Abstract
Aortic dissection is a relatively rare but dreadful illness, often presenting with tearing chest pain and acute hemodynamic compromise. Early and accurate diagnosis and treatment are essential for survival. In the present review, a rare case of an asymptomatic ascending aortic dissection is reported. The general clinical manifestations, diagnosis and management of aortic dissection will also be reviewed.
Keywords: Aortic dissection, Asymptomatic aortic dissection, DeBakey classification, Dissection of the thoracic aorta, Stanford classification
The primary event in aortic dissection is a tear in the aortic intima. Data concerning the incidence in the general population are limited; estimates range from 2.6 to 3.5 per 100,000 person-years. The most important predisposing factor for acute aortic dissection is systemic hypertension. Other predisposing factors include disorders of collagen (Marfan syndrome, Ehlers-Danlos syndrome, annuloaortic ectasia), bicuspid aortic valve, aortic coarctation, Turner syndrome, coronary artery bypass graft surgery, previous aortic valve replacement, crack cocaine use, strenuous resistance training and trauma (1–4).
Aortic dissection is generally suspected based on a patient’s history and physical examination. Patients with an aortic dissection typically present with severe, sharp or ‘tearing’ back pain (in dissection distal to the left subclavian artery) or anterior chest pain (in ascending aortic dissection). Painless dissection has been reported (5), but is relatively rare. In an analysis of 977 patients from the International Registry of Acute Aortic Dissection (5), only 63 patients (6.4%) had no pain. Patients with painless dissection were slightly older (mean age 67 years versus 62 years) and had a type A dissection more often (75% versus 61%). A history of diabetes, aortic aneurysm or cardiovascular surgery was more common in patients with painless dissection. Presenting symptoms of syncope, heart failure or stroke were seen more often in this group. In-hospital mortality was significantly higher than for patients presenting with pain (33% versus 23%) (6,7).
Differential diagnosis (8)
Myocardial ischemia
Pericarditis
Pulmonary embolus
Aortic regurgitation without dissection
Aortic aneurysm without dissection
Musculoskeletal pain
Mediastinal tumours
Pleuritis
Cholecystitis
Atherosclerotic or cholesterol embolism
Peptic ulcer disease or perforating ulcer
Acute pancreatitis
Routine blood tests are generally nondiagnostic and imaging studies are not performed until the patient is stabilized medically. In general terms, bedside transesophageal echocardiography (TEE) is performed in the emergency room for patients who present with acute chest pain and/or are thermodynamically unstable. Magnetic resonance imaging (MRI) is preferred for patients with chronic chest pain and for those who are hemodynamically stable, or are seen for follow-up of a chronic dissection. Computed tomography (CT) scan with contrast is reserved for situations in which both TEE and MRI are unavailable or contraindicated. Aortography is used when ascending aortic dissection is strongly suspected, but noninvasive tests are unavailable or inconclusive. Finally, coronary angiography is generally safe in stable patients, although some retrospective data suggest no in-hospital benefit from coronary angiography (9–13).
Acute dissections involving the ascending aorta are considered surgical emergencies. In contrast, dissections confined to the descending aorta are treated medically unless the patient demonstrates progression or continued hemorrhage into the pleural or retroperitoneal space. Patients with suspected aortic dissection should be admitted to an intensive care unit as rapidly as possible for confirmation of the diagnosis, pain control and reduction of systolic blood pressure to between 100 mmHg and 120 mmHg. Patients who are hemodynamically unstable should be intubated. All patients should receive lifelong therapy with an oral beta-blocker to reduce systemic blood pressure and the rate of rise in systolic pressure, both of which will minimize aortic wall stress. Avoidance of strenuous physical activity is also recommended as another method to minimize aortic shear stress. We generally perform a baseline thoracic magnetic resonance scan before discharge, with serial follow-up examinations at three, six and 12 months, even if the patient remains asymptomatic (5,14–22).
CASE PRESENTATION
A 54-year-old African American woman presented to the out-patient department for a routine health screen for employment. She had no complaints before presentation. Her medical history was significant for hypertension and stage III chronic kidney disease. Her surgical history was significant for two caesarean sections in the past, the first in 1975 and the second in 1977. She had no known allergies. Her medications included Diovan HCT 160 mg/25 mg (Novartis Pharmaceutical Corporation, USA) and Norvasc 10 mg (Pfizer Inc, USA). She had two pregnancies and two full-term births. She was a single mother with two children. She admitted to social alcohol use but denied use of cigarettes or recreational drugs. She denied having multiple sexual partners or a history of sexually transmitted diseases.
On presentation she was afebrile with a temperature of 36.7°C, blood pressure reading of 130/90 mmHg, a pulse rate of 88 beats/min and a respiratory rate of 18 breaths/min. She was alert and oriented to person, place and time. On physical examination, her pupils were round, equal, and reactive to light and accommodation. There was no jugular venous distension or hepatojugular reflux. There were no carotid bruits. The chest wall was symmetric and there was no deviation of the trachea. There was good air entry bilaterally with clear breath sounds. The precordium was normodynamic. The point of maximal impulse was located in the left fifth intercostal space anterior axillary line. Her heart sounds had a regular rate and rhythm, with a grade III/VI holosystolic murmur located in the apex and radiating to the axilla. There were no audible gallops or clicks. The heart sounds were not muffled and there was no pulsus paradoxus. The second heart sound was physiologically split. The abdomen was obese with no visible pulsations. The bowel sounds were normoactive. She had no palpable masses. Her abdomen was soft and nontender. Her extremities were warm to the touch, with no pallor, finger clubbing or cyanosis. Pulses were symmetrical and there was no peripheral edema. There was no femoral bruit. Muscle strength was 5/5 in both upper and lower extremities. Deep tendon reflexes were normal.
Because of the systolic murmur noted on physical examination, she was referred to a cardiologist for evaluation. An electrocardiogram and echocardiogram were ordered as preliminary tests. The electrocardiogram showed a normal sinus rhythm with a rate of 80 beats/min, left atrial abnormality and left ventricular hypertrophy. The echocardiogram showed a dilated aortic root and left atrium, concentric left ventricular hypertrophy, normal wall motion, an ejection fraction of 80% and diastolic dysfunction. The patient did not keep the cardiology appointment and was lost to follow-up. The following year, the patient presented for another health screening for employment. She again had no complaints. Because of the pre-existing heart murmur she was again referred to a cardiologist. A repeat echocardiogram was ordered, and this time it showed a dilated left atrium and left ventricle, concentric left ventricular hypertrophy, dilated aortic root with a mobile flap noted in the ascending aorta and the aortic arch, normal wall motion with normal systolic function, an ejection fraction of 78% and diastolic dysfunction. On evaluation by the cardiologist, a detailed history directed at symptoms and risk factors for aortic dissection was taken. She denied any episode of chest pain at rest or on exertion. She denied any diaphoresis, syncope or near-syncope episodes. She denied any periods of altered mental status. There were no symptoms suggestive of stroke. She denied any dyspnea, orthopnea, paroxysmal nocturnal dyspnea, leg swelling or cough. There were no symptoms suggestive of compression of the esophagus such as dysphagia, or symptoms suggestive of compression of the superior vena cava such as flushed facies, facial and arm edema, or a feeling of fullness in the head. She denied symptoms indicative of abdominal vasculature compromise such as abdominal pain or bloody stools.
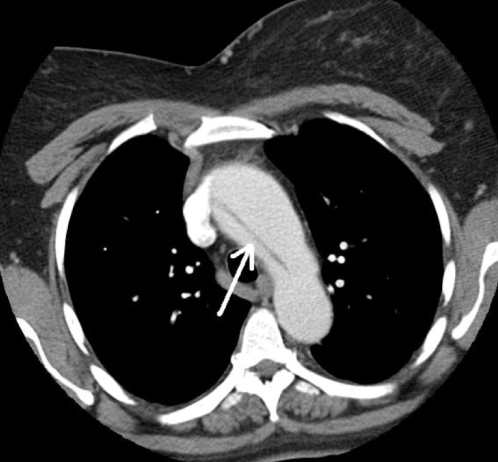
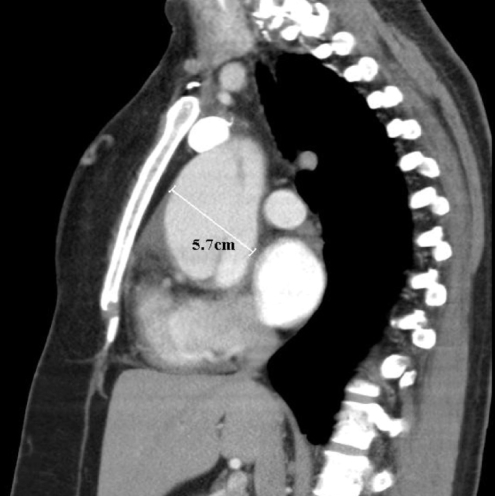
With regard to risk factors, she admitted having hypertension for over 30 years. She claimed compliance to her medications and denied any periods of poorly controlled blood pressure. She was not pregnant at the time the dissection was seen on the routine echocardiogram. She denied any history of chest wall trauma or being involved in any deceleration injury. She had no previous cardiac catheterization, intra-aortic balloon pump or any cardiac surgery such as valve replacement. A physical examination was not different from her initial presentation. A contrast CT scan of the chest was performed that showed an aortic dissection extending from the aortic root along the right lateral wall of the aortic arch (Figure 1). The ascending aorta measured 5.7 cm transversely (Figure 2). The descending aorta appeared normal, measuring 2.5 cm at its widest diameter. There was a small pericardial effusion. She was referred for cardiac surgery.
Figure 1).
The intimal flap (arrow) extending along the right lateral wall of the aortic arch
Figure 2).
The ascending aorta measured 5.7 cm transversely
DISCUSSION
The prevalence and incidence of thoracic aortic disease is increasing, as are the number of operations for thoracic aortic disease (1,2,16). In one study (1), the overall incidence rate of 10.4 per 100,000 person-years between 1980 and 1994 was more than threefold higher than the rate from 1951 to 1980.
Sixty per cent of thoracic aneurysms involve the aortic root or the ascending aorta, and 40% involve the descending aorta. Ten per cent involve the arch and 10% involve the thoracoabdominal aorta, with some involving more than one segment (22,23).
An aortic aneurysm is a localized dilation of the aorta (22). There are two types of aneurysms: a true aneurysm, in which the dilated segment involves all three layers of the vessel, and a false or pseudoaneurysm, which is a contained hematoma outlined by adventitia or surrounding tissue (22). Aortic aneurysms can be further classified according to their morphology into fusiform or saccular categories (22). Aneurysms can affect different locations of the aorta: the aortic root, ascending aorta, aortic arch or the descending aorta (22,23). The natural history of thoracic aneurysms is progressive expansion, subsequent increased aneurysm wall stress and eventual rupture (1,3).
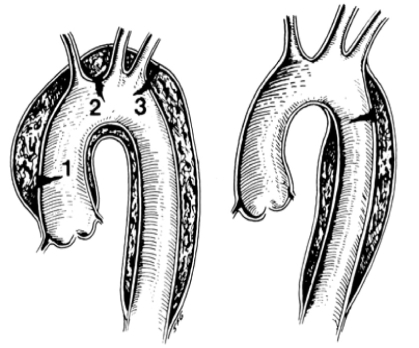
There are two widely known classifications of dissections, Stanford (Table 1) and DeBakey (Table 2). Stanford type A includes dissections that involve the ascending thoracic aorta, whereas type B dissections do not involve the ascending thoracic aorta. DeBakey type 1 dissections involve the whole aorta, type 2 dissections involve the ascending aorta and type 3 dissection involves the descending aorta. Thus, Stanford type A dissection includes DeBakey types 1 and 2, and Stanford type B equals DeBakey type 3 (Figure 3) (22). A new differentiation of aortic dissection is presented below (22).
TABLE 1.
Stanford classification
| Type | Characteristic |
|---|---|
| Type A | Dissection involving the ascending aorta, regardless of the site of the primary tear |
| Type B | Dissection of the descending aorta |
Data from reference 25
TABLE 2.
DeBakey classification
| Type | Characteristic |
|---|---|
| Type 1 | Dissection of the ascending and descending thoracic aorta |
| Type 2 | Dissection of the ascending aorta |
| Type 3 | Dissection of the descending aorta |
Data from reference 25
Figure 3).
Stanford type A dissections involve the ascending aorta (1) regardless of the site of the primary tear. 2 Aortic arch. Stanford type B dissections involve the descending aorta (3). Type A dissections include DeBakey type 1 and 2 dissections, while type B dissections correspond to DeBakey type 3 dissections
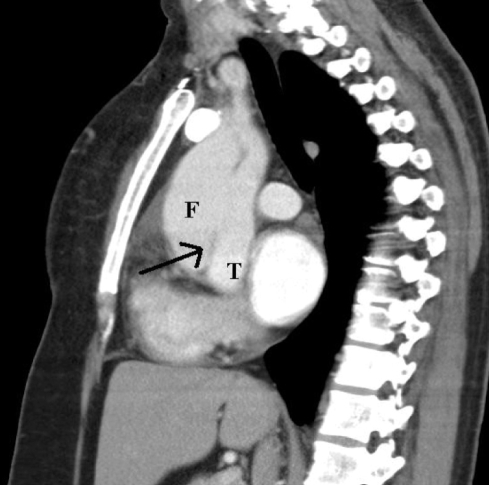
Class 1: Classic aortic dissection
Classic aortic dissection is characterized by the presence of an intimal flap that separates the true and false lumens (Figure 4). This category can be further divided into communicating and noncommunicating dissections. Communicating dissection has an intimal tear with unidirectional or multidirectional flow between the true and false lumen. On the other hand, in non-communicating dissection, no flow and no intimal tear can be detected. That dissection can spread either in an antegrade fashion, with involvement of the distal part of the aorta, or extend to different branches, such as the carotid, subclavian and renal arteries. It can also spread in a retrograde fashion to involve the coronary arteries.
Figure 4).
The intimal flap (arrow) separating the true lumen (T) from the false lumen (F)
Class 2: Intramural hematoma or hemorrhage
Intramural hematoma or hemorrhage may be the result of the rupture of the vasa vasorum and may be the initial lesion in cases of cystic medial degeneration. It often coexists with or progresses to class 1 dissection.
Class 3: Subtle-discrete aortic dissection
This form of dissection is characterized by a stellate or linear intimal tear with the exposure of the underlying media and adventitia, but without progression to separation of medial layers.
Class 4: Plaque rupture and ulceration
Ulceration of aortic plaques can lead to aortic dissection or aortic rupture.
Class 5: Traumatic and iatrogenic aortic dissection
Blunt trauma may cause dissection at the level of the aortic isthmus. Iatrogenic dissection may be seen after aortic angioplasty for aortic coarctation or after cross-clamping of the aorta during heart surgery.
It is believed that an imbalance of the equilibrium between matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) can contribute to the formation of an abdominal aortic aneurysm (15). It is known that altered expressions of MMPs and their TIMPs influence formation of atherosclerotic aneurysms in the abdominal aorta (15). A thoracic aortic aneurysm (TAA) is characterized by degradation of elastic fibre, suggesting the involvement of MMP-2 and MMP-9, the activation of which is regulated by TIMP types 1 and 2 (18). One study (18) reported upregulation of MMP-2 and MMP-9 during TAA formation in Marfan syndrome. This would cause a loss of elastic fibre and structural integrity. This elastic fibre degeneration, with deterioration of aortic contractile and mechanical properties, may explain the pathogenesis of TAA.
Many conditions can cause aneurysmal formation. The etiology may differ depending on the location of the aneurysm. The most common cause of descending aortic aneurysm is atherosclerosis, whereas the etiology for aortic root aneurysm may be associated with connective tissue disorders such as Marfan syndrome, Ehlers-Danlos syndrome and bicuspid aortic valve disease. Other etiologies include infection, inflammation, trauma, dissection and idiopathy (22).
Marfan syndrome is a heritable disorder of the connective tissue, with an estimated prevalence of one in 5000 individuals and no predilection for either sex. The syndrome is inherited as an autosomal dominant trait with complete penetrance, but with phenotypic expression that varies considerably both among and within families (19). The cause of the syndrome is a mutation in one of the genes for fibrillin-1, which is the major component of elastin (22).
Ehlers-Danlos syndrome is another connective tissue disorder and is associated with aortic aneurysm, articular hypermobility, skin hyperextensibility and tissue fragility (22).
Familial TAA syndrome is associated with cystic medial degeneration of the aorta but no other connective tissue abnormalities. It is believed to be an autosomal dominant disorder, with marked variability in expression and penetration (22).
Bicuspid aortic valves are also associated with aortic aneurysmal formation. Not only are they related to post-stenotic dilation, but interestingly, it was found that up to 52% of patients with a normally functioning bicuspid aortic valve have aortic dilation. Inadequate production of fibrillin-1 is thought to be the underlying pathology (22).
Inflammation may also lead to aneurysm formation (22). Syphilitic aortitis is usually associated with aortic aneurysm, aortic regurgitation and coronary ostial stenosis (20). Cardiovascular syphilis mainly occurs in the third stage of syphilis, and is likely to appear in the ascending aorta and aortic arch. Syphilitic aortitis is followed by aortic aneurysm (40%), rupture (14%), aortic valve regurgitation (29%) and coronary ostial stenosis (26%) (20,22).
Isolated necrotizing aortitis may be forme fruste Takayasu arteritis. The pathological features of isolated aortitis are similar to those of aortitis caused by Takayasu arteritis, giant cell arteritis, rheumatoid arthritis and other autoimmune syndromes. Isolated aortitis is characterized by medial laminar necrosis, a surrounding inflammatory infiltrate of macrophages, multinucleated giant cells and lymphocytes, elastic destruction, and fibrosis of the adjacent intimal and media (17).
Other inflammatory disorders include Takayasu arteritis, which occurs most often in women with a mean age of 29 years (22). Takayasu arteritis is characterized by destruction of the external elastic lamella, thickening of the intima, adventitial and external medial fibrosis, and inflammation of the media and adventitia (17). It mainly causes obstructive lesions, but aortic dilation is present in 15% of cases (22).
Giant cell arteritis is another rare disease that commonly affects the temporal artery, but also may affect the ascending aorta (22). The histological hallmark of giant cell arteritis is infiltration of chronic inflammatory cells initially along the internal elastic lamina, but often extending into the intima, media and adventitia. Necrosis is uncommon (17).
Trauma may lead to dissection or transection, with subsequent formation of a pseudoaneurysm that commonly affects the descending aorta distal to the origin of the left subclavian artery. Chronic dissection can dilate with time, causing aneurysmal formation (22).
Most patients are asymptomatic, and the aneurysm is discovered incidentally by chest radiography, echocardiography or CT. Aortic aneurysms may manifest with symptoms late in the course of the disease (22).
Progressive dilation of the ascending aneurysm may cause dilation of the aortic annulus, with resultant aortic regurgitation. This represents a significant volume overload on the left ventricle, resulting in progressive left ventricular dilation and failure. Compression of the adjacent structure may lead to chronic chest pain (22).
Acute sudden onset of severe pain is the typical manifestation of aortic dissection, but a wide variety of symptoms can be present. The patient may have symptoms suggestive of congestive heart failure, stroke, shock or loss of distal pulse (22). Other features may include diastolic murmur from aortic regurgitation and neurological deficits. If the dissection causes bleeding into the pericardium, distant heart sounds secondary to pericardial effusion may be noted, and symptoms and signs of tamponade may be seen in extreme cases (22).
In 591 patients with acute type A aortic dissection, the mean (± SD) patient age was 60.8±14.4 years. Two-thirds were men, and 71.2% had a history of hypertension (10).
Presenting symptoms in acute type A aortic dissection among the patients with aortic diameters of less than 5.5 cm were back pain, radiating pain, abrupt onset of pain and neurological deficits. On presentation, 32% of patients had hypertension, 12.8% were in shock and 26% had clinical signs of pulse deficits (10). Among the signs of aortic dissection, there was little to distinguish between patients with smaller or larger diameters of the ascending aorta, apart from more symptoms of cerebral malperfusion in the patients with smaller aortic diameters, and more congestive heart failure in the patients with larger aortic diameters (both P=0.05) (10).
A very unusual presentation of ruptured TAA was reported (5) in a 50-year-old patient who was almost asymptomatic and in a stable clinical condition. The chest radiograph and CT scan revealed a right-sided rupture of a previously undiagnosed TAA. The patient was treated successfully with an emergency surgical procedure.
There are many imaging modalities for aortic aneurysmal disease. Transthoracic two-dimensional echocardiography is very effective in evaluating the aortic root, but the mid and distal ascending aorta, aortic arch and descending aorta are not seen. Thus, it is a valuable and useful noninvasive test to evaluate and follow up on patients with an aortic root aneurysm (eg, Marfan syndrome). It also provides important information on aortic valvular regurgitation, and the function and dimensions of the left ventricle (22).
TEE allows better visualization of the aortic root, ascending aorta, aortic arch and descending aorta. Images are usually accurate, with the exception of part of the aortic arch and the distal part of the ascending aorta due to the position of the left bronchus between the probe and aorta. TEE allows near-complete evaluation of the aorta due to the proximity of the high-frequency probe to the aorta (22).
Helical CT and CT angiography allow for the full evaluation of the entire aorta and the extension of the aneurysm. They are also superior in evaluating the aortic branches. Three-dimensional reconstruction allows optimal measurement of the aneurysm size, especially when the aorta is tortuous. Another important tool that CT provides is the visualization and localization of the artery of Adamkiewicz, which supplies the anterior spinal artery. Most causes of paraplegia after repair of thoracoabdominal aneurysm or dissection are related to interrupted blood supply to the anterior spinal artery. Detection of the artery of Adamkiewicz can help in the surgical decision and may prevent postoperative paraplegia. In one study (22), Nienaber et al were able to detect the artery of Adamkiewicz in 63 of 70 patients (90%). The main disadvantage of CT is the need for contrast administration and radiation exposure. It is not the optimal test for critically ill patients who are not stable for transfer to the radiology suite.
MRI is another modality for evaluating aortic aneurysms. It is very sensitive and requires no radiation exposure, but is expensive and time-consuming, and may not be suitable for critically ill patients. It is also possible to detect the artery of Adamkiewicz on MRI angiography with great sensitivity (22).
Chest radiography may show an enlarged cardiac silhouette, aortic knob and tracheal deviation in large aneurysms; smaller aneurysms are not shown (22).
In aortic dissection, early diagnosis is critical because early intervention can decrease the mortality rate, which is estimated to increase by 1% to 2% per hour in the first 48 h of ascending aortic dissection (22).
The modalities for diagnosing aortic dissection are the same for aortic aneurysms. Aortography is another method for making the diagnosis, but is invasive and requires the use of contrast. Its use is also limited in critically ill and unstable patients. Nevertheless, it remains the method of choice in diagnosing class 3 dissection (22).
A recent meta-analysis by Shiga et al (24) that reviewed published studies of the diagnosis of aortic dissection by TEE, helical CT and MRI showed that these tests have equal and reliable diagnostic value. TEE had 99% sensitivity and 95% specificity, helical CT had 100% sensitivity and 98% specificity, and MRI had 98% sensitivity and 98% specificity (22,25).
Echocardiography provides important information not only regarding the function of the heart, but also the presence of complications of aortic dissection, such as pericardial effusion and mediastinal hematoma (22).
It is important to differentiate between different classes of aortic dissection because treatment and prognosis vary accordingly. For example, classic type A dissection needs rapid surgical intervention, whereas classic type B dissection needs medical management. It is important to localize the tear, if possible, because the main goal of intervention is to occlude the entry point. Using two-dimensional echocardiography, the intimal flap, point of entry, and true and false lumens can easily be seen (22).
CT is the most frequent first imaging modality performed, with very high sensitivity and specificity (10,22). MRI has the highest accuracy and sensitivity for detection of all types of dissection, with the exception of class 3, which can only be diagnosed with aortography (22). Chest x-rays normally show a widened mediastinum. In one study (10), 69% of patients with aortic dissection were reported to have a widened mediastinum. Significantly more patients with dissections that have diameters of less than 5.5 cm had a normal chest x-ray (12.1% versus 6.8%, P=0.05).
Aortic aneurysm is usually a progressive disease that needs to be monitored closely or treated. As aneurysms grow in size, there is an increased incidence of rupture, dissection and death. Ascending aortic aneurysms grow an average of 1 mm to 4 mm every year, but in patients with bicuspid aortic valves and Marfan syndrome, the rate of growth is more rapid (22).
The cumulative risk of rupture was 20% after five years. Seventy-nine per cent of ruptures occurred in women (P=0.01). The five-year risk of rupture as a function of aneurysm size at recognition was 0% for aneurysms less than 4 cm in diameter, 16% for those between 4 cm and 5.9 cm, and 31% for aneurysms 6 cm or larger (2,6).
Once ruptured, emergent repair is extremely challenging with an associated mortality in the mid 90% range (1,4). Overall survival for TAA has improved significantly in the past 15 years (2). Overall five-year survival improved to 56% (95% CI 48% to 66%) between 1980 and 1994 compared with only 19% between 1951 and 1980 (P<0.01) (2).
Aortic rupture and older age were risk factors for operative mortality, but the only variable associated with long-term mortality was increasing age. The patients who underwent surgery had an actuarial survival at one, five and 10 years of 92% (95% CI 91% to 93%), 77% (95% CI 75% to 80%) and 57% (95% CI 53% to 61%), respectively (16).
Before the evolution of open-heart surgery, Marfan patients usually died from acute aortic dissection or rupture, and thus had an average life expectancy of only 32 years. Today, management by expert centres has extended the life expectancy of Marfan patients to older than 60 years of age (19).
The mainstay of prevention of aortic dissection, aside from treatment of hypertension, is elective aortic surgery in patients with dilated ascending aortas. Guidelines for timing of aortic root repair are based on clinical observations by experienced clinicians and surgeons, and a consensus based on clinical series and patient characteristics. There is a consensus that surgery to prevent rupture or dissection of the ascending TAA should be recommended when the ascending aortic diameter reaches 5.5 cm for non-Marfan patients and 4.5 cm in Marfan patients (10–14,16).
Indications for prophylaxis surgery of the aortic root in adults (at least one criterion must be met) (19)
Aortic root diameter greater than 55 mm (50 mm according to other authors)
- Aortic root diameter greater than 50 mm (45 mm to 50 mm according to other authors) in patients at high risk for aortic complications
- Family history of aortic dissection
- Growth of the aortic root of more than 10 mm/year
- Dilation of the aortic sinus involving the ascending aorta
- More than mild aortic regurgitation
- Severe mitral regurgitation
- Patient scheduled to undergo major noncardiovascular surgery
- Woman planning pregnancy
Ratio of the diameters of the aortic root and the descending aorta of greater than 2
The standard for managing cardiovascular manifestations of Marfan syndrome recommends counselling on lifestyle modification including moderate restriction of physical activity, endocarditis prophylaxis, serial imaging of the aorta, beta-blocker medication for aortic protection and prophylactic replacement of the aortic root (19).
Aortic size is not a sufficient marker of risk for aortic dissection. The majority of patients with acute type A aortic dissection in a registry presented with aortic diameters less than 5.5 cm. Current surgical guidelines for TAA repair (5.5 cm or greater) would fail to prevent the majority of acute aortic dissections seen in this cohort. Even with more aggressive guidelines (less than 5 cm), pre-emptive aneurysm surgery would fail to prevent 40% of acute aortic dissections seen in this register (10).
Open repair of descending TAA has traditionally been a procedure associated with high morbidity and mortality, and has been offered only to good surgical candidates. Perioperative death rates exceeding 10%, a risk of paraplegia of 4% to 5%, and a long recovery from thoracotomy make this a weighty undertaking for surgeon and patient alike (6,26).
Endovascular stent grafting of aneurysmal disease processes of the thoracic aorta is feasible and relatively safe (1). In phase 2 multicentre data used by the United States Food and Drug Administration, the Gore TAG device (WL Gore & Associates Inc, USA) was demonstrated to be a safe alternative with relatively low morbidity (4% stroke and 3% temporary or permanent paraplegia) and excellent freedom from aneurysm-related death of 97% at two years (1,21,26).
Between 1996 and 2005, 45 patients (mean age 68±11 years) with aneurysms of the descending thoracic aorta underwent endovascular repair. They were included in a monocentric, non-randomized, prospective study (21). No patients died, and no surgical intervention was required during the procedures. Three patients (6.7%) died during the first month and six patients (14.7%) died later on. The main complications were stroke (13.3%), vascular access complication (8.9%), aortic complication (6.6%), paraplegia (4.4%) and sudden death (4.4%). Actuarial survival estimates at one, three and five years were 87.6%±5.3%, 76.9%±7.4% and 70.6%±9.2%, respectively.
More recently, repair of aortic arch aneurysms has been accomplished using both ‘hybrid’ (open and endovascular) and totally endovascular techniques (1).
In hybrid arch repair, the strategy of extra-anatomical bypass of the brachiocephalic vessels followed by endovascular stent graft deployment has been reported with promising early results. The avoidance of deep hypothermic circulatory arrest and the ability to avoid manipulation of the brachiocephalic origins in the aneurysmal arch may reduce perioperative morbidity and mortality (1,8).
In total endovascular arch repair, the reconstruction is performed without open surgery. Both single- and triple-branch stent grafts were inserted using a retrograde insertion technique (1,9).
REFERENCES
- 1.Larson EW, Edwards WD. Risk factors for aortic dissection: A necropsy study of 161 cases. Am J Cardiol. 1984;53:849–55. doi: 10.1016/0002-9149(84)90418-1. [DOI] [PubMed] [Google Scholar]
- 2.Bickerstaff LK, Pairolero PC, Hollier LH, et al. Thoracic aortic aneurysms: A population-based study. Surgery. 1982;92:1103–8. [PubMed] [Google Scholar]
- 3.Mészáros I, Mórocz J, Szlávi J, et al. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117:1271–8. doi: 10.1378/chest.117.5.1271. [DOI] [PubMed] [Google Scholar]
- 4.Clouse WD, Hallett JW, Jr, Schaff HV, et al. Acute aortic dissection: Population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc. 2004;79:176–80. doi: 10.4065/79.2.176. [DOI] [PubMed] [Google Scholar]
- 5.Tsai TT, Evangelista A, Nienaber CA, et al. Long-term survival in patients presenting with type A acute aortic dissection: Insights from the International Registry of Acute Aortic Dissection (IRAD) Circulation. 2006;114(1 Suppl):I350–6. doi: 10.1161/CIRCULATIONAHA.105.000497. [DOI] [PubMed] [Google Scholar]
- 6.von Kodolitsch Y, Schwartz AG, Nienaber CA. Clinical prediction of acute aortic dissection. Arch Intern Med. 2000;160:2977–82. doi: 10.1001/archinte.160.19.2977. [DOI] [PubMed] [Google Scholar]
- 7.Mehta R, O’Gara P, Bossone E, et al. Acute type A aortic dissection in the elderly: Clinical characteristics, management, and outcomes in the current era. J Am Coll Cardiol. 2002;40:685–92. doi: 10.1016/s0735-1097(02)02005-3. [DOI] [PubMed] [Google Scholar]
- 8.Erbel R, Alfonso F, Boileau C, et al. Diagnosis and management of aortic dissection. Eur Heart J. 2001;22:1642–81. doi: 10.1053/euhj.2001.2782. [DOI] [PubMed] [Google Scholar]
- 9.Cigarroa JE, Isselbacher EM, DeSanctis RW, Eagle KA. Diagnostic imaging in the evaluation of suspected aortic dissection. Old standards and new directions. N Engl J Med. 1993;328:35–43. doi: 10.1056/NEJM199301073280107. [DOI] [PubMed] [Google Scholar]
- 10.Erbel R, Engberding R, Daniel W, Roelandt J, Visser C, Rennollet H. Echocardiography in diagnosis of aortic dissection. Lancet. 1989;1:457–61. doi: 10.1016/s0140-6736(89)91364-0. [DOI] [PubMed] [Google Scholar]
- 11.Bansal RC, Chandrasekaran K, Ayala K, Smith DC. Frequency and explanation of false negative diagnosis of aortic dissection by aortography and transesophageal echocardiography. J Am Coll Cardiol. 1995;25:1393–401. doi: 10.1016/0735-1097(94)00569-C. [DOI] [PubMed] [Google Scholar]
- 12.White RD, Lipton MJ, Higgins CB, et al. Noninvasive evaluation of suspected thoracic aortic disease by contrast-enhanced computed tomography. Am J Cardiol. 1986;57:282–90. doi: 10.1016/0002-9149(86)90906-9. [DOI] [PubMed] [Google Scholar]
- 13.Nienaber CA, von Kodolitsch Y, Nicolas V, et al. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med. 1993;328:1–9. doi: 10.1056/NEJM199301073280101. [DOI] [PubMed] [Google Scholar]
- 14.Vasile N, Mathieu D, Keita K, et al. Computed tomography of thoracic aortic dissection: Accuracy and pitfalls. J Comput Assist Tomogr. 1986;10:211–5. doi: 10.1097/00004728-198603000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Sommer T, Fehske W, Holzknecht N, et al. Aortic dissection: A comparative study of diagnosis with spiral CT, multiplanar transesophageal echocardiography, and MR imaging. Radiology. 1996;199:347–52. doi: 10.1148/radiology.199.2.8668776. [DOI] [PubMed] [Google Scholar]
- 16.Hamada S, Takamiya M, Kimura K, Imakita S, Nakajima N, Naito H. Type A aortic dissection: Evaluation with ultrafast CT. Radiology. 1992;183:155–8. doi: 10.1148/radiology.183.1.1549663. [DOI] [PubMed] [Google Scholar]
- 17.Sebastià C, Pallisa E, Quiroga S, Alvarez-Castells A, Dominguez R, Evangelista A. Aortic dissection: Diagnosis and follow-up with helical CT. Radiographics. 1999;19:45–60. doi: 10.1148/radiographics.19.1.g99ja0945. [DOI] [PubMed] [Google Scholar]
- 18.DeBakey ME, Henly WS, Cooley DA, Morris GC, Jr, Crawford ES, Beall AC., Jr Surgical management of dissecting aneurysms of the aorta. J Thorac Cardiovasc Surg. 1965;49:130–49. [PubMed] [Google Scholar]
- 19.Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann Thorac Surg. 1970;10:237–47. doi: 10.1016/s0003-4975(10)65594-4. [DOI] [PubMed] [Google Scholar]
- 20.Pansini S, Gagliardotto PV, Pompei E, et al. Early and late risk factors in surgical treatment of acute type A aortic dissection. Ann Thorac Surg. 1998;66:779–84. doi: 10.1016/s0003-4975(98)00555-4. [DOI] [PubMed] [Google Scholar]
- 21.Kawahito K, Adachi H, Yamaguchi A, Ino T. Preoperative risk factors for hospital mortality in acute type A aortic dissection. Ann Thorac Surg. 2001;71:1239–43. doi: 10.1016/s0003-4975(00)02654-0. [DOI] [PubMed] [Google Scholar]
- 22.Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340:1539–45. doi: 10.1056/NEJM199905203402003. [DOI] [PubMed] [Google Scholar]
- 23.Liao MF, Jing ZP, Bao JM, et al. Role of nitric oxide and inducible nitric oxide synthase in human abdominal aortic aneurysms: A preliminary study. Chin Med J. 2006;119:312–8. [PubMed] [Google Scholar]
- 24.Shiga T, Wajma Z, Aptel CC, Inove T, Ohe Y. Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection. Symptomatic review and meta-analysis. Arch Intern Med. 2006;166:1350–6. doi: 10.1001/archinte.166.13.1350. [DOI] [PubMed] [Google Scholar]
- 25.Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: A best-evidence systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;142:203–11. doi: 10.7326/0003-4819-142-3-200502010-00012. [DOI] [PubMed] [Google Scholar]
- 26.Tsai TT, Nienaber CA, Eagle KA. Acute aortic syndromes. Circulation. 2005;112:3802–13. doi: 10.1161/CIRCULATIONAHA.105.534198. [DOI] [PubMed] [Google Scholar]