Abstract
Prostate cancer prognosis may be improved by healthy behaviors; however, little is known regarding whether prostate cancer survivors make health behavior changes post-diagnosis, and there is no data on racial/ethnic differences. This study explored patterns of, and factors that influence healthy behavior changes in diet, physical activity, and dietary supplement use among whites and African Americans (n=30), 45–70 years, ≅1 year after diagnosis with localized prostate cancer. Data were collected by telephone using semi-structured qualitative interviews. The mean participant age was 59.6 years, 77% had attended college, 87% were married, and 22% were retired. The majority (58%) had improved their diet since diagnosis, defined as eating more fruits/vegetables and less fat. Although 77% reported regular use of at least one dietary supplement before diagnosis, several discontinued use post-diagnosis. Sixty-seven percent exercised regularly before diagnosis and most of these (75%) continued post-diagnosis; however, time and health constraints were barriers. Physician recommendation and family support strongly influenced positive changes. Except for more post-diagnosis dietary improvements in African Americans, there were few racial differences in patterns/motives for behavior changes. Most respondents were motivated to maintain and/or adopt healthy behavioral changes post-diagnosis. Nurses/physicians are encouraged to inform their prostate cancer patients about the benefits of healthy eating and regular exercise and the absence of scientific evidence regarding the benefits/risks of most supplements, particularly herbal formulations.
Keywords: cancer survivors, diet, dietary supplements, health behaviors, lifestyle factors, physical activity, prostate cancer, qualitative
INTRODUCTION
Prostate cancer is the most common non-dermatological cancer and the second leading cause of cancer-related mortality in United States (U.S.) men.1,2 African Americans have much higher prostate cancer incidence and mortality rates, and considerably lower survival rates, compared to men of other racial and ethnic groups.1–5 Nonetheless, prostate cancer has a very high survival rate (almost 100% within the first five years), particularly when detected and treated at early stages.1–5 Consequently, the population of prostate cancer survivors is growing, and it is increasingly important to pay attention to their health-related needs.1,2,6,7
Prostate cancer prognosis (recurrence and survival) may be improved by healthy behaviors, such as diet, physical activity, and vitamin/mineral supplement use.2,5,8–12 Also, prostate cancer survivors (and cancer survivors in general) are at elevated risk for other lifestyle-related health conditions, such as obesity, diabetes, high blood pressure, stroke, cardiovascular disease, and second cancers. Reasons for this increased risk may be related to genetics, cancer treatment, the fact that lifestyle factors tend to increase the risk for more than one disease, because many of these medical conditions affect older persons, and because cancer is an age-related disease.2,5,6,7,11–15 Consequently, adoption of healthy diet and physical activity habits may be beneficial for men diagnosed with and successfully treated for prostate cancer.6,7,13,14 Therefore, it is important to understand factors that affect healthy lifestyle behavior modifications following a prostate cancer diagnosis in order to design appropriate intervention and education programs.
Available evidence generally suggests that cancer survivors are motivated to make changes in health behaviors. These changes are often self-initiated or may be prompted by participation in behavioral interventions.6,7,13–19 However, there is relatively little published information regarding whether men diagnosed with prostate cancer make changes in health behaviors post-diagnosis, and there are even fewer data on possible racial differences.15,16,18–20 This is because few studies have focused exclusively on prostate cancer survivors, and most have not stratified results by tumor type.15,16,18–20 In one of the few studies for which there are data specific to men with prostate cancer, Demark-Wahrenfried et al. found that behaviors of prostate cancer survivors were less healthy (they consumed fewer than 5 fruits and vegetables per day, consumed more fat, and were less likely to engage in routine exercise) than those of breast cancer survivors.14 This study population was primarily white. Likewise, very little is known regarding ways in which psychosocial factors, such as beliefs, attitudes, self-efficacy, cost, availability, social support, etc. are related to healthy behavior change in this population. Such information is crucial to the design and implementation of culturally appropriate and relevant interventions in prostate cancer survivors. Moreover, diagnosis of a cancer diagnosis can represent a “teachable” moment, in which survivors may be more receptive to counseling regarding health behaviors, such as diet and physical activity.6,7,15,16,18–20
The overall goal of the research study described in this report was to understand patterns of, and factors that impact, healthy behavior change in prostate cancer survivors. The specific aims were to use telephone-administered semi-structured qualitative interviews to explore patterns of health behaviors (dietary intake, physical activity, and dietary supplement use) among white and African American prostate cancer survivors, and to determine reasons for changing or not changing behaviors, including motivations, barriers, attitudes, perceived benefits, and perceived risks associated with engaging in healthy lifestyle behaviors. The focus of this report is not on detailing the specific health behavior changes made by each respondent, nor on whether national recommendations are being met. Rather, we were interested in respondents’ self-perceptions regarding these behaviors. To our knowledge, this is one of the first studies to use a qualitative approach to examine these issues in both white and African American men diagnosed with prostate cancer. Results of this study can be used in the design and implementation of interventions and education programs to improve diet, supplement use, and physical activity habits among men who have been diagnosed with prostate cancer.
METHODS
Study Design, Population, and Recruitment
Data on health behaviors (dietary intake, dietary supplement use, and physical activity), reasons for changing or not changing these behaviors, and factors that influence these behaviors in white and African American prostate cancer survivors were collected by telephone using semi-structured qualitative interviews. Eligible participants were white and African American men, aged 45–70 years, with localized prostate cancer approximately one year post-diagnosis, who had been treated at the University of North Carolina-Chapel Hill (UNC-CH) Hospital. Those who were still in treatment were ineligible, and if anyone had been deemed to be cognitively impaired and/or had language or hearing problems that would hinder the conduct of the telephone interview, he would have been ineligible. Participants were recruited so as to have a comparable distribution of urban and rural respondents.
Prospective participants were identified using the Web Clinical Information System (WebCIS). WebCIS is administered by the Information Services Division, the technical support group for the UNC-CH Hospitals. WebCIS electronically stores medical records, such as patient demographics, medications, allergies, laboratory results and health care history, and contact information. The WebCIS program is available to UNC-CH researchers following approval of a formal application.
The study project manager sent a letter requesting participation to potentially eligible persons, with instructions to contact the study team by telephone if interested. Those who expressed interest were contacted by a trained interviewer by telephone to confirm eligibility, explain the study, and answer any questions. Prospective participants who did not respond within two weeks of receiving the approach letter were contacted by telephone. The study received Institutional Review Board approval.
Conceptual Framework
The PRECEDE (Predisposing, Reinforcing, and Enabling Constructs in Educational Diagnosis and Evaluation) model was used as the conceptual framework for the study; and specifically, as a guide to identifying psychosocial and other factors that may influence (facilitate or hinder) healthy behavior changes following a prostate cancer diagnosis. PRECEDE is based on the premise that the factors important to an outcome must be diagnosed before the intervention is designed.21–24 According to PRECEDE, factors affecting behavior can be broadly grouped as predisposing, reinforcing, and enabling. Predisposing factors such as attitudes, beliefs, and values provide the rationale or motivation for a behavior. Reinforcing factors include variables such as social support, which provide intrinsic rewards for a behavior. Enabling factors are skills and resources that facilitate change.21–25 Unlike many models, PRECEDE assumes that factors affecting behavior are culturally determined and can vary across populations.21–25 Therefore, this is an excellent model to use in cross-cultural research, as in the present project.
Data Collection
Upon receipt of verbal consent, telephone-administered (one-on-one) semi-structured qualitative interviews were conducted with eligible and consenting participants. The interviewers used a structured set of questions to guide the interview. A semi-structured interview approach was used to ensure consistency in questions asked across participants while allowing for some flexibility depending on the participants’ level of interest in a particular topic.26,27 The interview guide was created in a series of steps to ensure that it was culturally and linguistically appropriate. Initially, the principal investigator developed a series of topics to be addressed during each interview. These topics and items were then reviewed and revised by the mixed gender and racial (white/African American) research team, which included an nutritional and cancer epidemiologist, a health behaviorist, a social scientist, and three experienced interviewers. A schematic description of the interview guide is included in Figure 1. The interviewers employed cues and probes to elicit more detailed information from study participants. All interviews were tape-recorded (with the participant’s permission) and ranged between 45 and 60 minutes, depending on respondent loquacity. Participants were sent a $20 gift card by mail for their time and effort upon completion of the telephone interview.
Figure 1.
Standard interview guide for the telephone-administered semi-structured qualitative interviews
Measures
Demographic characteristics
We collected information on age, race/ethnicity, educational attainment, employment, and marital status.
Health behaviors
Detailed information was collected on whether participants had made changes in health behaviors (diet, supplement use, and physical activity) since or as a result of their prostate cancer diagnosis, as well as on a wide array of factors that may have influenced any such changes.28–31 Specifically, we were interested in: 1) fruits, vegetables, and dietary fat consumption; 2) dietary supplements, such as use of multivitamins, single supplements, and herbal products, e.g.., prostate health formulas; and 3) frequency and types of exercise. Examples of the factors that may have influenced the changes include self-efficacy, beliefs and attitudes, perceived benefits, perceived risks, and social support. In particular, respondents were asked whether they thought adopting a healthy behavior post-diagnosis would affect their cancer prognosis, and to delineate what helped them to make or maintain make healthy changes and what barriers they had to overcome to continue doing so. The definition of “change” was left to the discretion of the respondent. The interviewers used cues and probes to elicit more detailed information (Figure 1).
Diet
Participants were queried on whether their dietary habits had changed or remained the same since diagnosis. Those who had made a change were asked what factors motivated the change, how easy it had been to make the change, and facilitators and barriers to making the change. Those who had not made any changes were queried on why they did not, whether they had considered making a change, and factors that would have enabled them to or prevented them from making the change.
Dietary supplements
Participants were asked whether they were taking any dietary supplements (vitamins, minerals, or herbal supplements) before their prostate cancer diagnosis, and if yes, what benefit they expected from taking the supplement. For those who reported currently using supplements, information was collected on the type of supplement being used, how they decided which supplement to take, and the perceived benefits associated with use of the supplement(s). Questions would have been similar for those who started taking supplements after diagnosis, but no respondent had done so. Finally, respondents who did not or were not currently taking supplements were asked whether they ever considered doing so, and if not, why they made that choice.
Physical activity
Participants were asked to indicate whether they currently exercised regularly (at least twice a week) and if they had done so before diagnosis. If yes, they were probed regarding what kinds of exercise they engaged in, how often, and for how long; and what factors encouraged them to start exercising. Men who were still exercising post-diagnosis were asked whether the level of activity had changed since diagnosis and what factors may have contributed to the change. Those who formerly exercised but no longer did were asked why they had stopped. Participants who neither exercised currently nor did so pre-diagnosis were probed for reasons why they did not exercise regularly.
Other behavioral changes
Participants were also queried regarding whether they had adopted any other behaviors or activities designed to improve their health since their diagnosis. These queries were open-ended and no specific behaviors were mentioned.
Statistical Analyses
Quantitative Data
Descriptive statistics were computed to describe participant demographic characteristics and quantifiable changes in the health behaviors, such as eating healthfully before diagnosis (yes/no), whether health behavior change(s) was/were associated with their diagnosis (yes/no), whether the change has been maintained (yes/no), etc. Frequencies and percentages were calculated for categorical variables and means and standard deviations for continuous variables. Most analyses were performed for the combined sample and stratified by race as appropriate.
Qualitative Data
All interviews were audio-taped, and later transcribed verbatim by a professional transcriptionist. A percentage of transcripts were reviewed with the original tapes to assure accuracy. For all interviews, textual responses were coded for relevant themes by the same staff member. This same staff member also documented the quantitative information, which was cross-checked and confirmed by the principal investigator. The limited number of interviews and the “yes/no” format of the majority of interview questions made this approach more efficient than the use of a qualitative analysis tool, such as ATLAS ti software.
RESULTS
Participant characteristics
We completed interviews with 31 men who had been diagnosed with prostate cancer; 20 whites (64.5%) and 11 African Americans (35.5%). The sampling frame included 69 men. Two men were ineligible; we were unable to schedule interviews with 30 men for various reasons (wrong number, left numerous messages without reply, etc.), and there were six refusals. We originally intended to enroll 20 whites and 20 African Americans; however, because of difficulties with recruiting eligible African Americans and the fact we reached theoretical saturation, we completed interviews with 20 whites and 11 African Americans. Due to the loss of one tape from an African American respondent, the following data are based on interviews from 30 respondents.
Demographic characteristics of study participants are given in Table 1. Participants were between 45 and 70 years of age, with a mean age of 59.6 (5.76 SD) years. White respondents were somewhat older (mean age for whites = 60.3 years; mean age for Blacks = 58.2 years). Only two respondents were in their 40s, and both were African American. Seven were 65 years and older, of whom five were white and two were African American. Most respondents (77%) had attended some college, attained a 4-year degree, or attained an advanced degree, and more than half (57%) had full-time employment. Most of the respondents (87%) were married. There were no appreciable differences in employment or marital status by race, but a higher percentage of African Americans had not attended college and a higher percentage of whites had advanced degrees. All participants were within approximately one year of diagnosis and all had completed prostate cancer treatment when the interviews were conducted.
Table 1.
Selected demographic characteristics of study participants (n=30)
| Participant Characteristic | Whites (n=20) |
African Americans (n=10) |
All participants (n=30) |
|---|---|---|---|
| N (%) | |||
| Age group (years) | |||
| 40–50 | 0 (0%) | 2 (20%) | 2 (6.7%) |
| 51–60 | 11 (55%) | 5 (50%) | 16 (53.3%) |
| 61–70 | 9 (45%) | 3 (30%) | 12 (40.0%) |
| mean (SD) | 60.3 (5.07) | 58.2 (7.04) | 59.6 (5.76) |
| Education | |||
| ≥High school | 3 (15%) | 4 (40%) | 7 (23.3%) |
| Some college | 5 (25%) | 3 (30%) | 8 (26.7%) |
| Completed college (4-year degree) | 5 (25%) | 2 (20%) | 7 (23.3%) |
| Advanced degree (MS, PhD, MD, JD) | 7 (35%) | 1 (10%) | 8 (26.7%) |
| Employment | |||
| Full-time | 11 (55%) | 6 (60%) | 17 (56.7%) |
| Part-time | 1 (5%) | 0 (0%) | 1 (3.3%) |
| Self-employed | 1 (5%) | 0 (0%) | 1 (3.3%) |
| Retired | 6 (35%) | 4 (40%) | 10 (22.3%) |
| Unemployed | 1 (5%) | 0 (0%) | 1 (3.3%) |
| Marital status | |||
| Married | 18 (90%) | 8 (80%) | 26 (86.7%) |
| Single/Never married | 2 910%) | 2 (20%) | 4 (13.3%) |
Diet
Current dietary behaviors and changes in dietary habits among study participants are shown in Table 2. The majority of respondents (n=18, 60%) stated that they had been eating healthfully (more fruits/vegetables and less fat) prior to diagnosis, and they all continued to eat healthfully after diagnosis and treatment. Of the other 12, seven (6 African Americans and 1 white) had improved their diets post-diagnosis, and they all indicated that their dietary improvements were connected to the diagnosis. Six of the seven had maintained their change successfully; one was having difficulty but indicated that he was aware of the need to “persevere.” Thus, 25 out of 30 survivors (83%) reported eating healthfully post-diagnosis.
Table 2.
Dietary habits before and after diagnosis among prostate cancer survivors (n=30)
| Whites (n=20) |
African Americans (n=10) |
All participants (n=30) |
|
|---|---|---|---|
| N (%) | |||
| Were eating healthfully before diagnosis | 15 (75%) | 3 (30%) | 18 (60%) |
| Were eating healthfully before diagnosis and have maintained the healthy dietary habits | 15 (75%) | 3 (30%) | 18 (60%) |
| Were not eating healthfully before diagnosis | 5 (25%) | 7 (70%) | 12 (40%) |
| Were not eating healthfully before diagnosis, but have improved since diagnosis | 1 (20%) | 6 (86%) | 7 (58%) |
| Change connected to diagnosis | 1 (100%) | 6 (100%) | 7 (100%) |
| Change maintained since diagnosis | 1 (100%) | 5 (83%) | 6 (100%) |
| Were not eating healthfully before diagnosis and have not improved post-diagnosis | 3 | 1 | 4 (33) |
| Did not provide any information on diet | 1 | 0 | 1 |
| Total number of men who were eating healthfully at the time of the interview | 16 (80%) | 9 (90%) | 25 (83%) |
The most common dietary changes reported by respondents included eating more vegetables and greens (n=5), eating more fruit (n=3), and not eating or eating less fried food (n=3). It is interesting to note that all these (healthy) changes were only reported by African American respondents. Using less salt, fat, or white bread, not eating red meat, or eating more fish, pasta, or fiber were each reported by one participant (data not shown).
Of the five respondents who had not improved their diets, one gave no information regarding his diet – he simply stated that he was eating as he always had and that no one had told him otherwise. One had begun using vegetable oil instead of pig fat in cooking prior to diagnosis, but had not made any other changes and did not consider himself to be eating healthfully. The other three men had not eaten healthfully before diagnosis and had not changed their dietary habits since diagnosis.
Use of dietary supplements (vitamin, mineral, and herbal formulas)
Seventy-seven percent of respondents (n=23) were taking at least one dietary (vitamin, mineral, or herbal) supplement prior to diagnosis, but only slightly more than half (n=16) were taking them when they were interviewed (Table 3). Of the 16 respondents who were still taking supplements, 14 were taking one to several, but one person was taking 27 different supplements. Vitamin and mineral supplements were more commonly used than herbal products; only five individuals were taking herbal supplements prior to diagnosis, and four had discontinued use after they were diagnosed with the disease. Somewhat surprisingly, no respondent indicated that he started taking supplements after diagnosis.
Table 3.
Use of dietary supplements before and after diagnosis among prostate cancer survivors(n=30)
| Whites (n=20) |
African Americans (n=10) |
All participants (n=30) |
|
|---|---|---|---|
| N (%) | |||
| Were taking vitamin and mineral supplements before diagnosis | 17 (85%) | 6 (60%) | 23 (77%) |
| Continued to take supplements after diagnosis | 11 (65%) | 4 (67%) | 15 (65%) |
| Were taking herbal supplements before diagnosis | 3 (15%) | 2 (20%) | 5 (17%) |
| Continued to take herbal supplements after diagnosis | 1 (33%) | 0 (0%) | 1 (20%) |
| Started taking a dietary supplement after diagnosis | 0 (0%) | 0 (0%) | 0 (0%) |
| Total number of men taking a dietary supplement at the time of the interview | 11 (55%) | 4 (40%) | 16 (53%) |
The most commonly used dietary supplements were multivitamins (n=9), fish oil (n=7), prostate health formulas, vitamin E and calcium (n=5 for each), and vitamin C and 81 mg. aspirin (n=4 for each). For all supplements except prostate health formulas, use was higher among whites than African Americans (data not shown). Among the herbal supplements, the most frequently used, although used by just 3 or fewer participants, were glucosamine/chondroitin, saw palmetto, grape seed extract, ginseng, MSM, and two herbal prostate formulas: “herbs for prostate” and “Prostat-9.”
Physical activity
Physical activity habits before and since diagnosis are shown in Table 4. Two-thirds of respondents were engaging in physical activity two or more days per week prior to diagnosis (n=20, 67%). Of these 20 men, 15 (75%) had continued to exercise regularly after diagnosis, while five had stopped. The five prior exercisers did not express an intention to resume regular exercise and did not indicate why they stopped. Of the 10 men who were not exercising regularly prior to diagnosis, three had started a regular exercise program since their diagnosis, while seven had not. Five indicated that they had exercised regularly at one time in their lives but not in the immediate period prior to diagnosis. In total, 18 of 30 respondents (60%) were exercising regularly at the time of the interview.
Table 4.
Physical activity habits before and after diagnosis among prostate cancer survivors (n=30)
| Whites (n=20) |
African Americans (n=10) |
All participants (n=30) |
|
|---|---|---|---|
| N (%) | |||
| Engaged in regular physical activity (2 or more times per week) before diagnosis | 15 (75%) | 5 (50%) | 20 (67%) |
| Engaged in regular physical activity before diagnosis and continued to exercise after diagnosis | 12 (80%) | 3 (60%) | 15 (75%) |
| Did not engage in regular physical activity before diagnosis | 5 (25%) | 5 (50%) | 10 (33%) |
| Did not engage in regular physical activity before diagnosis but started to exercise after diagnosis | 1 (20%) | 2 (40%) | 3 (30%) |
| Total number of men who were engaging in regular physical activity at the time of the interview | 13 (65%) | 5 (50%) | 18 (60%) |
The most frequently mentioned specific types of physical activity engaged in by respondents included walking (n=13), weight training (n=8), jogging/running, treadmill, tennis (n=4 for each), and biking (n=3). Other activities reported by one or two respondents included use of elliptical and rowing machines, golf, and stretching. There were few appreciable differences by race with regard to types of physical activity (e.g., tennis and golf were mentioned only by whites) (data not shown).
Other health behaviors
A few of the respondents volunteered information regarding other health-related behaviors they engaged in either before or after diagnosis. Two respondents noted that they did not smoke, and four mentioned drinking rarely or moderately, drinking red wine, or only drinking “lite” beer. Additional pre-diagnosis behaviors that were mentioned include getting proper sleep, going to check-ups and for prostate and other health screenings, and reading on health issues. Only one respondent mentioned adopting a new health behavior since treatment: getting outside more to get sunshine and vitamin D.
Factors influencing behavior changes
Motivations for making healthy behavior changes
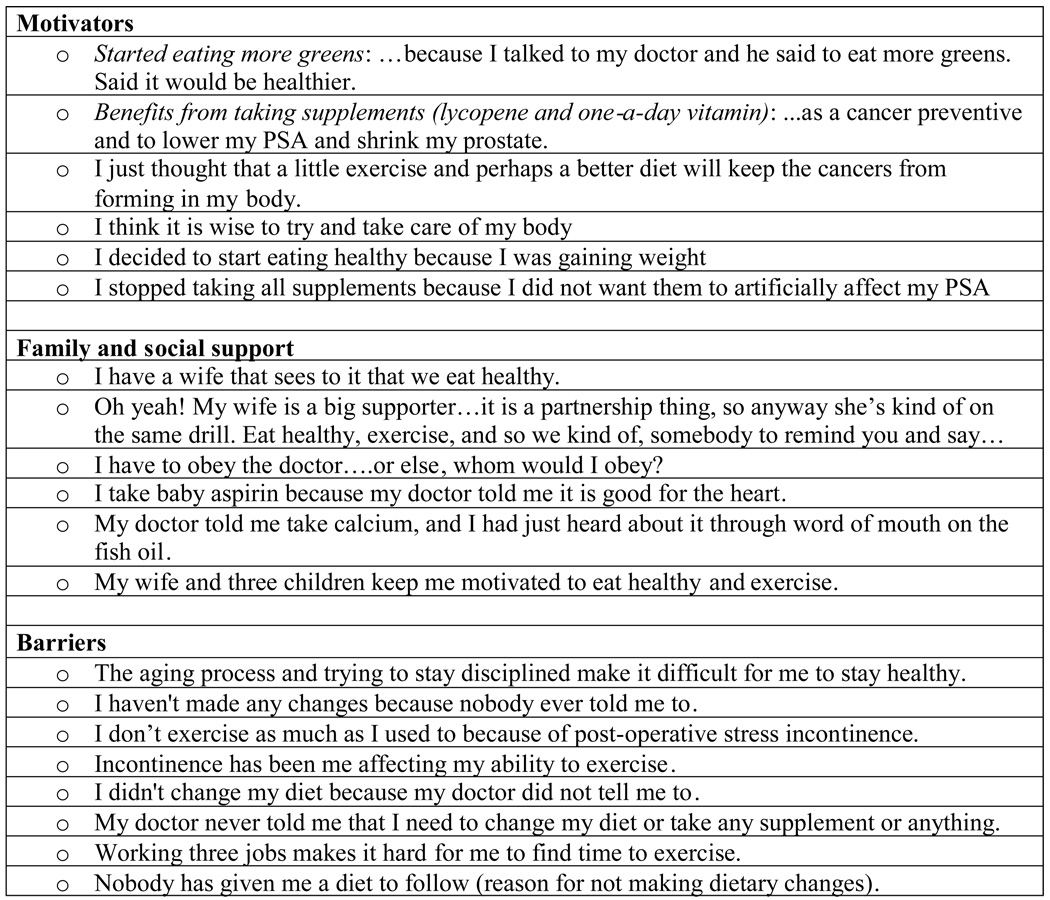
We specifically queried on factors that motivated respondents to make healthy behavior changes. All 30 participants responded to the question on motivation. Twenty-four (80%) said they were motivated by wanting to maintain health/avoid disease, ensure quality of life or length of life, recover their fitness/vitality, and/or control their weight. Nine (30%) discussed staying around for, spending time with, or taking care of family (wife, children, and/or grandchildren). One mentioned paying the bills and another indicated that it was important that he “stay around” for his family and friends. A number of men said they improved their diet or started to use dietary supplements based on their physicians’ recommendations. One respondent stated that “I have to obey him, or I don’t know who to obey.” The respondent who took 27 supplements made lengthy comments about observing what happened to others who didn’t take as much care of themselves as he did. Other quotes from respondents regarding motivators for healthy behavior changes are given in Figure 2.
Figure 2.
Selected quotes from study participants regarding factors that influenced health behavior changes
Other factors influencing healthy behavioral changes
Of the 30 respondents, only eight stated that they had received support from spouses, family members, and friends, in making healthful changes. Six of the eight mentioned that their wives were supportive by cooking more healthy meals for the family and/or participating in a similar health regimen themselves. Two respondents mentioned the support of other family members, and one noted friends who also had prostate cancer, although the latter was in the context of dealing with the diagnosis rather than making behavioral changes.
Barriers to making healthy behavior changes
One of the respondents who stopped regular exercise after diagnosis indicated that he was inhibited from doing so by incontinence and later by the heat, but noted that he intended to resume regular exercise. Interestingly, socioeconomic factors did not appear to influence or affect respondents’ ability or willingness to make health behavior changes. Selected quotes from study participants regarding other factors that influenced their behavior are given in Figure 2.
Surprisingly, there were few mentions of barriers to making healthy behavior changes. A few respondents noted that health issues, particularly incontinence, as well as lack of time made exercise more difficult. No respondent mentioned financial issues (e.g., inability to afford healthy foods or vitamin/mineral supplements) as a barrier to behavior change. Some quotes respondents regarding barriers to making healthy behavior changes are given in Figure 2.
Summary of positive and negative health behavior changes
Positive health behavior changes were defined as making dietary improvements since diagnosis, specifically increased consumption of fruits and vegetables, reduced fat intake (e.g., reduced consumption of fried foods) and beginning some type of physical activity since diagnosis when one hadn’t been exercising before. Initiating use of a vitamin or mineral (but not an herbal) supplement post-diagnosis was considered a positive change, although no respondent indicated he had done this. The number of respondents, by race, who had made one or more positive changes is shown in Table 5. Two white and five African American respondents made one positive change and two African Americans made two positive changes, largely due to the fact that African Americans made more dietary improvements. No respondent made three or more positive changes.
Table 5.
Proportion of respondents who made positive and negative health behavior changes after diagnosis with prostate cancer (n=30)
| Whites (n=20) |
African Americans (n=10) |
All participants (n=30) |
|
|---|---|---|---|
| N (%) | |||
| One positive change | 2 (10%) | 5 (50%) | 7 (23%) |
| Two positive changes | 0 (0%) | 2 (20%) | 2 (7%) |
| Three or more positive changes | 0 (0%) | 0 (0%) | 0 (0%) |
| One negative change | 4 (20%) | 4 (40%) | 8 (27%) |
| Two negative changes | 3 (15%) | 0 (0%) | 3 (10%) |
| Three or more negative changes | 0 (0%) | 1 (10%) | 1 (3%) |
Negative behavior changes were defined as having changed one’s previously healthy diet to a less healthy one after diagnosis (no respondent indicated he had done this), having discontinued supplement or vitamin or mineral supplement use (considered separately), and having stopped exercising since diagnosis when one had been doing so before. As shown in Table 5, equal numbers of whites and African Americans made one negative change, three whites (and no African Americans) made two negative changes, and one respondent (an African American) made three or more negative changes. The majority of negative changes were discontinuation of vitamin and/or mineral supplements, and the rest were discontinuation of exercise.
DISCUSSION
This study describes changes in diet, dietary supplement use, and physical activity among 30 whites and African Americans diagnosed with prostate cancer, and sought to identify factors that impacted their decision-making processes. We found that most participants (83%) reported having a healthy diet post-diagnosis, and that more African Americans than whites made healthful dietary changes post-diagnosis. Most respondents engaged in regular physical activity before diagnosis (67%), and the large majority of these men (75%) continued to exercise after diagnosis. However, few participants started exercising regularly after diagnosis. With regard to supplement use, half of the study sample reported regular use of at least one dietary supplement, and none had adopted this behavior post diagnosis; in fact, several others had stopped using dietary supplements after they were diagnosed with prostate cancer.
In general, our results are in agreement with the few other studies that have examined patterns of health behavior changes in cancer survivors. In a cross-sectional survey of breast, colorectal, and prostate cancer survivors in Washington state, 66.3% of patients reported making lifestyle changes: 40.4% made one or more dietary changes, 20.8% added new physical activity, and 48.0% started taking new dietary supplements.19 In a qualitative study (semi-structured interviews) of 143 cancer survivors in Hawaii by Maskarinec et al., about half of the respondents reported that they had changed their diet after learning about their disease and were still maintaining those modifications almost two years after their initial diagnosis.32 Also, the survivors who had changed their diets reported using some form of complementary and alternative medicine, such as herbal supplements, meditation, etc.
Of particular interest are the factors that influenced health behaviors among our study participants. Many respondents noted similar motivations for making healthful changes with regard to diet and physical activity. The majority indicated that they changed their diet or physical activity habits to maintain health, ensure quality or length of life, recover their fitness and vitality, reduce their prostate specific antigen (PSA) levels/shrink the prostate, and control their weight. About a third noted that they wanted to be able to spend time with and take care of their family members. Several respondents indicated that they did so based on advice from their physician, and, interestingly, another noted that he needed to stay alive to pay the family bills. These themes are comparable to those reported in the survey of cancer survivors by Maskarinec and colleagues,32 which found that respondents changed their diet based on the hope that doing so would increase well-being, maintain health, and prevent cancer recurrence.
It is reassuring that a number of respondents mentioned physician recommendation as a important factor influencing their decisions to make behavior changes, given that many studies have found that patients tend to take the advice given by their doctors.32–37 On the other hand, many men who did not make changes indicated that no one had suggested that they do so. Given that there is ample research evidence that recommendations from physicians, nurses, and other health professionals have a strong influence on preventive health practices, it would be beneficial for medical professionals, including nurse-oncologists, to communicate more directly with their prostate cancer patients regarding this important facet of their care. It was somewhat surprising that health-related and other “beliefs” were not a more prominent decision-making factor. In other studies, such as that by Patterson et al., a belief in a relationship between diet and disease was associated with a higher likelihood of making positive and healthy behavioral changes post-diagnosis.19 Similarly, respondents in the study by Maskarinec et al.32 noted that certain foods that cause or prevent cancer should be avoided or increased after diagnosis, respectively. Finally, it is worth noting that none of our respondents mentioned an impact of religiosity or genetic factors on their decision-making. Overall, participants seemed to believe that changes in health behaviors can impact prostate cancer outcomes, which may be one reason why the majority was engaging in healthy behaviors post-diagnosis. This suggests that they view health behaviors as one way to maintain control over and take responsibility for their health and well-being.
Our findings with regard to dietary supplement use were somewhat surprising. Although more than half of the participants reported using supplements pre-diagnosis, none adopted this behavior post-diagnosis; in fact, several stopped taking supplements after they were diagnosed with the disease. This finding differs somewhat from other investigations, which have generally reported that persons diagnosed with cancer tend to start using dietary supplements, in spite of the absence of scientific data regarding the benefits and risks of most supplements, particularly herbal formulations.14–19,32 For example, Maskarinec et al. reported that cancer survivors who changed their diets post-diagnosis reported very high supplement use, including a large number of products whose efficacy has not been proven or that may possibly be harmful.32. In the Washington state survey that included 114 prostate cancer survivors, 48% of respondents started taking new dietary supplements post-diagnosis.19 Nonetheless, we note that in that study, prostate cancer patients were only half as likely as colorectal cancer patients to take new dietary supplements (p<.05). Possibly, as noted by a few respondents, the men in our study may have been concerned that use of those products may interfere with prostate cancer treatment regimens and/or prognosis.
Participants in the present study mentioned few barriers to making behavior changes, and for the most part, the barriers noted pertained to physical activity. Specifically, a number of men indicated that they were unable to exercise regularly due to post-treatment incontinence, fatigue, and lack of time. Nonetheless, it was encouraging that most participants who were regular exercisers pre-diagnosis continued to engage in some kind of physical activity after treatment, and many were aware of the benefits of regular activity, even those who did not do so regularly. Interestingly, no respondent mentioned the costs of healthy foods as a barrier to healthy eating, and many men had active support from their spouses to eat more healthfully.
Because of its breadth and ease of cultural adaptation, the PRECEDE model was a practical conceptual framework to use to organize the themes identified in this present study. It was possible to capture a range of predisposing factors (e.g., motivators, beliefs, perceived benefits and risks), reinforcing factors (e.g., family and social support), and enabling factors (e.g., physician recommendation, economic concerns) that were salient to both white and African American prostate cancer survivors. Furthermore, because the factors/concept within PRECEDE are relatively wide-ranging, it can accommodate unanticipated factors. For example, we had not expected that “staying alive to take care of one’s family” would arise as a relevant theme; nonetheless, it fit within the predisposing factors as an important motivator. Results from this study suggest that the PRECEDE model is a sensible and useful option for similar investigations of health-related behaviors in cancer survivors, particularly those that include different demographic sub-populations.
There were few differences between whites and African Americans with regard to changes in health behaviors. Except for diet, for which we observed that more African Americans than whites (86% vs. 20%) made healthful changes post-diagnosis, there were no other appreciable differences in patterns of healthy behavior change by race. Similarly, there were no noticeable differences in the motivations or barriers influencing change between whites and African Americans. We are not aware of other studies to which we can compare our results. While these findings suggest that there may be relatively few differences between whites and African Americans with regards to health behaviors and factors influencing these practices, the small sample size and somewhat narrow scope of this study limits our ability to draw wider inferences.
This study has several limitations. First, although our response rate was good (71%), we cannot ensure that our respondents have the same behaviors as those who chose not to participate. It is possible that patients with less favorable behavior change profiles might be less likely to participate in a study focused on health behaviors; therefore, our estimates of health behavior change may be somewhat inflated. Because prostate cancer survival rates are quite high, motivations for making lifestyle changes among prostate cancer patients may be different than for patients having cancers with a poor prognosis, such as lung or pancreatic cancers; therefore, our findings may not be generalizable to other tumor types. In addition, the demographics of this study population reflect higher educational levels; additional studies are needed to assess lifestyle patterns and motives for behavior changes in populations of men with lower socioeconomic status. Finally, lifestyle changes were entirely based on self-report and were collected based on patient memories; it is possible that the experiences of cancer diagnosis, treatment, and coping may have affected patient recall.
CONCLUSIONS
In summary, this is one of the first studies to examine whether both white and African Americans diagnosed with prostate cancer make changes in diet, dietary supplement use, and physical activity. Data were collected using semi-structured qualitative interviews, which provided more detailed information than might have been generated from a more quantitative survey. The findings suggest that the majority of patients were motivated to adopt and/or maintain healthy behavioral changes post-diagnosis, and that physician/health professional recommendations and family support are key factors influencing positive behavioral changes. Barriers to engaging in healthy behaviors were primarily related to physical activity, and were largely health- and time-related constraints.
Based on these results, nurse-oncologists, physicians, and other health professionals should encourage their patients who are diagnosed and treated for prostate cancer to initiate or maintain healthful diet and physical activity-related behaviors, and should ensure that their patients are aware of the fact that there is limited scientific evidence regarding the benefits and possible risks of most dietary supplements, particularly herbal formulations. Other efforts to improve post-diagnosis health behaviors in prostate cancer survivors should include encouragement of family support and efforts to reduce or ameliorate barriers to exercise. Finally, studies are urgently needed to test whether changing health behaviors after diagnosis and treatment improves prognosis.
ACKNOWLEDGMENTS
We are grateful to the participants in our study who kindly provided us their time and valuable information. Grateful acknowledgement is also made to Ms. Carol Carr and Amanda Velasquez, Mr. Herbert Thibodeaux Jr., and Dr. Linda Ko for their valuable contributions to the implementation of this study.
FUNDING DISCLOSURE
This study was funded by grants K22CA096556, 5P60 MD000244-05, DK056350 from the National Institutes of Health, Department of Health and Human Services. The study sponsors had no role in the conduct of the study, in the collection, management, analysis, or interpretation of data, or in the preparation, review, or approval of the manuscript.
REFERENCES
- 1.Surveillance, Epidemiology, and End Results (SEER) [Accessed June 30, 2008]; Available from: URL www.seer.cancer.gov.
- 2.American Cancer Society. [Accessed June 15, 2008];Cancer facts and figures. Available from: URL www.cancer.org.
- 3.Ghafoor A, Jemal A, Cokkinides V, et al. Cancer statistics for African Americans. CA Cancer J Clin. 2002;52:326–341. doi: 10.3322/canjclin.52.6.326. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975-2001, with a special feature regarding survival. Cancer. 2004 Jul 1;101(1):3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 5.Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Front Biosci. 2006 May 1;11:1388–1413. doi: 10.2741/1891. [DOI] [PubMed] [Google Scholar]
- 6.Aziz N. Cancer survivorship research: challenge and opportunity. J Nutr. 2002;132 suppl 2:3494S–3503S. doi: 10.1093/jn/132.11.3494S. [DOI] [PubMed] [Google Scholar]
- 7.Rowland J, Mariotto A, Aziz N. Cancer survivorship--United States, 1971–2001. MMWR Morb Mortal Wkly Rep. 2004 Jun 25;53(24):526–529. [PubMed] [Google Scholar]
- 8.Boyle P, Severi G, Giles GG. The epidemiology of prostate cancer. Urol Clin North Am. 2003 May;30(2):209–217. doi: 10.1016/s0094-0143(02)00181-7. [DOI] [PubMed] [Google Scholar]
- 9.Wolk A. Diet, lifestyle and risk of prostate cancer. Acta Oncol. 2005;44(3):277–281. doi: 10.1080/02841860510029572. [DOI] [PubMed] [Google Scholar]
- 10.Chan JM, Gann PH, Giovannucci EL. Role of diet in prostate cancer development and progression. J Clin Oncol. 2005 Nov 10;23(32):8152–8160. doi: 10.1200/JCO.2005.03.1492. [DOI] [PubMed] [Google Scholar]
- 11.Nelson WG. Prostate cancer prevention. Curr Opin Urol. 2007 May;17(3):157–167. doi: 10.1097/MOU.0b013e3280eb110f. [DOI] [PubMed] [Google Scholar]
- 12.Friedenreich CM, Thune I. A review of physical activity and prostate cancer risk. Cancer Causes Control. 2001 Jun;12(5):461–475. doi: 10.1023/a:1011210121901. [DOI] [PubMed] [Google Scholar]
- 13.Demark-Wahnefried W, Moyad MA. Dietary intervention in the management of prostate cancer. Curr Opin Urol. 2007 May;17(3):168–174. doi: 10.1097/MOU.0b013e3280eb10fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demark-Wahnefried W, Peterson B, et al. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000 Feb 1;88(3):674–684. [PubMed] [Google Scholar]
- 15.Pinto BM, Trunzo JJ. Health behaviors during and after a cancer diagnosis. Cancer. 2005 Dec 1;104(11 Suppl):2614–2623. doi: 10.1002/cncr.21248. [DOI] [PubMed] [Google Scholar]
- 16.Demark-Wahnefried W, Aziz NM, Rowland JH, et al. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005 Aug 20;23(24):5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinto BM, Maruyama NC, Clark MM, et al. Motivation to modify lifestyle risk behaviors in women treated for breast cancer. Mayo Clin Proc. 2002 Feb;77(2):122–129. doi: 10.4065/77.2.122. [DOI] [PubMed] [Google Scholar]
- 18.Blanchard CM, Denniston MM, Baker F, et al. Do adults change their lifestyle behaviors after a cancer diagnosis? Am J Health Behav. 2003 May–Jun;27(3):246–256. doi: 10.5993/ajhb.27.3.6. [DOI] [PubMed] [Google Scholar]
- 19.Patterson RE, Neuhouser ML, Hedderson MM, et al. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J Am Diet Assoc. 2003 Mar;103(3):323–328. doi: 10.1053/jada.2003.50045. [DOI] [PubMed] [Google Scholar]
- 20.Demark-Wahnefried W, Jones LW. Promoting a healthy lifestyle among cancer survivors. Hematol Oncol Clin North Am. 2008 Apr;22(2):319–342. doi: 10.1016/j.hoc.2008.01.012. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gielen AC, McDonald EM. The PRECEDE-PROCEED Planning Model. In: Glanz K, Lewis FM, Rimer BK, editors. Health behavior and health education: theory, research, and practice. 2nd ed. San Francisco, CA: Jossey-Bass Inc; 1997. pp. 359–383. [Google Scholar]
- 22.Kegler MC, Miner K. Environmental health promotion interventions: considerations for preparation and practice. Health Educ Behav. 2004 Aug;31(4):510–525. doi: 10.1177/1090198104265602. [DOI] [PubMed] [Google Scholar]
- 23.Chang MW, Brown RL, Nitzke S, et al. Development of an instrument to assess predisposing, enabling, and reinforcing constructs associated with fat intake behaviors of low-income mothers. J Nutr Educ Behav. 2004 Jan–Feb;36(1):27–34. doi: 10.1016/s1499-4046(06)60125-5. [DOI] [PubMed] [Google Scholar]
- 24.Miilunpalo S. Evidence and theory based promotion of health-enhancing physical activity. Public Health Nutr. 2001 Apr;4(2B):725–728. doi: 10.1079/phn2001163. [DOI] [PubMed] [Google Scholar]
- 25.Satia-Abouta J, Patterson RE, Kristal AR, et al. Psychosocial predictors of diet and acculturation in Chinese American and Chinese Canadian women. Ethn Health. 2002 Feb;7(1):21–39. doi: 10.1080/13557850220146975. [DOI] [PubMed] [Google Scholar]
- 26.Session Y, Denzin NK, editors. Handbook of qualitative research. Second Edition. Sage Publications; 2000. [Google Scholar]
- 27.Williamson GR. Illustrating triangulation in mixed-methods nursing research. Nurse Res. 2005;12(4):7–18. doi: 10.7748/nr2005.04.12.4.7.c5955. Review. [DOI] [PubMed] [Google Scholar]
- 28.Van Duyn MA, Kristal AR, Dodd K, et al. Association of awareness, intrapersonal and interpersonal factors, and stage of dietary change with fruit and vegetable consumption: a national survey. Am J Health Promot. 2001;16(2):69–78. doi: 10.4278/0890-1171-16.2.69. [DOI] [PubMed] [Google Scholar]
- 29.Pomerleau J, Lock K, Knai C, et al. Interventions designed to increase adult fruit and vegetable intake can be effective: a systematic review of the literature. J Nutr. 2005;135(10):2486–2495. doi: 10.1093/jn/135.10.2486. [DOI] [PubMed] [Google Scholar]
- 30.Watters JL, Satia JA, Galanko JA. Associations of psychosocial factors with fruit and vegetable intake among African Americans. Public Health Nutr. 2007 Jul;10(7):701–711. doi: 10.1017/S1368980007662284. [DOI] [PubMed] [Google Scholar]
- 31.Satia JA, Galanko J. Intrinsic and extrinsic motivations for dietary change in African Americans. Am J Health Behav. 2007;31(6):643–656. doi: 10.5555/ajhb.2007.31.6.643. [DOI] [PubMed] [Google Scholar]
- 32.Maskarinec G, Murphy S, Shumay DM, et al. Dietary changes among cancer survivors. Eur J Cancer Care (Engl) 2001 Mar;10(1):12–20. doi: 10.1046/j.1365-2354.2001.00245.x. [DOI] [PubMed] [Google Scholar]
- 33.Beydoun HA, Beydoun MA. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer Causes Control. 2008 May;19(4):339–359. doi: 10.1007/s10552-007-9100-y. [DOI] [PubMed] [Google Scholar]
- 34.Kelly KM, Dickinson SL, Degraffinreid CR, et al. Colorectal cancer screening in 3 racial groups. Am J Health Behav. 2007 Sep–Oct;31(5):502–513. doi: 10.5555/ajhb.2007.31.5.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satia JA, Galanko J. Demographic, behavioral, psychosocial, and dietary correlates of cancer screening in African Americans. J Health Care Poor Underserved. 2007;18(4A):146–164. doi: 10.1353/hpu.2007.0114. [DOI] [PubMed] [Google Scholar]
- 36.Tasaki K, Maskarinec G, Shumay DM, et al. Communication between physicians and cancer patients about complementary and alternative medicine: exploring patients' perspectives. Psychooncology. 2002 May–Jun;11(3):212–220. doi: 10.1002/pon.552. [DOI] [PubMed] [Google Scholar]
- 37.Schouten BC, Meeuwesen L. Cultural differences in medical communication: a review of the literature. Patient Educ Couns. 2006 Dec;64(1–3):21–34. doi: 10.1016/j.pec.2005.11.014. [DOI] [PubMed] [Google Scholar]