Abstract
BACKGROUND:
While considered simple and effective, crystalloid antegrade cardioplegia solutions have had few prospective multicentre comparison trials.
METHODS:
A commercial intracellular-type histidine-tryptophan-ketoglutarate (HTK) cardioplegia solution (Custodiol HTK; Köhler Chemie GmbH, Germany) designed for 4 h of protection after a single administration was compared with a standard extracellular multidose product (Plegisol [PL]; Hospira Inc, USA) in an open-label, randomized, prospective seven-institution trial. A total of 136 isolated coronary bypass patients were randomly assigned into two groups and stratified by ejection fraction into categories of 40% or greater (n=118) and 20% to 39% (n=18).
RESULTS:
The mean age of the study cohort was 62 years, of which 94% were men. Seventy per cent of patients had Canadian Cardiovascular Society class III angina and 75% had three-vessel disease anatomy. Cross-clamp times were nearly identical for patients in both cardioplegia groups; however, defibrillation was needed less often for patients who were treated with HTK (64% versus 91%, P<0.01). Hospital and intensive care unit stays, creatine kinase isoenzyme MB curves, cardiac outputs, inotrope levels, and deaths or serious adverse events (PL=13, HTK=14) were very similar between groups. Logistic regression showed that myocardial infarction or possible treatment-related adverse events were associated with high cardiac troponin I (cTn-I) levels 6 h after the procedure (P=0.001), and HTK treatment (OR 3.5, P=0.01). The primary study end point (6 h post-ischemia cTn-I) favoured PL (16.7±13.2 μg/L versus 20.3±13.5 μg/L, P=0.01). Patients who underwent circumflex grafting had higher cTn-I levels with HTK (P<0.001) and 48% required reinfusions due to cardiac warming. Longer intervals between doses correlated with high cTn-I levels (P=0.02). HTK provided prolonged protection with low cTn-I release (10 μg/L or less), although this occurred less frequently than with PL (17 versus 27 patients, P=0.06).
CONCLUSIONS:
HTK caused more structural protein release and adverse events than PL, even when reinfusion was implemented.
Keywords: Myocardial protection in coronary bypass (HTK versus Plegisol)
Myocardial preservation is influenced by many complex perioperative factors other than the choice of cardioplegia. These diverse factors are managed by surgeons, anesthesiologists and perfusionists, yielding an institutional ‘fingerprint.’ As an element of this complex system, detecting suboptimal cardioplegia may be difficult. Furthermore, preoperative ischemia and cardiac compensatory mechanisms mask failures caused by inadequate protection of the heart.
Even during prolonged ischemic challenges (eg, cardiac transplantation), data from numerous patients were needed to detect an advantage for intracellular (low sodium) solutions using crude measurements such as mortality (1). Such differences were replicated experimentally and included a histidine-tryptophan-ketoglutarate solution (HTK) (Custodiol HTK; Köhler Chemie GmbH, Germany) (Table 1) (2). Given its buffering capacity, it showed promise as a single-dose, antegrade, crystalloid alternative to the popular, commercially produced multidose product, Plegisol (PL; Hospira Inc, USA) and numerous blood cardioplegia admixtures.
Table 1.
Composition of the histidine-tryptophan-ketoglutarate solution
| Component | Amount, mmol/kg |
|---|---|
| NaCl | 15.0 |
| KCl | 9.0 |
| MgCl2•H2O | 4.0 |
| Tryptophan | 2.0 |
| α-ketoglutarate | 1.0 |
| Mannitol | 30.0 |
| L-histidine | 180.0 |
| L-histidine HCl•H2O | 18.0 |
| CaCl2 | 0.015 |
| Osmolality | 295–325 |
Solution administered in 1 kg H2O; pH adjusted to between 7.0 and 7.2
METHODS
General design and eligibility
The present study was an open-label, multicentre, randomized, phase 3 comparison intended to demonstrate equivalence in surgical outcome between HTK and PL, as determined by cellular leak of cardiac troponin I (cTn-I; a primary efficacy and safety end point), postoperative morbidity (eg, myocardial infarction [MI]) and mortality. Given the limited specificity of clinical outcome parameters, a biochemical measure of cardiac injury was chosen as the primary outcome parameter. Postoperative practice variables that could obscure differences in solution performance were monitored (3). All research was approved by the respective local Institutional Review Boards and informed consent from all patients was documented. These and other regulatory documents were verified during site visits by the core investigative team.
A total of 138 patients were randomly assigned to a cardioplegia solution group before isolated coronary bypass surgery; 136 patients were treated (68 in each group). The outcomes of each group were studied with an intent-to-treat efficacy and safety analysis. Eighteen patients were stratified at the time of random assignment to an impaired ejection fraction (EF) group (EF 20% to 39%), which included 10 patients in the HTK group and eight patients in the PL group. The remaining 120 patients had a preserved EF (EF of 40% or greater) – 59 patients in the HTK group and 61 patients in the PL group. Left ventricle EF was determined in each case by one of the following: multiple-gated acquisition scan (MUGA) two-dimensional echocardiogram or contrast ventriculogram. Eligible patients were between 35 and 80 years of age, had coronary artery disease that required isolated coronary artery bypass grafting (CABG) and did not have recent MI (less than six weeks before baseline).
Patients were excluded from the present study for the following reasons: need for valve surgery; left ventricle EF less than 20%, need for mechanical circulatory support, previous CABG surgery, planned or current use of aprotinin, participation in investigational studies within 30 days of the operation, cardiogenic shock or severe chronic obstructive lung disease (defined as a forced expiratory volume in 1 s of less than 1.2 L/s). Hospital admission occurred at least 6 h before the operation and the follow-up lasted 30 days.
After 71 patients completed the treatment, an interim analysis was performed to confirm that the two treatment groups were equivalent with respect to the occurrence of safety end points. This analysis did not indicate a need to terminate the study.
Operative and solution delivery techniques
Both solutions were administered at a temperature of between 4°C and 6°C. Surgeons checked myocardial temperature with a needle temperature probe positioned in the ventricular septum. Patients received HTK through an initial antegrade infusion of 4000 mL over approximately 6 min to 7 min. Perfusion pressure in the line was not to exceed 80 mmHg. Additional 200 mL doses of HTK were administered only when myocardial temperature climbed above 15°C at temperature check intervals or if there was evidence of electrical activity. All HTK solution returning to the right atrium was vented to wall suction and discarded; none remained in the patient. The other patients received antegrade PL 1000 mL at the initial infusion; routine reinfusions occurred every 20 min. Perfusion pressure in the line was planned to be approximately 80 mmHg until cardiac arrest and approximately 40 mmHg during arrest. Infusions lasted between 1 min and 4 min.
Both groups received bicaval cannulation between 28°C and 32°C to provide some standardization between groups, but the HTK group had caval tape occlusions so the solution could be drained through a right atriotomy and discarded. The PL remained systemic, as it normally does.
Routine surgical techniques for CABG were used at all sites. Body temperature was maintained between 30°C and 32°C. A Swan-Ganz catheter was placed to measure cardiac index and cardiac output.
Body rewarming to 37°C began during the last distal coronary anastomosis. Patients were weaned from cardiopulmonary bypass once a stable cardiac rhythm and inotropic state were established.
Patients received two epicardial temporary pacing wires per chamber (atrial and ventricular) that were used when necessary. Investigators followed an inotrope escalation protocol; however, low-dose infusions to increase blood pressure to reach an acceptable cardiac index (greater than 2.2) were monitored, but left to investigator discretion.
Study end points and data collection methods
The cTn-I concentration 6 h following the release of the aortic cross-clamp was defined as the primary parameter to demonstrate equivalence in surgical outcome between HTK and PL. Creatine kinase isoenzyme MB (CK-MB) and cTn-I were determined at a central laboratory.
High-priority (objective) secondary end points were CK-MB levels at 6 h, 12 h, 24 h and 48 h following the release of the cross-clamp, and area under the curve (AUC); cTn-I levels at 12 h, 24 h and 48 h following the release of the cross-clamp, and AUC; Q wave MI; and time to spontaneous cardiac activity following aortic cross-clamp removal. The amount of inotropic support was categorized by level. Levels 1a, 1b and 2 were defined as pressor units of less than 5 μg/kg/min, 5 μg/kg/min to 9 μg/kg/min, and 10 μg/kg/min or more, respectively. These units represent μg/kg/min of dopamine or conversion of the general therapeutic range if other inotropic drugs were used. The use of an intra-aortic balloon pump (IABP) or LV assist device constituted level 3.
Safety parameters included vital signs, 12-lead electrocardiography (ECG) interpretations and adverse events (AEs). Blood samples for determination of the CK-MB and cTn-I levels were obtained immediately before induction of anesthesia and cross-clamp removal, as well as at the time intervals previously noted.
The time to cardiac arrest was measured in seconds from the initiation of infusion of cardioplegic solution to the cessation of electrical activity on the limb lead ECG. If the surgeon witnessed mechanical activity despite electrical quiescence, this was noted and the cardioplegic solution infusion was extended until movement of the heart could no longer be seen.
If the patient’s heart developed ventricular fibrillation after cross-clamp release, direct internal cardioversion by protocol was performed and the number of defibrillation episodes was noted. Once preoperative rhythm was achieved for 5 min, further clinically significant ventricular or atrial arrhythmias were recorded as adverse postoperative or intra-operative events.
Statistical methods
The sample size for the present study was determined to yield approximately 80% power for establishing clinical equivalence. Clinical equivalence was established if the HTK peak cTn-I level was no more than 0.55 μg/L greater than the peak cTn-I level obtained with PL. This value was chosen based on single-centre studies with similar infusion schemes that used troponin as an index of cardiac injury. It was not known whether this narrow interval would be valid in a multi-institutional setting or using the different infusion schemes required by these solutions.
Continuously distributed variables were expressed as mean ± SD. CIs were calculated for mean values based on the least squared means in the ANOVA models (LSMEANS statement in PROC GLM; SAS statistical software, SAS Instititute Inc, USA) if the assumption of normally distributed data was fulfilled. CIs for the shift between two distributions in stratified nonparametric analyses were based on the duality of statistical tests and CIs. The set (interval) of all shifts between the two distributions with nonsignificant results at the level α was used to calculate CI (1-α)100%.
Categorical variables were analyzed with Cochran-Mantel-Haenszel χ2 tests stratified on centre, except for comparisons with respect to centre. Continuous parameters were analyzed using analysis of covariance, with adjustment for centre when assumptions were met. Parameters that did not satisfy the analysis of covariance assumptions were analyzed using stratified Cochran-Mantel-Haenszel methods.
The statistical analysis of the efficacy parameter was performed using stratification by centre, including an analysis between the one high accrual centre and the other centres. Furthermore, the primary analysis was performed with stratification by the categorized EF (20% to 39% versus 40% or greater) at baseline. The event ‘MI and death’ was compared between the two solutions using logistic regression with the following covariates: 6 h cTn-I level, binary variable left main artery stenosis greater than 50% and categorized EF. Missing efficacy data were replaced using the last observation carried forward approach (carrying forward only post-baseline data).
RESULTS
There were 61 male patients in the PL group (89.7%) and 67 in the HTK group (98.5%); Caucasians comprised over 94% of each cohort.
There were differences between groups after cross-clamp removal. First spontaneous activity (generally ventricular fibrillation, then postdefibrillation bradycardia) varied enormously. More defibrillation was necessary for PL patients than for HTK patients, and one-half of all patients required pacing. The length of pacing was relatively inhomogeneous. Lidocaine was given to 52.6% of the patients and only five of these patients received various other antiarrhythmics.
Most patients (61.7% of the PL group, 47.0% of the HTK group) did not require inotropic support (Table 2), and 25% of the patients from the PL group and 45.5% of the patients from the HTK group were categorized as being level 1a. Only eight patients (five from the PL group and three from the HTK group) were categorized as level 1b. Three patients (two PL and one HTK) were categorized as level 2 and three patients (two PL and one HTK) were categorized as level 3. Regarding the duration of the inotropic support of level 1b or higher, the nine patients in the PL group needed a longer period of inotropic support than the five patients from the HTK group (however, this was not significant, given the wide range of values).
Table 2.
Level of inotropic support required in each patient (P not significant)
| Level | Plegisol, n | HTK, n |
|---|---|---|
| No support | 42 | 32 |
| 1a | 17 | 31 |
| 1b | 5 | 3 |
| 2 | 2 | 1 |
| 3 | 2 | 1 |
| Total | 68 | 68 |
HTK Custodiol histidine-tryptophan-ketoglutarate solution
Because of the institutional variations in ECG interpretation, the diagnoses were grouped into categories of increasing concern for detectable injury or ischemia (Table 3). HTK had more combined ischemia and injury ECG changes attributable to the operations (P<0.05).
Table 3.
Occurence of postoperative electrocardiogram (ECG) events
| Plegisol (%) | HTK (%) | |
|---|---|---|
| ECG | ||
| No changes | 48.5 | 50.8 |
| Changes observed | 51.5 | 49.2 |
| ECG changes observed | ||
| Nonspecific* | 18.2 | 13.8 |
| Atrial fibrillation† | 39.7 | 45.6 |
| Infarction pattern | 1.5 | 4.6 |
No differences were significant between Plegisol and Custodiol histidine-tryptophan-ketoglutarate solution (HTK).
Nonspecific included premature atrial contractions, premature ventricular contractions, T wave, ST segments, and brady- and tachycardias;
Some overlap with nonspecific changes
Preoperatively and on arrival to the intensive care unit (ICU), there were no clinically or statistically discernable differences between the two groups with respect to their presentation and evolution.
Primary end point
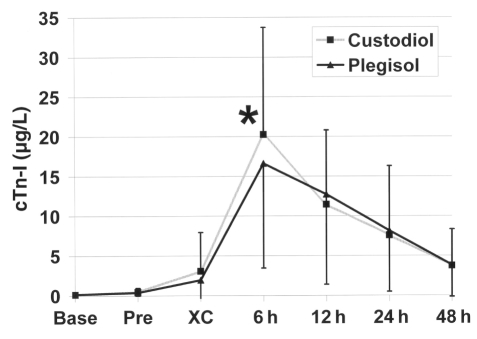
The cTn-I concentration 6 h following aortic cross-clamp release favoured PL (16.6±13.1 μg/L versus HTK 20.3±13.5 μg/L, P<0.01; Figure 1). This difference persisted for the subset of patients with an EF of 40% or greater. The cTn-I levels after 6 h for the low EF group (20% to 39%) also tended toward favouring PL (17.3±13.9 μg/L versus HTK 24.5±12.9 μg/L, P=0.08). HTK did provide prolonged protection with low cTn-I release (less than 10 μg/L), although this occurred less frequently than with PL (17 versus 27 patients, P=0.06). The cTn-I AUCs of both treatment groups were comparable (406±330 versus 406±336). The heterogeneity and inconsistency of the centres were reflected in the reported cTn-I values. While centres 1, 4 and 6 had wide ranges of cTn-I values, centre 7 had a relatively narrow range. There were differences between the centres that reported low (less than 10 μg/L) cTn-I release and other parameters described in earlier tables. It is interesting that the centre with the lowest mean cTn-I release had the longest mean hospital stay (because of several outlier cases), but the median ICU and hospital stay was no shorter than the mean hospital and ICU stays for centres with higher mean cTn-I release levels.
Figure 1).
Time course of cardiac troponin I (cTn-I) release. The graph shows the expected peak of cTn-I release at 6 h (*P=0.01), which is the only statistically different time point. Base indicates the time of study registration, typically the day before surgery; Pre indicates immediately before incision; XC Cross-clamp release
A greater cTn-I release was observed when the heart required retraction for circumflex (Cx) grafting. The cTn-I release was greater for 78 patients who had Cx grafting than the 58 other patients (19.9±13.4 μg/L versus 16.3±13.3 μg/L, P=0.08). This difference is related to the type of cardioplegia solution. Cx grafting patients who received HTK had much higher cTn-I levels than patients who received PL (23.5±12.4 μg/L versus 16.7±13.4 μg/L, P<0.001). Alternatively, non-Cx grafting patients had similar cTn-I levels between the PL and HTK cardioplegic groups (16.4±12.9 μg/L versus 16.2±13.8 μg/L, P not significant). The difference for Cx grafting is not apparent when centre 1 was excluded from the analysis. Similar results were obtained when the patients were grouped by the number of grafts they underwent – one or two grafts versus three or more grafts.
While the mechanical effect of cardiac retraction could have caused a higher cTn-I release, longer intervals between doses correlated with high cTn-I levels (P=0.02), which suggests that myocardial warming may have been the cause. There was no interaction between the release of cTn-I and the need for vasopressor agents. Also, year of study and release of cTn-I showed no correlation that suggested a learning benefit.
Secondary end points
The CK-MB levels at 6 h (64.1±55.8 μg/L versus 60.5±34.1 μg/L), 12 h, 24 h and 48 h following the release of the cross-clamp were comparable at each time interval, as were the AUCs (1653±1041 versus 1690±955). Typically, CK-MB rose in parallel with cTn-I and reached diagnostic levels between approximately 4 h and 6 h after the cardiac intervention.
Five operative MIs (three HTK and two PL) were diagnosed by Q wave (Table 3) and other acute injury patterns on ECG.
Table 2 also lists other postoperative ECG AEs that the investigators (who were not blinded to the identity of the solution) felt were possibly related to the study drug. ORs for MI and related AEs based on various parameters were 3.51 (P=0.0094) for HTK versus PL, 0.74 for centres 2 to 7 versus centre 1, 1.72 for EF 40% or greater versus EF 20% to 39%, 0.67 for left main artery lesion versus lesion not in the left main artery and 1.05 (P=0.0014) for cTn-I level after 6 h. Only significant P values were cited; the others did not approach significance. Factors such as bypassing the Cx and higher inotropic levels were not predictive.
DISCUSSION
Rather than equivalency between PL and HTK, the biochemical primary end point favoured PL, probably because its multidose infusion scheme yielded more consistent myocardial exposure. Variable results between centres shown in the present study emphasize the importance of a multicentre trial in a validation effort, although this type of trial has been reported infrequently in the cardiac surgical literature. Patients randomly assigned to HTK were comparable with the PL group in most traditional clinical outcomes.
We compared HTK with PL because crystalloid cardioplegia is considered effective in routine cases and the technique is easier to standardize between centres than in many popular blood cardioplegia admixtures that have variable delivery strategies (4).
PL is a modification of St Thomas’ Hospital Solution that was developed in London in 1975. HTK cardioplegic solution was formulated by Bretschneider (5) in 1975, introduced clinically in 1977 and modified (Table 1) in 1980. The solution has a high extracellular buffer capacity at low temperatures because its low sodium content allows more solute capacity for a high concentration of histidine and tryptophan residues (6,7). The 4 L used in the present study to achieve buffer equilibration between intravascular and interstitial spaces was successfully modified to a more practical 1 L to 2 L for up to 3 h of ischemic protection (6). Single-centre investigations have shown HTK to be equivalent or superior to common cardioplegia solutions and it has been used in hundreds of thousands of cases worldwide (manufacturer tabulation) (8,9).
Other than the potential convenience of a single-dose infusion scheme, HTK crystalloid formulation could be advantageous over a blood solution because it does not obscure the operative field for CABG or heart valve replacements. It was also interesting that, as a single stocked product, it may be used for both organ transplantation needs and routine clinical myocardial preservation.
The choice of cTn-I levels 6 h post cross-clamp release as the primary end point constitutes a potential limitation of the present study if it is unrelated to the ECG findings. There appeared to be a statistically significant relation between cTn-I and related AEs; however, the assessment of its relationship to the study medication may be biased by the surgeons’ knowledge of the administered solution in the present open-label research. Also, there are few reported studies measuring troponin in which the methods of solution delivery are so different. For instance, the differences in troponin levels may have occurred because the single-dose infusion scheme led to a higher spike in the troponin value at the 6 h point, rather than a blunted or delayed peak resulting from a multidose strategy. Additional measurements around the 6 h time point may have addressed this concern.
Despite attempts to limit variation, surgeons in the present study may have caused cTn-I release through preferences in anastomosis, heart retraction, endarterectomy, graft de-airing and other techniques. Treatments used by anesthesiologists and perfusionists may affect results as well (10,11). For instance, most centres had difficulty infusing the volume of HTK at the lower pressure defined by the protocol in a practical time frame. It seems unlikely that this affected cTn-I release, because cTn-I release was lowest in the centre with the highest mean infusion pressure. Another criticism of the present trial may be its reliance on a pure antegrade technique, although all patients achieved sufficient cooling. Supplementation of delivery by retrograde methods may be used in future designs.
Given the favourable past experimental and clinical results of HTK for much longer periods of ischemia, it seems likely that the differences observed are from variations in solution delivery or retention rather than composition. Our study suggests that intermittent reinfusion of PL is safer than reinfusion of HTK when rewarming of the heart occurs.
The present experience is one of only a few scientific projects studying myocardial preservation using a multi-institutional design. Single-centre investigations yield fewer process variations and better controlled exploratory trials for the aforementioned reasons. However, because the present study design introduced the HTK solution to new practice environments, it was more powerful in revealing unforeseen safety concerns caused by institutional practices that were not used at the original sites of HTK development. The lessons learned in the present study may be useful for planning future research projects.
Acknowledgments
Funded in part by Köhler Chemie GmbH, Alsbach-Hähnlein, Germany.
REFERENCES
- 1.Demmy TL, Biddle JS, Bennett LE, Walls JT, Schmaltz RA, Curtis JJ. Organ preservation solutions in heart transplantation – patterns of usage and related survival. Transplantation. 1997;63:262–9. doi: 10.1097/00007890-199701270-00015. [DOI] [PubMed] [Google Scholar]
- 2.Demmy TL, Turpin TA, Wagner-Mann CC. Laboratory confirmation of clinical heart allograft preservation variability. Ann Thorac Surg. 2001;71:1312–9. doi: 10.1016/s0003-4975(00)02659-x. [DOI] [PubMed] [Google Scholar]
- 3.Etievent JP, Chocron S, Toubin G, et al. Use of cardiac troponin I as a marker of perioperative myocardial ischemia. Ann Thorac Surg. 1995;59:1192–4. doi: 10.1016/0003-4975(95)00129-9. [DOI] [PubMed] [Google Scholar]
- 4.Ovrum E, Tangen G, Tollofsrud S, Oystese R, Ringdal MA, Istad R. Cold blood cardioplegia versus cold crystalloid cardioplegia: A prospective randomized study of 1440 patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2004;128:860–5. doi: 10.1016/j.jtcvs.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Bretschneider HJ. Myocardial protection. Thorac Cardiovasc Surg. 1980;28:295–302. doi: 10.1055/s-2007-1022099. [DOI] [PubMed] [Google Scholar]
- 6.Heinemeyer D, Belles G, Stapenhorst K. Intracellular pH measurement during cardiac arrest in ventricular myocardium by Bretschneider’s cardioplegic solution HTK and St Thomas Hospital solution with and without procaine. Thorac Cardiovasc Surg. 1987;35:48–52. doi: 10.1055/s-2007-1020195. [DOI] [PubMed] [Google Scholar]
- 7.Preusse CJ, Gebhard MM, Bretschneider HJ. Interstitial pH value in the myocardium as indicator of ischemic stress of cardioplegically arrested hearts. Basic Res Cardiol. 1982;77:372–87. doi: 10.1007/BF02005338. [DOI] [PubMed] [Google Scholar]
- 8.Sakata J, Morishita K, Ito T, Koshino T, Kazui T, Abe T. Comparison of clinical outcome between histidine-tryptophan-ketoglutalate solution and cold blood cardioplegic solution in mitral valve replacement. J Card Surg. 1998;13:43–7. doi: 10.1111/j.1540-8191.1998.tb01053.x. [DOI] [PubMed] [Google Scholar]
- 9.Gallandat Huet RC, Karliczek GF, van der Heide JN, et al. Clinical effect of Bretschneider-HTK and St. Thomas cardioplegia on hemodynamic performance after bypass measured using an automatic datalogging database system. Thorac Cardiovasc Surg. 1988;36:151–6. doi: 10.1055/s-2007-1020064. [DOI] [PubMed] [Google Scholar]
- 10.Ebel D, Preckel B, You A, Mullenheim J, Schlack W, Thamer V. Cardioprotection by sevoflurane against reperfusion injury after cardioplegic arrest in the rat is independent of three types of cardioplegia. Br J Anaesth. 2002;88:828–35. doi: 10.1093/bja/88.6.828. [DOI] [PubMed] [Google Scholar]
- 11.Aldea GS, Soltow LO, Chandler WL, et al. Limitation of thrombin generation, platelet activation, and inflammation by elimination of cardiotomy suction in patients undergoing coronary artery bypass grafting treated with heparin-bonded circuits. J Thorac Cardiovasc Surg. 2002;123:742–55. doi: 10.1067/mtc.2002.120347. [DOI] [PubMed] [Google Scholar]