Abstract
Activation of the hypothalamic-pituitary-adrenal (HPA) axis is a basic response of animals to environmental perturbations that threaten homeostasis. These responses are regulated by neurones in the paraventricular nucleus of the hypothalamus (PVN) that synthesise and secrete corticotrophin-releasing hormone (CRH). Other PVN neuropeptides, such as arginine vasopressin and oxytocin, can also modulate activity of CRH neurones in the PVN and enhance CRH secretagogue activity of the anterior pituitary gland. In rodents, sex differences in HPA reactivity are well established; females exhibit a more robust activation of the HPA axis after stress than do males. These sex differences primarily result from opposing actions of sex steroids, testosterone and oestrogen, on HPA function. Ostreogen enhances stress activated adrenocorticotrophic hormone (ACTH) and corticosterone (CORT) secretion, whereas testosterone decreases the gain of the HPA axis and inhibits ACTH and CORT responses to stress. Data show that androgens can act directly on PVN neurones in the male rat through a novel pathway involving oestrogen receptor (ER)β, whereas oestrogen acts predominantly through ERα. Thus, we examined the hypothesis that, in males, testosterone suppresses HPA function via an androgen metabolite that binds ERβ. Clues to the neurobiological mechanisms underlying such a novel action can be gleaned from studies showing extensive colocalisation of ERβ in oxytocin-containing cells of the PVN. Hence, in this review, we address the possibility that testosterone inhibits HPA reactivity by metabolising to 5α-androstane-3β,17β-diol, a compound that binds ERβ and regulates oxytocin containing neurones of the PVN. These findings suggest a re-evaluation of studies examining pathways for androgen receptor signalling.
Keywords: androgen, 5α-androstane-3β, 17β-diol, dihydrotestosterone, oestrogen receptor β, corticosterone, ACTH, vasopressin, oestrogen response element
There exists a close relationship and overlapping function between, testosterone and oestradiol; a relationship based on numerous observations that testosterone can be converted to oestradiol in a number of tissues, including brain (1), by the aromatase enzyme. Testosterone can also be converted to a more potent androgen, dihydrotestosterone (DHT), by the 5-α reductase enzyme (2). DHT has been considered a prototypical androgen receptor (AR) agonist, with potent activity at the AR but with no ability to be aromatised to oestrogen-like metabolites. By contrast, recent data indicate that DHT may likewise be converted to products with oestrogen-like activity, but by enzymes other than aromatase. These DHT metabolites could play an important role in regulating nonreproductive functions such as stress reactivity in the male rodent. Such a paradigm shift may have important ramifications regarding how we interpret previous and future studies concerning gonadal steroid hormone regulation of brain function.
The hypothalamic-pituitary-adrenal (HPA) axis
In rodents, adrenal corticosterone (CORT) secretion is controlled by the activity of a neuroendocrine axis that involves the hypothalamus, the anterior pituitary and the adrenal gland. This HPA axis represents a cascade of neural and humoral signals driven by both the circadian pacemaker as well as the environment. Changing environmental conditions or perceived threats to homeostasis activate the HPA by funneling information through neurones located in the paraventricular nucleus of the hypothalamus (PVN), a critical brain region that integrates excitatory and inhibitory inputs. Central to HPA axis regulation are neurones in the parvicellular part of the PVN that contain corticotrophin-releasing hormone (CRH). The release of CRH to the hypophyseal portal system enhances synthesis and release of adrenocorticotrophic hormone (ACTH) from the anterior pituitary. In turn, ACTH acts on the adrenal cortex to stimulate synthesis and secretion of CORT. Circulating CORT subsequently acts at the level of the pituitary, hypothalamus and higher brain areas to limit further hormone secretion (3, 4).
Sex differences in the HPA axis response to stress
Sex differences in the ACTH and CORT response to a stressor have been consistently reported in the literature (5, 6). The neuroendocrine response of female rodents to acute stress is reportedly more robust than that of males. This enhanced reactivity of the female stress response is characterised by a greater and prolonged secretion of ACTH and CORT, suggesting a greater stimulus as well as a reduced negative feedback (7). Consistent with these findings, females also have higher levels of corticosteroid binding globulin, a liver-derived plasma protein that binds and sequesters corticosterone from its receptor (8). Moreover, gonadectomy of both males and females reduces the sex difference, whereas treatment of gonadectomised animals with oestradiol enhances, and testosterone treatment inhibits, HPA reactivity. Currently, the mechanisms by which testosterone and oestradiol act to influence HPA function have not been completely resolved. Evidence of oestradiol and testosterone acting at the adrenal gland (9), anterior pituitary (10–12) and hypothalamus (13–16) have been reported. Although contributions of each level of the axis likely mediate the sex differences in HPA function, in this review, we focus our attention on the hypothalamic effects of the androgenic component of this regulation.
Androgen regulation of the neuroendocrine response to stress
Testosterone acts upon the HPA axis in an opposing fashion to that of oestradiol (5, 11), which seemingly rules out a role for aromatisation of testosterone to oestradiol as an underlying mechanism. Gonadectomy of male rats increases CORT and ACTH responses to stress, and correspondingly, c-fos mRNA expression in the PVN is elevated (13, 15, 17). Hormone replacement of gonadectomised rats with either testosterone or the non-aromatisable androgen, DHT, returns stress-responsive plasma CORT and ACTH levels back to that of the intact male (17). Treatment of gonadectomised animals with DHT also inhibits the stress-induction of c-fos mRNA in the PVN (14, 15, 18, 19) further demonstrating that the effects of testosterone are likely not due to the aromatisation of testosterone to oestradiol. Evidence that androgen can regulate the HPA axis also comes from studies examining the HPA response to stress in pre- and post-pubertal male rats. Prior to puberty, when testosterone levels are low, the CORT response to acute and chronic stress is high relative to the response seen after puberty (20, 21), which corresponds to elevations in testosterone that occur during the pubertal transition of males. However, the involvement of testosterone in this mechanism is not absolute because Romeo et al.(22) have demonstrated that testosterone alone cannot shift the pattern of HPA regulation in pre-pubertal males to that of post-pubertal males.
Further evidence for AR involvement in controlling HPA axis function comes from studies utilising rodents with the testicular feminisation mutation (Tfm), which lack functional ARs (23, 24). Compared to their wild-type male siblings, Tfm mice show increased CORT levels both at baseline and after exposure to an acute stressor. However, Tfm mice also have low levels of circulating testosterone, so these differences in CORT cannot be directly attributed to AR deficiency (25). A more compelling argument for AR regulation of the HPA axis comes from studies in Tfm rats. Tfm male rats have higher testosterone levels than wt males but, similar to Tfm male mice, they show elevated CORT levels after acute stress (Zuloaga et al., unpublished data).
Although androgens can inhibit HPA axis function (13) and reduce CRH immunoreactivity in the PVN (26), ARs are not localised in CRH or arginine vasopressin (AVP) neurones within the PVN (26). AR immunoreactivity (-ir) has been found in some PVN neurones, but these AR-ir neurones are in the dorsal parvocellular and the ventral medial parvocellular parts of the PVN. These regions contain non-neurosecretory neurones that project to spinal cord and brain-stem autonomic nuclei (27). Consequently, it has been hypothesised that androgens regulate PVN neuropeptide expression and secretion trans-synaptically (16). Data supporting this hypothesis comes from studies showing that implantation of testosterone into the medial preoptic area (MPOA) and bed nucleus of the stria terminalis (BnST), brain regions that provide afferent input to the PVN, can reduce the CORT response to acute stress (16). Further, retrograde tracing studies have shown that AR-ir can be found in neurones of the BnST, but not the septum, that project to the PVN (28). The BnST and MPOA, are two areas that provide inhibitory signals to PVN function (16). The distribution of AR overlaps considerably with glutamic acid decarboxylase (GAD) immunoreactivity in the MPOA and BnST. Because GAD is an enzyme necessary for production of the inhibitory neurotransmitter, gamma-aminobutyric acid (GABA), these data suggest this as a potential mechanism for androgenic inhibition of PVN reactivity to stress.
Androgens may also regulate HPA reactivity by direct action at the level of the PVN. Stereotaxic application of DHT to a region just above the PVN (to prevent mechanical disruption of the PVN) is as effective as peripherally administered DHT in inhibiting HPA function (14). Zhou et al. (29) have shown that AR-ir is present in neurones in the parvicellular part of the PVN and that a small population of AR-ir neurones contain AVP, although another study failed to see AR-ir in AVP and CRH neurones (30). Alternatively, it is also possible that androgens can work though a membrane AR to inhibit HPA reactivity. However, few studies to date have explored such rapid membrane associated effects of androgens in any neural function (31). Nonetheless, the possibility that androgens can work at multiple levels to inhibit the neuroendocrine responses to stress must be considered.
Given the limited distribution of AR in the PVN, it is likely that the inhibitory effect observed for androgen occurs through a multisynaptic pathway that involves activation/inhibition of pathways controlling the autonomic nervous system and the resulting feedback loops that inhibit activity of neurosecretory PVN neurones. An additional hypothesis is that DHT does not act through ARs found in PVN neurones, but rather activates another type of receptor found in or near the PVN. Although DHT has been historically viewed as a pure AR agonist, recent studies suggest that, similar to testosterone, DHT can be metabolised to compounds that can bind oestrogen receptors (ERs), particularly ERβ.
Neural oestrogen receptor distribution relative to HPA axis function
ARs and ERs belong to a superfamily of ligand activated transcription factors that are characterised by their ability to directly alter gene transcription by binding to hormone response elements in DNA (32, 33). The classical DNA target for ER is the oestrogen response element and, for AR, the androgen response element; however, many hormone responsive genes lack these elements (34). Alternate sites in some gene promoters, such as activator protein-1 and selective promoter factor-1 could serve as targets through which some of these hormone receptors can modify transcription through protein–protein interactions (35–37).
Several types of oestrogen receptors have been described in the literature, with ERα and ERβ being the most widely explored. Following early reports describing ERβ (38), its mRNA was shown to be expressed at high levels by neurones within the PVN (39) and this localisation corresponded with ERβ-ir (40–42). A large percent of ERβ-ir cells in PVN are AVP positive, but ERβ is also found in most oxytocin and prolactin (43–46) neurones and some CRH containing neurones of the PVN (40, 46, 47). This suggests that, by binding to ERβ, oestradiol could directly alter the function of PVN neuropeptide neurones. By contrast, ERα is found at low levels and only in the periventricular PVN and rarely in CRH, AVP or oxytocin neurones (46, 48, 49), thereby ruling out a direct action of oestradiol as mediated through ERα on PVN responses to stress. However, ERα is colocalised with GAD67-ir, one of two enzymes involved in the synthesis of the inhibitory neurotransmitter, GABA, in neurones surrounding the PVN. One interpretation from this localisation would be that ERα can modulate inhibitory input to the PVN, thus affecting HPA axis function through trans-synaptic mechanisms (50).
Androgen metabolism
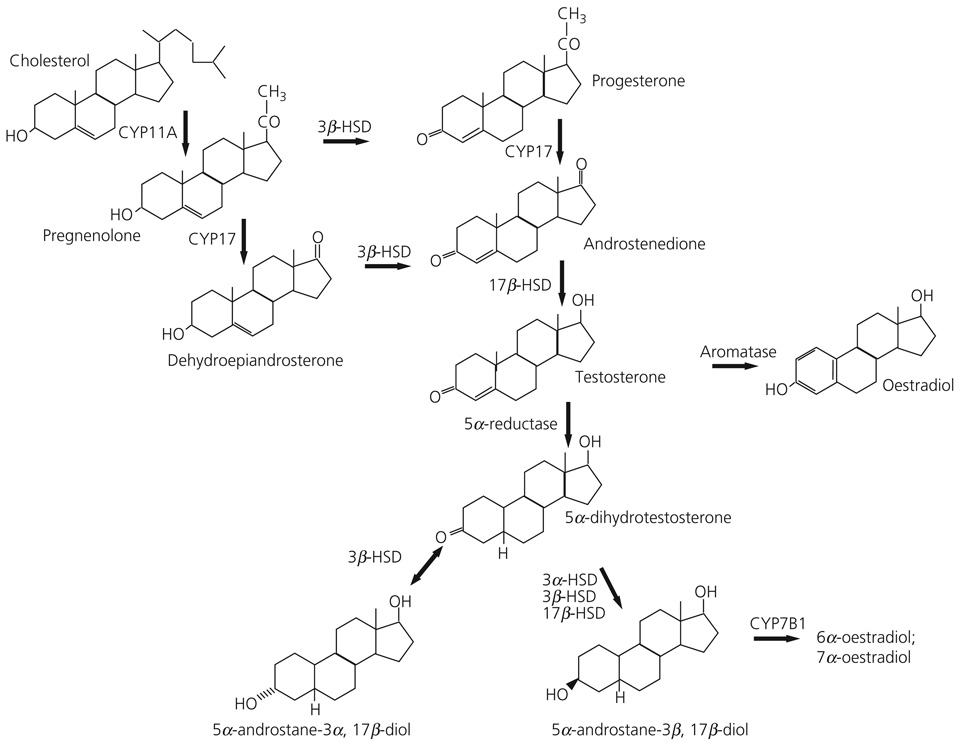
The metabolism of steroid hormones in both central and peripheral tissues has been extensively studied. Figure 1 shows the pathway of androgen synthesis and some of the main enzymes involved in the synthesis of testosterone and oestradiol from the steroid precursor, cholesterol. In both males and females, testosterone serves, not only as a ligand for AR, but as a precursor for other steroids. It is well established that testosterone can be intracellularly converted in brain tissue to oestradiol by the aromatase enzyme (51), or to DHT by the 5α-reductase (5αR) enzyme (52). DHT has historically been used as an agonist for androgen receptors since it is not a substrate for aromatisation to oestradiol. Furthermore, whereas oestradiol binds both ERα and ERβ with an affinity in the sub-nanomolar range (53), it does not bind well to AR (54). Correspondingly, DHT binds with high affinity to AR but does not bind ER (54, 55).
Fig. 1.
Diagram depicting synthetic pathway for androgens from cholesterol precursor. Bold lettering denotes the common or chemical name of the hormone, italics indicates enzyme required for the conversion of precursor to product. Arrows indicate the direction in which the reaction proceeds.
The selectivity of the steroid metabolising enzymes is not absolute. For example, DHT can be further metabolised to 5α-androstane-3α,17β-diol (3α-diol) or 5α-androstane-3β,17β-diol (3β-diol) by the actions of a number of enzymes of the aldo-keto reductase superfamily including 3α hydroxysteroid dehydrogenase (3α-HSD), 3β hydroxysteroid dehydrogenase (3β-HSD) and 17β-hydroxysteroid dehydrogenase (17β-HSD) (56–60). All of these enzymes are also involved in the pathways for synthesis and metabolism of other steroids.
Androgen metabolites 3α-diol and 3β-diol possess only weak androgen receptor binding activity, but they may initiate responses through other receptor types. For example, 3α-diol is a neuroactive steroid and, similar to other 3α tetrahydrosteroids, is a potent allosteric modulator of GABAa receptors. As a result, 3α-diol has been implicated in the regulation of a several behaviors (61–64). By contrast, 3β-diol cannot bind the benzodiazepine receptor (M. J. Weiser and R. J. Handa, unpublished data) but, if its actions are similar to other 3β-tetrahydrosteroids, it may be an antagonist of 3α-tetrahydrosteroids at the GABAA receptor (58). Importantly, 3β-diol has been reported to preferentially bind ERβ, whereas 3α-diol has much lower affinity for ERβ or ERα (53). Moreover, the conversion of DHT to 3α-diol is reversible, and therefore 3α-diol can serve as a sink for subsequent DHT synthesis (65). This is not the case for 3β-diol where conversion is unidirectional. Ultimately, 3β-diol is converted to inactive 6α- or 7α-triols by the actions of the enzyme CYP7B1, thereby providing another potential site of regulation for this system (60, 66).
The brain contains the necessary steroid metabolising enzymes to convert DHT to 3β-diol (67) and we have hypothesised that in some brain areas the actions of testosterone could be mediated by its 5α reduction to DHT and its subsequent conversion to 3β-diol. The net result is a product that can bind to and activate ERβ and not AR. This endocrine pathway exists in numerous tissues, but its functional significance was first suggested for the prostate gland (60), where it has been proposed that 3β-diol is the predominant endogenous oestrogen. Moreover, our data indicate that the mRNAs for 5αR, 3α-HSD,17β-HSD and CYP7B1 are present in the PVN of male rats (14). Curiously, we did not detect 3β-HSD mRNA in the microdissected PVN (14); however, enzyme activity assays indicate that cells within the microdissected PVN are capable of producing 3β-diol from a 3H-DHT precursor in vitro (R. J. Handa, unpublished data). Such results indicate that other members of the aldo-keto reductase superfamily, such as 3α-HSD or 17β-HSD, may be responsible for this conversion (58).
3β-Diol regulation of the HPA axis
To address the hypothesis that the inhibitory effects of DHT on HPA axis reactivity to stress might be mediated by 3β-diol, Lund et al. (17) examined the ability of peripherally administered 3β-diol to alter stress-responsive CORT and ACTH secretion in castrated adult male mice. Peripheral 3β-diol treatment was as effective as peripheral DHT administration in reducing the rise in CORT and ACTH after restraint stress. These effects of 3β-diol were blocked by co-administration of the ER antagonist, tamoxifen, but not by the AR antagonist, flutamide. Furthermore, the ERβ agonist, diarylpropionitrile (DPN), was also capable of inhibiting HPA reactivity in a fashion similar to DHT and 3β-diol. Taken together, these results provide correlative evidence that 3β-diol mediates the effects of DHT on CORT and ACTH secretion by binding ERβ.
Further evidence as to the site of the HPA inhibiting activity of 3β-diol was provided recently by Lund et al. (14). Using small pellets of beeswax as a carrier for hormone, they discovered that the stereotaxic application of 3β-diol to the PVN of castrated male rats mimics the actions of both central and peripherally administered DHT. Furthermore, local application of an ERβ selective agonist, DPN, also mimics the actions of DHT. These inhibitory actions of 3β-diol and DPN can be blocked by the co-administration of the ER antagonist, tamoxifen, whereas the AR antagonist, flutamide has little effect. By contrast, both tamoxifen and flutamide only partially block the inhibitory actions of local DHT application, suggesting that there still exists some role of androgen receptors in the actions of DHT. Such data indicate that local synthesis of 3β-diol by cells in or around the PVN can profoundly impact HPA reactivity to stressors. Furthermore, these data indicate that compounds that bind ERβ can act in an inhibitory fashion. This latter point appears to be counter-intuitive to results demonstrating that oestradiol treatment of female rats increases their CORT response to stress. However, it is important to note that oestradiol appears to act through ERα to augment HPA reactivity. The ERα selective agonist propylpyrazole triol has an action opposite that of ERβ agonists, causing an increase in HPA reactivity to restraint stress (14). This raises the possibility that oestrogen increases HPA reactivity by binding ERα in females and that 3β-diol works to inhibit HPA reactivity by binding ERβ in males.
How then, can the HPA axis distinguish the enhancing from inhibiting actions of compounds, such as oestradiol, that bind equivalently to both ERα and ERβ? Our studies show the presence of aromatase mRNA in or near the PVN (14), and this fact presents a potential interpretive problem for the oestradiol regulation of the HPA axis in both males (as a result of aromatisation of testosterone to oestradiol) and females. One possibility lies in the ratio of ERα to ERβ that exists within neurones in and around of the PVN. One could argue that a greater ratio of ERα to ERβ results in a shift towards greater oestradiol-induced stimulation and the opposite would be true under conditions where ERβ was greater than ERα. Unfortunately, changes in the ratios of ERα and ERβ have not been described in any brain region to date, although it has been demonstrated that levels of receptor might change in response to circulating concentrations of hormones, particularly adrenal steroids. For example, stress and adrenalectomy can increase ERβ mRNA levels in the PVN (68) and the effect of adrenalectomy can be partially blocked by corticosterone, and another study indicated that adrenalectomy decreases ERβ mRNA levels and corticosterone prevents this response (69). Furthermore, glucocorticoid receptor stimulation with dexamethasone increases ERβ mRNA and immunoreactive cell numbers without altering ERα (42), thus shifting the balance toward inhibition. By contrast, oestradiol appears to reduce ERβ immunoreactivity in neurones around the PVN (42), thereby shifting the balance toward activation.
An emerging alternative explanation comes from the observation that transactivation of a gene promoter sequence by ERβ, after binding oestradiol, does not completely mimic the activation of the same promoter by 3β-diol. For example, using in vitro reporter gene assays, Pak et al. (70) have shown that, in the presence of ERβ, 3β-diol is a more potent activator of the human AVP promoter than is oestradiol, even though oestradiol has much greater binding affinity for ERβ than does 3β-diol. Hence, there is the potential for ligand identity to control the inhibitory actions of ERβ, and this may be a unique feature for 3β-diol gene activation, which is different from that observed after oestradiol binding. On a molecular level, these differences may be in part explained by the recruitment of different coregulatory proteins based on how the ligand fits within the ligand binding domain and alters the folding of the receptor; however, these studies await to be performed.
A third explanation may be found in the interactions of ERα and ERβ. ERβ has been shown to form heterodimers with ERα and, in doing so, are thought to act in a dominant negative fashion (71). Although possible for hormonal regulatory systems where ERα and ERβ are coexpressed by regulatory cells, this does not appear to be the case in the PVN, where ERα and ERβ are found in non-overlapping neuronal populations (46).
Oxytocin as a neuropeptide mediating the actions of ERβ
Oxytocin is a neuropeptide synthesised by neurones in the PVN and supraoptic nucleus (SON), which classically functions as a mediator of parturition and lactation. A number of studies report that oestrogen can increase oxytocin expression in brain (72–74). This effect appears to be mediated by ERβ because ERβ knockout mice, unlike wild-type mice, do not show increases after treatment with oestrogen (73, 75). Furthermore, a number of anatomical studies have identified ERβ in oxytocin neurones of the PVN of rats and mice (43–46). Interestingly, oxytocin neurones in the SON are devoid of ERβ (46).
Increasing evidence shows that oxytocin possesses anxiolytic properties and brain oxytocin can inhibit the activity of the HPA axis of both sexes (76–78). This effect is characterised by altered responses of parvocellular PVN neurones similar to those observed after ERβ activation (14). For example, intracerebroventricular (i.c.v.) administration of oxytocin decreased anxiety-related behaviors, the CORT and ACTH response to stress, and the induction of c-fos expression in PVN neurones (78–81). By contrast, peripheral administration of oxytocin enhances HPA responses to stress, acting at the level of the anterior pituitary (82, 83). Moreover, oxytocin knockout enhances CORT secretion after shaking stress (84) and blockade of oxytocin receptor, also enhances HPA reactivity (77). Furthermore, synaptic contacts have been reported between CRH and oxytocin neurones in the PVN (85).
Our previous results raise the question as to the mechanism whereby oxytocin/ERβ neurones can influence HPA reactivity. One possibility would be that oxytocin is released locally to PVN neurones to inhibit responses. Neurones within the PVN are sensitive to oxytocin (86), and oxytocin receptors are found in PVN (87, 88). Furthermore, oxytocin release in the PVN during stress has been demonstrated (89–91), and it results from dendritic release from magnocellular oxytocin neurones (92). The above possibility is further supported by microdialysis experiments showing a release of oxytocin and/or AVP within the hypothalamus that is dissociated from neurohypophysial secretion (91). More direct evidence for an action of oxytocin within the PVN comes from the observation that infusion of an oxytocin antagonist directly into the PVN enhances the stress response (76, 77). However, if oxytocin does have its primary action within the PVN, it is not necessarily a direct effect on the production of corticotrophin-releasing factor by parvocellular neurones because the majority of neuronal responses to oxytocin are excitatory (86), which would implicate an inhibitory intermediary.
Alternatively, axons from oxytocin neurones (that likely contain ERβ) arise from the parvocellular parts of the PVN and project to a number of extrahypothalamic brain regions, including limbic areas and autonomic centres such as the septum, amygdala, hippocampus, ventrolateral medulla and nucleus of the solitary tract (93–95). These oxytocin projections make synaptic contacts based on electron microscopy studies (94). Moreover, ERβ containing neurones in the PVN project to brainstem regions, such as the ventrolateral medulla (96) and preautonomic nuclei of the spinal cord (27). Cells in these same regions of the PVN also project trans-synaptically to the adrenal gland (97, 98). Such anatomical studies are supported by studies showing i.c.v. infusion of an oxytocin antagonist can prevent the actions of DPN and 3β-diol on hormonal responses to stress (A. Kudwa and R. J. Handa, unpublished data). Furthermore, we have shown that the c-fos mRNA response to stress is muted by both ERβ agonist and 3β-diol administration (14); thus, the possibility exists that inhibition of the PVN occurs via activation of ERβ in oxytocin containing neurones.
Summary
Increasing data indicate that the potent androgen, DHT, can be metabolised to 3β-diol, a steroid that can selectively bind ERβ. Because of its ability to bind ERβ, 3β-diol can act to inhibit hormonal responses to stress and stress related behaviors. It remains to be determined whether androgen metabolites that selectively bind ERβ, but have limited androgenic properties, may be useful pharmacological tools in the treatment of behavioral disorders that involve hyper-reactivity of the HPA axis. Nonetheless, increasing data have now demonstrated that the activity of DHT may not occur solely through its activation of ARs. Further consideration of potential oestrogenic actions of DHT metabolites are likely to be important for interpretating the growing literature regarding the nonreproductive actions of sex steroid hormones.
Acknowledgement
These studies were supported by the USPHS grant 5R01-NS 039951 (R.J.H.).
References
- 1.Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, Kuhn M, White RJ, Takaoka Y, Wolin L. The formation of estrogens by central neuroendocrine tissues. Recent Prog Horm Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- 2.Selmanoff MK, Brodkin LD, Weiner RI, Siiteri PK. Aromatization and 5alpha-reduction of androgens in discrete hypothalamic and limbic regions of the male and female rat. Endocrinology. 1977;101:841–848. doi: 10.1210/endo-101-3-841. [DOI] [PubMed] [Google Scholar]
- 3.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 4.Holsboer F, Barden N. Antidepressants and hypothalamic-pituitary-adrenocortical regulation. Endocr Rev. 1996;17:187–205. doi: 10.1210/edrv-17-2-187. [DOI] [PubMed] [Google Scholar]
- 5.Gaskin JH, Kitay JI. Adrenocortical function in the hamster. Sex differences and effects of gonadal hormones. Endocrinology. 1970;87:779–786. doi: 10.1210/endo-87-4-779. [DOI] [PubMed] [Google Scholar]
- 6.Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 7.Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- 8.McCormick CM, Linkroum W, Sallinen BJ, Miller NW. Peripheral and central sex steroids have differential effects on the HPA axis of male and female rats. Stress. 2002;5:235–247. doi: 10.1080/1025389021000061165. [DOI] [PubMed] [Google Scholar]
- 9.Kitay JI. Depression of adrenal corticosterone production in oophorectomized rats. Endocrinology. 1965;77:1048–1052. doi: 10.1210/endo-77-6-1048. [DOI] [PubMed] [Google Scholar]
- 10.Coyne MD, Kitay JI. Effect of ovariectomy on pituitary secretion of ACTH. Endocrinology. 1969;85:1097–1102. doi: 10.1210/endo-85-6-1097. [DOI] [PubMed] [Google Scholar]
- 11.Coyne MD, Kitay JI. Effect of orchiectomy on pituitary secretion of ACTH. Endocrinology. 1971;89:1024–1028. doi: 10.1210/endo-89-4-1024. [DOI] [PubMed] [Google Scholar]
- 12.Viau V, Meaney MJ. Testosterone-dependent variations in plasma and intrapituitary corticosteroid binding globulin and stress hypothalamic-pituitary-adrenal activity in the male rat. J Endocrinol. 2004;181:223–231. doi: 10.1677/joe.0.1810223. [DOI] [PubMed] [Google Scholar]
- 13.Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol Behav. 1994;55:117–124. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 14.Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viau V, Lee P, Sampson J, Wu J. A testicular influence on restraint-induced activation of medial parvocellular neurons in the paraventricular nucleus in the male rat. Endocrinology. 2003;144:3067–3075. doi: 10.1210/en.2003-0064. [DOI] [PubMed] [Google Scholar]
- 16.Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci. 1996;16:1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lund TD, Munson DJ, Haldy ME, Handa RJ. Dihydrotestosterone may inhibit hypothalamo-pituitary-adrenal activity by acting through estrogen receptor in the male mouse. Neurosci Lett. 2004;365:43–47. doi: 10.1016/j.neulet.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 18.Kerr JE, Beck SG, Handa RJ. Androgens selectively modulate C-fos messenger RNA induction in the rat hippocampus following novelty. Neuroscience. 1996;74:757–766. doi: 10.1016/0306-4522(96)00219-9. [DOI] [PubMed] [Google Scholar]
- 19.Lund TD, Munson DJ, Haldy ME, Handa RJ. Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2004;16:272–278. doi: 10.1111/j.0953-8194.2004.01167.x. [DOI] [PubMed] [Google Scholar]
- 20.Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- 21.Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146:137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- 22.Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology. 2004;79:125–132. doi: 10.1159/000077270. [DOI] [PubMed] [Google Scholar]
- 23.He WW, Kumar MV, Tindall DJ. A frame-shift mutation in the androgen receptor gene causes complete androgen insensitivity in the testicular-feminized mouse. Nucleic Acids Res. 1991;19:2373–2378. doi: 10.1093/nar/19.9.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yarbrough WG, Quarmby VE, Simental JA, Joseph DR, Sar M, Lubahn DB, Olsen KL, French FS, Wilson EM. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J Biol Chem. 1990;265:8893–8900. [PubMed] [Google Scholar]
- 25.Zuloaga DG, Morris JA, Jordan CL, Breedlove SM. Mice with the testicular feminization mutation demonstrate a role for androgen receptors in the regulation of anxiety-related behaviors and the hypothalamic-pituitary-adrenal axis. Horm Behav. 2008;54:758–766. doi: 10.1016/j.yhbeh.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Bingaman EW, Magnuson DJ, Gray TS, Handa RJ. Androgen inhibits the increases in hypothalamic corticotropin-releasing hormone (CRH) and CRH-immunoreactivity following gonadectomy. Neuroendocrinology. 1994;59:228–234. doi: 10.1159/000126663. [DOI] [PubMed] [Google Scholar]
- 27.Bingham B, Williamson M, Viau V. Androgen and estrogen receptor-beta distribution within spinal-projecting and neurosecretory neurons in the paraventricular nucleus of the male rat. J Comp Neurol. 2006;499:911–923. doi: 10.1002/cne.21151. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki S, Lund TD, Price RH, Handa RJ. Sex differences in the hypothalamo-pituitary-adrenal axis: novel roles for androgen and estrogen receptors. Recent Res Dev Endocrinol. 2001;2:69–86. [Google Scholar]
- 29.Zhou L, Blaustein JD, De Vries GJ. Distribution of androgen receptor immunoreactivity in vasopressin- and oxytocin-immunoreactive neurons in the male rat brain. Endocrinology. 1994;134:2622–2627. doi: 10.1210/endo.134.6.8194487. [DOI] [PubMed] [Google Scholar]
- 30.Bingaman EW, Baeckman LM, Yracheta JM, Handa RJ, Gray TS. Localization of androgen receptor within peptidergic neurons of the rat fore-brain. Brain Res Bull. 1994;35:379–382. doi: 10.1016/0361-9230(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 31.Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrinol. 2008;29:169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- 33.Beato M, Klug J. Steroid hormone receptors: an update. Hum Reprod Update. 2000;6:225–236. doi: 10.1093/humupd/6.3.225. [DOI] [PubMed] [Google Scholar]
- 34.Massie CE, Adryan B, Barbosa-Morais NL, Lynch AG, Tran MG, Neal DE, Mills IG. New androgen receptor genomic targets show an interaction with the ETS1 transcription factor. EMBO Rep. 2007;8:871–878. doi: 10.1038/sj.embor.7401046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim K, Barhoumi R, Burghardt R, Safe S. Analysis of estrogen receptor alpha-Sp1 interactions in breast cancer cells by fluorescence resonance energy transfer. Mol Endocrinol. 2005;19:843–854. doi: 10.1210/me.2004-0326. [DOI] [PubMed] [Google Scholar]
- 36.Price RH, Jr, Butler CA, Webb P, Uht R, Kushner P, Handa RJ. A splice variant of estrogen receptor beta missing exon 3 displays altered subnuclear localization and capacity for transcriptional activation. Endocrinology. 2001;142:2039–2049. doi: 10.1210/endo.142.5.8130. [DOI] [PubMed] [Google Scholar]
- 37.Webb P, Lopez GN, Uht RM, Kushner PJ. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol. 1995;9:443–456. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- 38.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 40.Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol. 1998;36:357–378. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 41.Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436:64–81. [PubMed] [Google Scholar]
- 42.Suzuki S, Handa RJ. Regulation of estrogen receptor-beta expression in the female rat hypothalamus: differential effects of dexamethasone and estradiol. Endocrinology. 2004;145:3658–3670. doi: 10.1210/en.2003-1688. [DOI] [PubMed] [Google Scholar]
- 43.Alves SE, Lopez V, McEwen BS, Weiland NG. Differential colocalization of estrogen receptor beta (ERbeta) with oxytocin and vasopressin in the paraventricular and supraoptic nuclei of the female rat brain: an immuno-cytochemical study. Proc Natl Acad Sci USA. 1998;95:3281–3286. doi: 10.1073/pnas.95.6.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hrabovszky E, Kallo I, Hajszan T, Shughrue PJ, Merchenthaler I, Liposits Z. Expression of estrogen receptor-beta messenger ribonucleic acid in oxytocin and vasopressin neurons of the rat supraoptic and paraventricular nuclei. Endocrinology. 1998;139:2600–2604. doi: 10.1210/endo.139.5.6024. [DOI] [PubMed] [Google Scholar]
- 45.Somponpun SJ, Sladek CD. Depletion of oestrogen receptor-beta expression in magnocellular arginine vasopressin neurones by hypovolaemia and dehydration. J Neuroendocrinol. 2004;16:544–549. doi: 10.1111/j.1365-2826.2004.01200.x. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki S, Handa RJ. Estrogen receptor-beta, but not estrogen receptor-alpha, is expressed in prolactin neurons of the female rat paraventricular and supraoptic nuclei: comparison with other neuropeptides. J Comp Neurol. 2005;484:28–42. doi: 10.1002/cne.20457. [DOI] [PubMed] [Google Scholar]
- 47.Miller WJ, Suzuki S, Miller LK, Handa R, Uht RM. Estrogen receptor (ER)beta isoforms rather than ERalpha regulate corticotropin-releasing hormone promoter activity through an alternate pathway. J Neurosci. 2004;24:10628–10635. doi: 10.1523/JNEUROSCI.5540-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Estacio MA, Yamada S, Tsukamura H, Hirunagi K, Maeda K. Effect of fasting and immobilization stress on estrogen receptor immunoreactivity in the brain in ovariectomized female rats. Brain Res. 1996;2:55–61. doi: 10.1016/0006-8993(96)00022-4. [DOI] [PubMed] [Google Scholar]
- 49.Simerly RB, Young BJ. Regulation of estrogen receptor messenger ribo-nucleic acid in rat hypothalamus by sex steroid hormones. Mol Endocrinol. 1991;5:424–432. doi: 10.1210/mend-5-3-424. [DOI] [PubMed] [Google Scholar]
- 50.Weiser MJ, Handa RJ. Estrogen impairs glocococricoid dependent negative feedback on the hypothalamic-pitoitacy-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009 Jan; doi: 10.1016/j.neuroscience.2008.12.058. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roselli CE, Horton LE, Resko JA. Distribution and regulation of aromatase activity in the rat hypothalamus and limbic system. Endocrinology. 1985;117:2471–2477. doi: 10.1210/endo-117-6-2471. [DOI] [PubMed] [Google Scholar]
- 52.Lephart ED, Lund TD, Horvath TL. Brain androgen and progesterone metabolizing enzymes: biosynthesis, distribution and function. Brain Res Brain Res Rev. 2001;3:25–37. doi: 10.1016/s0165-0173(01)00111-4. [DOI] [PubMed] [Google Scholar]
- 53.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 54.Handa RJ, Stadelman HL, Resko JA. Effect of estrogen on androgen receptor dynamics in female rat pituitary. Endocrinology. 1987;121:84–89. doi: 10.1210/endo-121-1-84. [DOI] [PubMed] [Google Scholar]
- 55.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 56.Gangloff A, Shi R, Nahoum V, Lin SX. Pseudo-symmetry of C19 steroids, alternative binding orientations, and multispecificity in human estrogenic 17beta-hydroxysteroid dehydrogenase. FASEB J. 2003;17:274–276. doi: 10.1096/fj.02-0397fje. [DOI] [PubMed] [Google Scholar]
- 57.Jin Y, Penning TM. Steroid 5alpha-reductases and 3alpha-hydroxysteroid dehydrogenases: key enzymes in androgen metabolism. Best Pract Res Clin Endocrinol Metab. 2001;15:79–94. doi: 10.1053/beem.2001.0120. [DOI] [PubMed] [Google Scholar]
- 58.Steckelbroeck S, Jin Y, Gopishetty S, Oyesanmi B, Penning TM. Human cytosolic 3alpha-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3beta-hydroxysteroid dehydrogenase activity: implications for steroid hormone metabolism and action. J Biol Chem. 2004;279:10784–10795. doi: 10.1074/jbc.M313308200. [DOI] [PubMed] [Google Scholar]
- 59.Torn S, Nokelainen P, Kurkela R, Pulkka A, Menjivar M, Ghosh S, Coca-Prados M, Peltoketo H, Isomaa V, Vihko P. Production, purification, and functional analysis of recombinant human and mouse 17beta-hydroxysteroid dehydrogenase type 7. Biochem Biophys Res Commun. 2003;305:37–45. doi: 10.1016/s0006-291x(03)00694-6. [DOI] [PubMed] [Google Scholar]
- 60.Weihua Z, Lathe R, Warner M, Gustafsson JA. An endocrine pathway in the prostate, ERbeta, AR, 5alpha-androstane-3beta,17beta-diol, and CYP7B1, regulates prostate growth. Proc Natl Acad Sci USA. 2002;99:13589–13594. doi: 10.1073/pnas.162477299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernandez-Guasti A, Martinez-Mota L. Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology. 2005;30:762–770. doi: 10.1016/j.psyneuen.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 62.Reddy DS. Anticonvulsant activity of the testosterone-derived neurosteroid 3alpha-androstanediol. Neuroreport. 2004;15:515–518. doi: 10.1097/00001756-200403010-00026. [DOI] [PubMed] [Google Scholar]
- 63.Rosellini RA, Svare BB, Rhodes ME, Frye CA. The testosterone metabolite and neurosteroid 3alpha-androstanediol may mediate the effects of testosterone on conditioned place preference. Brain Res Brain Res Rev. 2001;3:162–171. doi: 10.1016/s0165-0173(01)00116-3. [DOI] [PubMed] [Google Scholar]
- 64.Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- 65.Bauman DR, Steckelbroeck S, Williams MV, Peehl DM, Penning TM. Identification of the major oxidative 3alpha-hydroxysteroid dehydrogenase in human prostate that converts 5alpha-androstane-3alpha, 17beta-diol to 5alpha-dihydrotestosterone: a potential therapeutic target for androgen-dependent disease. Mol Endocrinol. 2006;20:444–458. doi: 10.1210/me.2005-0287. [DOI] [PubMed] [Google Scholar]
- 66.Sundin M, Warner M, Haaparanta T, Gustafsson JA. Isolation and catalytic activity of cytochrome P-450 from ventral prostate of control rats. J Biol Chem. 1987;262:12293–12297. [PubMed] [Google Scholar]
- 67.Guennoun R, Fiddes RJ, Gouezou M, Lombes M, Baulieu EE. A key enzyme in the biosynthesis of neurosteroids, 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase (3 beta-HSD), is expressed in rat brain. Brain Res Mol Brain Res. 1995;30:287–300. doi: 10.1016/0169-328x(95)00016-l. [DOI] [PubMed] [Google Scholar]
- 68.Somponpun SJ, Holmes MC, Seckl JR, Russell JA. Modulation of oestrogen receptor-beta mRNA expression in rat paraventricular and supraoptic nucleus neurones following adrenal steroid manipulation and hyperosmotic stimulation. J Neuroendocrinol. 2004;16:472–482. doi: 10.1111/j.1365-2826.2004.01190.x. [DOI] [PubMed] [Google Scholar]
- 69.Isgor C, Cecchi M, Kabbaj M, Akil H, Watson SJ. Estrogen receptor beta in the paraventricular nucleus of hypothalamus regulates the neuroendocrine response to stress and is regulated by corticosterone. Neuroscience. 2003;121:837–845. doi: 10.1016/s0306-4522(03)00561-x. [DOI] [PubMed] [Google Scholar]
- 70.Pak T, Chung W, Handa R. Estrogen receptor-beta mediates DHT-induced stimulation of the arginine vasopressinpromoter in neuronal cells. Endocrinology. 2007;148:3371–3382. doi: 10.1210/en.2007-0086. [DOI] [PubMed] [Google Scholar]
- 71.Pettersson K, Delauney F, Gustafsson JA. Estrogen receptor beta acts as a dominant regulator of estrogen signaling. Oncogene. 2000;19:4970–4978. doi: 10.1038/sj.onc.1203828. [DOI] [PubMed] [Google Scholar]
- 72.Dellovade TL, Zhu YS, Pfaff DW. Thyroid hormones and estrogen affect oxytocin gene expression in hypothalamic neurons. J Neuroendocrinol. 1999;11:1–10. doi: 10.1046/j.1365-2826.1999.00250.x. [DOI] [PubMed] [Google Scholar]
- 73.Nomura M, McKenna E, Korach KS, Pfaff DW, Ogawa S. Estrogen receptor-beta regulates transcript levels for oxytocin and arginine vasopressin in the hypothalamic paraventricular nucleus of male mice. Brain Res Mol Brain Res. 2002;2:84–94. doi: 10.1016/s0169-328x(02)00525-9. [DOI] [PubMed] [Google Scholar]
- 74.Shughrue PJ, Dellovade TL, Merchenthaler I. Estrogen modulates oxytocin gene expression in regions of the rat supraoptic and paraventricular nuclei that contain estrogen receptor-beta. Prog Brain Res. 2002;13:915–929. doi: 10.1016/s0079-6123(02)39004-6. [DOI] [PubMed] [Google Scholar]
- 75.Patisaul HB, Scordalakes EM, Young LJ, Rissman EF. Oxytocin, but not oxytocin receptor, is rRegulated by oestrogen receptor beta in the female mouse hypothalamus. J Neuroendocrinol. 2003;15:787–793. doi: 10.1046/j.1365-2826.2003.01061.x. [DOI] [PubMed] [Google Scholar]
- 76.Neumann ID, Kromer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept. 2000;2:31–38. doi: 10.1016/s0167-0115(00)00197-x. [DOI] [PubMed] [Google Scholar]
- 77.Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraven-tricular nucleus. J Neuroendocrinol. 2000;12:235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- 78.Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 79.Windle RJ, Gamble LE, Kershaw YM, Wood SA, Lightman SL, Ingram CD. Gonadal steroid modulation of stress-induced hypothalamo-pituitary-adrenal activity and anxiety behavior: role of central oxytocin. Endocrinology. 2006;147:2423–2431. doi: 10.1210/en.2005-1079. [DOI] [PubMed] [Google Scholar]
- 80.Ochedalski T, Subburaju S, Wynn PC, Aguilera G. Interaction between oestrogen and oxytocin on hypothalamic-pituitary-adrenal axis activity. J Neuroendocrinol. 2007;19:189–197. doi: 10.1111/j.1365-2826.2006.01525.x. [DOI] [PubMed] [Google Scholar]
- 81.Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J Neurosci. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muir JL, Brown R, Pfister HP. A possible role for oxytocin in the response to a psychological stressor. Pharmacol Biochem Behav. 1986;25:107–110. doi: 10.1016/0091-3057(86)90238-8. [DOI] [PubMed] [Google Scholar]
- 83.Petersson M, Hulting AL, Uvnas-Moberg K. Oxytocin causes a sustained decrease in plasma levels of corticosterone in rats. Neurosci Lett. 1999;3:41–44. doi: 10.1016/s0304-3940(99)00159-7. [DOI] [PubMed] [Google Scholar]
- 84.Mantella RC, Vollmer RR, Rinaman L, Li X, Amico JA. Enhanced corticosterone concentrations and attenuated Fos expression in the medial amygdala of female oxytocin knockout mice exposed to psychogenic stress. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1494–R1504. doi: 10.1152/ajpregu.00387.2004. [DOI] [PubMed] [Google Scholar]
- 85.Hisano S, Fukui Y, Chikamori-Aoyama M, Aizawa T, Shibasaki T. Reciprocal synaptic relations between CRF-immunoreactive- and TRH-immunoreactive neurons in the paraventricular nucleus of the rat hypothalamus. Brain Res. 1993;620:343–346. doi: 10.1016/0006-8993(93)90178-p. [DOI] [PubMed] [Google Scholar]
- 86.Inenaga K, Yamashita H. Excitation of neurones in the rat paraventricular nucleus in vitro by vasopressin and oxytocin. J Physiol. 1986;370:165–180. doi: 10.1113/jphysiol.1986.sp015928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brinton RE, Wamsley JK, Gee KW, Wan YP, Yamamura HI. [3H]Oxytocin binding sites in the rat brain demonstrated by quantitative light microscopic autoradiography. Eur J Pharmacol. 1984;102:365–367. doi: 10.1016/0014-2999(84)90270-x. [DOI] [PubMed] [Google Scholar]
- 88.Freund-Mercier MJ, Stoeckel ME. Somatodendritic autoreceptors on oxytocin neurones. Adv Exp Med Biol. 1995;395:185–194. [PubMed] [Google Scholar]
- 89.Nishioka T, Anselmo-Franci JA, Li P, Callahan MF, Morris M. Stress increases oxytocin release within the hypothalamic paraventricular nucleus. Brain Res. 1998;2:56–60. doi: 10.1016/s0006-8993(97)01159-1. [DOI] [PubMed] [Google Scholar]
- 90.Engelmann M, Ebner K, Landgraf R, Holsboer F, Wotjak CT. Emotional stress triggers intrahypothalamic but not peripheral release of oxytocin in male rats. J Neuroendocrinol. 1999;11:867–872. doi: 10.1046/j.1365-2826.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- 91.Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- 92.Ludwig M, Leng G. Intrahypothalamic vasopressin release. An inhibitor of systemic vasopressin secretion? Adv Exp Med Biol. 1998;449:163–173. [PubMed] [Google Scholar]
- 93.Buijs RM, Swaab DF. Immuno-electron microscopical demonstration of vasopressin and oxytocin synapses in the limbic system of the rat. Cell Tissue Res. 1979;204:355–365. doi: 10.1007/BF00233648. [DOI] [PubMed] [Google Scholar]
- 94.Voorn P, Buijs RM. An immuno-electronmicroscopical study comparing vasopressin, oxytocin, substance P and enkephalin containing nerve terminals in the nucleus of the solitary tract of the rat. Brain Res. 1983;270:169–173. doi: 10.1016/0006-8993(83)90809-0. [DOI] [PubMed] [Google Scholar]
- 95.Landgraf R, Malkinson T, Horn T, Veale WL, Lederis K, Pittman QJ. Release of vasopressin and oxytocin by paraventricular stimulation in rats. Am J Physiol. 1990;2:R155–R159. doi: 10.1152/ajpregu.1990.258.1.R155. [DOI] [PubMed] [Google Scholar]
- 96.Stern JE, Zhang W. Preautonomic neurons in the paraventricular nucleus of the hypothalamus contain estrogen receptor beta. Brain Res. 2003;2:99–109. doi: 10.1016/s0006-8993(03)02594-0. [DOI] [PubMed] [Google Scholar]
- 97.Kerman IA, Akil H, Watson SJ. Rostral elements of sympatho-motor circuitry: a virally mediated transsynaptic tracing study. J Neurosci. 2006;26:3423–3433. doi: 10.1523/JNEUROSCI.5283-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Strack AM, Sawyer WB, Platt KB, Loewy AD. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res. 1989;491:274–296. doi: 10.1016/0006-8993(89)90063-2. [DOI] [PubMed] [Google Scholar]