Summary
In budding yeast, vacuole inheritance is tightly coordinated with the cell cycle. The movement of vacuoles and several other organelles is actin-based and is mediated by interaction between the yeast myosin V motor Myo2, and organelle-specific adaptors. Myo2 binds to vacuoles via the adaptor protein, Vac17, which binds to the vacuole membrane protein, Vac8. Here we show that the yeast cyclin-dependent kinase, Cdk1 phosphorylates Vac17 and that phosphorylation of Vac17 parallels cell-cycle dependent movement of the vacuole. Substitution of the Cdk1 sites in Vac17 decreases its interaction with Myo2 and causes a partial defect in vacuole inheritance. This defect is enhanced in the presence of Myo2 with mutated phosphorylation sites. Thus, Cdk1 appears to control the timing of vacuole movement. The presence of multiple predicted Cdk1 sites in other organelle-specific myosin V adaptors suggests that the inheritance of other cytoplasmic organelles may be regulated by a similar mechanism.
Keywords: cyclin-dependent kinase, organelle inheritance, phosphorylation, cell cycle, Vac17, Myo2
Introduction
The movement of the nucleus and cytoplasmic organelles during cell division is spatially and temporally coordinated with the cell cycle. In the budding yeast S. cerevisiae, two class V myosins, Myo2 and Myo4, move several cytoplasmic components. Attachment of each motor protein with its diverse cargoes is differentially regulated to ensure that each cargo arrives at the correct time and at its proper destination. This regulation is achieved in part through recognition of distinct cargoes by different regions of the myosin globular tail. For example, the region of Myo2 that binds the vacuole is distinct from the region that binds secretory vesicles (Catlett et al., 2000; Pashkova et al., 2005; Pashkova et al., 2006; Schott et al., 1999). The cargo-binding region of Myo2 is phosphorylated at residues T1132, S1134 and S1135 (Legesse-Miller et al., 2006). Surprisingly, mutation of these residues to alanine or aspartic acid has no effect on known functions of Myo2, suggesting that regulation of Myo2 by phosphorylation is redundant with other types of regulation. The kinase(s) that phosphorylate Myo2 remain to be determined.
Regulation of cargo binding to Myo2 is achieved through cargo-specific myosin V adaptors. Some adaptors have been identified. Vac17-Vac8 connects the yeast vacuole with Myo2 (Ishikawa et al., 2003; Tang et al., 2003). Kar9-Bim1 links Myo2 to the microtubles that orient the nucleus (Miller et al., 2000; Miller and Rose, 1998). Inp2 connects Myo2 to peroxisomes (Fagarasanu et al., 2006; Itoh et al., 2004). Mmr1 and Ypt11 are required for the interaction of Myo2 with mitochondria (Altmann et al., 2008; Boldogh et al., 2004; Itoh et al., 2004; Itoh et al., 2002).
Components of the vacuole-specific Myo2 adaptor, Vac17 and Vac8, were identified from mutants defective in vacuole inheritance (Ishikawa et al., 2003; Tang et al., 2003; Wang et al., 1998). Vac17 interacts with Myo2 and Vac8, a vacuole membrane protein (Tang et al., 2003). Vac8 attaches to the vacuole via myristoylation and palmitoylation (Peng et al., 2006; Wang et al., 1998). The formation of the Myo2-Vac17-Vac8 complex connects the vacuole to Myo2. Vac8 participates in four additional vacuole-related processes and thereby plays a role in coordinating vacuole inheritance with other vacuole-related pathways (Tang et al., 2006).
Vac17 is composed of a Myo2-binding domain, a PEST sequence, and a Vac8- binding domain. PEST sequences, stretches of at least 12 amino acids enriched in proline (P), glutamate (E), serine (S) and threonine (T), are often found in short-lived proteins (Rechsteiner and Rogers, 1996). Deletion of the Vac17 PEST sequence results in elevated Vac17 levels, suggesting that the PEST sequence serves as a signal for the rapid turnover of Vac17. In wild type cells, Myo2 localizes to sites of polarized growth: the presumptive bud site before budding, the bud tip early in the cell cycle and the mother-bud neck at cytokinesis (Lillie and Brown, 1994). Stabilization of Vac17 by removal of its PEST sequence results in the continued attachment Myo2 to the vacuole, and leads to an inappropriate “backward” movement of the bud vacuole to the mother-bud neck. The above evidence suggests that the degradation of Vac17 plays a key role in releasing Myo2 from the vacuole and depositing the vacuole at its proper location (Tang et al., 2003).
Here we show that Vac17 is a phosphoprotein and that its phosphorylation is coordinated with the cell cycle and parallels vacuole inheritance. Phosphorylation of Vac17 occurs on the vacuole membrane in the presence of Vac8. Cdc28, the yeast Cdk1, phosphorylates Vac17. Substitution of the four putative Cdk1 phosphorylation sites in Vac17 results in a defect in vacuole inheritance and in the ability of Vac17 to bind to Myo2. The defect caused by mutation of the Cdk1 sites on Vac17 is enhanced in the presence of Myo2 with mutated phosphorylation sites. These findings provide molecular insight into how vacuole inheritance is coordinated with the cell cycle.
Results
Phosphorylation of Vac17 is coordinated with vacuole inheritance
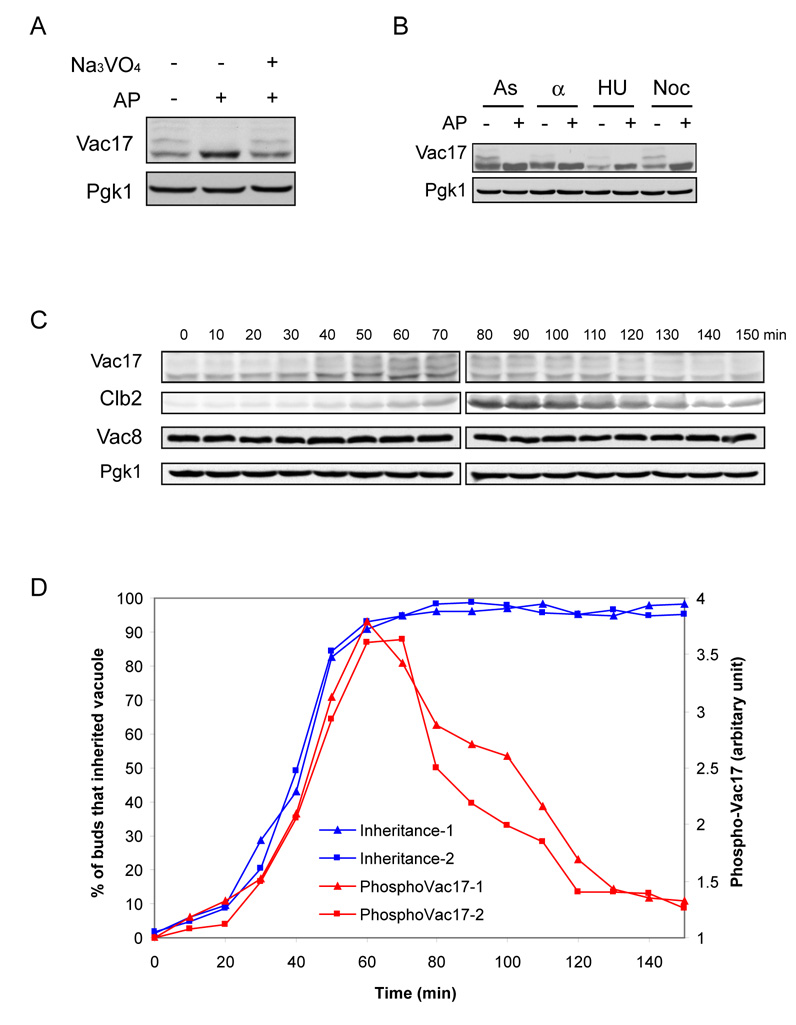
Vac17 migrates as several bands on SDS-PAGE, which result from its phosphorylation (Figure 1A). Upon treatment with alkaline phosphatase, Vac17 collapses into a single fast-migrating band, an effect blocked by addition of sodium orthovanadate, an alkaline phosphatase inhibitor. The presence of multiple slow-migrating bands indicates that multiple residues of Vac17 are phosphorylated.
Figure 1. Phosphorylation of Vac17 is coordinated with vacuole inheritance.
A. Vac17 is phosphorylated. 15 µl of water, alkaline phosphatase (AP), and/or sodium orthovanadate were added to cell extracts (1 ml) and incubated for at 30°C 1 hour. Immunoblot analysis was performed using anti-Vac17 and anti-Pgk1 antibodies. B–C. Phosphorylation of Vac17 is cell cycle-dependent. B. Wild type cells were arrested in G1 phase with α-factor (α), S phase with hydroxyurea (HU), or M phase with nocodazole (Noc). As: untreated asynchronous culture. Cell extracts analyzed by immnunoblot. C. Wild type cells were synchronized by α-factor arrest and release. Samples were collected at indicated times after release to monitor phosphorylation of Vac17 during the cell cycle. D. Phosphorylation of Vac17 parallels vacuole inheritance. Wild type cells labeled with FM4-64, 1 hour, arrested with α-factor for 3 hours, then released. Percentage of buds with inherited vacuoles (blue) at the indicated times after release. 80–200 cells scored for each time point. Phosphorylated Vac17 levels (red) were quantified using Labworks 4.0 software. Data from two independent experiments.
Both mRNA and protein levels of Vac17 oscillate during the cell cycle (Spellman et al., 1998; Tang et al., 2003). Phosphorylation of Vac17 is also cell cycle-dependent. Based on the relative abundance of the shifted bands in extracts from arrested cultures, Vac17 was least phosphorylated in G1, and reached maximal phosphorylation in M phase (Figure 1B). To further monitor changes in phosphorylation of Vac17 during the cell cycle, wild type cells were synchronized by α-factor arrest and release. The level of phosphorylated Vac17 was examined every 10 minutes (Figure 1C). As the cell cycle progressed, the proportion of phosphorylated Vac17 increased, reaching a peak at 60 minutes (Figure 1D). Then it decreased, reaching its lowest levels at 150 minutes, the point where the first cell division is finished and the next cycle initiates.
Notably, the timing of phosphorylation of Vac17 directly parallels vacuole inheritance. Wild type cells labeled with FM4–64 were synchronized. At each time point, aliquots of cells were examined for Vac17 phosphorylation and for the arrival of an inherited vacuole in the bud. The rise in the levels of phosphorylated Vac17 corresponded with the extent of vacuole inheritance (Figure 1D). Vacuole inheritance started immediately after α-factor release, and by 60 minutes nearly all buds inherited vacuoles. Phosphorylation of Vac17 paralleled vacuole inheritance and reached its peak at 60 minutes. The tight correlation of phosphorylation of Vac17 with vacuole inheritance strongly suggests that Vac17 phosphorylation plays a key role in the regulation of vacuole movement during the cell cycle.
Vac17 is a direct target of Cdk1
Many yeast proteins whose phosphorylation is cell cycle-dependent, are phosphorylated by Cdc28, the S. cerevisiae Cdk1 (Verma et al., 1997; Xu et al., 2000). To test whether the kinase activity of Cdc28 is required for Vac17 phosphorylation, we examined a cdc28 temperature-sensitive mutant, cdc28–4 (Surana et al., 1991), whose kinase activity is compromised at the restrictive temperature (36°C). In cdc28–4, Vac17 phosphorylation was strongly reduced at 36°C. In contrast, the levels of phosphorylated Vac17 in an isogenic wild type strain were similar at 24°C and 36°C (Figure 2A). Since phosphorylation of Vac17 is cell cycle-dependent, a decrease in the level of phosphorylated Vac17 in cdc28–4 at 36°C may be due to cell cycle arrest. To estimate cell cycle progression, we measured the ratio of the diameter of the bud versus the mother cell. The majority of cdc28–4 cells accumulated in G1 phase at 36°C (Figure S1) (Surana et al., 1991), suggesting that the decrease of Vac17 phosphorylation is due to a defect in cell cycle progression.
Figure 2. Vac17 is a direct target of Cdk1.
A. Phosphorylation of Vac17 is decreased in cdc28-4ts. cdc28-4ts and wild type (W303) strains grown at the permissive temperature (24°C) in log phase, then shifted to the restrictive temperature (36°C). Cells were harvested before and 90 minutes after the shift. Cell extracts were treated with alkaline phosphatase and analyzed by immunoblot. B. Inhibition of Cdc28 kinase activity results in a decrease in Vac17 phosphorylation. 1NM-PP1 (10 mM in DMSO) or DMSO alone were added to a final concentration of 10 nM to log phase cdc28-as1 cells. Samples were collected after 30 min and analyzed by immunoblot. C. Activation of Cdc28 via constitutive overexpression of Clb2 or Clb5 results in hyper-phosphorylation of Vac17. Cells expressing either Gal-CLB2-TAP or Gal-CLB5-TAP were grown in YEP + 2% glycerol + 2% ethanol media in log phase. Galactose was added to a final concentration of 2% to induce expression of Clb2-TAP or Clb5-TAP. Cells were harvested before and 3 hours after induction. Cell extracts were subjected to immunoblot analysis. D. Vac17 contains four Cdk1 consensus sites. Full consensus sites (red), minimum consensus sites (blue). Myo2 BD: Myo2-binding domain; PEST: PEST sequence; Vac8 BD: Vac8-binding domain. E. Direct phosphorylation of Vac17 by Clb2-Cdc28 or Clb5-Cdc28 in vitro. 1 µg of histone H1 or 2 µg recombinant Vac17 fragments purified from E. coli were incubated with [γ32P]-ATP in the presence or absence of Clb2-Cdc28 or Clb5- Cdc28. Phosphorylation was analyzed by autoradiography following SDS-PAGE. F. Phosphorylation sites of the Vac17 peptides determined by mass spectrometry. Phosphorylated peptides with phosphorylation sites underlined.
Thus, we tested an ATP analogue-sensitive cdc28 mutant (cdc28-as1), whose kinase activity is specifically inhibited within 30 minutes by the designed inhibitor, 1NM-PP1 (Bishop et al., 2000; Ubersax et al., 2003). At 30 minutes the inhibition of Cdc28 activity had a modest effect on cell cycle progression (Figure S2). However, phosphorylation of Vac17 was dramatically reduced (Figure 2B). Note that inhibition of the Cdc28 kinase also resulted in a reduction in the level of the Vac17 protein. Given that VAC17 transcription levels are regulated during the cell cycle (Spellman et al., 1998), it is likely that inhibition of the kinase activity of Cdc28 also decreased VAC17 transcription.
The kinase activity of Cdc28 is regulated via its binding to a series of cyclins: Cln1, Cln2 and Cln3 at G1 phase; Clb5 and Clb6 at S phase; and Clb1, Clb2, Clb3 and Clb4 at mitosis (Mendenhall and Hodge, 1998). To further test whether Cdc28 is responsible for Vac17 phosphorylation, we constitutively overexpressed an M phase cyclin (CLB2) or an S phase cyclin (CLB5) using a galactose-inducible promoter. Overexpression of CLB2 had a slight effect, whereas overexpression of CLB5 had no obvious effect on the cell cycle (Figure S3). Notably, overexpression of either cyclin increased Vac17 phosphorylation (Figure 2C). The above results strongly suggest that Cdk1 kinase activity is required for phosphorylation of Vac17.
Vac17 contains four predicted Cdk1 phosphorylation sites, two full-consensus sites T149 and S178 (S/T-P-x-K/R, where x = any amino acid), and two minimal-consensus sites S119 and T248 (S/T-P) (Figure 2D). To test if Cdc28 phosphorylates Vac17 directly, the Clb2-Cdc28 and Clb5-Cdc28 complexes were purified from yeast. Both complexes were active and phosphorylated histone H1 (a known Cdk1 substrate) as well as two recombinant Vac17 peptides (Figure 2E). The phosphorylated Vac17 peptides were subjected to mild proteolysis and analyzed by tandem mass spectrometry. Four predicted Cdk1 sites, S119, T149, S178, and T248 or possibly the adjacent site S247 were phosphorylated (Figure 2F).
Vac17 is less phosphorylated and dissociates from the vacuole membrane in the absence of Vac8
To test whether Vac17 association with Myo2, Vac8 and/or the vacuole membrane is required for its phosphorylation, we examined the phosphorylation and localization of Vac17 in the myo2-N1304S and vac8Δ mutants. myo2-N1304S interaction with Vac17 is defective (Catlett et al., 2000), however, the degree of Vac17 phosphorylation resembled that of wild type. Conversely, in the vac8Δ mutant, Vac17 was significantly less phosphorylated (Figure 3A). These findings suggest that the association of Vac17 with Vac8, rather than Myo2, is crucial for phosphorylation of Vac17.
Figure 3. Phosphorylation of Vac17 in vivo requires the presence of Vac8.
A. Vac17 phosphorylation is impaired in vac8Δ cells. Cell extracts from wild type, vac8Δ, myo2-N1304S and vac17Δ cells grown to log phase, were treated with alkaline phosphatase (AP). Because Vac17 levels in vac8Δ and myo2 are 10-fold higher than wild-type, extracts from the mutants were diluted 10-fold using cell extracts from the vac17Δ mutant. Aliquots (15 µl) analyzed by immunoblot. B. Localization of Vac17-GFP in wild type, vac8 and myo2 mutant cells. Cells carrying the VAC17-GFP plasmid were labeled with FM4–64 for 1.5 hours, and chased for one doubling time (~3 hours). Overlay images indicate vacuoles (red) and Vac17-GFP (green). >250 cells scored for the mutant cells. 9.7% vac8Δ and 20.6% myo2-N1304S cells had detectable Vac17-GFP. Localization of Vac17-GFP in cells where fluorescence was detectable was the same as the examples shown. C. Vac17 and Myo2 co-localize in vac8Δ cells. Wild type VAC17-GFP was visualized in a vac8Δ MYO2-mRFP strain. Scale bars, 5 µm.
To determine the localization of Vac17 in wild-type, myo2-N1304S, and vac8Δ strains, we examined the localization of Vac17-GFP. Due to the low abundance of Vac17, Vac17-GFP in wild type cells was not detected (Figure 3B). However Vac17-GFP is detectable in vac8 and myo2 mutants; Vac17 levels are elevated about 10-fold in these strains (Figure 3A). Consistent with a defect in the interaction of Vac17 with myo2-N1304S, Vac17-GFP appears dispersed throughout the vacuole membrane (Figure 3B). In contrast, in the vac8Δ mutant, Vac17-GFP no longer localized on the vacuole membrane, and instead was concentrated at the bud tip in cells with small buds, and the bud neck in large-budded cells (Figure 3B). These are the sites where Myo2 accumulates (Figure 3C). The above results strongly support the postulate that phosphorylation of Vac17 either requires its association with Vac8 and/or the vacuole membrane, but does not require Vac17 interaction with Myo2.
A vac17 mutant missing the Cdk1 phosphorylation sites causes a defect in vacuole inheritance
Mutations at either of the full-consensus sites, T149A and S178A, reduced the slow-migrating bands of Vac17, and in vac17-2A (T149A/S178A), these bands were no longer detected (Figure S4A). To test if T149 and S178 were the only phosphorylation sites, we utilized a stabilized vac17 mutant (vac17-F225S); whose protein levels are about 10-fold higher than wild type but whose phosphorylation pattern, as measured by migration on SDS-PAGE, closely resembles wild type Vac17 (Figure S4B). The slow-migrating bands of Vac17 were diminished when all four putative Cdk1 sites were mutated. Thus, all four Cdk1 sites are phosphorylated in vivo. Substitution of the Cdk1 sites had no effect on the cell cycle-dependent synthesis of Vac17. In addition, these mutations do not alter the localization of Vac17, Myo2 or Vac8 (Figure S4C–E).
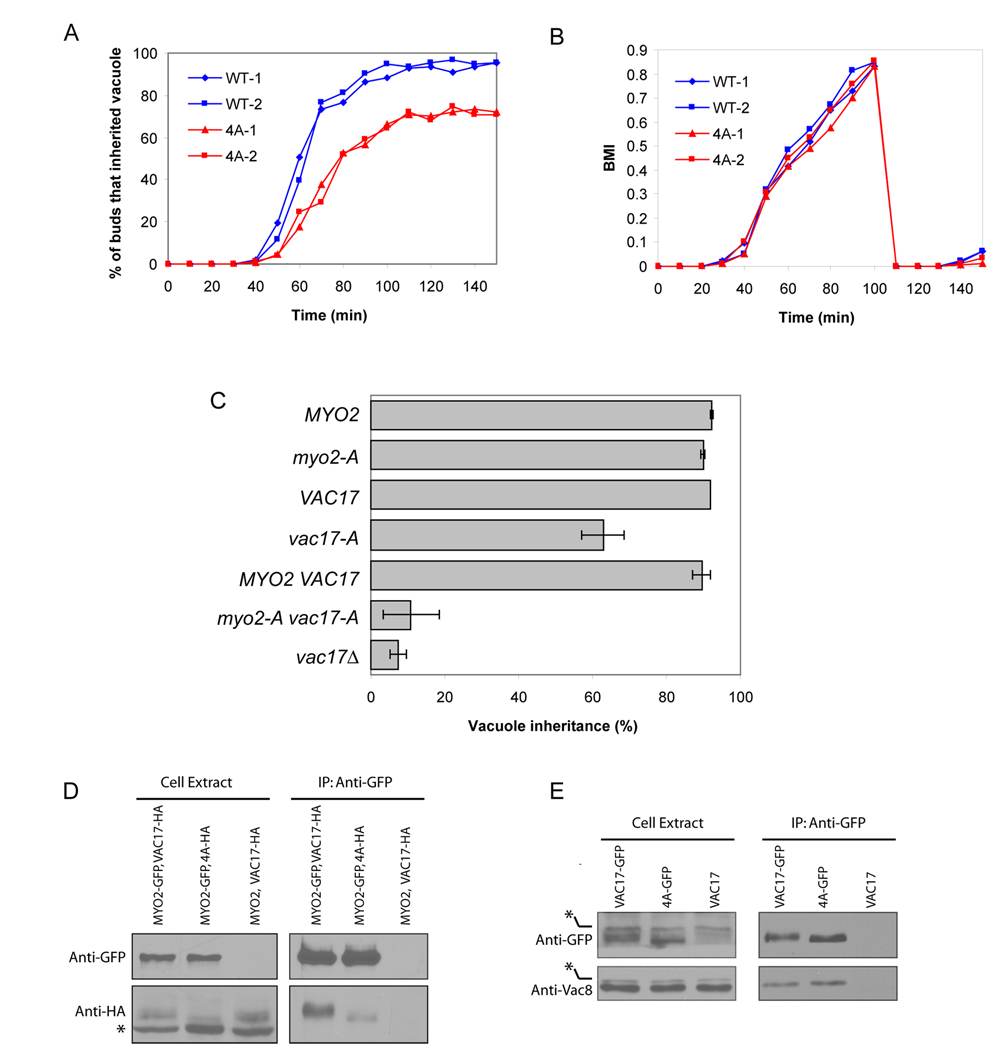
Notably, the vac17-4A mutant caused a delay in vacuole inheritance (Figure 4A). By 70 minutes after alpha-factor release, 80% of wild type buds had received a vacuole. In contrast, at the same time-point less than 30% of mutant buds had inherited a vacuole. In addition, while nearly 95% of the wild type buds ultimately inherit vacuoles from mother cells, only ~70% of vac17-4A buds inherit vacuoles (Figure 4A). This delay and defect was not due to a disturbance of the cell cycle (Figure 4B).
Figure 4. Cdk1 phosphorylation of Vac17 regulates vacuole inheritance via increasing the interaction of Vac17 and Myo2.
A. Mutation of the four Cdk1 consensus sites of Vac17 results in a partial defect in vacuole inheritance. Vacuole inheritance of wild type and vac17-4A cells were scored at each time point as described in Figure 1D. Two independent cell cycles shown. B. Mutation of four Cdk1 consensus sites in Vac17 does not affect the cell cycle. Cell cycle progression was estimated from the bud-mother index (BMI, ratio of bud to mother cell diameter). >80 cells scored for each time point. Two independent experiments shown. C. Vacuole inheritance is further impaired in the myo2-3A vac17-4A double mutant. >200 cells scored for each sample. Averages and standard deviations from three independent experiments. D. Substitution of the Cdk1 sites of Vac17 decrease its binding to Myo2. Myo2-GFP was immunoprecipitated with an anti-GFP antibody. Co-precipitation of Vac17-HA or vac17-4A-HA assayed by immunoblot. C. Substitution of the Cdk1 sites of Vac17 does not affect its ability to bind Vac8. Vac17-GFP or vac17-4A-GFP was immunoprecipitated with an anti-GFP antibody. Co-precipitation of Vac8 assayed by immunoblot. Asterisks: non-specific bands.
The fact that vac17-4A retains partial function in vacuole inheritance suggested that regulation of Vac17 via Cdk1-dependent phosphorylation is partially redundant with additional regulation of the transport complex. One potential second target is Myo2. Therefore, we tested whether regulation of vacuole movement via Cdk1-dependent phosphorylation of Vac17 was partially redundant with phosphorylation of the cargo-binding domain of Myo2. Indeed, when the myo2–3A mutation (Legesse-Miller et al., 2006) was combined with vac17-4A mutant, there was a dramatic reduction in vacuole inheritance. Approximately 10% of the double mutant cells (myo2-3A, vac17-4A) showed normal vacuole inheritance, whereas, in wild type, nearly 90% of buds inherited a vacuole (Figure 4C). Thus, phosphorylation of both Vac17 and Myo2 regulate the formation of the vacuole specific, Myo2-transport complex. This would account for the partial defect observed in vac17-4A, and the lack of an observable defect in myo2–3A. These findings strongly support the hypothesis that phosphorylation of Vac17 by Cdk1 is part of the mechanism that coordinates vacuole inheritance with the cell cycle.
Cdk1 phosphorylation of Vac17 promotes vacuole inheritance via an increase in the interaction of Vac17 and Myo2
Two of the four Cdk1 sites, S119 and T149, reside in the Myo2-binding domain (Figure 2D). Thus phosphorylation of Vac17 by Cdk1 may promote vacuole inheritance by enhancing the interaction between Vac17 and Myo2. Indeed, Myo2-GFP pulled down approximately 75% less vac17-4A-HA compared with wild type Vac17 (Figure 4D). Moreover, based on the mobility shift, it appears that phosphorylated Vac17 preferentially binds to Myo2. These findings strongly suggest that Cdk1-dependent phosphorylation of Vac17 regulates vacuole inheritance by enhancing the interaction between Vac17 and Myo2.
Loss of Myo2 phosphorylation does not cause a further decrease in the interaction between Vac17 and Myo2. The myo2–3A mutant has no defect in its ability to pull down Vac17 (Figure S5, lane 3 vs 5). Similarly, there was no further defect in the interaction of vac17-4A-HA with myo2–3A (Figure S5, lane 4 vs 6). vac17-4A interacted with Myo2 and myo2–3A to similar extents. Thus phosphorylation of Myo2 likely promotes its interaction with the vacuole via an as yet unidentified mechanism. In fact, previous genetic studies had suggested that in addition to Vac17 and Vac8 at least one other as yet unidentified protein is important for the interaction of Myo2 with the vacuole (Ishikawa et al., 2003). Alternatively, phosphorylation of the globular tail of Myo2 may recruit a protein with a role in the attachment of Myo2 to all cargoes. Despite the fact that phosphorylation of Myo2 does not increase the interaction of Myo2 with Vac17, the above results demonstrate that Cdk1 phosphorylation of Vac17 increases the association of Vac17 with Myo2, and thereby promotes vacuole inheritance and coordinates vacuole movement with the cell cycle.
None of the Cdk1 sites are in the Vac8-binding domain of Vac17. As expected, Cdk1 phosphorylation did not affect Vac17-Vac8 interactions; similar amounts of Vac8 were co-immunoprecipitated with Vac17-GFP or vac17-4A-GFP (Figure 4E).
Discussion
Cytoplasmic organelles of S. cerevisiae are distributed to the bud in coordination with the cell cycle. The molecular basis of this coordination was unknown. In fact, the inheritance of diverse cytoplasmic organelles has often been viewed as secondary to other processes controlled by the cell cycle. This is likely due in part to the fact that distinct organelles are moved by different mechanisms. Moreover, the intrinsic importance of coordinating DNA replication or transcription with the cell cycle is more apparent. Our finding that the key players that control yeast cell cycle progression, Cdk1 and its cyclins, also play a direct regulatory role in vacuole inheritance is of critical importance. This study demonstrates that Vac17, a component of a vacuole specific adaptor for Myo2, is a direct substrate of Cdk1. Phosphorylation of Vac17 enhances the interaction of Vac17 with Myo2 and promotes vacuole inheritance. These results provide insight into how vacuole inheritance is coordinated with the cell cycle.
Several lines of evidence indicate that Vac17 is a substrate of Cdk1. Substitution of the Cdk1 consensus sites dramatically reduced Vac17 phosphorylation as measured by the disappearance of the slow-migrating species. Likewise, inhibition of Cdk1 kinase activity led to a decrease in Vac17 phosphorylation. Moreover, overexpression of the Cdk1 cyclins, Clb2 and Clb5, resulted in hyperphosphorylation of Vac17. Finally, Cdk1 phosphorylates Vac17 in vitro at the predicted Cdk1 sites.
Multiple Cdk1 cyclins might be required for Vac17 phosphorylation; deletion of a single cyclin does not affect Vac17 phosphorylation (not shown). Phosphorylation of Vac17 increases early in the cell cycle and continues to increase through S phase. In addition, the levels of the M phase cyclin, Clb2, peak after the peak of Vac17 phosphorylation. It is likely that the G1 and/or S phase cyclins are required for Vac17 phosphorylation. In an earlier study, deletion of CLN3, resulted in a partial defect in vacuole inheritance (Han et al., 2003). However, using the same strains, we observed little difference between wild type and mutant cells (86.3% wild type vs 78.8% cln3Δ cells have normal vacuole inheritance, n>200). In a second strain background (LWY3250), cln3Δ had no effect on vacuole inheritance (95.7% of wild type vs 95.5% of cln3Δ cells have normal vacuole inheritance, n> 200). Thus, the cyclins required for coordination of vacuole movement with the cell cycle are unknown.
Notably, another Myo2-binding protein, Kar9, is a substrate of Cdk1. Phosphorylation of Kar9 prevents its binding to the spindle pole that is distal from the bud, and therefore ensures that only one pole is oriented toward the bud (Liakopoulos et al., 2003; Maekawa et al., 2003; Moore and Miller, 2007). Our studies of Vac17 raise the possibility that Cdk1 phosphorylation of Kar9 may regulate its attachment to Myo2. Likewise, other yeast myosin V adaptors, such as Mmr1 and Inp2 contain multiple putative Cdk1 sites (Table S1). It is possible that these myosin V adaptors are phosphorylated by Cdk1, and like Vac17, may regulate a cell cycle-dependent interaction with myosin V. The fact that each myosin V adaptor is dedicated to the movement of a single organelle suggests that they are ideal candidates to regulate movement of each cargo. Thus, Cdk1 phosphorylation may be a common regulatory mechanism for coordination of organelle inheritance with the cell cycle.
Experimental Procedures
Strains (Table S2) and plasmids (Table S3) are listed. Strains were grown at 24°C unless specified.
Alkaline phosphatase treatment and immunoblot analysis of Vac17
Log phase cells (20 OD600 units) were suspended in 1 ml ice cold 0.2 M NaOH, 0.2% β-mercaptoethanol (v/v), and incubated on ice for 10 min. 50 µl trichloroacetic acid (TCA) was added. Protein pellets were resuspended in 140 µl of 0.3 M sorbitol, 10 mM Tris, pH 7.5, 0.1 M NaCl, 1.0 mM MgCl2, 1.0 mM EDTA, then 60 µl of 1 M Tris base, 133.4 µl 10% SDS and 7 µl of β-mercaptoethanol were added. 100 µl of resultant lysates were brought to 1.0 ml with 50 mM Tris-HCl, pH 8.5. Phosphatase reactions were started with 15 µl of water (mock) or calf intestine alkaline phosphatase (Roche, 1 U/µl), incubated at 30°C, 1 h, and terminated with TCA; final concentration 10%. After 10 min on ice, proteins were pelleted; 12,000 g, 5 min. Protein pellets were dissolved in 100 µl 2 × SDS loading buffer and 20 µl 1 M Tris base. The resultant samples were subjected to immunoblot analysis. Blots were visualized with a CCD camera (BioChemi System, UVP BioImaging), and quantified using LabWorks 4.0 (UVP BioImaging).
Live-cell imaging
Vacuoles were labeled with FM4-64 (Molecular Probes) (Wang et al., 1998). Images were obtained with an Axioscope 2 (Carl Zeiss) or Olympus IX-71 microscope, and processed using MetaMorph 4.5 software or Photoshop.
Subcellular fractionation
50 OD600 of log phase cells were frozen in liquid N2. Cells were lysed in 500 µl lysis buffer (50 mM HEPES-KOH pH7.6, 150 mM KCl, 1 mM EGTA, 20 mM sodium pyrophosphate, 10 mM NaN3, 20 mM NaF, 1mM sodium orthovandate, 100 mM β-glycerophosphate, 1 × Sigma™ yeast protease inhibitor cocktail) using a beadbeater (Biospec). Lysates were centrifuged; 300 × g for 1 min, 4°C. Resultant cell extracts were centrifuged at 13,000 × g for 10 min; 4°C. Supernatants (S13) and pellets (P13) were resuspended in the same volume of lysis buffer.
In vitro kinase assays
Cells carrying pGal-CLB2-TAP or pGal-CLB5-TAP were grown to log phase in YEP containing 2% glycerol, 2% ethanol. Galactose was added to 2% and cells grown 4 h. The Clb2-TAP and Clb5-TAP proteins were purified (Loog and Morgan, 2005; Puig et al., 2001), and their kinase activities assessed using histone H1. His-Vac17 (97–260) and His-Vac17 (1–195) were purified from bacteria using Ni-NTA resin. Equivalent activities of kinase complexes were mixed with 2 µg of Vac17 peptides, 10 ×buffer (200 mM HEPES-KOH, pH7.5, 100 mM MgCl2, 10 mM DTT), 2 µCi of [γ32P]ATP, cold ATP (final concentration 30 µM). Reactions incubated at room temperature, 30 min and stopped with 4 × SDS loading buffer.
Phosphorylation-site mapping by tandem mass spectrometry
Phosphorylated His-Vac17 peptides (10 µg) were separated on SDS-PAGE, gel bands were digested with trypsin or endoproteinase AspN and prepared for mass spectrometry (Jensen et al., 1999; Shevchenko et al., 1996). Samples were analyzed by reverse-phase liquid chromatography-tandem mass spectrometry. Data was acquired in Xcalibur v2.0 using a hypothesis driven neutral loss method. Target phosphopeptides predicted from theoretical digestion of the protein sequence using MS Digest (http://prospector.ucsf.edu/ucsfhtml4.0/msdigest.htm). Spectra were converted to peak lists using BioWorks Browser v3.2 and searched against S. cerevisiae sequences available from NCBI (http://www.ncbi.nlm.nih.gov/; downloaded on May 31, 2005) using SEQUEST. Identifications were considered positive if the protein probability was <1e−3 and the Xcor was >2 for a 1+ ion. When digested with trypsin, Vac17 (1–195) and Vac17 (98–260) produced three phosphorylated peptides; the masses indicated that three of the predicted Cdk1 sites, S119, T149, and S178, are phosphorylated. Digestion of Vac17 (98–260) with AspN, produced a fourth phosphorylated peptide; most likely the fourth predicted Cdk1 site T248 or possibly the adjacent site S247 (Figure 2F).
Synchronization of cell culture and measurement of vacuole inheritance
400 ml cells (OD600 ~0.4) were harvested, resuspended in 3.5 ml YEPD, labeled with FM4-64 (40 µg/ml), 1 h, 24°C. Cells were washed twice with YEPD and synchronized with 2.5 µM α-factor (Zymo Research); 3 h, 24°C. Arrested cells were washed twice with YEPD; then resuspended in 400 ml of YEPD, 24°C. 20 ml of cells were harvested every 10 min. Cell extracts were prepared and subjected to immunoblot. At each time point, cells were examined by fluorescence microscopy (Axioscope 2, Carl Zeiss MicroImaging, Inc.). Diameters of bud and mother cells were measured using MetaMorph 4.5 (Universal Imaging Corporation).
Co-immunoprecipitation
Immunoprecipitation (Mortensen et al., 2002) was performed with modifications. 70 OD600 log phase cells were frozen in liquid N2. Cells were lysed in 700 µl lysis buffer in a beadbeater. Extracts were centrifuged at maximum speed in a microcentrifuge for 10 min, 4°C. Supernatants were incubated 3 h, 4°C with protein G sepharose beads (GE Healthcare) coupled with anti-GFP antibody (Roche). Beads were washed with lysis buffer supplemented with 10% glycerol. Co-immunoprecipitation of Vac17-HA or Vac8 was assayed by immunoblot.
Supplementary Material
Acknowledgements
We thank Drs Anthony Bretscher, David Pruyne, Mike Tyers, Roger Tsien and David Morgan for sharing strains and reagents. We are grateful to Drs David Drubin, Peter Rubenstein, Robert Piper, Robert Cohen, Lori Wallrath, Arthur Spector, and Rajeshwari Valiathan for helpful discussions. We thank Doug Whitten, MSU Proteomics Center, for performing mass spectrometry; Dr. Gili Bitan-Banin for her participation in preparing Vac17 antibodies; Emily Kauffman for technical assistance; and Taylor Eves for sharing unpublished information. This work was supported by NIH R01-GM62261 (to LSW) and an American Heart Association Predoctoral Fellowship 0410029Z (to YP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altmann K, Frank M, Neumann D, Jakobs S, Westermann B. The class V myosin motor protein, Myo2, plays a major role in mitochondrial motility in Saccharomyces cerevisiae. J Cell Biol. 2008;181:119–130. doi: 10.1083/jcb.200709099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, Shimizu E, Tsien JZ, Schultz PG, Rose MD, et al. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 2000;407:395–401. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- Boldogh IR, Ramcharan SL, Yang HC, Pon LA. A type V myosin (Myo2p) and a Rab-like G-protein (Ypt11p) are required for retention of newly inherited mitochondria in yeast cells during cell division. Mol Biol Cell. 2004;15:3994–4002. doi: 10.1091/mbc.E04-01-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonangelino CJ, Catlett NL, Weisman LS. Vac7p, a novel vacuolar protein, is required for normal vacuole inheritance and morphology. Mol Cell Biol. 1997;17:6847–6858. doi: 10.1128/mcb.17.12.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett NL, Duex JE, Tang F, Weisman LS. Two distinct regions in a yeast myosin-V tail domain are required for the movement of different cargoes. J Cell Biol. 2000;150:513–526. doi: 10.1083/jcb.150.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagarasanu A, Fagarasanu M, Eitzen GA, Aitchison JD, Rachubinski RA. The peroxisomal membrane protein Inp2p is the peroxisome-specific receptor for the myosin V motor Myo2p of Saccharomyces cerevisiae. Dev Cell. 2006;10:587–600. doi: 10.1016/j.devcel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Han BK, Aramayo R, Polymenis M. The G1 cyclin Cln3p controls vacuolar biogenesis in Saccharomyces cerevisiae. Genetics. 2003;165:467–476. doi: 10.1093/genetics/165.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Catlett NL, Novak JL, Tang F, Nau JJ, Weisman LS. Identification of an organelle-specific myosin V receptor. J Cell Biol. 2003;160:887–897. doi: 10.1083/jcb.200210139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Toh EA, Matsui Y. Mmr1p is a mitochondrial factor for Myo2p-dependent inheritance of mitochondria in the budding yeast. Embo J. 2004;23:2520–2530. doi: 10.1038/sj.emboj.7600271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Watabe A, Toh EA, Matsui Y. Complex formation with Ypt11p, a rab-type small GTPase, is essential to facilitate the function of Myo2p, a class V myosin, in mitochondrial distribution in Saccharomyces cerevisiae. Mol Cell Biol. 2002;22:7744–7757. doi: 10.1128/MCB.22.22.7744-7757.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen ON, Wilm M, Shevchenko A, Mann M. Sample preparation methods for mass spectrometric peptide mapping directly from 2-DE gels. Methods Mol Biol. 1999;112:513–530. doi: 10.1385/1-59259-584-7:513. [DOI] [PubMed] [Google Scholar]
- Legesse-Miller A, Zhang S, Santiago-Tirado FH, Van Pelt CK, Bretscher A. Regulated phosphorylation of budding yeast's essential myosin V heavy chain, Myo2p. Mol Biol Cell. 2006;17:1812–1821. doi: 10.1091/mbc.E05-09-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liakopoulos D, Kusch J, Grava S, Vogel J, Barral Y. Asymmetric loading of Kar9 onto spindle poles and microtubules ensures proper spindle alignment. Cell. 2003;112:561–574. doi: 10.1016/s0092-8674(03)00119-3. [DOI] [PubMed] [Google Scholar]
- Lillie SH, Brown SS. Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae. J Cell Biol. 1994;125:825–842. doi: 10.1083/jcb.125.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loog M, Morgan DO. Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature. 2005;434:104–108. doi: 10.1038/nature03329. [DOI] [PubMed] [Google Scholar]
- Maekawa H, Usui T, Knop M, Schiebel E. Yeast Cdk1 translocates to the plus end of cytoplasmic microtubules to regulate bud cortex interactions. Embo J. 2003;22:438–449. doi: 10.1093/emboj/cdg063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendenhall MD, Hodge AE. Regulation of Cdc28 cyclin-dependent protein kinase activity during the cell cycle of the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1998;62:1191–1243. doi: 10.1128/mmbr.62.4.1191-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RK, Cheng SC, Rose MD. Bim1p/Yeb1p mediates the Kar9p-dependent cortical attachment of cytoplasmic microtubules. Mol Biol Cell. 2000;11:2949–2959. doi: 10.1091/mbc.11.9.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RK, Rose MD. Kar9p is a novel cortical protein required for cytoplasmic microtubule orientation in yeast. J Cell Biol. 1998;140:377–390. doi: 10.1083/jcb.140.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JK, Miller RK. The cyclin-dependent kinase Cdc28p regulates multiple aspects of Kar9p function in yeast. Mol Biol Cell. 2007;18:1187–1202. doi: 10.1091/mbc.E06-04-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen EM, McDonald H, Yates J, 3rd, Kellogg DR. Cell cycle-dependent assembly of a Gin4-septin complex. Mol Biol Cell. 2002;13:2091–2105. doi: 10.1091/mbc.01-10-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashkova N, Catlett NL, Novak JL, Weisman LS. A point mutation in the cargo-binding domain of myosin V affects its interaction with multiple cargoes. Eukaryot Cell. 2005;4:787–798. doi: 10.1128/EC.4.4.787-798.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashkova N, Jin Y, Ramaswamy S, Weisman LS. Structural basis for myosin V discrimination between distinct cargoes. Embo J. 2006;25:693–700. doi: 10.1038/sj.emboj.7600965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Tang F, Weisman LS. Palmitoylation plays a role in targeting Vac8p to specific membrane subdomains. Traffic. 2006;7:1378–1387. doi: 10.1111/j.1600-0854.2006.00472.x. [DOI] [PubMed] [Google Scholar]
- Puig O, Caspary F, Rigaut G, Rutz B, Bouveret E, Bragado-Nilsson E, Wilm M, Seraphin B. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods. 2001;24:218–229. doi: 10.1006/meth.2001.1183. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- Schott D, Ho J, Pruyne D, Bretscher A. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J Cell Biol. 1999;147:791–808. doi: 10.1083/jcb.147.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Spellman PT, Sherlock G, Zhang MQ, Iyer VR, Anders K, Eisen MB, Brown PO, Botstein D, Futcher B. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol Biol Cell. 1998;9:3273–3297. doi: 10.1091/mbc.9.12.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surana U, Robitsch H, Price C, Schuster T, Fitch I, Futcher AB, Nasmyth K. The role of CDC28 and cyclins during mitosis in the budding yeast S. cerevisiae. Cell. 1991;65:145–161. doi: 10.1016/0092-8674(91)90416-v. [DOI] [PubMed] [Google Scholar]
- Tang F, Kauffman EJ, Novak JL, Nau JJ, Catlett NL, Weisman LS. Regulated degradation of a class V myosin receptor directs movement of the yeast vacuole. Nature. 2003;422:87–92. doi: 10.1038/nature01453. [DOI] [PubMed] [Google Scholar]
- Tang F, Peng Y, Nau JJ, Kauffman EJ, Weisman LS. Vac8p, an armadillo repeat protein, coordinates vacuole inheritance with multiple vacuolar processes. Traffic. 2006;7:1368–1377. doi: 10.1111/j.1600-0854.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- Ubersax JA, Woodbury EL, Quang PN, Paraz M, Blethrow JD, Shah K, Shokat KM, Morgan DO. Targets of the cyclin-dependent kinase Cdk1. Nature. 2003;425:859–864. doi: 10.1038/nature02062. [DOI] [PubMed] [Google Scholar]
- Verma R, Annan RS, Huddleston MJ, Carr SA, Reynard G, Deshaies RJ. Phosphorylation of Sic1p by G1 Cdk required for its degradation and entry into S phase. Science. 1997;278:455–460. doi: 10.1126/science.278.5337.455. [DOI] [PubMed] [Google Scholar]
- Wang YX, Catlett NL, Weisman LS. Vac8p, a vacuolar protein with armadillo repeats, functions in both vacuole inheritance and protein targeting from the cytoplasm to vacuole. J Cell Biol. 1998;140:1063–1074. doi: 10.1083/jcb.140.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Huang HK, Kaiser P, Latterich M, Hunter T. Phosphorylation and spindle pole body localization of the Cdc15p mitotic regulatory protein kinase in budding yeast. Curr Biol. 2000;10:329–332. doi: 10.1016/s0960-9822(00)00382-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.