Abstract
Rationale
There are complex relationships between stress and smoking; smoking may reduce the emotional discomfort of stress, yet nicotine activates stress systems and may alter responses to acute stress. It is important to understand how smoking affects physiological and psychological outcomes after stress and how these may interact to motivate smoking.
Objectives
This study aimed to examine the magnitude and time course of hormonal, cardiovascular, and psychological responses to acute psychosocial stress in smokers and non-smokers to investigate whether responses to acute stress are altered in smokers.
Materials and methods
Healthy male non-smokers (n=20) and smokers (n=15) participated in two experimental sessions involving a standardized public speaking stress procedure and a control non-stressful task. The outcome measures included self-reported mood, cardiovascular measures (heart rate and blood pressure), and plasma hormone levels (noradrenaline, cortisol, progesterone, and allopregnanolone).
Results
Smokers exhibited blunted increases in cortisol after the Trier Social Stress Test, and they reported greater and more prolonged subjective agitation than non-smokers. Stress-induced changes in progesterone were similar between smokers and non-smokers, although responses overall were smaller among smokers. Stress did not significantly alter levels of allopregnanolone, but smokers exhibited lower plasma concentrations of this neurosteroid.
Conclusions
These findings suggest that smoking dampens hormonal responses to stress and prolongs subjective discomfort. Dysregulated stress responses may represent a breakdown in the body’s ability to cope efficiently and effectively with stress and may contribute to smokers’ susceptibility to acute stress, especially during abstinence.
Keywords: Anxiety, Cardiovascular, Stress, Drug abuse, HPA axis, Nicotine
Introduction
There are complex bidirectional relations between stress and drug use. Stress is thought to be a contributing factor to the development and maintenance of drug use and dependence (Sinha 2001), and drug use itself appears to alter responses to stress. An acute physical or psychological stressor activates two main physiological systems, the hypothalamic pituitary adrenal (HPA) axis and the sympathoadrenal system, resulting in a range of hormonal, cardiovascular, subjective cognitive, and emotional responses, which can vary independently of one another. Many drugs of abuse, including nicotine and alcohol, also stimulate the same physiological systems as acute stressors. Laboratory studies conducted with both animals and humans have shown that repeated drug exposure can alter certain responses to external stressors (Kirschbaum et al. 1993a, 1994; Roy et al. 1994; Tsuda et al 1996; al’Absi et al. 2003; Mantsch et al. 2007; Chen et al. 2008). However, the specificity of alterations in different components of the stress response, i.e., hormonal, cardiovascular, and subjective, is not known, partly because few studies have examined the full profile of stress responses in a single study. It is important to fully understand how smoking affects each of the different physiological and psychological outcomes of acute stress and how these may interact to motivate smoking during stressful times. In this study, we sought to characterize alterations in hormonal, cardiovascular, and subjective responses to acute stress between male smokers and non-smokers.
The relationships between acute stress and smoking are particularly complicated. First, smoking appears to reduce emotional discomfort after acute stress (Perkins et al. 1992). Paradoxically, however, nicotine also activates the systems that mediate stress responses, and smoking may prolong the hormonal and cardiovascular response to stress (Fuxe et al. 1989; Pomerleau and Pomerleau 1990). Second, there is some evidence that smokers exhibit atypical responses to acute stress (Kirschbaum et al. 1993a, 1994; Roy et al. 1994; Tsuda et al. 1996; al’Absi et al. 2003). Finally, smoking abstinence, like smoking itself, may also be considered a stressor. Smokers undergoing acute withdrawal exhibit negative mood and tension (Swan et al. 1996), which may render them especially vulnerable to the perceived stress-relieving properties of smoking and thus at risk for relapse. An appreciation of how the different physiological and psychological responses to acute stress are altered in regular cigarette smokers will advance an understanding of why stress is such a powerful motivator of cigarette use.
Several studies have demonstrated that cigarette smokers exhibit altered responses to acute stress; however, these effects vary between studies and depend in part on the particular measure that is assessed. In most studies, smokers have exhibited blunted HPA axis responses (i.e., cortisol and ACTH) to a psychological stressor, compared to non-smokers (Kirschbaum et al. 1993a, 1994; Roy et al. 1994; Tsuda et al 1996; al’Absi et al. 2003; but see Back et al. 2008). These findings are consistent with animal data indicating that restraint stress-induced increases in plasma corticosterone are attenuated after a chronic regimen of nicotine administration (Kiritsy-Roy et al. 1990; Cheng et al. 2005). On the other hand, another study reported that smoking immediately after a stressful task prolonged stress-induced increases in cortisol (Pomerleau and Pomerleau 1990). Similarly, there is mixed evidence of altered cardiovascular responses to stress between smokers and non-smokers. Several studies have reported no differences in heart rate and blood pressure responses between smokers and non-smokers (Perkins et al. 1992; Kirschbaum et al. 1993a; Tsuda et al. 1996), while others report enhanced (MacDougall et al. 1988) or diminished cardiovascular responses to stress among smokers (Roy et al. 1994; al’Absi et al. 2003). With regard to subjective responses to stress, smokers tend to exhibit greater and more prolonged emotional reactions to stressful tasks than non-smokers (Perkins et al. 1992; Tsuda et al. 1996), although smoking immediately after stress appears to counteract emotional discomfort (Tsuda et al. 1996). Many of these studies focused upon only one measure of the stress response, usually HPA axis responses, making it difficult to visualize how the full range of stress responses may be altered in smokers. In addition, some of these studies lacked a non-stressful control condition with which to compare stress responses, which makes it difficult to fully interpret the results. Together, these factors have contributed to the discrepancy in findings between studies; however, overall, it appears that a history of smoking blunts hormonal response to stress, but may exacerbate subjective responses. Because smoking appears to differentially affect separate physiological and psychological components of stress responses, it is important to fully characterize the specific changes in each modality that occur in regular cigarette smokers in a single study. These alterations may influence long-term health and may have implications during early smoking abstinence.
In this study, we measured hormonal, cardiovascular, and subjective responses to a standardized acute psychosocial stressor, the Trier Social Stress Test (TSST, Kirschbaum et al. 1993b), in healthy male smokers and non-smokers. We examined the magnitude and time course of changes in a range of physiological and psychological measures in order to fully characterize responses to acute stress, including activation of the HPA axis (cortisol) and sympathoadrenal systems (plasma noradrenaline, heart rate, blood pressure) and emotional changes (self-reported mood). Recent evidence has indicated that progesterone and allopregnanolone, a metabolite of progesterone, may be important regulators of responses to acute stress (Barbaccia et al. 1996), and so, we also measured plasma levels of these hormones. By examining a wide range of measures, we could identify whether alterations are specific to each system or whether there is a more general dysregulation of the stress response in smokers. Participants also underwent a separate non-stressful control session with which we could compare responses in order to fully interpret the findings. We did not include women in this study of smokers due to concerns of variability in mood and hormonal measures across the menstrual cycle, which we are investigating in a separate experiment. We hypothesized that in line with previous studies, smokers would exhibit blunted hormonal responses, e.g., cortisol, to stress yet more pronounced subjective discomfort, e.g., anxiety, than non-smokers. In addition, we hypothesized that stress would increase plasma levels of the neurosteroids progesterone and allopregnanolone but that these responses would be smaller in smokers.
Materials and methods
Participants
Male non-smokers (n=20) and daily cigarette smokers (n= 15, ≥10 cigarettes per day) aged 18–32 years were recruited without regard to race or ethnicity. The exclusion criteria included serious medical conditions; current or prior diagnosis of Axis I disorder (APA 1994), including drug abuse or dependence except nicotine dependence; less than a high school education; a body mass index outside of 19–26 kg/m2; high or low blood pressure; abnormal electrocardiogram; or night shift work. Before the study began, participants signed a consent form, which stated that the aim of study was to investigate the effects of alertness upon mood and physiology. They were told that they would complete short verbal tasks during each session but were not told the specific nature of the tasks.
Design
This study used a mixed within- (Stress vs. No stress) and between-subjects (Smokers vs. Non-smokers) design. Volunteers participated in two 4 h experimental sessions, which always commenced at 9:00 a.m., at least 48 h apart, in which they underwent the TSST or a control task in randomized order.
Procedure
The University of Chicago Hospital’s Institutional Review Committee for the use of human subjects approved the protocol, and the experiments were therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Participants were tested individually in patient rooms at the University of Chicago Hospital’s GCRC. They stayed overnight at the GCRC before each session to standardize sleeping, eating, and smoking. Volunteers were woken at 7:30 a.m., and smokers smoked one cigarette at 8:00 a.m. Participants were provided with a standard breakfast prepared by the GCRC nutritionist and could consume their usual amounts of caffeine. Individuals submitted breath and urine samples to test for recent use of drugs or alcohol; no one tested positive. At 8:30 a.m., an intravenous catheter was placed into a vein in the subject’s non-dominant arm for collection of blood samples. At 9:00 a.m., levels of CO in expired air were recorded, and a blood sample was drawn for DNA extraction. Participants were given a heart rate monitor and Polar chest band to wear, which measured heart rate continuously (Mini-Logger® system). At 9:40 a.m., baseline measures were obtained [blood and saliva samples, blood pressure (Critikon Dinamap® Plus Vital Signs Monitor), and mood questionnaires]. At 9:50 a.m., the research assistant read instructions to the participant. For the TSST, the instructions stated that they had 10 min to prepare a 5 min speech about themselves for a mock job interview that would be videotaped and performed in front of two examiners trained to monitor non-verbal behavior. They would also be required to perform 5 min of arithmetic (serial subtraction). For the control task, the instructions stated that participants would speak to the research assistant about neutral topics for 10 min (not videotaped). Participants remained alone in a room for 10 min before each task and could make notes about their speech, although they could not bring their notes into the examination. At 10:00 a.m., after the 10 min preparatory period had elapsed, participants were escorted to an adjacent room for the tasks. Throughout the TSST, participants could see their own image portrayed on a television monitor. At the end of the task, the participant was escorted back to the patient room. Both the TSST and control tasks lasted for 10 min each. Blood pressure was measured, and blood and saliva samples were obtained immediately and then again at 5, 10, 20, 30, 60, 90, and 120 min after the tasks. Participants also completed mood questionnaires immediately and then again at 10, 20, 30, 60, 90, and 120 min after completing the tasks. After the last measures, participants were given a meal and then allowed to leave. At the end of the second session, participants were fully debriefed about the study procedures and received payment.
Hormonal measures
Saliva samples were collected using Salivette® cotton wads. The GCRC Core Laboratory at The University of Chicago determined levels of cortisol in saliva and plasma (saliva= Salimetrics LLC, sensitivity=0.003 µg/dL; plasma=Immulite®/Immulite® 1000 Cortisol, sensitivity=0.2 µg/dL). The University of Chicago Medical Endocrinology Laboratory determined levels of progesterone in plasma (Immulite® 2000 Progesterone, sensitivity=0.1 ng/mL). Dr. Richard Hauger at The University of California, San Diego, determined levels of plasma allopregnanolone according to the methods of Purdy et al. (1990). Mayo Medical Laboratories determined levels of plasma catecholamines [Test code 8532, sensitivity: NA=70 pg/mL, Adr=110 pg/mL, DA 30 pg/mL]. Unfortunately, plasma samples collected for ACTH assay were spoiled after a freezer malfunction, and insufficient data were obtained for meaningful statistical analysis.
Subjective measures
Subjective states were measured using the State version of the STAI (Spielberger et al. 1970), VAS (Folstein and Luria 1973), and an experimental version of the POMS (McNair et al. 1971). Smokers also completed the BQsu (Tiffany and Drobes 1991) to assess cigarette craving.
Statistical analysis
Differences in demographics between smokers and non-smokers were analyzed using independent samples t test (continuous variables) and Pearson’s Chi-Square (categorical variables). The effects of demographic characteristics upon responses to the TSST were analyzed using two-factor [task (TSST vs. control) × characteristic] ANOVA for repeated measures (categorical variables) or one-factor (Task) ANCOVA with the characteristic as a covariate (continuous variables).
We examined group differences in the measures at baseline using independent samples t test. Baseline values were expressed as the average of the pre-task values during the stress and no stress sessions (since there were no significant differences between the values at baseline before each task or effects of session order).
Physiological, hormonal, and subjective data were expressed as changes from the pre-task baseline at each time-point during the stress and no stress sessions. Peak change scores were expressed as the maximum change within 30 min after the task. First, we assessed the effects of task order upon responses to the TSST using one-factor (peak change scores) or two-factor (changes across time) ANOVA for repeated measures with task (TSST vs. control) and time (repeated time points during each session) as within-subjects factors and task order as between-subjects factor. Any measures that were significantly influenced by task order were not analyzed further.
Peak change scores were analyzed using two-factor ANOVA for repeated measures with task as within-subjects factor and group (smokers vs. non-smokers) as between-subjects factor. The time course of changes in dependent measures was analyzed by three-factor ANOVA for repeated measures with task and time as within-subjects factors and group as between-subjects factor. Significant baseline differences between groups were addressed by (1) including the average baseline value (of stress and no stress session) as a covariate in peak change score analyses and (2) analyzing absolute values of that measure for time course analysis.
All analyses were conducted using SPSS version 14 for Windows. Missing cases (due to equipment malfunction, problems with sample collection) were deleted list wise, leading to smaller sample sizes for some analyses. Due to the large number of different measures assessed and analyses performed, we selected several qualitatively different outcome measures as our primary variables, i.e., STAI anxiety, cortisol, heart rate, and blood pressure. We did not correct these primary measures for multiple comparisons, because they are expected to reflect separate and independent underlying processes, and thus, differences were considered to be significant if p<0.05. For the other secondary measures, p values were adjusted by Bonferroni correction to limit experiment-wise error; changes in POMS measures were considered significant if p<0.005 (corrected for ten empirically defined scales), for VAS items p<0.006 (corrected for eight items), and for plasma hormones p< 0.016 (corrected for three measures). Effect sizes are reported throughout: Cohen’s d for Student’s t tests and partial eta-squared for (ηP2) ANOVAs.
Results
Demographic characteristics
Most participants were European-American full-time students in their early twenties (Table 1). Smokers were older, less likely to be full-time students, and more likely to report previous use of other drugs, including stimulants, sedatives, hallucinogens, and inhalants. No demographic characteristic significantly influenced responses to the TSST. Age was included in later analyses as a covariate but was dropped if there were no significant main effects or interactions with group.
Table 1.
Demographic characteristics of participants
| Demographic characteristic | Non-smokers | Smokers |
|---|---|---|
| N | 20 | 15 |
| Age (mean±SEM) | 20.5±.6 | 23.2±1.2* |
| BMI (kg/m2) | 22.1±.4 | 22.9±.5 |
| Height (inches) | 70.7±.6 | 70.3±.8 |
| Weight (lbs) | 157.6±3.9 | 160.7±5.3 |
| Race | ||
| % European-American | 55 | 53.3 |
| % African-American | 20 | 20 |
| % Asian-American | 15 | 6.7 |
| % Other | 10 | 20 |
| Current drug use (mean±SEM) | ||
| Alcoholic drinks/week | 4.4±1.0 | 7.5±1.5 |
| Caffeine cups/week | 4.5±1.5 | 12.7±4.6 |
| Cigarettes/week | 0.0 ± 0.0 | 95.9±10.1*** |
| Marijuana times/month | 1.25±0.5 | 3.1±1.3 |
| Lifetime drug use (% ever used) | ||
| Stimulants | 5 | 80*** |
| Sedatives | 0 | 33.3** |
| Hallucinogens | 10 | 46.7* |
| Opiates | 45 | 73.3 |
| Marijuana | 90 | 100 |
| Inhalants | 0 | 46.7** |
| Full time student (%) | 90 | 47** |
Data indicate mean±SEM. Asterisks indicate a significant difference between the groups
p<0.05;
p<0.01;
p<0.001 (Pearson Chi-square for categorical variables; independent samples t test for continuous variables)
Baseline differences
At baseline, smokers exhibited significantly higher levels of plasma cortisol than non-smokers [t(33)=−2.8, p=0.01, Cohen’s d=−0.97, r=0.44], while non-smokers exhibited higher levels of allopregnanolone than smokers [t(32)=3.4, p<0.01, Cohen’s d=1.2, r=0.44; see Table 2 for values].
Table 2.
Average pre-task baseline and changes in dependent measures after the TSST and control tasks for smokers (n=15) and non-smokers (n=20)
| Dependent measure | Non-smokers | Smokers | Statistics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Control | TSST | Baseline | Control | TSST | Task | Group | Task×group | ||
| STAI | 34.8±2.0 | −2.1±1.4 | 5.4±2.2 | 35.9±2.0 | −2.5±2.7 | 6.3±2.5 | 12.9 (0.29) | 0.0 (0.00) | 0.1 (0.00) | |
| POMS | Anger | 1.9±0.6 | 0.1±0.6 | 3.6±1.3 | 2.9±0.8 | −1.6±0.9 | 4.0±1.4 | 21.0 (0.40) | 0.3 (0.01) | 1.1 (0.03) |
| Anxiety | 4.9±0.8 | −0.6±0.9 | 3.4±1.4 | 5.5±1.0 | −0.5±1.0 | 3.2±1.7 | 7.6 (0.19) | 0.0 (0.00) | 0.02 (0.00) | |
| Positive mood | 7.4±1.3 | 1.1±1.3 | −4.9±1.2 | 4.8±1.6 | 3.1±1.5 | −2.7±2.9 | 9.9 (0.24) | 0.0 (0.00) | 1.8 (0.05) | |
| VAS | Anxious | 23.8±6.3 | −0.6±4.1 | 5.2±6.5 | 25.6±5.0 | −4.3±5.5 | 14.3±7.0 | 4.4 (0.12) | 0.2 (0.01) | 1.2 (0.04) |
| Jittery | 14.3±4.3 | 0.9±4.6 | 3.0±4.9 | 12.6±3.1 | −0.6±5.2 | 22.9±6.3 | 6.4 (0.17) | 2.8 (0.08) | 4.5 (0.12) | |
| Restless | 23.4±4.5 | 4.8±3.8 | −4.9±6.9 | 22.2±4.3 | 1.5±6.0 | 26.6±7.7 | 1.6 (0.05) | 4.8 (0.14) | 8.3 (0.22) | |
| Heart rate (bpm) | 70.9±2.1 | 20.6±2.1 | 32.9±3.8 | 70.1±2.5 | 17.4±2.2 | 26.7±3.3 | 19.7 (0.43) | 1.6 (0.06) | 0.4 (0.01) | |
| Systolic BP (mm Hg) | 126.1±2.0 | −10.4±4.0 | 12.0±3.7 | 123.7±3.3 | −5.1±3.2 | 4.9±4.4 | 16.5 (0.33) | 0.1 (0.00) | 2.5 (0.07) | |
| Diastolic BP (mm Hg) | 63.6±1.3 | 4.2±2.4 | 7.5±2.0 | 65.5±1.5 | −3.5±2.1 | 6.6±2.1 | 7.7 (0.19) | 4.5 (0.12) | 2.0 (0.06) | |
| Plasma cortisol (µg/dL) | 11.3±0.6 | 2.9±0.8 | 5.4±0.8 | 13.4±0.5 | 1.0±1.5 | 2.0±1.7 | 2.8 (0.08) | 4.2 (0.11) | 0.5 (0.02) | |
| Salivary cortisol (µg/dL) | 0.46±0.07 | 0.13±0.08 | 0.58±0.18 | 0.55±0.05 | −0.07±0.09 | −0.08±0.10 | 4.8 (0.13) | 6.9 (0.18) | 5.4 (0.15) | |
| Progesterone (ng/mL) | 0.42±0.02 | 0.05±0.04 | 0.19±0.03 | 0.50±0.04 | −0.03±0.05 | 0.12±0.07 | 13.8 (0.30) | 2.0 (0.06) | 0.01 (0.00) | |
| Allopregnanolone (ng/mL) | 1.6±0.2 | −0.02±0.17 | 0.22±0.12 | 0.9±0.1 | 0.13±0.09 | −0.05±0.08 | 0.5 (0.01) | 2.5 (0.07) | 2.8 (0.08) | |
| Noradrenaline (pg/mL) | 205.0±17.8 | 52.5±30.2 | 207.4±48.9 | 243.7±17.8 | 99.4±30.2 | 159.3±36.4 | 10.4 (0.24) | 0.0 (0.00) | 2.0 (0.06) | |
Data represent the mean±SEM average pre-task baseline and peak change from pre-task baseline within 30 min after each task. Statistics column presents the F value and effect size in parentheses for two-factor (task×group) ANOVA with repeated measures. Significant findings are indicated in bold. For allopregnanolone, average baseline values were included as covariate (see “Results” section)
Subjective responses to stress
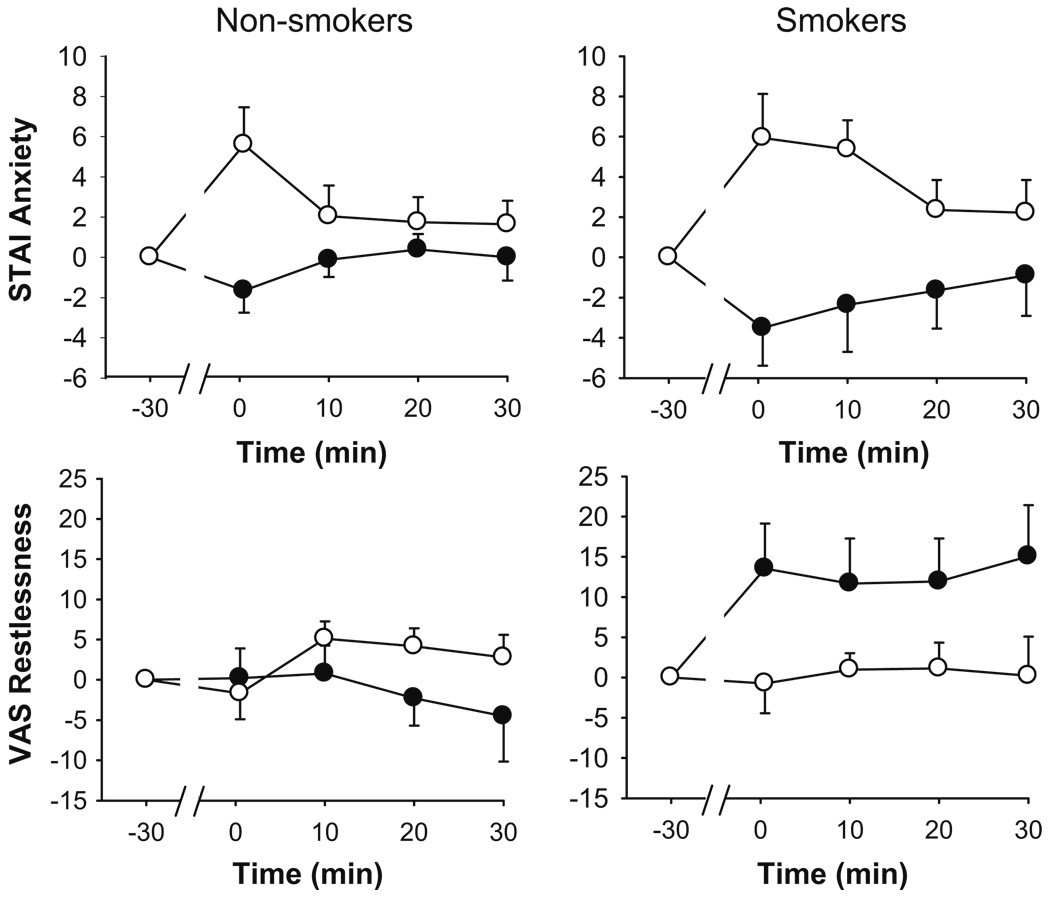
In comparison to the control task, the TSST significantly increased reports of anxiety (STAI) and frustration (POMS Anger). Stress also significantly increased mood disturbance (POMS Confusion) and decreased positive mood (POMS Positive Mood; see Table 2 for values and significance). Only the smokers exhibited elevated feelings of psychomotor agitation (VAS Restlessness and Jitteriness), although in general, the magnitude of subjective responses to the tasks did not differ between smokers and non-smokers. The effects of the TSST upon mood were short-lived; for example, peak changes in anxiety were exhibited immediately after the task ended and had returned to baseline 10 min later (see Fig. 1). However, analysis of changes in Restless and Jittery across each session (see Fig. 1) revealed a trend to prolonged TSST-induced increases among smokers (task × Group, p<0.05, ). Simple effects analyses revealed a significant main effect of stress among smokers (p<0.05, ).
Fig. 1.
Changes in self-reported anxiety and restlessness after the TSST (filled symbols) and control tasks (open symbols). Data represent mean±SEM changes from pre-task baseline values as a function of time after completion of the tasks (task preparation from −20 to −10 min; task performance from −10 to 0 min)
Cigarette craving (BQSU global scores) increased nonspecifically throughout the session in smokers (time, p< 0.05, ); however, there was no significant main effect of the TSST or interaction with time upon craving scores.
Cardiovascular responses to stress
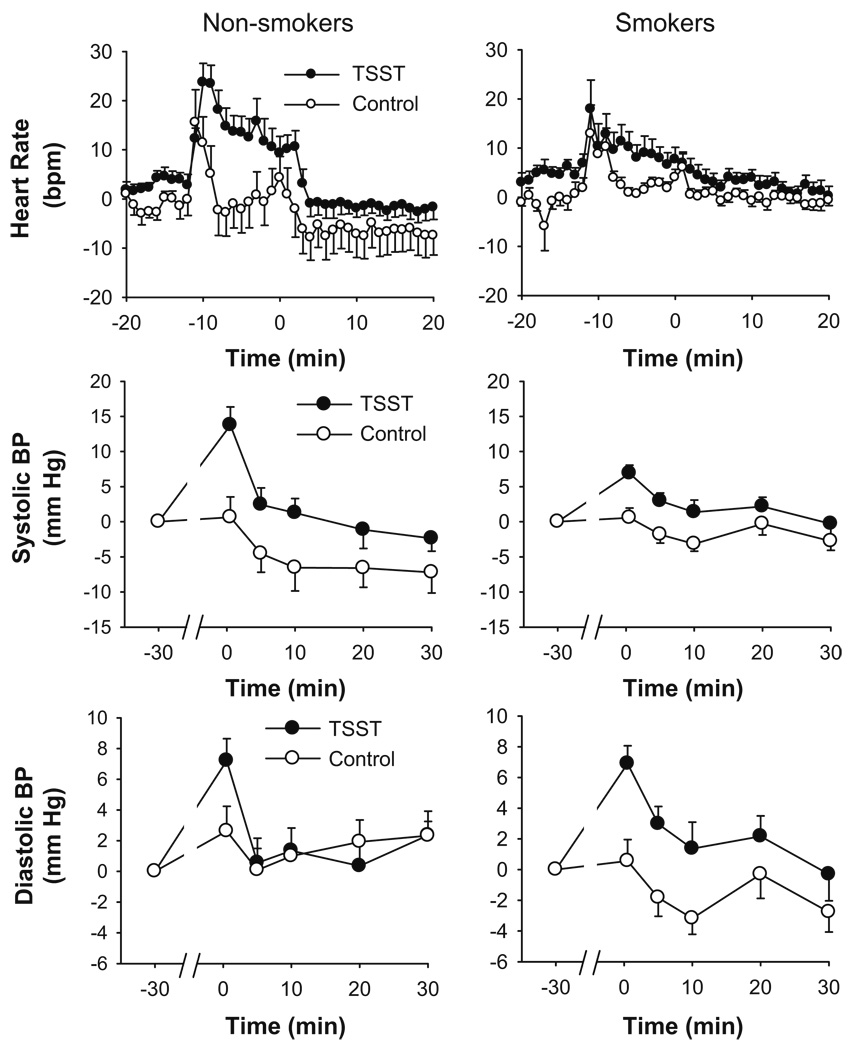
Compared to the control task, the TSST significantly increased heart rate and systolic and diastolic blood pressure. Mean peak increases in heart rate and systolic blood pressure were similar in each group, although overall, changes in diastolic blood pressure were smaller amongst smokers. Analysis of heart rate responses across each session revealed subtle differences between the groups in the pattern of changes (group × time, p=0.001, ; see Fig. 2). Simple effects analysis (separate task × time ANOVAs for each group) revealed that the TSST selectively influenced heart rate only among the non-smokers (task × time, p<0.001, ). Stress significantly increased heart rate among smokers (task p=0.02, ); however, there was no significant interaction of the task with time (p=0.7, ).
Fig. 2.
Changes in cardiovascular measures after the TSST (filled symbols) and control tasks (open symbols). Data represent mean±SEM changes from pre-task baseline as a function of time after completion of the tasks (task preparation from −20 to −10 min; task performance from −10 to 0 min)
Hormonal responses to stress
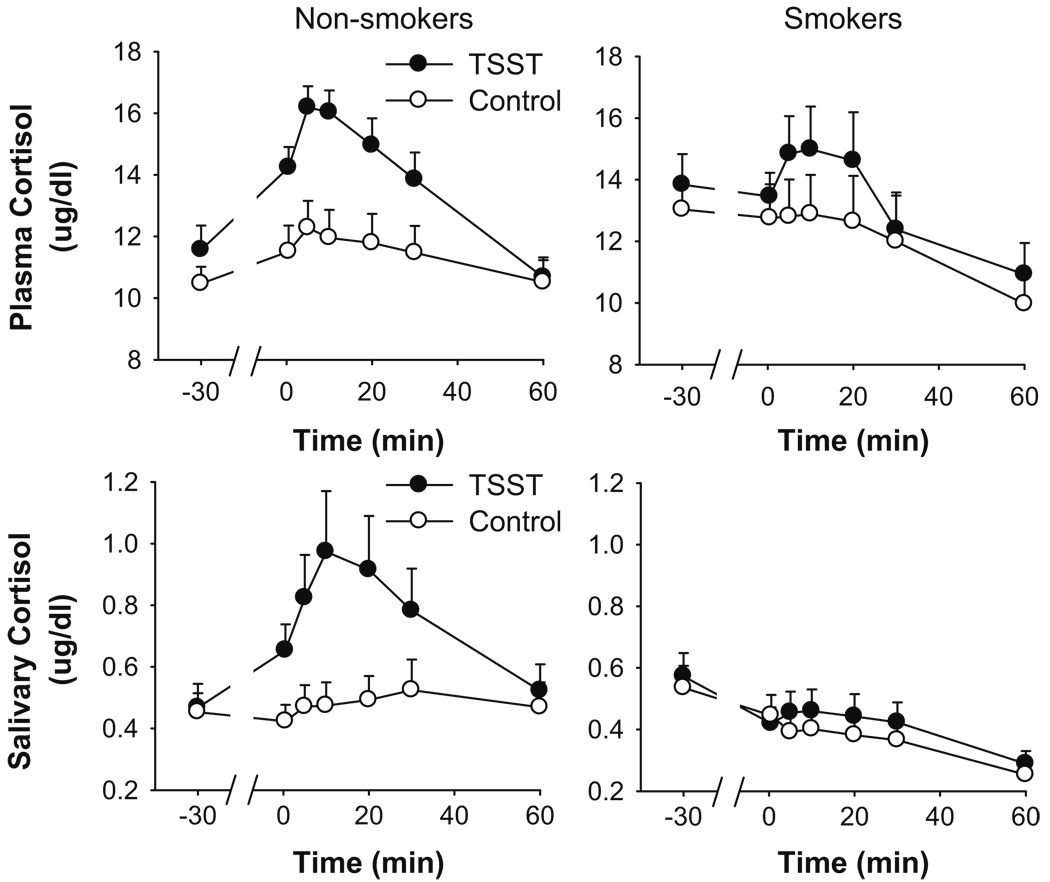
Compared to the control task, the TSST significantly increased salivary free cortisol only among non-smokers (see Table 2 for peak change scores; Fig. 3 for time course analysis task × time × group, p<0.01, ). Simple effects analysis revealed a significant task × time interaction amongst the non-smokers (p<0.001, ) but not amongst smokers (p=0.8, ).
Fig. 3.
Changes in plasma and salivary cortisol after the TSST (filled symbols) and control tasks (open symbols). Data represent mean±SEM absolute values of plasma and salivary cortisol as a function of time in minutes after completion of the tasks (task preparation from −20 to −10 min; task performance from −10 to 0 min)
For total plasma cortisol, the magnitude of change was smaller and more variable among the smokers (Table 2). Including average baseline values, which were higher among smokers, as a covariate in the analysis did not significantly alter the result. Analysis of the time course of changes in plasma cortisol across each session revealed a significant interaction between group and time (p<0.05, ; Fig. 3). Analysis of simple effects (separate task × time ANOVAs for each group) revealed that the TSST increased plasma cortisol in non-smokers (task × time, p<0.001, ) but not in smokers (p=0.7, ). In fact, among smokers, levels of plasma cortisol declined across each session.
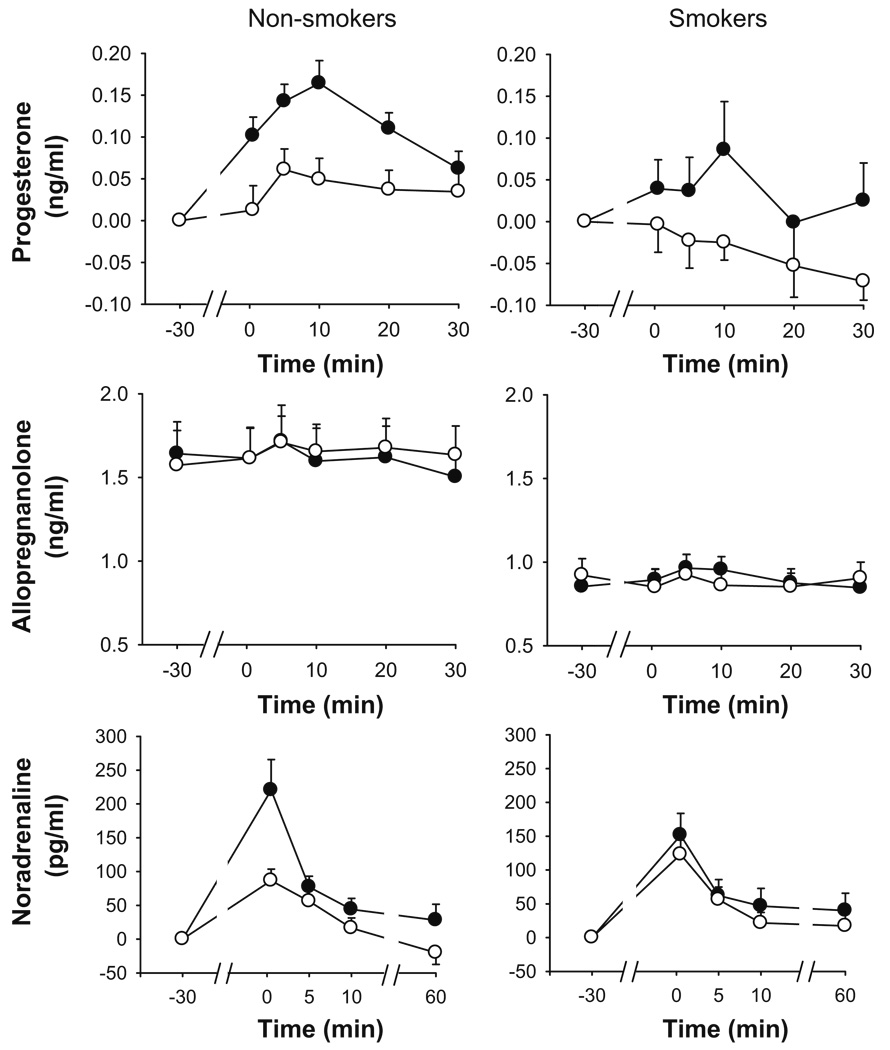
The TSST significantly increased levels of plasma progesterone to a similar extent in smokers and non-smokers (Table 2). Analysis of changes across each session showed that peak increases were exhibited 10 min after the task among both groups (Fig. 4) and that on the whole, smokers exhibited smaller progesterone responses than smokers [group, p=0.014, ].
Fig. 4.
Changes in plasma hormones after the TSST (filled symbols) and control tasks (open symbols). Data represent mean±SEM (1) change from pre-task baseline for progesterone and noradrenaline, (2) absolute values for allopregnanolone, across time in min after completion of the tasks (task preparation from −20 to −10 min; task performance from −10 to 0 min)
The TSST did not significantly influence mean peak changes in allopregnanolone in either group (Table 2), although baseline values did affect task-induced changes . Specifically, there was a negative association between baseline levels of allopregnanolone and changes after the tasks.
The TSST significantly increased plasma noradrenaline similarly in each group (Table 2). Analysis of changes across time showed that peak changes were exhibited immediately after the task. There were group differences in the pattern of changes across each session, although this was not significant after correction for multiple comparisons [task × group × time, p<0.05, ; Fig. 4].
Discussion
There are complex relationships between stress and smoking. This study aimed to characterize the magnitude and time course of physiological and psychological responses to acute psychosocial stress in smokers and non-smokers. Healthy male non-smokers (N=20) and smokers (N=15) participated in a stress session and a no stress session. In comparison to the non-smokers, smokers exhibited blunted cortisol responses to acute stress yet reported more psychomotor agitation. Stress produced increases in plasma progesterone in both smokers and non-smokers, and overall, responses were smaller among smokers. Allopregnanolone, a neurosteroid that has been implicated in mediating stress responses, was not significantly altered by the stressful task, but plasma levels were lower among smokers. These findings highlight important differences in the acute response to stress and mediators of the response between cigarette smokers and non-smoking controls. These alterations may have implications for long-term health and may underlie why stress is such a potent motivator of smoking behavior.
There were few differences between the smokers and non-smokers in the measures before exposure to the stress task. Smokers exhibited higher baseline levels of cortisol that is consistent with previous reports in minimally deprived (up to 3 h) smokers (Kirschbaum et al. 1992; Field et al. 1994; Baron et al. 1995; al’Absi et al. 2003; Steptoe and Ussher 2006). It is thought that nicotine directly activates the HPA axis via stimulation of nicotinic receptors upon noradrenergic neurons in the nucleus tract solitarius, which project to CRH-containing neurons in the paraventricular nucleus of the hypothalamus (Fuxe et al. 1989; Matta et al. 1998). Stimulation of these neurons induces increases in plasma ACTH and corticosterone in animals (Balfour et al. 1975; Cam and Bassett 1983a, b) and cortisol in humans (Newhouse et al. 1990). In our study, smokers were required to smoke a single cigarette 2 h before the first samples were obtained in order to standardize the time since last smoking, and evidence indicates that smoking a single high-nicotine-yield cigarette acutely increases cortisol levels (Mendelson et al. 2005). However, a recent study found that awakening cortisol response and total cortisol release during waking hours were significantly greater among smokers than non-smokers (Badrick et al. 2007). Awakening cortisol response but not total cortisol release was related to the level of smoking, which suggests that mechanisms other than the direct effects of nicotine are responsible for elevated cortisol secretion in smokers. However, in our study, it is not definitively clear whether elevated baseline cortisol is a long-term consequence of smoking or reflects transient increases related to the pre-session cigarette (see later for further discussion of this point).
In comparison to non-smokers, smokers exhibited significantly lower plasma levels of the neurosteroid allopregnanolone at baseline. A recent study reported that serum allopregnanolone was positively correlated with salivary cotinine in smokers enrolling in a cessation study (Marx et al. 2006); however, in that study, the time since last cigarette and quantity of smoking prior to sample collection was not controlled. In addition, in that study, serum allopregnanolone was measured, while in our study, we measured plasma allopregnanolone, and there may be differences between the two measures. In rats, acute nicotine administration dose dependently increases allopregnanolone in the cerebral cortex (Porcu et al. 2003); however, the effects of chronic nicotine administration have not been investigated. In our study, we cannot differentiate between the effects of habitual smoking and those of the cigarette smoked before the session, but together, the evidence suggests an association between nicotine intake or level of smoking and allopregnanolone, although the specific relationship is unclear from the available evidence.
Overall, the magnitudes of subjective responses to the TSST were similar in both groups. However, only smokers reported elevated subjective agitation: jitteriness and restlessness. Thus, stress appeared to produce more symptoms of anxiety among smokers. In addition, increases in these measures were long lasting and remained elevated up to 90 min after the task (data not shown). This is in line with others’ findings that negative subjective responses to stress are prolonged in smokers (Perkins et al. 1992; Tsuda et al. 1996). Smokers report that smoking relieves stress-induced tension, and there is also empirical evidence to support this (Tsuda et al. 1996). Therefore, the present finding that stress produced prolonged psychomotor agitation in smokers who were not able to smoke after the stressor may explain why stress is such a powerful motivator of smoking behavior, i.e., to relieve subjective distress. It would be interesting to assess whether smoking after stress alters the time course of increases in subjective agitation. Indeed, prolonged emotional responses to stress among abstinent smokers may increase their susceptibility to relapse especially if they are unable to manage or relieve their heightened agitation by other methods. These findings highlight the importance of coaching smokers in alternative ways of managing the emotional effects of stress before they begin abstinence, which may improve success rates.
In line with the results of some studies (Perkins et al. 1992; Kirschbaum et al. 1993a; Tsuda et al. 1996), the magnitude of peak heart rate responses to the TSST did not differ significantly among groups. However, there were subtle differences in the pattern of heart rate responses to stress between smokers and non-smokers; there was a clear distinction between the periods of the stressful session for non-smokers, while smokers exhibited a more measured pattern of changes across time and heart rate returned to baseline more slowly. There is evidence that the rapid return of heart rate to resting levels following a physical stressor is indicative of physical fitness (Astrand and Rodahl 1977; Cooper et al. 1977; Peters et al. 1983), and our findings may extend this relationship to cardiovascular responses to acute psychosocial stress. In addition, diastolic blood pressure responses were smaller overall among smokers. This has not previously been reported and should be interpreted with caution until replicated.
In line with previous studies, salivary free cortisol responses to the tasks were blunted in smokers (al’Absi et al. 2003; Kirschbaum et al. 1993a, b, 1994; Roy et al. 1994; Tsuda et al. 1996). The mean peak total plasma cortisol response did not differ between the groups, which may have been due to large variation in responses amongst smokers. However, analysis of the time course of changes in absolute values of plasma cortisol revealed that only non-smokers mounted a significant plasma cortisol response to the TSST. Smokers exhibited significantly higher levels of plasma cortisol at baseline, and this may have influenced adrenocortical responses to the TSST. First, the high prestress baseline may have produced a ceiling effect upon stress-induced cortisol production. Second, overall, there was a decline in plasma cortisol from the beginning to the end of experimental sessions among smokers; however, levels at the end did not differ from non-smokers. Nevertheless, the findings indicate altered adrenocortical responses to psychosocial stress in minimally deprived smokers. Altered adrenocortical sensitivity to acute psychosocial stress in smokers may be mediated at the level of the hypothalamus, pituitary, or adrenal. Unfortunately, due to sample loss, we were not able to collect sufficient data to analyze levels of ACTH in plasma. Thus, it is unclear whether ACTH responses are similarly blunted in smokers or whether pituitary responsiveness to psychosocial stress remains intact, and instead, the adrenals are hyposensitive to ACTH signal.
Stress significantly increased levels of progesterone in both smokers and non-smokers alike. This is one of the first demonstrations of stress-induced increases in progesterone in humans. Animal studies have previously shown that levels of progesterone are elevated in response to acute stressors, including restraint and swim stress (Purdy et al. 1991; Barbaccia et al. 1996), and in humans in response to emotion arousing stimuli (Schultheiss et al. 2004; Wirth and Schultheiss 2006; Wirth et al. 2007). It is thought that stress-induced progesterone release is stimulated by ACTH (Genazzani et al. 1998) and may represent a homeostatic mechanism to counteract the subjective effects of stress and down-regulate HPA axis activity (Barbaccia et al. 1996). Allopregnanolone, a metabolite of progesterone, is a highly efficacious positive modulator of GABAA receptor function with potency at least equal to or greater than benzodiazepines and barbiturates (Paul and Purdy 1992). Thus, progesterone, via its metabolite allopregnanolone, produces sedative effects in humans (de Wit et al. 2001; Soderpalm et al. 2004) and decreases anxiety-like behaviors in animals (Wieland et al. 1991; Bitran et al. 1991, 1993, 1995). In addition, animal studies indicate that allopregnanolone attenuates elevations in CRH and corticosterone induced by stress and pharmacological agents (Purdy et al. 1991; Patchev et al. 1994, 1996). This effect is likely mediated by actions of allopregnanolone at GABAA receptors upon CRH-containing neurons. Thus, stress-induced increases in progesterone may, via allopregnanolone, serve to counteract increases in anxiety and also limit HPA axis activation. Overall, smokers exhibited lower progesterone responses, which could underlie the prolonged mood disturbances (increased jitteriness and restlessness) exhibited by these individuals.
Allopregnanolone is a major metabolite of progesterone, yet it is also itself released from the adrenals and synthesized by brain glial cells in response to stress (Patchev et al. 1996). Overall, the TSST did not significantly alter levels of allopregnanolone in this experiment, and there was quite large variability between subjects in each group. However, there were clear differences in plasma concentrations of allopregnanolone between smokers and non-smokers. As discussed, allopregnanolone produces anxiolytic and sedative effects via actions at GABAA receptors, and lower concentrations of allopregnanolone in cerebrospinal fluid have been related to depressive symptoms in humans (Uzunova et al. 1998). However, it is unclear whether plasma levels of allopregnanolone reflect central allopregnanolone concentrations. Although there were no significant differences in mood between smokers and non-smokers at baseline, it will be interesting to further investigate whether there is dysregulated neurosteroid function in smokers, particularly of anxiolytic neurosteroids, and whether this is related to symptoms of anxiety and depression in withdrawal.
The TSST significantly increased plasma noradrenaline, and mean peak increases did not differ amongst the groups. However, the time course of changes differed between smokers and non-smokers; stress significantly potentiated task-induced increases only amongst the non-smokers. It is interesting that smokers did not show a significantly larger noradrenaline response after the TSST in comparison to the control task, and this should be investigated further. The large increase in noradrenaline after the control task was likely a result of physical and mental exertion during this task.
Limitations of the study include the relatively small sample size, the large number of statistical analyses performed, and the small variation in level of smoking between subjects. Together, the first two factors highlight that the results should be interpreted with caution; however, in support of our results are that several of the findings replicate previous reports and that we did correct significance levels for experiment-wise error among the secondary measures. It would be interesting to further investigate the major findings, i.e., altered cortisol and subjective responses, in smokers with a greater variation in the level of habitual smoking to assess whether these alterations are related to the effects of the pre-session cigarette or to the chronic effects of smoking. In this study, we particularly wanted to avoid the influences of nicotine withdrawal upon the measures at baseline and in response to stress. In addition, previous studies have assessed stress responses in smokers after a minimal period of deprivation (usually 2–3 h from last smoking to participation in a task). Thus, we felt it important to standardize the time since last smoking among individuals in the smoking group to remove variation due to this factor. Others have investigated stress responses between deprived and non-deprived smokers; however, this may only differentiate the acute effects of smoking from the acute effects of nicotine withdrawal. One further limitation was that caffeine intake before the tasks was not controlled. Again, we particularly wanted to avoid the effects of caffeine withdrawal upon the measures at baseline and after stress, and thus, we felt it important that individuals participate in the tasks in their “normal” state, i.e., not caffeine-deprived, as they would experience stress in every day life. There was no effect of level of caffeine consumption upon outcomes to the tasks, and there was also no difference in caffeine intake between the groups; however, we recognize that acute effects of caffeine may have contributed to variability in the effects observed.
A strength of our study is that we measured both peak responses to and also the time course of changes in measures after acute stress. The TSST is a procedure that has more significance than other laboratory manipulations to stressful encounters in real life. However, there is often large inter-individual variation in the magnitude of responses to the TSST. Thus, our findings highlight the importance of also considering the time course of responses to stress in order to fully characterize alterations in stress responses between experimental groups.
In conclusion, the present findings reveal blunted cortisol responses and more prolonged psychological agitation in response to psychosocial stress among cigarette smokers. Altered responses to acute stress in smokers may denote a breakdown in the body’s ability to cope efficiently and effectively with stress. These alterations may also underlie why stress is such a potent motivator of smoking behavior, causing high rates of stress-induced relapse in abstinent smokers. It is unclear whether chronic nicotine administration by smoking induces adaptations in stress systems that underlie altered responses to stress or whether there are certain pre-existing disturbances, which render individuals vulnerable to smoking. If these system alterations are induced by nicotine, then it is also uncertain whether these systems return to normal functioning upon continued abstinence. Overall, the present findings highlight the importance of coaching smokers in alternative methods, besides smoking, to cope with the effects of psychological stress during early abstinence.
Acknowledgments
These experiments complied with current US laws. The authors declare that they have no conflicts of interest. This research was supported by NIDA (DA02812) and the University of Chicago Hospital’s GCRC (USPHS MO1RR000555). We thank Ben Cunningham, Stephen Sittler, Lisa Vicini, Heather Phillips, and Nicholas Van Dam for their technical assistance.
Contributor Information
Emma Childs, Department of Psychiatry and Behavioural Neuroscience, The University of Chicago, 5841 S. Maryland Ave., MC 3077, Chicago, IL 60637, USA, e-mail: echilds@uchicago.edu.
Harriet de Wit, Department of Psychiatry and Behavioural Neuroscience, The University of Chicago, 5841 S. Maryland Ave., MC 3077, Chicago, IL 60637, USA.
References
- al’Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol Biochem Behav. 2003;74:401–410. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- APA. American psychiatric association diagnostic and statistical manual of psychiatry. 4th edn. Arlington: APA; 1994. [Google Scholar]
- Astrand P, Rodahl K. Physiological basis of exercise. 2nd ed. New York: McGraw-Hill Book Co.; 1977. Textbook of work physiology. [Google Scholar]
- Back SE, Waldrop AE, Saladin ME, Yeatts SD, Simpson A, McRae AL, Upadhyaya HP, Contini Sisson R, Spratt EG, Allen J, Kreek MJ, Brady KT. Effects of gender and cigarette smoking on reactivity to psychological and pharmacological stress provocation. Psychoneuroendocrinology. 2008;33:560–568. doi: 10.1016/j.psyneuen.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. J Clin Endocrinol Metab. 2007;92(3):819–824. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- Balfour DJ, Khullar AK, Longden A. Effects of nicotine on plasma corticosterone and brain amines in stressed and unstressed rats. Pharmacol Biochem Behav. 1975;3:179–184. doi: 10.1016/0091-3057(75)90145-8. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, Biggio G. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996;63:166–172. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- Baron JA, Comi RJ, Cryns V, Brinck-Johnsen T, Mercer NG. The effect of cigarette smoking on adrenal cortical hormones. J Pharmacol Exp Ther. 1995;272:151–155. [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3 alpha-hydroxy-5 alpha[beta]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Bitran D, Purdy RH, Kellogg CK. Anxiolytic effect of progesterone is associated with increases in cortical allopregnanolone and GABAA receptor function. Pharmacol Biochem Behav. 1993;45:423–428. doi: 10.1016/0091-3057(93)90260-z. [DOI] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol. 1995;7:171–177. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Cam GR, Bassett JR. The effect of acute nicotine administration on plasma levels of the thyroid hormones and corticosterone in the rat. Pharmacol Biochem Behav. 1983a;19:559–561. doi: 10.1016/0091-3057(83)90135-1. [DOI] [PubMed] [Google Scholar]
- Cam GR, Bassett JR. The plasma levels of ACTH following exposure to stress or nicotine. Arch Int Pharmacodyn Ther. 1983b;264:154–167. [PubMed] [Google Scholar]
- Chen H, Fu Y, Sharp BM. Chronic nicotine self-administration augments hypothalamic–pituitary–adrenal responses to mild acute stress. Neuropsychopharmacology. 2008;33:721–730. doi: 10.1038/sj.npp.1301466. [DOI] [PubMed] [Google Scholar]
- Cheng SY, Glazkova D, Serova L, Sabban EL. Effect of prolonged nicotine infusion on response of rat catecholamine biosynthetic enzymes to restraint and cold stress. Pharmacol Biochem Behav. 2005;82:559–568. doi: 10.1016/j.pbb.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Cooper KH, Meyer BU, Blide R, Pollock M, Gibbons L. The important role of fitness determination and stress testing in predicting coronary incidence. Ann N Y Acad Sci. 1977;301:642–652. doi: 10.1111/j.1749-6632.1977.tb38235.x. [DOI] [PubMed] [Google Scholar]
- de Wit H, Schmitt L, Purdy R, Hauger R. Effects of acute progesterone administration in healthy postmenopausal women and normally-cycling women. Psychoneuroendocrinology. 2001;26:697–710. doi: 10.1016/s0306-4530(01)00024-5. [DOI] [PubMed] [Google Scholar]
- Field AE, Colditz GA, Willett WC, Longcope C, McKinlay JB. The relation of smoking, age, relative weight, and dietary intake to serum adrenal steroids, sex hormones, and sex **hormone-binding globulin in middle-aged men. J Clin Endocrinol Metab. 1994;79:1310–1316. doi: 10.1210/jcem.79.5.7962322. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Luria R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol Med. 1973;3:479–486. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Andersson K, Eneroth P, Harfstrand A, Agnati LF. Neuroendocrine actions of nicotine and of exposure to cigarette smoke: medical implications. Psychoneuroendocrinology. 1989;14:19–41. doi: 10.1016/0306-4530(89)90054-1. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83:2099–2103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- Kiritsy-Roy JA, Mousa SA, Appel NM, Van Loon GR. Tolerance to nicotine-induced sympathoadrenal stimulation and cross-tolerance to stress: differential central and peripheral mechanisms in rats. Neuropharmacology. 1990;29:579–589. doi: 10.1016/0028-3908(90)90071-x. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Strasburger CJ. ‘Normal’ cigarette smoking increases free cortisol in habitual smokers. Life Sci. 1992;50:435–442. doi: 10.1016/0024-3205(92)90378-3. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Strasburger CJ, Langkrar J. Attenuated cortisol response to psychological stress but not to CRH or ergometry in young habitual smokers. Pharmacol Biochem Behav. 1993a;44:527–531. doi: 10.1016/0091-3057(93)90162-m. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993b;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Scherer G, Strasburger CJ. Pituitary and adrenal hormone responses to pharmacological, physical, and psychological stimulation in habitual smokers and nonsmokers. Clin Investig. 1994;72:804–810. doi: 10.1007/BF00180552. [DOI] [PubMed] [Google Scholar]
- MacDougall JM, Musante L, Castillo S, Acevedo MC. Smoking, caffeine, and stress: effects on blood pressure and heart rate in male and female college students. Health Psychol. 1988;7:461–478. doi: 10.1037//0278-6133.7.5.461. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Cullinan WE, Tang LC, Baker DA, Katz ES, Hoks MA, Ziegler DR. Daily cocaine self-administration under long-access conditions augments restraint-induced increases in plasma corticosterone and impairs glucocorticoid receptor-mediated negative feedback in rats. Brain Res. 2007;1167:101–111. doi: 10.1016/j.brainres.2007.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx CE, Trost WT, Shampine L, Behm FM, Giordano LA, Massing MW, Rose JE. Neuroactive steroids, negative affect, and nicotine dependence severity in male smokers. Psychopharmacology (Berl) 2006;186:462–472. doi: 10.1007/s00213-005-0226-x. [DOI] [PubMed] [Google Scholar]
- Matta SG, Fu Y, Valentine JD, Sharp BM. Response of the hypothalamo–pituitary–adrenal axis to nicotine. Psychoneuroendocrinology. 1998;23:103–113. doi: 10.1016/s0306-4530(97)00079-6. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. Profile of mood states. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Mendelson JH, Sholar MB, Goletiani N, Siegel AJ, Mello NK. Effects of low- and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology. 2005;30:1751–1763. doi: 10.1038/sj.npp.1300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse PA, Sunderland T, Narang PK, Mellow AM, Fertig JB, Lawlor BA, Murphy DL. Neuroendocrine, physiologic, and behavioral responses following intravenous nicotine in nonsmoking healthy volunteers and in patients with Alzheimer’s disease. Psychoneuroendocrinology. 1990;15:471–484. doi: 10.1016/0306-4530(90)90070-p. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Shoaib M, Holsboer F, Almeida OF. The neurosteroid tetrahydroprogesterone counteracts corticotropinreleasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience. 1994;62:265–271. doi: 10.1016/0306-4522(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Hassan AH, Holsboer DF, Almeida OF. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology. 1996;15:533–540. doi: 10.1016/S0893-133X(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. Faseb J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Fonte C, Breus M. “Paradoxical” effects of smoking on subjective stress versus cardiovascular arousal in males and females. Pharmacol Biochem Behav. 1992;42:301–311. doi: 10.1016/0091-3057(92)90531-j. [DOI] [PubMed] [Google Scholar]
- Peters RK, Cady LD, Jr., Bischoff DP, Bernstein L, Pike MC. Physical fitness and subsequent myocardial infarction in healthy workers. Jama. 1983;249:3052–3056. [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS. Cortisol response to a psychological stressor and/or nicotine. Pharmacol Biochem Behav. 1990;36:211–213. doi: 10.1016/0091-3057(90)90153-9. [DOI] [PubMed] [Google Scholar]
- Porcu P, Sogliano C, Cinus M, Purdy RH, Biggio G, Concas A. Nicotine-induced changes in cerebrocortical neuroactive steroids and plasma corticosterone concentrations in the rat. Pharmacol Biochem Behav. 2003;74:683–690. doi: 10.1016/s0091-3057(02)01065-1. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Moore PH, Jr, Rao PN, Hagino N, Yamaguchi T, Schmidt P, Rubinow DR, Morrow AL, Paul SM. Radioimmunoassay of 3 alpha-hydroxy-5 alpha-pregnan-20-one in rat and human plasma. Steroids. 1990;55:290–296. doi: 10.1016/0039-128x(90)90031-6. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy MP, Steptoe A, Kirschbaum C. Association between smoking status and cardiovascular and cortisol stress responsivity in healthy young men. Int J Behav Med. 1994;1:264–283. doi: 10.1207/s15327558ijbm0103_6. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, Wirth MM, Stanton SJ. Effects of affiliation and power motivation arousal on salivary progesterone and testosterone. Horm Behav. 2004;46:592–599. doi: 10.1016/j.yhbeh.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Soderpalm AH, Lindsey S, Purdy RH, Hauger R, Wit de H. Administration of progesterone produces mild sedative-like effects in men and women. Psychoneuroendocrinology. 2004;29:339–354. doi: 10.1016/s0306-4530(03)00033-7. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. Manual for the State-Trait Anxiety Inventory. Washington, DC: Consulting Psychologist Press; 1970. [Google Scholar]
- Steptoe A, Ussher M. Smoking, cortisol and nicotine. Int J Psychophysiol. 2006;59:228–235. doi: 10.1016/j.ijpsycho.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Swan GE, Ward MM, Jack LM. Abstinence effects as predictors of 28-day relapse in smokers. Addict Behav. 1996;21:481–490. doi: 10.1016/0306-4603(95)00070-4. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Tsuda A, Steptoe A, West R, Fieldman G, Kirschbaum C. Cigarette smoking and psychophysiological stress responsiveness: effects of recent smoking and temporary abstinence. Psychopharmacology (Berl) 1996;126:226–233. doi: 10.1007/BF02246452. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland S, Lan NC, Mirasedeghi S, Gee KW. Anxiolytic activity of the progesterone metabolite 5 alpha-pregnan-3 alpha-o1-20-one. Brain Res. 1991;565:263–268. doi: 10.1016/0006-8993(91)91658-n. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Schultheiss OC. Effects of affiliation arousal (hope of closeness) and affiliation stress (fear of rejection) on progesterone and cortisol. Horm Behav. 2006;50:786–795. doi: 10.1016/j.yhbeh.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Meier EA, Fredrickson BL, Schultheiss OC. Relationship between salivary cortisol and progesterone levels in humans. Biol Psychol. 2007;74:104–107. doi: 10.1016/j.biopsycho.2006.06.007. [DOI] [PubMed] [Google Scholar]