Abstract
Objective
To assess effects of male circumcision on female genital symptoms, and vaginal infections.
Methods
HIV-negative men enrolled in a trial were randomized to immediate or delayed circumcision (control arm). Genital symptoms, BV and trichomonas were assessed in HIV-negative wives of married participants. Adjusted prevalence risk ratios (adjPRR) and 95% confidence intervals (95%CI) were assessed by multivariable log-binomial regression, intent-to-treat analyses.
Results
783 wives of control and 825 wives of intervention arm men were comparable at enrollment. BV at enrollment was higher in control (38.3%) than intervention arm spouses (30.5%, p=0.001). At one year follow up, intervention arm wives reported lower rates of genital ulceration (adjPRR 0.78, 95%CI 0.63–0.97), but there were no differences in vaginal discharge or dysuria. The risk of trichomonas was reduced in intervention arm wives (adjPRR 0.52, 95%CI 0.05–0.98), as were the risks of any BV (adjPRR 0.60, 95%CI 0.38–0.94) and severe BV (PRR = 0.39, 95%CI 0.24–0.64).
Conclusions
Male circumcision reduces the risk of ulceration, trichomonas and BV in female partners.
Introduction
Three randomized trials and multiple observational studies demonstrate that male circumcision reduces the risk of HIV infection in men,1–3 and WHO has recommended that circumcision be promoted for HIV prevention.4 However, the effects of male circumcision on male STIs are more equivocal. In observational studies5,6 and two randomized trials,1,7 circumcision was associated with reduced symptomatic genital ulcer disease (GUD) in men, but had no effects on symptoms of urethral discharge or dysuria in male participants.
If circumcision becomes widely adopted for HIV prevention in men, it is possible that there may be derivative benefits for female partners if the procedure reduces male carriage of HIV and STIs or directly affects male-to-female transmission. One observational study suggested that there may be a reduction of HIV, bacterial vaginosis (BV), and Trichomonas vaginalis infections in female partners of circumcised men.8 However, two US studies observed no association between a man’s circumcision status and female BV.9,10 Therefore, we examined data on vaginal infections from female partners of men enrolled in a randomized trial of male circumcision for HIV prevention, in Rakai District, Uganda.
Methods
The trial of male circumcision, supported by the National Institutes of Health has been described elsewhere.1 In brief, 4996 HIV-negative men aged 15–49 who accepted voluntary counseling and receipt of their HIV results were enrolled and randomized to immediate circumcision (the intervention arm, n = 2474), or circumcision delayed for two years (the control arm, n = 2522). The trial was closed early on December 12, 2006 because an interim analysis showed evidence of circumcision efficacy for male HIV acquisition. At enrolment, male trial participants were asked to identify their wives or long-term consensual partners. Consenting men who provided this information were then linked to their female partners who were enrolled and followed up in a separate study supported by the Bill & Melinda Gates Foundation. Because the male circumcision trial was closed, we felt it appropriate to assess the potential effects of male circumcision on the health of female partners, since this could be of relevance to future policy decisions regarding promotion of male circumcision.
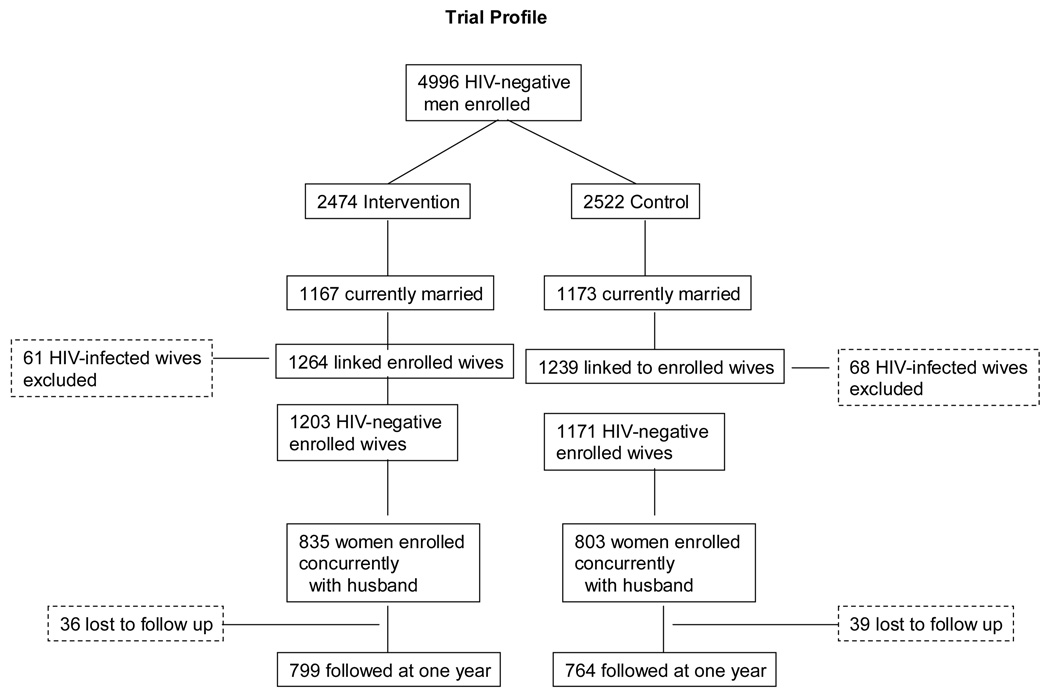
The trial profile is given in Figure 1. There were 1264 wives currently married or in long-term union with 1167 men enrolled in the intervention arm (1.08 wives per married man), and there were 1239 female spouses of 1173 men enrolled in the control arm of the trial (1.06 wives per married man). The number of linked, enrolled women exceeded the number of enrolled men because of polygamous relationships. At time of female enrollment, 1203 wives of intervention arm men were HIV-negative (95.2%); and in the control arm 1171 were uninfected with HIV (94.5%). Among these HIV-negative married women, 835 (69.4%) were enrolled concurrently with their husbands in the intervention arm (i.e., before the man’s circumcision surgery), and 803 (68.6%) were enrolled concurrently with their control arm husbands. These HIV-uninfected women who were enrolled at the same time as their husbands constitute the primary analysis sample for this study (Figure 1). A minority of women (368 in the intervention arm and 368 in the control arm) were enrolled six or more months after their husband’s enrollment date. These women were excluded from the primary intent-to-treat analysis because, if circumcision affected female vaginal symptoms or infections, the baseline information for these women could have been biased by the interval of exposure between their male partner’s circumcision and the woman’s initial enrollment visit.
Figure 1.
Among the 835 HIV-negative concurrently enrolled HIV-negative female partners of HIV-uninfected intervention arm men, 799 (95.7%) were followed up at one year post-enrollment, and among the 803 concurrently enrolled female partners of control arm men 764 (95.1%) were followed up at one year. At the time of the NIH male trial closure, 90% of men had completed 12 months follow up, but only 44% of male participants had the opportunity to complete 24 months follow up. Therefore, the female follow up was truncated at one year.
The married women were visited after their husbands had enrolled in the trial, and were then followed up at annual intervals. At each study visit, women were interviewed to ascertain sociodemographic characteristics, sexual risk behaviors and health status, including symptoms of genital tract infections (genital ulcer disease (GUD), vaginal discharge and dysuria). Symptomatic women were treated syndromically. At each visit, women were asked to provide a self-collected vaginal swab for diagnosis of bacterial vaginosis (BV) and Trichomonas vaginalis. BV was detected by Gram stained slides from vaginal swabs using the Nugent criteria11, a quantitative morphology that has a standardized 0 to 10 point scoring system, whereby 0–3 is normal, 4–6 is intermediate (transitional state), and 7–10 is BV. Severe BV was defined as a score of 9–10. Enrollment information on BV was available for 825 out of 835 concurrently enrolled wives of intervention arm men (98.8%), and 783 out of 803 wives of control arm men (97.5%). Twelve month follow up data on BV were available for 785 intervention arm women (98.7% of women followed), and 751 control arm wives (98.4%) of women followed. The number of women with information on BV was less than the number of women seen, because some participants declined to provide self-collected vaginal swabs. Trichomonas vaginalis was detected from cultures of vaginal swabs using the InPouch TV™ culture method. Due to financial constraints, trichomonas culture was only initiated late in the study and there were limited data available at time of female enrollment. At the 12 month follow up visit, data on trichomonas were available for 408 intervention arm wives (51.1% of those followed), and for 402 control arm wives (52.7% of those followed). Assays for humanpapilloma virus (HPV) and herpes simplex virus type 2 (HSV-2) are ongoing, so this report focuses on vaginal infections with trichomonas and BV.
We conducted an intent-to-treat analysis to assess the prevalence of symptoms of genital tract infections and diagnoses of BV and trichomonas during follow up in concurrently enrolled HIV-negative wives of male participants in the intervention compared to the control arm of the randomized trial. STI symptoms reported over the 6 month period preceding the one year follow up visit were used to estimate the period prevalence of symptoms per 100 women. Diagnoses of BV and trichomonas at the 12 month visit were used to estimate the point prevalence of these conditions per 100 women. In addition, tabulations of BV scores at follow up were stratified by BV scores at enrollment to determine rates of progression to BV from normal or intermediate baseline flora scores, and among women with BV at enrollment we determined persistence of BV at follow up. Unadjusted prevalence risk ratios (PRR) and 95% confidence intervals (95%CI) were estimated. Adjusted prevalence risk ratios (adj PRR) were estimated using multiple log-binomial regression, with adjustment for age (15–19, 20–24, 25–29, 30+), monogamous or polygamous marriage at enrollment, self-reported numbers of sexual partners (one, two, three or more), and use of condoms (none, consistent or inconsistent) during the one year follow up interval. Because the prevalence of BV differed between study arms at enrollment, we also adjusted the follow up prevalence of BV for the presence of BV at enrollment. The percent efficacy of circumcision was estimated as (1-adj PRR) x 100%. We also conducted an as-treated analysis whereby men allocated to the intervention arm but who failed to return for surgery within 6 months of enrollment were classified as uncircumcised (n=36). Two control participants received circumcision from other sources during the first year of follow up and were circumcised crossovers.
Enrollment of the HIV-negative male trial participants was funded by the National Institutes of Health and the trial was registered with ClinicalTrials.Gov number NCT00425984. The enrollment and follow up of female partners of trial participants was funded by the Gates Foundation and is registered with Clinical.Trials.Gov number NCT00124878. The study was reviewed and approved by two Institutional Review Boards (IRBs) in Uganda (The Scientific and Ethics Committee of the Uganda Virus Research Institute and the Uganda National Committee of Science and Technology), and two IRBs in the U.S. (The Johns Hopkins Bloomberg School of Public Health IRB, and Western IRB). A Community Advisory Board provided guidance for the study, and two Data Safety Monitoring Boards, one for the NIH and the other for the Gates funded study, provided oversight.
Results
Table 1 shows the characteristics of the women at enrollment. Randomization of male trial participants produced a high degree of comparability in female partner enrollment characteristics and sexual behaviors between arms. There was also comparability with respect to STI symptoms. Only a small minority of women had enrollment cultures for trichomonas because these tests were initiated late in the study, so the overall baseline prevalence cannot be assessed. BV at enrollment was less frequent among women married to intervention arm men (30.6%) than women married to control men (38.3%), and this difference was statistically significant (p = 0.001). Severe BV (scores 9–10) were also lower in the intervention than control arm wives (1.8% versus 2.6%), but this difference was not statistically significant (p = 0.4).
Table 1.
Enrollment characteristics of HIV-negative women by their husband’s study arm
| Control | Intervention | |||
|---|---|---|---|---|
| N | % | N | % | |
| All | 803 | 100 | 835 | 100 |
| Age | ||||
| 15–17 | 22 | 2.7% | 29 | 3.5% |
| 18–19 | 95 | 11.8% | 109 | 13.1% |
| 20–24 | 290 | 36.1% | 274 | 32.8% |
| 25–29 | 212 | 26.4% | 233 | 27.9% |
| 30–39 | 155 | 19.3% | 167 | 20.0% |
| 40–49 | 29 | 3.6% | 23 | 2.8% |
| Marital status | ||||
| Monogamous | 687 | 85.6% | 690 | 82.6% |
| Polygamous | 116 | 14.4% | 143 | 17.1% |
| Religion | ||||
| Catholic | 494 | 61.5% | 516 | 61.8% |
| Protestant | 218 | 27.1% | 230 | 27.5% |
| Saved | 68 | 8.5% | 66 | 7.9% |
| Muslim | 23 | 2.9% | 22 | 2.6% |
| Education | ||||
| None | 108 | 13.4% | 118 | 14.1% |
| Primary | 581 | 72.4% | 604 | 72.3% |
| Secondary | 98 | 12.2% | 96 | 11.5% |
| Tertiary | 16 | 2.0% | 16 | 1.9% |
| Sex partners past year | ||||
| 0 | 3 | 0.4% | 1 | 0.1% |
| 1 | 763 | 95.0% | 810 | 97.0% |
| 2 | 34 | 4.2% | 20 | 2.4% |
| 3+ | 3 | 0.4% | 4 | 0.5% |
| Condom use | ||||
| Consistent | 4 | 0.5% | 4 | 0.5% |
| Inconsistent | 143 | 17.8% | 127 | 15.2% |
| No use | 656 | 81.7% | 704 | 84.3% |
| Drank alcohol with sex | ||||
| Never | 543 | 67.6% | 565 | 67.7% |
| Sometimes | 260 | 32.4% | 269 | 32.2% |
| Always | 0 | 0.0% | 1 | 0.1% |
| STD past year | ||||
| GUD | 105 | 13.1% | 113 | 13.5% |
| Discharge | 360 | 44.8% | 386 | 46.2% |
| Dysuria | 164 | 20.4% | 160 | 19.2% |
| Vaginal Infections | ||||
| Trichomonas | 1/1 | 100% | 0/1 | 0% |
| Any BV | 300/783 | 38.3% | 252/825 | 30.5%* |
| Severe BV | 20/783 | 2.6% | 15/825 | 1.8% |
p = 0.001
As shown in Table 2, the rates of self-reported GUD were significantly lower in the wives of intervention arm men (12.8%), than in women with control arm partners (16.8%, p=0.03). However, there were no differences in the frequency of female symptoms of discharge or dysuria by their husband’s arm of randomization. The prevalence of trichomonas was significantly lower among women with intervention arm husbands (5.9%), compared to women with control arm husbands (11.2%, p = 0.01). BV prevalence was significantly lower among the wives of intervention arm men (40.3%) compared with wives of control arm men (50.6%, p=0.00006). The proportion of women reporting two or more sex partners during the follow up interval was higher in the control than intervention arm women (5.6% and 3.4%, respectively, p =0.02), but there were no differences between study arms in self-reported condom use or consistency of use during follow up.
Table 2.
Vaginal symptoms, Trichomonas and BV during follow up visits for HIV-negative women, by male partner’s randomization arm
| Outcomes at one year follow up and sexual behaviors | Control | Intervention | Unadjusted PRR (intervention/ control) | 95%CI | ||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| Vaginal symptoms | ||||||
| Genital ulceration | 128/763 | 16.8 | 102/798 | 12.8 | 0.76 | 0.60–0.97 |
| Discharge | 323/763 | 42.3 | 336/798 | 42.1 | 0.99 | 0.89–1.12 |
| Dysuria | 114/763 | 14.9 | 113/798 | 14.2 | 0.97 | 0.75–1.21 |
| Vaginal infections | ||||||
| Trichomonas | 45/402 | 11.2 | 24/408 | 5.9 | 0.53 | 0.33–0.85 |
| BV | 380/751 | 50.6 | 316/785 | 40.3 | 0.80 | 0.71–0.89 |
| Severe BV | 49/751 | 6.52 | 16/785 | 2.04 | 0.31 | 0.18–0.54 |
| Sexual behaviors | ||||||
| Number of sex partners | ||||||
| 1 | 715/760 | 94.1 | 786/795 | 96.6 | 1.03 | 1.00–1.05 |
| 2+ | 45/760 | 5.6 | 27/795 | 3.4 | 0.57 | 0.36–0.91 |
| Condom use | ||||||
| None | 607/763 | 79.6 | 655/798 | 82.1 | 1.03 | 0.98–1.08 |
| Inconsistent | 148/763 | 19.4 | 134/798 | 16.8 | 0.87 | 0.70–1.07 |
| Consistent | 8/763 | 1.1 | 9/798 | 1.1 | 1.08 | 0.42–2.77 |
Because BV prevalence differed significantly between study arms at enrollment with a higher prevalence in the control arm (Table 1) the differentials observed at follow up could reflect this pre-existing differential at enrollment. Therefore, we assessed BV at follow-up, stratified by enrollment vaginal flora scores. Our reasoning was that if circumcision affected BV, the effects should be observed among women without BV at enrollment, and this should not be affected by disparities in BV prevalence at enrollment. The results are shown in Table 3. Among women with normal flora (scores 0–3) at enrollment, progression to BV during follow up was significantly lower in the wives of intervention than control arm men (PRR = 0.80, 95%CI 0.65–0.97, p = 0.005). Progression to BV among women with intermediate flora scores (4–6) at enrollment was less in the intervention than the control arm, but this was not statistically significant (PRR = 0.83, 95%CI 0.65–1.06, p=0.2). However, in women who had BV at enrollment, persistent BV at one year’s follow up was significantly lower in intervention than control arm women (PRR = 0.83, 95%CI 0.72–0.96, p = 0.02.) We also assessed women with severe BV (scores 9–10) at follow up (Table 3). In the control arm wives with initially normal vaginal flora, 4.0% progressed to severe BV, whereas no intervention arm wives developed severe BV (p = 0.0002). Similarly, among women with intermediate flora at enrollment, progression to severe BV was lower in the intervention (0.7%) than in the control arm (5.6%) wives and this difference was of borderline statistical significance (PRR = 0.13, 95%CI 0.02–0.1.06, Fisher p=0.03). Among women with BV at enrollment, severe BV was lower in the wives of intervention than control arm at follow up, although the difference was not statistically significant (PRR = 0.61, 95%CI 0.33–1.12).
Table 3.
Bacterial Vaginosis at follow up by vaginal flora score at enrollment
| Vaginal flora score at enrollment | Control | Intervention | ||||
|---|---|---|---|---|---|---|
| BV at follow up/N at enrollment | BV at follow up % | BV at follow up/N at enrollment | BV at follow up % | PRR (intervention/control) | 95% CI | |
| 0–3 | 124/325 | 38.2 | 122/402 | 30.3 | 0.80 | 0.65–0.97 |
| 4–6 | 67/124 | 54.0 | 60/134 | 44.8 | 0.83 | 0.65–1.06 |
| 7–10 | 185/285 | 64.9 | 130/240 | 54.2 | 0.83 | 0.72–0.96 |
| Severe BV | Severe BV | |||||
| (9–10) | (9–10) | |||||
| 0–3 | 13/325 | 4.0 | 0/402 | 0.0 | 0.00 | 0.00–0.24* |
| 4–6 | 7/124 | 5.6 | 1/134 | 0.7 | 0.13 | 0.02–1.06 |
| 7–10 | 29/285 | 10.2 | 15/240 | 6.3 | 0.61 | 0.33–1.12 |
Unconditional exact interval (StatXact 6.0).
After adjustment for enrollment characteristics and sexual risk behaviors during follow up, the log binomial adjusted prevalence risk ratio of GUD among wives of intervention compared to control arm participants was 0.78, 95%CI 0.61–0.99, p = 0.04, suggesting a circumcision efficacy of 22%, (95%CI 1–39%). For trichomonas infection in the intervention relative to control arm wives the adj PRR was 0.55, 95%CI 0.34–0.89, p = 0.02 (efficacy 45%, 95%CI 11–66%). The adjusted risk of trichomonas was increased among women reporting two (adj PRR = 2.02, 95%CI 1.05–4.33), and three or more sex partners (adj PRR = 5.12, 95%CI 1.05–25.77) compared with 1 partner. For BV at follow up, the adj PRR was 0.82, 95%CI 0.74–0.91, p = 0.0003, with an efficacy of 18% (95%CI 9–26%.) Compared to women without BV at enrollment, the adjusted risk of BV at follow up was also significantly increased if the woman had BV at enrollment (adjPRR = 1.51, 95%CI 1.35–1.568).
The as treated analyses yield similar results to the intent to treat analyses because crossovers were few in number (n = 36). For trichomonas, the as treated adjusted PRR was 0.55, 95%CI 0.34–0.88, p = 0.14, and for BV the adj PRR was 0.82, 95%CI 0.74–0.92.
Discussion
This trial of HIV uninfected female partners of HIV uninfected men found that female partners of circumcised men had reduced risks of GUD (efficacy 22%), trichomonas (efficacy 45%) and BV (efficacy 18%). This strongly suggests that male circumcision may have direct benefits for prevention of GUD and vaginal infections in female partners.
The findings from this randomized trial are consistent with those from a prior observational study in Rakai which reported significantly reduced risks of GUD (PRR = 0.6, 95%CI 0.4–1.0), BV (PRR = 0.79, 95%CI 0.69–0.91) and trichomonas (PRR = 0.55, 95%CI CI 0.55–0.77).8 However, our findings are contrary to two small U.S. studies which found no association between male circumcision and BV in female partners, but a high proportion of men in these studies were circumcised and there was limited power to detect an effect relative to women with uncircumcised partners.9,10 We are not aware of other studies examining female GUD or vaginal infections associated with male circumcision.
The mechanisms for the protective effects of male circumcision on female GUD and vaginal infections are unknown. However, it is known that circumcised men are less likely to have symptomatic genital ulcer disease,12 and a meta-analysis suggested that circumcision is associated with reduced rates of ulceration due to HSV-2, H ducreyi and syphilis.13 Therefore, reduced male carriage of these pathogens may reduce transmission of these ulcerative STIs to women. In addition, the subpreputial space in uncircumcised men is moist14 and this may enhance survival of trichomonas and possibly the gram negative anaerobes associated with BV, so removal of the foreskin could reduce female exposures to these pathogens.
We considered potential biases which might affect these results. Our findings with respect to the effects of male circumcision on BV are complicated by the fact that BV was more common among control than intervention arm women at enrollment, so differentials observed at follow up could have been due to pre-existing differences between women in the two arms, rather than a direct effect of circumcision on BV per se. We do not know why randomization did not result in comparability of BV at enrollment, and we have been unable to identify any factor, from randomization to the selection of the final analysis set, that would have made a systematic difference in the proportions with BV at enrollment However, circumcision significantly reduced the risk of progression to BV among women with normal flora at enrollment, and reduced the risks of persistent BV among women with BV at enrollment (Table 3). This strongly suggests that male circumcision provides partial protection from BV in female partners.
Although there were no differentials in sexual risk behaviors reported by women at enrollment (Table 1), the wives of control arm men did report more sexual partners during follow up (Table 2), and this could have affected their risks of vaginal infections. This apparent disinhibition among wives of control arm men could have occurred by chance or might arise if uncircumcised men experienced more difficulties with intercourse (e.g., due to phimosis) which caused a minority of their wives to seek other partners. However, we adjusted for these differentials in behaviors between arms by multivariate analyses, and there were no differentials in sexual risk behaviors reported by male trial participants.1 Thus, confounding due to differential risk behaviors is unlikely. The questions regarding STI symptoms were asked prior to questions on the woman’s partner’s circumcision status, so interviewer bias is also unlikely. Moreover, if such bias occurred, it would probably have affected questions on all vaginal symptoms, whereas the only protective effects were observed with symptomatic GUD, but not with vaginal discharge or dysuria. A similar protective effect of circumcision specifically against GUD was also observed among men in the randomized trial.1 Laboratory bias in the diagnosis of trichomonas or BV is extremely unlikely because technicians were blinded to the male partner’s circumcision status. Retention rates were high and comparable in both study arms so selective loss to follow up cannot explain the study findings. Thus, we conclude that the protective effects of male circumcision on female GUD and vaginal infections is likely to be a valid observation.
These findings may have implications for future programs providing male circumcision for HIV prevention, since GUD and vaginal infections are potential cofactors for HIV acquisition,15–19 and reductions in these conditions due to circumcision may potentially protect women from HIV infection. Three observational studies suggest that male circumcision is associated with decreased risks of HIV in female partners.20, 21 Thus, male circumcision might protect women from HIV risk by lowering infectivity (e.g., reduced male HIV shedding from the preputial mucosa), by reducing HIV cofactors such as GUD in both men and women, and reducing vaginal infections in women. In addition, since circumcision prevents male HIV acquisition, it is also likely to have an indirect effect via reduced female exposures to the virus, thus lowering secondary HIV transmissions to women.
We conclude that male circumcision prevents genital ulceration, trichomonas and BV in female partners, and that this benefit to women should be considered when planning scale up of male circumcision programs for HIV prevention.
Acknowledgements
The study was primarily supported by grants (UO1 AI11171-01-02) from the National Institutes of Allergy and Infectious Disease (NIAD), Division of AIDS, National Institutes of Health (NIH), and grants # 2228 and 220062228 from the Bill & Melinda Gates Foundation. The laboratory component was supported in part by the Division of Intramural Research, NIAID, NIH. This publication was supported, in part, by a fellowships/grants from the Fogarty International Center/USNIH: Grant # 2 D 43 TW000010-19-AITRP and 5D43TW001508. We would like to acknowledge the members of the NIH and Gates Foundation DSMBs who monitored this trial, as well as the IRBs which provided oversight (the Scientific and Ethics Committee of the Uganda Virus Research Institute, the Committee for Human Research at Johns Hopkins and Western Institutional Review Board). We also are grateful for the advice provided by the Rakai Community Advisory Board. Finally, we wish to express our gratitude to study participants whose commitment and cooperation made the study possible.
Footnotes
Condensation : A randomized trial of male circumcision showed reduced genital ulceration, trichomonas and BV in female partners of circumcised men.
References
- 1.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, Kiwanuka N, Moulton LH, Chaudhary MA, Chen MZ, Sewankambo NK, Wabwire-Mangen F, Bacon MC, Williams CF, Opendi P, Reynolds SJ, Laeyendecker O, Quinn TC, Wawer MJ. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomized trial. Lancet. 2007;369(9562):657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 2.Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, Williams CF, Campbell RT, Ndinya-Achola JO. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomized controlled trial. Lancet. 2007;369(9562):643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 3.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265. Trial PLoS Med. 2005 Nov;2(11):e298. doi: 10.1371/journal.pmed.0020298. Epub 2005 Oct 25. Erratum in: PLoS Med. 2006 May;3(5)e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO/UNAIDS New Data on Male Circumcision and HIV Prevention. Policy and Programme Implications. Conclusions and recommendations. Geneva: WHO/UNAIDS; WHO/UNAIDS Technical Consultation, Montreux, March 6–8, 2007. 2007 March 28;:1–19.
- 5.Reynolds SJ, Shepherd ME, Risbud AR, Gangakhedkar RR, Brookmeyer RS, Divekar AD, et al. Male circumcision and risk of HIV-1 and other sexually transmitted infections in India. Lancet. 2004;363(9414):1039–1040. doi: 10.1016/S0140-6736(04)15840-6. [DOI] [PubMed] [Google Scholar]
- 6.Gray RH, Azire J, Serwadda D, Kiwanuka N, Kigozi G, Kiddugavu M, et al. Male circumcision and the risk of sexually transmitted infections and HIV in Rakai, Uganda. AIDS. 2004;18:2428–2430. [PubMed] [Google Scholar]
- 7.Bailey RC. Scaling up circumcision programmes: the road from evidence to practice. Plenary Talk: 4th International AIDS Society Conference on HIV Pathogenesis; July 24, 2007; Sydney, Australia. [Google Scholar]
- 8.Gray RH, Wawer M, Serwadda D, Thoma M, Nalugoda F, Li X, et al. Male Circumcision and the Risks of Female HIV and Sexually Transmitted Infections Acquisition in Rakai. Uganda, Denver Colorado, USA: 13 th CROI Conference on Retroviruses and Opportunistic Infection.2006. [Google Scholar]
- 9.Zenilman JM, Freisia A, Berger B, McCormack WM. Bacterial vaginosis is not associated with circumcision of the current male partner. Sex Transm Infect. 1999;75:347–348. doi: 10.1136/sti.75.5.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwebke JR, Desmond R. Risk factors for bacterial vaginosis in women at high risk for sexually transmitted diseases. Sex Transm Dis. 2005;32:654–658. doi: 10.1097/01.olq.0000175396.10304.62. [DOI] [PubMed] [Google Scholar]
- 11.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Micro. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thoma M, Gray RH. Male Circumcision and Genital Ulcer Disease: A meta-analysis. 17th International Society for Sexually Transmitted Disease Research, Seattle. 2007 Abstract #542. [Google Scholar]
- 13.Weiss HA, Thomas SL, Munabi SK, Hayes RJ. Male circumcision and risk of syphilis, chancroid, and genital herpes: a systematic review and meta-analysis. Sex Transm Infect. 2006;82(2):101–109. doi: 10.1136/sti.2005.017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Farrell, Morison L, Moodley P, Pilay K, Vanmali T, Quigley M, Hayes R, Sturm AW. Association between HIV and subpreputial penile wetness in uncircumcised men in South Africa. JAIDS. 2006;43:69–77. doi: 10.1097/01.qai.0000225014.61192.98. [DOI] [PubMed] [Google Scholar]
- 15.Serwadda D, Gray RH, Sewankambo NK, Wabwire-Mangen F, Chen MZ, Quinn TC, et al. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simplex virus type 2 infection: a nested case-control study in Rakai, Uganda. J Infect.Dis. 2003;188(10):1492–1497. doi: 10.1086/379333. [DOI] [PubMed] [Google Scholar]
- 16.Taha TE, Hoover DR, Dalabetta GA, Kumwebda NI, Mtimavalye LA, Yang LP, Liomba GN, Broadhead RL, Chiphangwi JD, Miotti PG. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998;12:1699–1706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Martin HL, Richardson BA, M Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya-Achola JO, Bwayo J, Kreiss J. Vaginal lactobacilli, microbial flora and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 18.Sewankambo N, Gray RH, Wawer MJ, Paxton L, McNaim D, Wabwire-Mangen F, Serwadda D, Li C, Kiwanuka N, Hillier SL, Rabe L, Gaydos CA, Quinn TC, Konde-Lule J. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:546–550. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 19.Gray RH, Kiwanuka N, Quinn TC, Sewankambo NK, Serwadda D, Mangen FW, et al. Male circumcision and HIV acquisition and transmission: cohort studies in Rakai, Uganda Rakai Project Team. AIDS. 2000;14(15):2371–2381. doi: 10.1097/00002030-200010200-00019. [DOI] [PubMed] [Google Scholar]
- 20.Hunter DJ, Maggwa BN, Mati JK, Tukei PM, Mbugua S. Sexual behavior, sexually transmitted diseases, male circumcision and risk of HIV infection among women in Nairobi, Kenya. AIDS. 2000;8(1):93–99. doi: 10.1097/00002030-199401000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Fonck K, Kidula N, Kirui P, Ndinya-Achola J, Bwayo J, Claeys P, et al. Pattern of sexually transmitted diseases and risk factors among women attending an STD referral clinic in Nairobi, Kenya. Sex Transm.Dis. 2000;27(7):417–423. doi: 10.1097/00007435-200008000-00007. [DOI] [PubMed] [Google Scholar]