Abstract
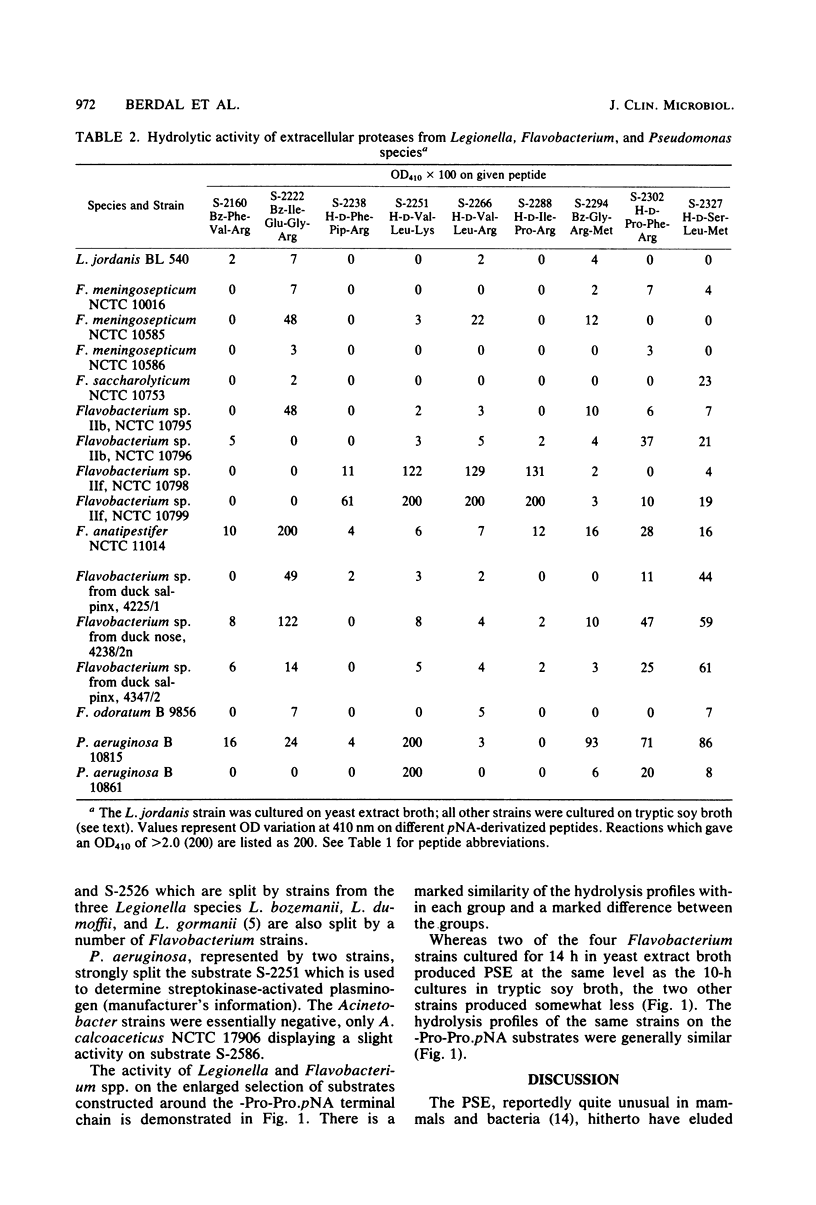
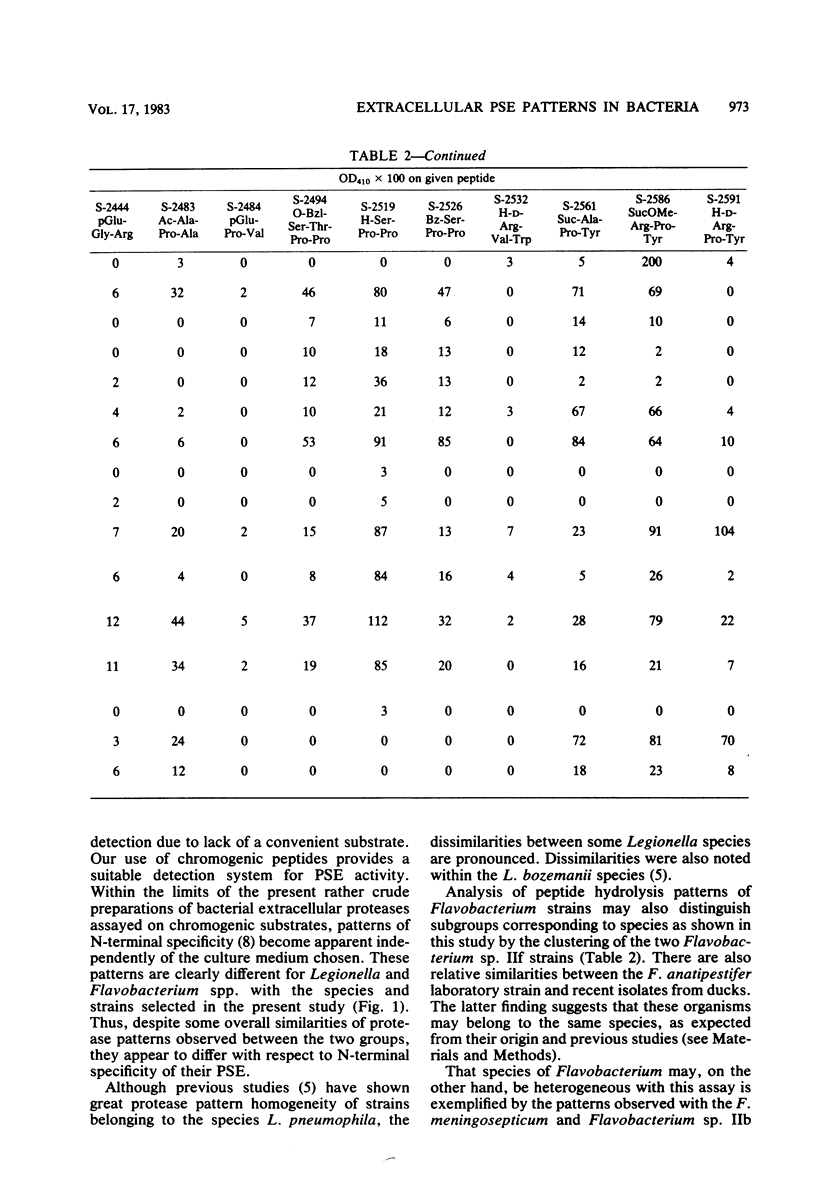
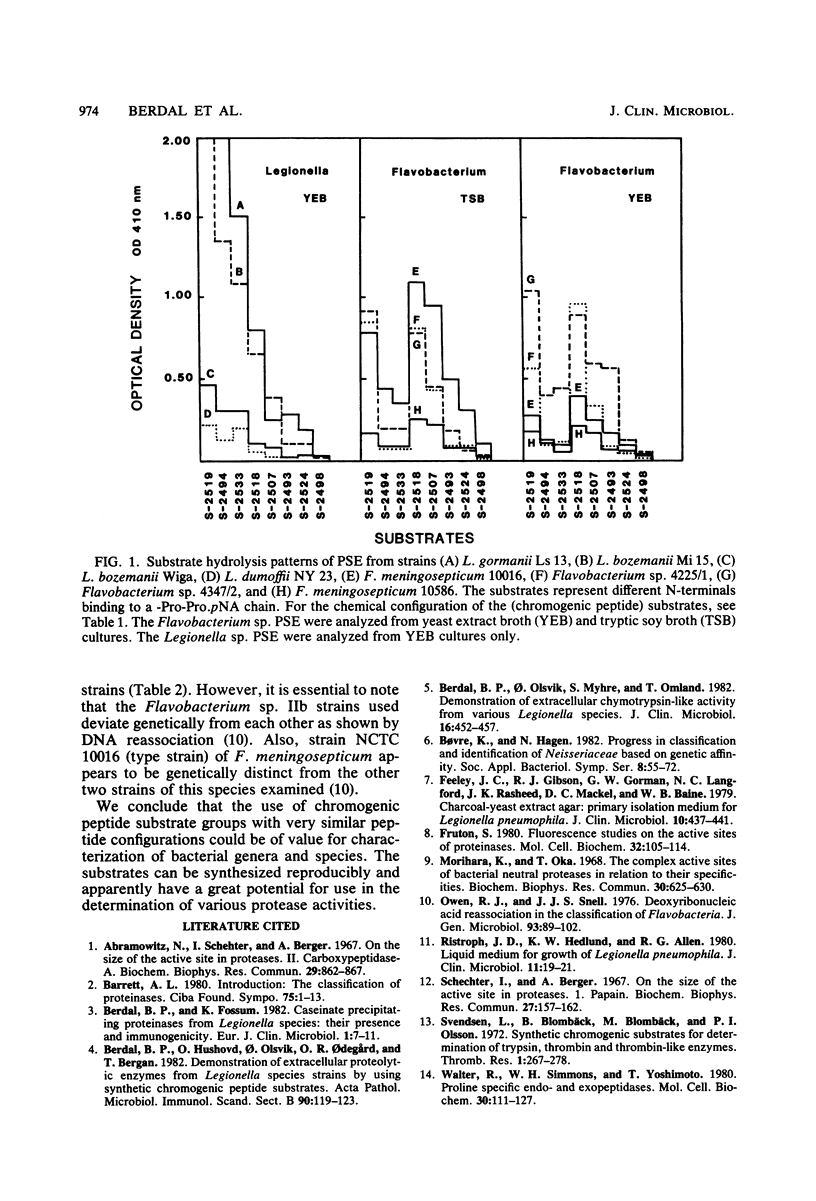
Some Legionella strains possess a strong extracellular proline-specific endopeptidase (PSE) activity. Using an enlarged selection of chromogenic peptides representing a variety of N-terminal amino-acids binding to a -prolyl-proline, paranitroanilide chain, PSE activity of Legionella and Flavobacterium strains was examined. Differences in PSE activity emphasized the importance of the chemical structure at the nonchromogenic end of the peptide substrates. There seem to be distinct patterns of N-terminal specificity of PSE in the two bacterial groups.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramowitz N., Schechter I., Berger A. On the size of the active site in proteases. II. Carboxypeptidase-A. Biochem Biophys Res Commun. 1967 Dec 29;29(6):862–867. doi: 10.1016/0006-291x(67)90299-9. [DOI] [PubMed] [Google Scholar]
- Berdal B. P., Fossum K. Occurrence and immunogenicity of proteinases from Legionella species. Eur J Clin Microbiol. 1982 Feb;1(1):7–11. doi: 10.1007/BF02014133. [DOI] [PubMed] [Google Scholar]
- Berdal B. P., Hushovd O., Olsvik O., odegård O. R., Bergan T. Demonstration of extracellular proteolytic enzymes from Legionella species strains by using synthetic chromogenic peptide substrates. Acta Pathol Microbiol Immunol Scand B. 1982 Apr;90(2):119–123. doi: 10.1111/j.1699-0463.1982.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Berdal B. P., Olsvik O., Myhre S., Omland T. Demonstration of extracellular chymotrypsin-like activity from various Legionella species. J Clin Microbiol. 1982 Sep;16(3):452–457. doi: 10.1128/jcm.16.3.452-457.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeley J. C., Gibson R. J., Gorman G. W., Langford N. C., Rasheed J. K., Mackel D. C., Baine W. B. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979 Oct;10(4):437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruton J. S. Fluorescence studies on the active sites of proteinases. Mol Cell Biochem. 1980 Sep 15;32(2):105–114. doi: 10.1007/BF00227803. [DOI] [PubMed] [Google Scholar]
- Morihara K., Oka T. The complex active sites of bacterial neutral proteases in relation to their specificities. Biochem Biophys Res Commun. 1968 Mar 27;30(6):625–630. doi: 10.1016/0006-291x(68)90558-5. [DOI] [PubMed] [Google Scholar]
- Owen R. J., Snell J. J. Deoxyribonucleic acid reassociation in the classification of flavobacteria. J Gen Microbiol. 1976 Mar;93(1):89–102. doi: 10.1099/00221287-93-1-89. [DOI] [PubMed] [Google Scholar]
- Ristroph J. D., Hedlund K. W., Allen R. G. Liquid medium for growth of Legionella pneumophila. J Clin Microbiol. 1980 Jan;11(1):19–21. doi: 10.1128/jcm.11.1.19-21.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Walter R., Simmons W. H., Yoshimoto T. Proline specific endo- and exopeptidases. Mol Cell Biochem. 1980 Apr 18;30(2):111–127. doi: 10.1007/BF00227927. [DOI] [PubMed] [Google Scholar]


