Abstract
The EPA reference dose for methyl mercury (MeHg) was established using data from populations with greater exposures than those typical of the US. Few data are available on potential adverse health effects at lower levels. We examined relationships between hair mercury (Hg) levels and neuropsychological outcomes in a population of US children. This study included data from 355 children ages 6–10 enrolled in the New England Children’s Amalgam Trial. Data on total hair Hg levels, sociodemographic information and neuropsychological function were collected. We evaluated associations between hair Hg and neuropsychological test scores with linear regression methods and used generalized additive models to determine the shape of associations that departed from linearity. Models controlled for relevant covariates, including the potential beneficial effects of consuming fish.
In adjusted models, we observed no significant linear relationships between hair Hg level and any test score. Significant departures from linearity were identified for WIAT Math Reasoning and WRAMVA Visual-Motor Composite scores. The association was positive for hair Hg levels below 0.5 μg/g and negative for levels between 0.5 and 1.0 μg/g. Overall, test scores of children with hair Hg levels ≥ 1.0 μg/g appeared to be lower than those of children with levels < 1.0 μg/g, but few children had levels in this upper range and these differences did not reach statistical significance. Hair Hg levels below 1.0 μg/g in US school-age children were not adversely related to neuropsychological function.
Keywords: mercury, children, neurodevelopment, semi-parametric models
INTRODUCTION
Intake guidelines for methyl mercury (MeHg), such as the reference dose (Rice, 2004) and the provisional tolerable weekly intake (JointFAO/WHO Expert Committee on Food Additives, 2004), have been derived on the basis of studies of adverse health effects in populations that consume large quantities of seafood (Kjellstrom et al., 1989; Grandjean et al., 1997; Myers et al., 2003). Although the findings of these epidemiologic studies are somewhat mixed, the weight-of-evidence indicates that higher prenatal exposures to MeHg are associated with neuropsychological deficits in children (National Research Council, 2000; Axelrad et al., 2007). MeHg exposures in the U.S. population, in which consumption of seafood is relatively modest, are considerably lower than those in these other studies. For example, the mean maternal hair Hg levels in the Faroese and Seychellois birth cohort studies were 4.3 and 6.9 μg/g, respectively (Grandjean et al., 1999; Myers et al., 2003). In contrast, among US women of child-bearing age, the mean, median, and 95th percentile of hair Hg levels are 0.47, 0.19 and 1.73 μg/g, respectively (McDowell et al., 2004). The median hair Hg level among children 1 to 5 years of age is 0.11μg/g (McDowell et al., 2004). MeHg is incorporated into hair in proportion to the MeHg in blood. Since hair grows approximately one cm per month, hair Hg reflects exposure during the past months or year, depending on hair length (McDowell et al., 2004).
Few data are available on potential adverse health effects of hair Hg levels typical of the US population. However, two recent studies did suggest that deficits in infant development are evident within the range of typical US maternal hair, maternal blood or cord blood Hg levels at delivery ( Oken et al., 2005; Jedrychowski et al., 2006; Oken et al., 2008). Furthermore, data on potential health effects associated with childhood, contrasted with fetal exposures, are very limited. Although the fetus is considered to be the most susceptible subgroup of the population (National Research Council, 2000), cross-sectional studies conducted on Amazonian children and children from French Guiana suggested that maternal hair and/or cord blood Hg levels greater than approximately 10 μg/g at the time of delivery or interview (used as a proxy for prenatal exposures) were associated with reduced neuropsychological performance in school-age children, but few associations were found in relation to children’s concurrent hair Hg levels (Grandjean et al., 1999; Cordier et al., 2002).
The goal of the present analyses was to evaluate the relationships between hair Hg levels measured in childhood and cognitive outcomes in a population with Hg exposures that are typical of those observed in the US population.
METHODS
Study design and participants
This study included baseline data from the 5-year New England Children’s Amalgam Trial (NECAT), a randomized trial conducted between 1999 and 2005 for the purpose of studying potential effects of dental amalgam on child neurodevelopment (Children’s Amalgam Trial Study Group 2003; Bellinger et al., 2006). Children aged 6–10 were eligible to participate if they spoke English, had at least two dental caries on posterior occlusal tooth surfaces, and did not have previous amalgam fillings. Children were also excluded if a parent/guardian reported they had medically diagnosed psychological, behavioral, neurological, immunosuppressive, or renal disease. Although data were originally collected for an amalgam trial to study elemental Hg, the focus of our analyses was on hair Hg, which reflects primarily MeHg.
Initially, 598 eligible children were identified from the Boston, Massachusetts area and Farmington, Maine, a rural area. Parental consent and child assent were obtained for 534 children (89%; 291 from Boston, 243 from Maine). The institutional review boards of the New England Research Institutes, the Forsyth Institute, and hospital-affiliated dental clinics (affiliated with Franklin Memorial Hospital in Maine, and the Cambridge Health Alliance, Boston University Medical Center, or Children’s Hospital in Massachusetts) approved this study. Total Hg level was determined in a sample of the child’s hair collected at baseline. Sociodemographic information was also collected from parents/guardians.
Measurement of Hg exposure
Approximately 50–100 strands of hair, cut as close to the scalp as possible, were collected at the child’s baseline visit. This visit was conducted before a child was randomized to the amalgam or non-amalgam group. Therefore, at the time hair Hg was measured none of the children had ever had any exposure to elemental Hg from amalgam fillings. Hair samples were sent to the University of Rochester Medical School (Department of Environmental Medicine, Rochester, NY) for analysis. Total Hg was measured in hair by cold vapor atomic absorption spectrometry (CVAAS) (Magos and Clarkson, 1972). The laboratory has performed well in inter- and intra-laboratory comparisons (Boischio and Cernichiari, 1998). Comparisons have also been performed between the CVAAS method and independent methods (X-ray fluorescence spectrometry and gas chromatography with atomic fluorescence detection) with good agreement between the three methods (Cernichiari et al. 1995).
The detection limit was 0.75 ng Hg. Hair samples were dissolved in 10 ml volume, with an aliquot (typically 3 ml) removed for analysis. Detectable concentration (μg Hg/g hair) varied with hair mass, with larger samples (e.g. longer hair) having a lower detectable concentration. Forty-seven percent of the samples were below the detectable concentration (0.22 μg/g on average). Samples below the detectable limit were imputed as detectable concentration/sqrt(2) (Horning and Reed, 1990). Due to inaccuracy of imputing from very high detection limits, samples with hair mass < 6.3 mg were excluded from all analysis and descriptive statistics. A hair mass of 6.3 mg corresponds to a detection limit of 0.40 μg/g, the median H-Hg content of the detectable samples. Of the 534 children enrolled, 16 did not have baseline hair samples and for 32 children the hair sample weighed <6.3 mg. Thus, for the purposes of analyses, a hair Hg level was available for 486 children (91% of 534).
Neuropsychological endpoints
With the aim of studying the effects of hair Hg on a broad range of neuropsychological endpoints, outcome variables included the Full-Scale, Verbal, and Performance IQ scores on the Wechsler Intelligence Scale for Children (WISC-III) (Wechsler, 1991); the Reading Composite, Basic Reading, Reading Comprehension, Math Composite, Math Reasoning, and Numerical Operations scores on the Wechsler Individual Achievement Test (WIAT) (Psychological Corporation 1992); the Visual Motor Composite, Drawing, Matching, and Pegboard scores on the Wide Range Assessment of Visual Motor Ability (WRAVMA) (Adams and Sheslow, 1995); the General Memory Index on the Wide Range Assessment of Memory and Learning (WRAML) (Sheslow and Adams, 1990); perseveration errors on the Wisconsin Card Sorting Test (WCST) (Heaton et al., 1993), the Stroop Test of Color-Word Interference (Trenerry et al., 1989); the time to complete Part B of the Trail-Making Test (Spreen and Strauss, 1998), and Finger Tapping counting the mean of 5 trials with the dominant hand (the WPS Electronic Tapping Test). With the exception of the Trail Making Test, higher scores on the neuropsychological tests indicated better performance. All data on neuropsychological endpoints were collected at baseline.
Statistical modeling
Because maternal IQ is an important predictor of child cognitive abilities, we restricted our study to include only the 379 children with information on maternal IQ. We also excluded 24 observations missing information on birthweight or lead exposure, covariates included in the analyses. This resulted in a sample size of 355. For a multiple linear regression model which already adjusts for a moderate number of covariates having an R2 of 10% to 30%, a sample size of 355 provides 80% power to detect an increase in R2 of 2% or greater, and 90% power to detect an increase in R2 of 3% or greater when adding a linear effect of hair Hg to the model (nQuery Advisor, Statistical Solutions, Saugus, MA).
For descriptive purposes, we estimated relationships between demographic factors and neuropsychological test scores of children with continuous hair Hg, by calculating group comparisons using a likelihood ratio test for categorical variables and Spearman’s correlations for continuous variables. Spearman’s correlations were also used to assess unadjusted associations between continuous hair Hg levels and neuropsychological outcomes. In adjusted models we used linear regression controlling for independent predictors and potential confounders, including child gender, child age (6–7, 8–9, 10–11 years), child race as reported by the caregiver (black, Hispanic, white, other/missing), caregiver education (< high school, high school, college or more), caregiver marital status (married or living as married vs. single, separated, divorced, or widowed), child birthweight, socio-economic status (calculated by the method of (Green, 1970)), primary caregiver IQ measured with the Kaufman-Brief Intelligence Test (K-BIT) (Kaufman & Kaufman 1990), and baseline blood lead level. To adjust for potential non-linear associations between birthweight, socio-economic status, primary caregiver IQ, and blood lead level, we used generalized additive models with four degrees of freedom for each covariate (Hastie and Tibshirani, 1990; SAS Procedures Guide 2006).
Generalized additive models were also used when there was statistically significant evidence of deviation from linearity (p<0.05 in a 3 degree of freedom test) in the association between hair Hg level and a neuropsychological test score. Because of concern about imbalance of race in our hair Hg categories, in additional analyses the ‘other’ race category, as well as the ‘other’ and ‘Hispanic’ race categories, were excluded. Separate addition of study site, birth order, parenting stress as measured with the Parenting Stress Index, maternal use of prenatal care, and maternal use of health care did not appreciably affect the associations between hair mercury and neurodevelopment outcomes.
Fish consumption was categorized as at least once a week, at least twice a month (but less than once a week), monthly or less, and never. Recent studies suggest that MeHg neurotoxicity is underestimated if analyses fail to address the fact that the primary pathway of MeHg exposure, the consumption of contaminated seafood, also provides macro- and micronutrients whose effects on cognition are antagonistic to those of MeHg (Oken et al., 2005; Budtz-Jorgensen et al., 2007; Davidson et al., 2008; Strain et al., 2008; Oken et al., 2008). Therefore, additional analyses were conducted in which adjustments were made for overall child fish consumption as a covariate. All analyses were conducted using SAS, Version 9.3.1 (SAS Institute, Cary NC).
RESULTS
Children’s hair Hg levels did not differ according to age, primary caregiver education, caregiver marital status, birthweight, socio-economic status, primary caregiver IQ, and blood lead level (Table 1). There was a difference in hair Hg exposure by race, with both Hispanic and ‘other’ race having higher mean hair Hg levels than those of whites and blacks. As expected, we also found reported quantity of fish consumed to be strongly associated with hair Hg exposure, in a dose-response fashion. The 355 children included in our analysis differed somewhat from the 131 who were excluded because of missing covariates; children with missing covariates were more likely to be from Boston, be of Hispanic and other races, have lower socio-economic status and birthweights, and have slightly higher hair Hg and blood lead levels.
Table 1.
Associations between hair mercury levels and demographic and other child characteristics (N=355)
| Hair Mercury Level (μg/g) | |||
|---|---|---|---|
| % | Mean (SD) | p-valuea | |
| Gender | 0.71 | ||
| Male | 46.8 | 0.31 (0.36) | |
| Female | 53.2 | 0.32 (0.24) | |
| Age (years) | 0.22 | ||
| 6–7 | 53.5 | 0.29 (0.27) | |
| 8–9 | 37.2 | 0.35 (0.35) | |
| 10–11 | 9.3 | 0.28 (0.23) | |
| Race | <0.001 | ||
| Non-Hispanic White | 72.4 | 0.29 (0.27) | |
| Non-Hispanic Black | 16.9 | 0.30 (0.22) | |
| Hispanic | 3.1 | 0.39 (0.36) | |
| Other | 7.6 | 0.52 (0.52) | |
| Education of primary caregiver | 0.72 | ||
| < High school | 10.4 | 0.28 (0.19) | |
| High school graduate | 80.3 | 0.32 (0.31) | |
| College graduate | 9.3 | 0.33 (0.27) | |
| Marital Status | 0.96 | ||
| Married | 37.2 | 0.30 (0.30) | |
| Not Married | 62.8 | 0.29 (0.30) | |
| Fish Consumption | 0.003 | ||
| At least once a week | 33.8 | 0.36 (0.32) | |
| At least twice a month | 22.3 | 0.37 (0.35) | |
| Monthly or less | 29.0 | 0.27 (0.27) | |
| Never | 14.9 | 0.21 (0.15) | |
| Mean (SD) | Spearman Correlation | p-value | |
| Birth weight (grams) | 3374 (538) | −0.05 | 0.31 |
| Socio-economic statusb | 52.9 (30.6) | 0.03 | 0.61 |
| Primary caregiver IQc | 96.5 (12.1) | −0.00 | 0.94 |
| Blood lead level (μg/dL) | 2.19 (1.63) | 0.04 | 0.47 |
| Hair mercury level (μg/g) | 0.31 (0.30) | -- | -- |
P-values measuring associations between hair mercury level and categorical variables were calculated with a likelihood ratio test comparing the groups.
Computed using the method of Green (1970).
Measured with the Kaufman-Brief Intelligence Test (K-BIT).
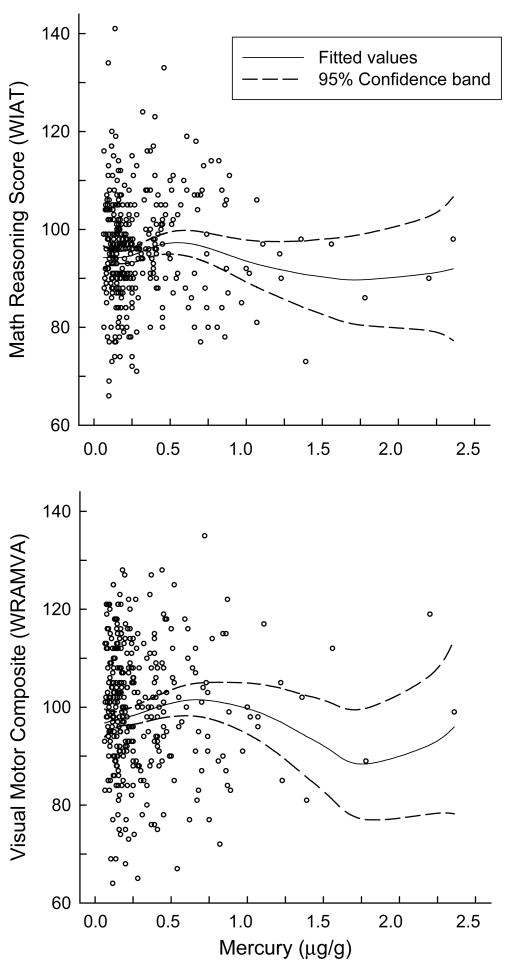
None of the children’s neuropsychological test scores were significantly correlated with hair Hg in unadjusted analyses (Table 2) and no significant linear relationships were observed between hair Hg level and the neuropsychological test scores in adjusted analyses (Table 3). This result held when fish consumption was included in models. Significant departures from linearity were identified in the relationships between hair Hg level and two test scores, WIAT Math Reasoning score and WRAMVA Visual-Motor Composite (p=0.03 for both). For three other test scores, the WIAT Mathematics Composite, WRAMVA Pegboard, and WCST perseveration errors, the deviations from linearity were marginally significant (all p=0.06) (Table 3). Use of smoothed generalized additive models suggested an inverted U-shaped function for both the WIAT Math Reasoning score and WRAMVA Visual-Motor Composite (Figure 1). The association appeared to be positive for hair Hg levels below 0.5 μg/g and negative for levels between 0.5 and 1.5 μg/g. For hair Hg levels >1.5 μg/g, the confidence intervals were wide due to the scarcity of data, making it difficult to draw conclusions about the shape of the dose-effect relationship.
Table 2.
Neurodevelopmental test scores and associations with hair mercury
| Hair mercury level (μg/g) | ||||
|---|---|---|---|---|
| N | Mean (sd) | Spearman Correlations with hair Hg | p-value | |
| Wechsler Intelligence Scale for Children (WISC-III) | 355 | |||
| Full-Scale IQ | 97.4 (12.9) | 0.005 | 0.93 | |
| Verbal IQ | 96.2 (13.4) | 0.009 | 0.86 | |
| Performance IQ | 99.4 (14.0) | −0.02 | 0.69 | |
| Wechsler Individual Achievement Test (WIAT) | 355 | |||
| Reading Composite | 97.4 (13.7) | 0.001 | 0.98 | |
| Basic Reading | 98.5 (13.4) | 0.02 | 0.67 | |
| Reading Comprehension | 96.6 (12.7) | −0.08 | 0.12 | |
| Math Composite | 95.1 (12.4) | 0.03 | 0.51 | |
| Math Reasoning | 96.2 (10.8) | 0.03 | 0.56 | |
| Numerical Operations | 95.2 (12.1) | 0.02 | 0.69 | |
| Wide Range Assessment of Visual Motor Ability (WRAVMA) | 354 | |||
| Visual Motor Composite | 100.4 (12.8) | 0.09 | 0.07 | |
| Drawing | 100.6 (12.0) | 0.04 | 0.51 | |
| Matching | 99.8 (13.1) | 0.07 | 0.19 | |
| Pegboard | 101.7 (15.4) | 0.08 | 0.15 | |
| Wide Range Assessment of Memory and Learning (WRAML) | ||||
| General Memory Index | 353 | 93.4 (14.5) | 0.001 | 0.98 |
| Wisconsin Card Sorting Test (WCST) | ||||
| Perseveration errors | 346 | 97.3 (14.5) | 0.02 | 0.69 |
| Stroop Test of Color-Word Interference | 285 | 20.7 (5.6) | 0.06 | 0.32 |
| Trail-Making Test a | ||||
| Time to complete Part B (seconds) | 340 | 79.4 (55.9) | 0.02 | 0.72 |
| Finger Tapping | ||||
| Mean of 5 trials with dominant hand | 353 | 38.3 (9.1) | 0.09 | 0.07 |
Higher scores indicate poorer performance.
Table 3.
Linear effects of the relationship between hair mercury level and 355 children’s neurodevelopmental outcomes
| Linear effect of hair Hg (μg/g) on outcomea | Test of departure from linearity (μg/g)b | ||
|---|---|---|---|
| β (se) | p-value | p-value | |
| Wechsler Intelligence Scale for Children (WISC-III) | |||
| Full-Scale IQ | 0.11 (2.13) | 0.96 | 0.18 |
| Verbal | 0.35 (2.22) | 0.87 | 0.10 |
| Performance | −1.14 (2.33) | 0.63 | 0.52 |
| Wechsler Individual Achievement Test (WIAT) | |||
| Reading Composite | −0.35 (2.31) | 0.88 | 0.46 |
| Basic Reading | 1.01 (2.32) | 0.66 | 0.52 |
| Reading Comprehension | −3.79 (2.16) | 0.08 | 0.52 |
| Mathematics Composite | −0.04 (2.12) | 0.98 | 0.06 |
| Math Reasoning | 0.25 (1.85) | 0.89 | 0.03 |
| Numerical Operations | −0.49 (2.12) | 0.82 | 0.18 |
| Wide Range Assessment of Visual Motor Ability (WRAVMA) | |||
| Visual-Motor Composite | 1.32 (2.24) | 0.56 | 0.03 |
| Drawing | 0.96 (2.18) | 0.66 | 0.22 |
| Matching | 2.03 (2.29) | 0.38 | 0.11 |
| Pegboard | −0.13 (2.70) | 0.96 | 0.06 |
| Wide Range Assessment of Memory and Learning (WRAML) | |||
| General Memory Index | 3.72 (2.56) | 0.15 | 0.46 |
| Wisconsin Card Sorting Test (WCST) | |||
| Perseveration errors | 1.03 (2.49) | 0.67 | 0.06 |
| Stroop Test of Color-word Interference | 0.80 (1.01) | 0.43 | 0.38 |
| Trail-Making Test | |||
| Time to complete Part B (transformed)c | 0.06 (0.09) | 0.50 | 0.50 |
| Finger Tapping | |||
| Mean of 5 trials with dominant hand | 0.82 (1.25) | 0.51 | 0.10 |
Models were adjusted for gender, child age (6–7, 8–9, 10–11), race (white, Hispanic, black, other/missing), primary caregiver education (< high school, high school, college+), marital status (married or living as married/single, separated, divorced or widowed) as well as birthweight, socio-economic status, caregiver IQ, and blood lead, as smoothed variables. All smoothed variables were modeled with four degrees of freedom.
Models adjusted for all covariates above. Hair Hg level was included as a smoothed variable.
The log transform of the Trail-Making Test Part B was used to normalize this variable.
Figure 1.
Generalized additive models of non-linear relationships between hair mercury exposure and WIAT Math Reasoning (top) and WRAMVA Visual Motor Composite (bottom). Solid lines indicate fitted values. Dotted lines indicate 95% Confidence Intervals.
Reanalysis of the data excluding the ‘other’ race category and the ‘other’ and ‘Hispanic’ races from the linear regression models resulted in only negligible changes in our results. One of our 18 endpoints, WIAT Reading Comprehension became statistically significant (<0.05) in the linear model when excluding the ‘other’ race, but was nonsignificant when excluding ‘other’ and ‘Hispanic’ races from the analysis (p=0.08) (data not shown). Likewise, two of our outcome variables showing marginal statistical significance for the test of departure from linearity, WRAML General Memory Index and WCST Perseveration Errors, became statistically significant with at least one of these two race exclusions (data not shown).
When fish consumption was included in the models of WIAT Math Reasoning and WRAVMA Visual Motor Composite scores, the p-values for departure from linearity remained statistically significant (p<0.05), and the shape of the relationship did not change perceptibly. Likewise, the shape of these associations did not change appreciably when ‘other’ race or ‘other’ and ‘Hispanic’ races were excluded.
DISCUSSION
Our study provides limited evidence of differences in neuropsychological functioning across the range of hair Hg exposures among the children enrolled in the NECAT. For no endpoint did we observe a significant inverse linear relationship between children’s scores and their hair Hg levels. For two endpoints, WIAT Math Reasoning and WRAMVA Visual-Motor Composite, significant deviations from linearity were observed. For both test scores, the relationship was positive at hair Hg levels below 0.5 μg/g and inverse between 0.5 and 1.5 μg/g. Given the limited information available about possible adverse effects of low Hg exposures in children, the lack of significant associations in our study adds important information to the existing literature.
Although fish consumption is the primary pathway of exposure to MeHg in the general population, it also provides nutrients that might counteract the adverse effects of Hg on child development. Indeed, several recent studies suggest that failure to adjust for fish consumption results in underestimates of the adverse effects of Hg (Oken et al. 2005; Budtz-Jorgensen et al., 2007; Davidson et al., 2008; Strain et al., 2008; Oken et al., 2008). In contrast, our results were largely unaffected by the inclusion of fish consumption in our models. Unfortunately, because we lacked data on the specific types of fish consumed, we could not estimate how well the quantity of fish consumed is likely to correspond to benefits from n-3 fatty acids. We can hypothesize, however, that the benefits of fish consumption might help to explain why, for the two test scores that bore nonlinear relationships to hair Hg levels, the relationships were in the positive direction at the lowest hair Hg levels. One explanation for this is that at low hair Hg levels there may appear to be positive effects, e.g., because of the beneficial effects of fish nutrients such as docosahexaenoic acid (DHA). But, at higher levels of fish intake, and thus higher levels of hair Hg, the adverse effects of mercury might outweigh the nutritional benefits.
Other studies have explored the possibility of nonlinear relationships between Hg biomarkers and children’s neurodevelopmental outcomes (Axtell et al., 1998; Axtell et al., 2000; Huang et al., 2005). Huang and colleagues reanalyzed data from the Seychelles cohort using semi-parametric additive models (Huang et al., 2005). They reported a non-linear effect for only one endpoint, the Grooved Pegboard using the dominant hand. The curve was flat in the range of maternal hair Hg levels below 12 μg/g total Hg but slightly increasing above this value. Interpretation of this finding was complicated by the fact that the outcome was transformed by the negative reciprocal function (Huang et al., 2005). Also, using the Seychelles data, Axtell et al used semi-parametric methods to explore the relationship between children’s hair Hg levels, measured at 66 months of age, and their General Cognitive Index score on the McCarthy Scales of Children’s Abilities (Axtell et al., 2000). Their generalized additive models, with four degrees of freedom, appeared to have the same general shape as ours did, although hair Hg levels were much higher for the Seychellois children than for the children enrolled in the NECAT. The relationships undulated upwards to 10 μg/g total Hg, slightly down above this value, and then slightly upwards near 20 μg/g, where there were relatively few data points (Axtell et al., 2000). The lowest hair Hg level among children in the Seychelles cohort was 0.8 μg/g, whereas 93% of the children in the NECAT had a level below this value. An earlier study by the same group used semi-parametric analyses to model prenatal Hg exposure and age at talking, but did not find any significant non-linear relationships (Axtell et al., 1998).
One of the main strengths of our study was a rigorous statistical approach, using both linear and semi-parametric models to evaluate possible adverse effects of low levels of Hg exposure. In addition, our sample consisted of school-age US children, a population on which research using hair Hg has not previously focused. Fish consumption is an important potential confounder because of the possible neurodevelopmental benefits of n-3 fatty acids (DHA) and other nutrients in fish (Mozaffarian and Rimm, 2006). We were able to control for the total amount of fish consumed, but we did not have detailed data on specific types of fish consumed. Thus although fish consumption and hair Hg were highly correlated, we were not able to estimate how close a proxy this variable was for n-3 fatty acids. Several other limitations should be noted. First, hair Hg was measured at only one time, precluding evaluation of how previous or cumulative exposures to Hg affected the outcomes. Second, a large fraction of hair samples had MeHg levels below the detection limit, requiring imputation. This is likely to have resulted in some exposure misclassification in these children, biasing our estimates of exposure-response relationships towards the null. Thus we are unable to draw any inferences about the presence of such relationships in the range of hair Hg levels below 0.22 μg/g. However, since we did not find consistent evidence of an association above this detection limit, it is unlikely that an association would have been observed below it.
Finally, in our multivariable regression analyses we dropped a fair number of subjects with missing covariates, particularly for maternal IQ, which could limit the generalizability of our results. Also, because hair Hg measurements in this study do not reflect a child’s exposure during the prenatal period of brain development, results of this study should not be generalized to exposures at other periods of development. There is evidence that low prenatal Hg levels have significant adverse neuropsychological consequences at the hair Hg levels that we found not to be associated with neuropsychological effects in children ages 6 to 10 (Oken et al., 2008).
The EPA derived the reference dose for MeHg (0.1μg/kg body weight per day) by applying uncertainty factors to an adverse effect level, the lower confidence limit of the benchmark dose for cord blood Hg, observed in the Faroe Islands cohort, a group of children with exposures that exceeded, by a considerable margin, those typical of the US population. The uncertainty factor was chosen to account for inter-individual pharmacokinetic and pharmacodynamic variability. The maternal hair Hg level that corresponds to the cord blood Hg levels used to derive the reference dose is approximately 1 μg/g. The reference dose should not result, over a lifetime of exposure, in adverse effects. The reference dose is not, therefore, based on the direct observation of adverse effects at hair Hg levels just above 1.0 μg/g. There are few empirical data showing whether or not 1.0 μg/g in fact provides an adequate margin of safety to children. Although the reference dose was based on prenatal exposures, it is used as a guideline for exposures in children of other ages as well, such as the school-age children in our study. Our study on hair Hg in children speaks to this issue, i.e. exposure in school-age children. In the NECAT, we did not observe adverse neuropsychological effects among children with hair Hg levels below 1.0 μg/g. We did observe some indications that the neuropsychological test scores of children with hair Hg levels greater than 1.0 μg/g were lower than the scores of children with levels less than 1.0 μg/g. However, despite a relatively large sample size, the number of children with hair Hg levels greater than 1.0 μg/g was limited, providing cause for caution in drawing any inferences about the dose-effect relationship in this range. Our findings, therefore, suggest that hair Hg levels below 1.0 μg/g are unlikely to produce adverse effects on neuropsychological function in school-age children.
Acknowledgments
Funding Sources: Supported by a cooperative agreement (U01 DE11886) between the New England Research Institutes and the National Institute of Dental and Craniofacial Research, National Institutes of Health, T32 MH073122 from the NIH, and P30 HD18655 from the National Institute of Child Health and Human Development.
We would like to acknowledge Annie Zhang for her help with the preliminary statistical analyses in preparation of this manuscript.
Abbreviations
- Hg
Mercury
- MeHg
Methylmercury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams W, Sheslow D. WRAMVA: Wide Range Assessment of Visual Motor Abilities. Wilimington, DE: Wide Range, Inc; 1995. [Google Scholar]
- Axelrad DA, Bellinger DC, Ryan LM, Woodruff TJ. Dose-response relationship of prenatal mercury exposure and IQ: An integrative analysis of epidemiologic data. Environ Health Perspect. 2007;115:609–615. doi: 10.1289/ehp.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell CD, Cox C, Myers GJ, Davidson PW, Choi AL, Cernichiari E, et al. Association between methylmercury exposure from fish consumption and child development at five and a half years of age in the Seychelles Child Development Study: An evaluation of nonlinear relationships. Environ Res. 2000;84:71–80. doi: 10.1006/enrs.2000.4082. [DOI] [PubMed] [Google Scholar]
- Axtell CD, Myers GJ, Davidson PW, Choi AL, Cernichiari E, Sloane-Reeves J, et al. Semiparametric modeling of age at achieving developmental milestones after prenatal exposure to methylmercury in the Seychelles child development study. Environ Health Perspect. 1998;106:559–563. doi: 10.1289/ehp.106-1533142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC, Trachtenberg F, Barregard L, Tavares M, Cernichiari E, Daniel D, et al. Neuropsychological and renal effects of dental amalgam in children: A randomized clinical trial. JAMA. 2006;295:1775–1783. doi: 10.1001/jama.295.15.1775. [DOI] [PubMed] [Google Scholar]
- Boischio AA, Cernichiari E. Longitudinal hair mercury concentration in riverside mothers along the Upper Madeira River (Brazil) Environ Res. 1998;77:79–83. doi: 10.1006/enrs.1998.3831. [DOI] [PubMed] [Google Scholar]
- Budtz-Jorgensen E, Grandjean P, Weihe P. Separation of risks and benefits of seafood intake. Environ Health Perspect. 2007;115:323–327. doi: 10.1289/ehp.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Children’s Amalgam Trial Study Group. The Children’s Amalgam Trial: Design and methods. Control Clin Trials. 2003;24:795–814. doi: 10.1016/s0197-2456(03)00105-3. [DOI] [PubMed] [Google Scholar]
- Cordier S, Garel M, Mandereau L, Morcel H, Doineau P, Gosme-Seguret S, et al. Neurodevelopmental investigations among methylmercury-exposed children in French Guiana. Environ Res. 2002;89:1–11. doi: 10.1006/enrs.2002.4349. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Cox C, Axtell C, Shamlaye C, Sloane-Reeves J, et al. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: outcomes at 66 months of age in the Seychelles Child Development Study. JAMA. 1998;280:701–707. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Strain JJ, Myers GJ, Thurston SW, Bonham MP, Shamlaye CF, et al. Neurodevelopmental effects of maternal nutritional status and exposure to methylmercury from eating fish during pregnancy. Neurotoxicology. 2008;29:767–75. doi: 10.1016/j.neuro.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Budtz-Jorgensen E, White RF, Jorgensen PJ, Weihe P, Debes F, et al. Methylmercury exposure biomarkers as indicators of neurotoxicity in children aged 7 years. Am J Epidemiol. 1999;150:301–305. doi: 10.1093/oxfordjournals.aje.a010002. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Green LW. Manual for scoring socioeconomic status for research on health behavior. Public Health Rep. 1970;85:815–827. [PMC free article] [PubMed] [Google Scholar]
- Hastie TJ, Tibshirani RJ. Generalized Additive Models. New York: Chapman and Hall; 1990. [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- Horning RW, Reed LD. Estimation of average concentration in the presense of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- Huang LS, Cox C, Myers GJ, Davidson PW, Cernichiari E, Shamlaye CF, et al. Exploring nonlinear association between prenatal methylmercury exposure from fish consumption and child development: Evaluation of the Seychelles Child Development Study nine-year data using semiparametric additive models. Environ Res. 2005;97:100–108. doi: 10.1016/j.envres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, Jankowski J, Flak E, Skarupa A, Mroz E, Sochacka-Tatara E, et al. Effects of prenatal exposure to mercury on cognitive and psychomotor function in one-year-old infants: Epidemiologic cohort study in Poland. Ann Epidemiol. 2006;16:439–447. doi: 10.1016/j.annepidem.2005.06.059. [DOI] [PubMed] [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives. Evaluation of certain food additives and contaminants - Sixty-first report of the Joint FAO/WHO Expert Committee on Food Additives. Report No. 922. Geneva: World Health Organization; 2004. [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intellegence Test. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- Kjellström T, Kennedy P, Wallis S, Stewart A, Friberg L, Lind B. Physical and mental development of children with prenatal exposures to mercury from fish: National Swedish Environmental Protection Board. 1989. [Google Scholar]
- Magos L, Clarkson TW. Atomic absorption determination of total, inorganic, and organic mercury in blood. J Assoc Off Anal Chem. 1972;55:966–971. [PubMed] [Google Scholar]
- McDowell MA, Dillon CF, Osterloh J, Bolger PM, Pellizzari E, Fernando R, et al. Hair mercury levels in U.S. children and women of childbearing age: reference range data from NHANES 1999–2000. Environ Health Perspect. 2004;112:1165–1171. doi: 10.1289/ehp.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari E, et al. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet. 2003;361:1686–1692. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Strain JJ. Nutrient and methyl mercury exposure from consuming fish. J Nutr. 2007;137:2805–2808. doi: 10.1093/jn/137.12.2805. [DOI] [PubMed] [Google Scholar]
- National Research Council. Toxicological Effects of Methylmercury. Washington, DC: National Academy Press; 2000. [Google Scholar]
- Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena CJ, Kleinman KP, et al. Maternal fish intake during pregnancy, blood mercury, and child cognition at age 3 years in a US cohort. Am J Epidemiol. doi: 10.1093/aje/kwn034. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, Hu H, et al. Maternal fish consumption, hair mercury, and infant cognition in a U.S. cohort. Environ Health Perspect. 2005;113:1376–1380. doi: 10.1289/ehp.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychological Corporation. Wechsler Individual Achievement Test. San Antonio, TX: Psychological Corporation; 1992. [Google Scholar]
- Rice DC. The US EPA reference dose for methylmercury: Sources of uncertainty. Environ Res. 2004;95:406–413. doi: 10.1016/j.envres.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Rice DC, Schoeny R, Mahaffey K. Methods and rationale for derivation of a reference dose for methylmercury by the U.S. EPA. Risk Anal. 2003;23:107–115. doi: 10.1111/1539-6924.00294. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. Base SAS 9.1.3 Procedures Guide. Second Edition. Volumes 1,2,3 and 4. GAM Procedure - Chapter 4. Cary, NC: The SAS Institute; 2006. [Google Scholar]
- Sheslow D, Adams W. Wide Range Assessment of Memory and Learning. Wilmington, DE: Wide Range, Inc; 1990. [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. 2. New York: Oxford University Press; 1998. [Google Scholar]
- Strain JJ, Davidson PW, Bonham MP, Duffy EM, Stokes-Riner A, Thurston SW, et al. Associations of maternal long-chain polyunsaturated fatty acids, methyl mercury, and infant development in the Seychelles Child Development Nutrition Study. Neurotoxicology. 2008;29:776–82. doi: 10.1016/j.neuro.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenerry MR, Crosson B, DeBoe J, Leber WR. Stroop Neuropsychological Screening Test Manual. Odessa, FL: Psychological Assessement Resources; 1989. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]