nM 17β-estradiol, 100 nM 17α-estradiol, 100 nM ENT E2, and/or 10 nM ZYC3. B) Caspase 3/7 activity was measured in cortical neurons 24 hr following simultaneously treatment with 50 nM okadaic acid, 50 μM glutamate, and/or 100 nM 17β-estradiol, 100 nM 17α-estradiol, 100 nM ENT E2, and/or 10 nM ZYC3. All data were normalized to % survival of non-treated control. Depicted are mean ± SEM for six independent experiments with two replicates per experiment for MTT reduction and six independent experiments for caspase 3/7 activity measurement. * P <0.05 vs. vehicle control. † P < 0.05 vs. glutamate treated group.
Inhibition of MAPK pathway in glutamate or okadaic acid induced neuronal cell death
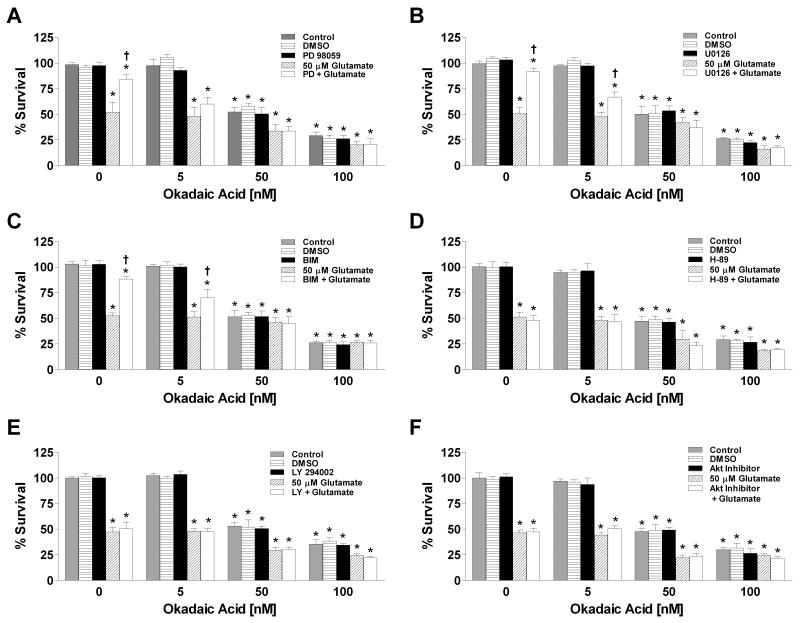
The MAPK pathway has been implicated in the determination of cell survival or death and estrogens are known to transiently activate (Singh et al., 2000) and chronically suppress ERK1/2 phosphorylation (Yi et al., 2008). We examined whether pharmacological inhibition of MEK1/2 using PD98059 or U0126 would be neuroprotective against glutamate- and OA-mediated neuronal cell death because MEK 1/2 is the upstream kinase that phosphorylates ERK1/2. MEK1/2 inhibition with PD98059 or U0126 was neuroprotective against glutamate toxicity but not OA toxicity (Fig 3A & 3B). In addition, the presence of non-lethal concentrations of OA attenuated the PD98059 or U0126 mediated neuroprotection against glutamate-induced neurotoxicity. Inhibition of PKC was also neuroprotective against glutamate, but not OA and the protection against glutamate toxicity was antagonized by OA (Fig 3C). We also assessed the ability of PKA, PI3K, and Akt inhibitors to protect cortical neurons against glutamate- and OA-induced cell death. As shown in figure 3D-3F, inhibition of PKA, PI3K, or Akt was not protective against either glutamate or OA-mediated death.
Figure 3. Effects of glutamate, okadaic acid and/or various protein kinase inhibitors on cell viability in primary cortical neurons.
Primary cortical neurons were seeded into 96-well plates at a density of 25,000 cells/well. A) Cells were treated simultaneously with 50 μM glutamate, 50 μM PD98059 and/or varying concentrations of okadaic acid. B) Cells were treated simultaneously with 50 μM glutamate, 10 μM U0126 and/or varying concentrations of okadaic acid. C) Cells were treated simultaneously with 50 μM glutamate, 2.5 μM Bis-indolylmaleimide (BIM) and/or varying concentrations of okadaic acid. D) Cells were treated simultaneously with 50 μM glutamate, 1 μM H-89 and/or varying concentrations of okadaic acid. E) Cells were treated simultaneously with 50 μM glutamate, 50 μM LY294002 and/or varying concentrations of okadaic acid. F) Cells were treated simultaneously with 50 μM glutamate, 100 μM Akt Inhibitor and/or varying concentrations of okadaic acid. Cell viability was determined by calcein AM assay (Molecular Probes, Eugene, OR) after 24 hr exposure to the various compounds. All data were normalized to % survival of non-treated control. Depicted are mean ± SEM for ten independent experiments with two replicates per experiment. * P <0.05 vs. vehicle control; † P < 0.05 vs. glutamate treated group.
MEK inhibition mediated neuroprotection against oxidative/excitotoxic stress
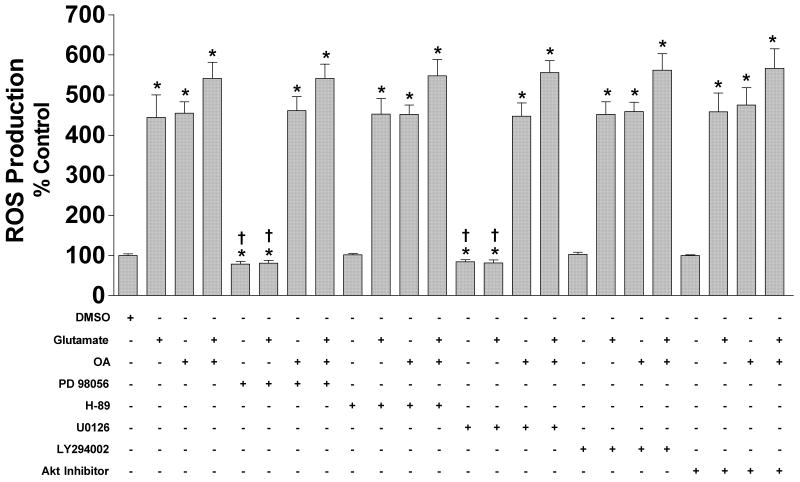
In order to determine the effects of MEK inhibition on ROS production induced by glutamate and okadaic acid treatment, we examined the cytosolic hydrogen peroxide content of primary cortical neurons following 24 hr simultaneous treatment of glutamate, OA, and/or PD98059, U0126, H-89, LY294002, or Akt inhibitor. Interestingly, the presence of a MEK inhibitor blocked the glutamate but not OA-induced production of ROS inhibition (Fig 4). In addition, the presence of OA abolished this ERK1/2 inhibition mediated down-regulation of glutamate-induced ROS production. Interestingly, inhibition of MEK1/2 with PD98059 or U0126 alone caused a decrease in basal ROS production. PKA, PI3K, or Akt inhibition had no effect on basal ROS production, and these inhibitors did not attenuate the ROS induction by glutamate or OA.
Figure 4. Effects of glutamate, okadaic acid and/or various protein kinase inhibitors on ROS production in primary cortical neurons.
Primary cortical neurons were seeded into 96-well plates at a density of 25,000 cells/well. A) Cells were treated simultaneously with 50 μM glutamate, 50 μM PD98059, 10 μM U0126, 1 μM H-89, 50 μM LY294002, 100 μM Akt Inhibitor, and/or 50nM of okadaic acid. ROS production was determined by DCFH-DA assay (Molecular Probes, Eugene, OR) after 24 hr exposure to the various compounds. All data were normalized to % survival of non-treated control. Depicted are mean ± SEM for ten independent experiments with two replicates per experiment. * P <0.05 vs. vehicle control.
Discussion
The present study demonstrates that OA-mediated neuronal cell death is similar to glutamate induced neurotoxicity in that both compound cause the production of reactive oxygen species, lipid peroxides and protein carbonyls, activation of caspase 3/7, and mitochondrial dysfunction. We also show that while estrogen and estrogen analogues mitigate or abolish the glutamate-induced oxidative/excitotoxic stress and subsequent cell death, they are not effective against the same events induced by OA. In addition, the presence of OA in non-lethal concentrations abolishes the estrogen and estrogen analogue-mediated neuroprotection against oxidative/excitotoxic stress induced by glutamate toxicity. Pharmacological inhibition of ERK1/2 phosphorylation also attenuated the ROS production mediated by glutamate but was ineffective against OA induced oxidative stress. Similarly, ERK1/2 and PKC inhibition rescued primary cortical neurons from cell death induced by glutamate toxicity but not OA toxicity. Collectively, these results are consistent with the hypothesis that protein phosphatase activity is a necessary component of estrogen-mediated neuroprotection and that estrogen-induced protein phosphatases cause dephosphorylation and inactivation of ERK1/2 and/or PKC.
OA-induced oxidative stress found in our study is consistent with the results of others (Schreck and Baeuerle, 1994; Schmidt et al., 1995; Tunez et al., 2003). Mitochondrial dysfunction and the consequential induction of caspase 3/7 are also in agreement with previous observations by other laboratories (Boe et al., 1991; Fernandez et al., 1991; Arias et al., 1993; Fernandez-Sanchez et al., 1996; Yoon et al., 2006). It has been shown in primary cortical neurons that mitochondrial swelling and decreased membrane potential as well as increased protein expression of caspase-3, Bad, Bax, and Bim are seen following OA treatment (Yoon et al., 2006). Since protein phosphatases are important modulators of cellular function, it is not surprising that inhibition of protein phosphatases by okadaic acid leads to cellular dysfunction that ultimately ends with cell death. We have previously shown that calyculin A, another protein phosphatase inhibitor, also induces cell death that is not protected by estrogens (Yi et al., 2005). In addition, inhibition of specific serine/threonine phosphatases do not induced cell death in and of itself; however, neurons treated with a combination of inhibitors are vulnerable to toxicity (Yi and Simpkins, 2008). Interestingly, the activities of various phosphatases including PP1, PP2A, and PP2B are found to be reduced in the brains of AD patients (Gong et al., 1995), and AD brains show increased oxidative stress (Butterfield, 2006), which is thought to be one mediator of AD pathology. In addition, serine/threonine phosphatases have been shown to play a role in hyperphosphorylation of tau and amyloid plaques (Sontag et al., 2004; Celsi et al., 2007). Therefore, it appears that serine/threonine phosphatases may have a multifaceted role in the pathology of neurodegenerative diseases.
We have previously shown that estrogen-mediated neuroprotection involves the maintenance of serine/threonine phosphatase protein levels and activities (Yi et al., 2005; Yi et al., 2008; Yi and Simpkins, 2008). Inhibition of phosphatases abrogated the estrogen-induced attenuation of persistent ERK 1/2 phosphorylation following oxidative/excitotoxic stress (Yi et al., 2008), suggesting that estrogens reduce MAPK signaling pathway during neurotoxic events. In agreement with this observation, we show in this study that pharmacological inhibition of ERK1/2 with PD98059 or U0126 leads to neuroprotection against oxidative/excitotoxic stress caused by glutamate, similar to that seen with estrogen treatment. Additionally, ERK1/2 inhibition, like E2 treatment, did not protect against OA induced cell toxicity, and non-lethal concentration of OA was able to attenuate ERK1/2 inhibition-mediated neuroprotection. It is interesting to note that ERK1/2 inhibitors, PD98059 and U0126, were able to attenuate the ROS production induced by glutamate toxicity and reduced basal ROS production. Our findings are contradicted by the findings of Levinthal and DeFranco in which ERK inhibition via U0126 did not alter the rate or extent of ROS production during glutamate-induced oxidative stress (Levinthal and DeFranco, 2004). This can be in part explained by differences in the culture and glutamate exposure, as we used mature cortical neurons and lower glutamate concentrations while Levinthal and DeFranco used immature cortical neurons that do not yet express glutamate receptors, as well as 100-fold higher glutamate concentrations. On the other hand, acute exposure to U0126 has been shown to decrease ROS formation and attenuated cell death caused by a hydrocarbon solvent (Myhre et al., 2004). Our data indicates that persistent ERK1/2 activation leads to ROS production.
There are at least three possible explanations for the effectiveness of estrogens and kinase inhibitors against glutamate-induced neuronal death and their complete lack of efficacy against OA toxicity. First, OA could have caused a more profound insult then glutamate at the concentrations of each toxin used. Our previous studies determined that the LD50 for OA is ∼ 40 nM and for glutamate is ∼ 50 μM (Yi et al., 2005), which is approximately a 1000 fold difference in potency. However, at the concentrations used in the present study, each toxin caused about a 50% cell death, and similar magnitude of oxidative damage, mitochondrial impairment and caspase activation. In brief, the extent of cell insult applied appeared to be similar. The difference in the insults is that OA is known to directly inhibit serine/threonine phosphatases, while glutamate probably indirectly causes the inactivation or inhibition of these phosphatases.
Second, it is possible that the mechanism by which glutamate causes these death-inducing events is different than that of OA. For example, it is well known that glutamate causes an excess influx and accumulation of calcium into neurons that eventually leads to mitochondrial dysfunction and subsequent cell death. However, Fernandez et al (1991) has shown that OA-induced mediated neurotoxicity in neurons is not mediated via voltage-gated calcium channels. Moreover, estrogens have been shown to attenuate glutamate-induced calcium overload (Nilsen et al., 2002; Simpkins et al., 2005), and we have shown that estrogens directly modulate L-type calcium channels (Sarkar et al., 2008). Therefore, estrogen neuroprotection could be a specific effect on the voltage-gated calcium channels.
Third, and most likely, multiple, interactive death-inducing kinase pathways are activated simultaneously following glutamate or OA treatment. It has been observed that both glutamate and OA (Yoon et al., 2006; Kraft et al., 2007) persistently activate JNK, p38 MAPK and ERK1/2 pathways. Activation of the different MAPK pathways occurs simultaneously, and there is crosstalk between ERK 1/2 and p38 that is mediated by various protein phosphatases (Rice et al., 2002; Wang et al., 2006). Moreover, it has been demonstrated that PKC phosphorylates both ERK1/2 and p38 MAPK (Schonwasser et al., 1998; Kim et al., 1999; Hu et al., 2007) providing a link between PKC and MAPK signaling. In the presence of functioning protein phosphatases, estrogens, via maintenance of protein phosphatase activity (Yi et al., 2005; Yi and Simpkins, 2008), or specific inhibitors of ERK1/2 or PCK (present study) prevent glutamate death signaling. However, in the face of broad protein phosphatase inhibition by OA, profound activation of multiple death-inducing kinases cannot be overcome with either estrogens or specific kinase inhibitors. Therefore, it is possible that direct inhibition of death inducing kinases is sufficient for neuronal survival, but inhibition of phosphatases leads to activation of multiple death signaling pathways that cannot be overcome by inhibition of ERK, p38, or PKC alone.
Estrogens have been shown to activate or inhibit different proteins that are involved in various signaling pathways such as the Akt, PKC, PKA, MAPK, and NFκB pathway, caspases, Bcl group of proteins, various other mitochondrial related proteins, and many others (Migliaccio et al., 1996; Kelly and Wagner, 1999; Singh et al., 1999; Zhang et al., 2001). Several groups have shown ER-dependent mechanism of estrogen-induced neuroprotection via activation of PKC with subsequent activiation of ERK 1/2 that in turn results in induction of antiapoptoic genes, Bcl-2 and Bcl-xl (for review see Zhao et al 2005). However, it is unlikely that the mode of action seen in our studies are ER-dependent in light of fact that all four estrogens used in our studies produced similar results even though these compounds vary broadly in binding to estrogen receptors (Perez et al., 2005) but have similar efficacies in protection against ischemic insult in vivo (Liu et al., 2002). Therefore, the preservation of protein phosphatases (Yi et al., 2005, Yi and Simpkins, 2008) and inhibition of persistent activation of ERK1/2 (Yi et al., 2005; Yi et al., 2008) appears to be an estrogen-receptor independent process. Estrogens are known to maintain mitochondrial function by stabilizing mitochondrial membrane potential by an unknown mechanism. It is likely that in the face of oxidative overload, protein phosphatases are damaged thus rendering them inactive. Therefore, estrogen-mediated neuroprotection is at least in part due to maintenance of normal protein phosphatase activities.
Acknowledgments
This work was supported by NIH grants AG10485 and AG22550
References
- Arias C, Sharma N, Davies P, Shafit-Zagardo B. Okadaic acid induces early changes in microtubule-associated protein 2 and tau phosphorylation prior to neurodegeneration in cultured cortical neurons. J Neurochem. 1993;61:673–682. doi: 10.1111/j.1471-4159.1993.tb02172.x. [DOI] [PubMed] [Google Scholar]
- Boe R, Gjertsen BT, Vintermyr OK, Houge G, Lanotte M, Doskeland SO. The protein phosphatase inhibitor okadaic acid induces morphological changes typical of apoptosis in mammalian cells. Exp Cell Res. 1991;195:237–246. doi: 10.1016/0014-4827(91)90523-w. [DOI] [PubMed] [Google Scholar]
- Butterfield DA. Oxidative stress in neurodegenerative disorders. Antioxid Redox Signal. 2006;8:1971–1973. doi: 10.1089/ars.2006.8.1971. [DOI] [PubMed] [Google Scholar]
- Celsi F, Svedberg M, Unger C, Cotman CW, Carri MT, Ottersen OP, Nordberg A, Torp R. Beta-amyloid causes downregulation of calcineurin in neurons through induction of oxidative stress. Neurobiol Dis. 2007;26:342–352. doi: 10.1016/j.nbd.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Fagan JM, Sleczka BG, Sohar I. Quantitation of oxidative damage to tissue proteins. Int J Biochem Cell Biol. 1999;31:751–757. doi: 10.1016/s1357-2725(99)00034-5. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sanchez MT, Garcia-Rodriguez A, Diaz-Trelles R, Novelli A. Inhibition of protein phosphatases induces IGF-1-blocked neurotrophin-insensitive neuronal apoptosis. FEBS Lett. 1996;398:106–112. doi: 10.1016/s0014-5793(96)01192-1. [DOI] [PubMed] [Google Scholar]
- Fernandez MT, Zitko V, Gascon S, Novelli A. The marine toxin okadaic acid is a potent neurotoxin for cultured cerebellar neurons. Life Sci. 1991;49:PL157–162. doi: 10.1016/0024-3205(91)90398-u. [DOI] [PubMed] [Google Scholar]
- Ferri A, Nencini M, Battistini S, Giannini F, Siciliano G, Casali C, Damiano MG, Ceroni M, Chio A, Rotilio G, Carri MT. Activity of protein phosphatase calcineurin is decreased in sporadic and familial amyotrophic lateral sclerosispatients. J Neurochem. 2004;90:1237–1242. doi: 10.1111/j.1471-4159.2004.02588.x. [DOI] [PubMed] [Google Scholar]
- Gong CX, Shaikh S, Wang JZ, Zaidi T, Grundke-Iqbal I, Iqbal K. Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem. 1995;65:732–738. doi: 10.1046/j.1471-4159.1995.65020732.x. [DOI] [PubMed] [Google Scholar]
- Green PS, Yang SH, Nilsson KR, Kumar AS, Covey DF, Simpkins JW. The nonfeminizing enantiomer of 17beta-estradiol exerts protective effects in neuronal cultures and a rat model of cerebral ischemia. Endocrinology. 2001;142:400–406. doi: 10.1210/endo.142.1.7888. [DOI] [PubMed] [Google Scholar]
- Ho Y, Logue E, Callaway CW, DeFranco DB. Different mechanisms account for extracellular-signal regulated kinase activation in distinct brain regions following global ischemia and reperfusion. Neuroscience. 2007;145:248–255. doi: 10.1016/j.neuroscience.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Kang C, Philp RJ, Li B. PKC delta phosphorylates p52ShcA at Ser29 to regulate ERK activation in response to H2O2. Cell Signal. 2007;19:410–418. doi: 10.1016/j.cellsig.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Wagner EJ. Estrogen Modulation of G-protein-coupled Receptors. Trends Endocrinol Metab. 1999;10:369–374. doi: 10.1016/s1043-2760(99)00190-3. [DOI] [PubMed] [Google Scholar]
- Kim JY, Yang MS, Oh CD, Kim KT, Ha MJ, Kang SS, Chun JS. Signalling pathway leading to an activation of mitogen-activated protein kinase by stimulating M3 muscarinic receptor. Biochem J. 1999;337(Pt 2):275–280. [PMC free article] [PubMed] [Google Scholar]
- Kins S, Kurosinski P, Nitsch RM, Gotz J. Activation of the ERK and JNK signaling pathways caused by neuron-specific inhibition of PP2A in transgenic mice. Am J Pathol. 2003;163:833–843. doi: 10.1016/S0002-9440(10)63444-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft CA, Efimova T, Eckert RL. Activation of PKCdelta and p38delta MAPK during okadaic acid dependent keratinocyte apoptosis. Arch Dermatol Res. 2007;299:71–83. doi: 10.1007/s00403-006-0727-4. [DOI] [PubMed] [Google Scholar]
- Levinthal DJ, DeFranco DB. Transient phosphatidylinositol 3-kinase inhibition protects immature primary cortical neurons from oxidative toxicity via suppression of extracellular signal-regulated kinase activation. J Biol Chem. 2004;279:11206–11213. doi: 10.1074/jbc.M314261200. [DOI] [PubMed] [Google Scholar]
- Liu R, Yang SH, Perez E, Yi KD, Wu SS, Eberst K, Prokai L, Prokai-Tatrai K, Cai ZY, Covey DF, Day AL, Simpkins JW. Neuroprotective effects of a novel non-receptor-binding estrogen analogue: in vitro and in vivo analysis. Stroke. 2002;33:2485–2491. doi: 10.1161/01.str.0000030317.43597.c8. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Pedersen WA, Culmsee C. Neurodegenerative disorders and ischemic brain diseases. Apoptosis. 2001;6:69–81. doi: 10.1023/a:1009676112184. [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. Embo J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- Myhre O, Sterri SH, Bogen IL, Fonnum F. Erk1/2 phosphorylation and reactive oxygen species formation via nitric oxide and Akt-1/Raf-1 crosstalk in cultured rat cerebellar granule cells exposed to the organic solvent 1,2,4-trimethylcyclohexane. Toxicol Sci. 2004;80:296–303. doi: 10.1093/toxsci/kfh166. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Chen S, Brinton RD. Dual action of estrogen on glutamate-induced calcium signaling: mechanisms requiring interaction between estrogen receptors and src/mitogen activated protein kinase pathway. Brain Res. 2002;930:216–234. doi: 10.1016/s0006-8993(02)02254-0. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Braak H, An WL, Winblad B, Cowburn RF, Iqbal K, Grundke-Iqbal I. Up-regulation of mitogen-activated protein kinases ERK1/2 and MEK1/2 is associated with the progression of neurofibrillary degeneration in Alzheimer's disease. Brain Res Mol Brain Res. 2002;109:45–55. doi: 10.1016/s0169-328x(02)00488-6. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Gong CX, An WL, Winblad B, Cowburn RF, Grundke-Iqbal I, Iqbal K. Okadaic-acid-induced inhibition of protein phosphatase 2A produces activation of mitogen-activated protein kinases ERK1/2, MEK1/2, and p70 S6, similar to that in Alzheimer's disease. Am J Pathol. 2003;163:845–858. doi: 10.1016/S0002-9440(10)63445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez E, Liu R, Yang SH, Cai ZY, Covey DF, Simpkins JW. Neuroprotective effects of an estratriene analog are estrogen receptor independent in vitro and in vivo. Brain Res. 2005;1038:216–222. doi: 10.1016/j.brainres.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Poppek D, Keck S, Ermak G, Jung T, Stolzing A, Ullrich O, Davies KJ, Grune T. Phosphorylation inhibits turnover of the tau protein by the proteasome: influence of RCAN1 and oxidative stress. Biochem J. 2006;400:511–520. doi: 10.1042/BJ20060463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Grundke-Iqbal I, Iqbal K. Phosphothreonine-212 of Alzheimer abnormally hyperphosphorylated tau is a preferred substrate of protein phosphatase-1. Neurochem Res. 2005;30:277–287. doi: 10.1007/s11064-005-2483-9. [DOI] [PubMed] [Google Scholar]
- Rice AB, Ingram JL, Bonner JC. p38 mitogen-activated protein kinase regulates growth factor-induced mitogenesis of rat pulmonary myofibroblasts. Am J Respir Cell Mol Biol. 2002;27:759–765. doi: 10.1165/rcmb.2002-0070OC. [DOI] [PubMed] [Google Scholar]
- Sarkar SN, Huang RQ, Logan SM, Yi KD, Dillon GH, Simpkins JW. Estrogens directly potentiate neuronal L-type Ca2+ channels. Proc Natl Acad Sci U S A. 2008;105(39):15148–53. doi: 10.1073/pnas.0802379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KN, Traenckner EB, Meier B, Baeuerle PA. Induction of oxidative stress by okadaic acid is required for activation of transcription factor NF-kappa B. J Biol Chem. 1995;270:27136–27142. doi: 10.1074/jbc.270.45.27136. [DOI] [PubMed] [Google Scholar]
- Schonwasser DC, Marais RM, Marshall CJ, Parker PJ. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R, Baeuerle PA. Assessing oxygen radicals as mediators in activation of inducible eukaryotic transcription factor NF-kappa B. Methods Enzymol. 1994;234:151–163. doi: 10.1016/0076-6879(94)34085-4. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Wang J, Wang X, Perez E, Prokai L, Dykens JA. Mitochondria play a central role in estrogen-induced neuroprotection. Curr Drug Targets CNS Neurol Disord. 2005;4:69–83. doi: 10.2174/1568007053005073. [DOI] [PubMed] [Google Scholar]
- Singh M, Setalo G, Jr, Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Setalo G, Jr, Guan X, Frail DE, Toran-Allerand CD. Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-alpha knock-out mice. J Neurosci. 2000;20:1694–1700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag E, Luangpirom A, Hladik C, Mudrak I, Ogris E, Speciale S, White CL., 3rd Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with Alzheimer disease pathology. J Neuropathol Exp Neurol. 2004;63:287–301. doi: 10.1093/jnen/63.4.287. [DOI] [PubMed] [Google Scholar]
- Sultana R, Perluigi M, Butterfield DA. Protein oxidation and lipid peroxidation in brain of subjects with Alzheimer's disease: insights into mechanism of neurodegeneration from redox proteomics. Antioxid Redox Signal. 2006;8:2021–2037. doi: 10.1089/ars.2006.8.2021. [DOI] [PubMed] [Google Scholar]
- Tunez I, Munoz Mdel C, Feijoo M, Munoz-Castaneda JR, Bujalance I, Valdelvira ME, Montilla Lopez P. Protective melatonin effect on oxidative stress induced by okadaic acid into rat brain. J Pineal Res. 2003;34:265–268. doi: 10.1034/j.1600-079x.2003.00039.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yang H, Tachado SD, Capo-Aponte JE, Bildin VN, Koziel H, Reinach PS. Phosphatase-mediated crosstalk control of ERK and p38 MAPK signaling in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:5267–5275. doi: 10.1167/iovs.06-0642. [DOI] [PubMed] [Google Scholar]
- Yi KD, Simpkins JW. Protein Phosphatase 1, Protein Phosphatase 2A, and Calcineurin Play a Role in Estrogen-Mediated Neuroprotection. Endocrinology. 2008;149(10):5235–43. doi: 10.1210/en.2008-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi KD, Chung J, Pang P, Simpkins JW. Role of protein phosphatases in estrogen-mediated neuroprotection. J Neurosci. 2005;25:7191–7198. doi: 10.1523/JNEUROSCI.1328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi KD, Cai ZY, Covey DF, Simpkins JW. Estrogen receptor-independent neuroprotection via protein phosphatase preservation and attenuation of persistent extracellular signal-regulated kinase 1/2 activation. J Pharmacol Exp Ther. 2008;324:1188–1195. doi: 10.1124/jpet.107.132308. [DOI] [PubMed] [Google Scholar]
- Yoon SY, Choi JE, Yoon JH, Huh JW, Kim DH. BACE inhibitor reduces APP-beta-C-terminal fragment accumulation in axonal swellings of okadaic acid-induced neurodegeneration. Neurobiol Dis. 2006;22:435–444. doi: 10.1016/j.nbd.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Zhang L, Rubinow DR, Xaing G, Li BS, Chang YH, Maric D, Barker JL, Ma W. Estrogen protects against beta-amyloid-induced neurotoxicity in rat hippocampal neurons by activation of Akt. Neuroreport. 2001;12:1919–1923. doi: 10.1097/00001756-200107030-00030. [DOI] [PubMed] [Google Scholar]
- Zhao L, O'Neill K, Brinton RD. Selective estrogen receptor modulators (SERMs) for the brain: Current status and remaining challenges for developing NeuroSERMs. Brain Res Rev. 2005;49:472–493. doi: 10.1016/j.brainresrev.2005.01.009. [DOI] [PubMed] [Google Scholar]