Abstract
Background
White matter hyperintensities (WMHs) on MRI have been associated with age, cardiovascular risk factors, and falls in the elderly. This study evaluated the relationship between WMHs and dynamics of postural control in older adults without history of falls.
Methods
We studied 76 community living subjects without history of falls (age 64.5±7.3 yrs). Brain and WMHs volume calculations and clinical rating were done on FLAIR and MP-RAGE MR images on 3 Tesla. Balance was assessed from the center of pressure displacement using the force platform during 3 minutes of quiet standing using traditional and dynamic measures (using stabilogram-diffusion analysis). Gait speed was measured from 12 minute walk.
Results
Age-adjusted periventricular and focal WMHs were associated with changes in certain dynamic balance measures, including reduced range of postural sway in anteroposterior direction (fronto-temporal WMHs, p=0.045; parieto-occipital WMHs, p=0.009) and more irregular longterm mediolateral fluctuations (p=0.046). Normal walking speed was not affected by WMHs.
Conclusions
Periventricul and focal WMHs affect long-term dynamics of postural control, which requires engagement of feedback mechanisms, and may contribute to mobility decline in the elderly.
INTRODUCTION
White matter hyperintensities (WMHs) are seen as multifocal and/or diffuse hyperintense areas on T2 weighted brain MRIs of older people. WMHs have been related pathologically to cerebral microangiopathy [1], [2], hypoperfusion [3], and neuronal loss in affected areas [4], [5]. WMHs are strongly associated with age, hypertension, and diabetes, thus supporting a vascular hypothesis of their origin. Clinical studies have suggested a link between WMHs and the age-related frontal lobe syndrome of cognitive decline [6, 7], balance disorders, and falls [8]. Specifically, it appears that the fronto-temporal cortex [9] and periventricular white matter [10] are particularly vulnerable to hypoperfusion and the development of WMHs. Because of the close proximity of frontal-subcortical circuits that control both motor and cognitive functions, it would not be surprising that periventricular WMHs may simultaneously cause dysfunction in both systems and that neuroanatomical changes in these structures have consequences for memory, executive functions, and balance in the older adults. We hypothesized that WMHs may affect feedback mechanisms controlling the dynamics of postural control. This study assessed the relationship between WMHs on MRI and postural control in community-living older people without history of falls.
METHODS
Participants
Postural control assessment and MRI studies were conducted in the SAFE (Syncope and Falls in the Elderly) Laboratory and at the Magnetic Resonance Imaging Center at the Beth Israel Deaconess Medical Center (BIDMC) in a GE 3 Tesla VHI scanner. We enrolled consecutively 104 participants, aged >50 years, who provided informed consent, approved by the Institutional Review Boards at the BIDMC and the Joslin Diabetes Center to participate in observational physiological studies about the effects of cerebromiscrovascular disease on perfusion and functional outcomes in older adults. The study protocol included anatomical and perfusion MRI measures, cardiovascular physiological measurements and functional outcomes of postural control, cognitive function and everyday activities. The study cohort includes community living older people with common cardiovascular risk factors and co-morbidities (hypertension, diabetes), without a history of recurrent falls. Demographic and clinical characteristics of 76 subjects who completed both MRI and balance studies are summarized in the Table 1. All subjects were screened with a medical history and physical examination. Laboratory chemistries included routine glucose, lipid and renal panels. Hypertension and diabetes were defined by clinical diagnostic criteria. Hypertensive patients were treated with beta blockers, angiotensin converting enzyme and arginin-renin inhibitors, and diuretics. Diabetic patients were treated with insulin, oral glucose-control agents, and their combinations or diet for at least one year, and for hypertension when clinically diagnosed. Excluded were participants with a history of stroke, myocardial infarction, congestive heart failure and other clinically important cardiac diseases, arrhythmias, significant nephropathy, kidney or liver transplant, renal or congestive heart failure, carotid artery stenosis (over 50% by medical history and MR angiography), neurological or other systemic disorders, recurrent falls and walking assisting devices. Vestibular function evaluation was not included but patients with known neurological and or movement disorders were excluded. Patients were allowed to wear glasses if needed. Participants with MRI-incompatible metal implants, pacemakers, arterial stents, and claustrophobia were also excluded.
Table 1.
Characteristics of the Study Population
| Group/Variable | All | Normotensive | Diabetes-normotensive | Diabetes-hypertensive | Hypertensive | P |
|---|---|---|---|---|---|---|
| (n=76) | (n=38) | (n=14) | (n=10) | (n=14) | ||
| Age | 64.7±7.2 | 64.9±6.8 | 59.8±5.0 | 63.6±8.0 | 69.8±6.2 | NS* |
| Men/women | 36/40 | 16/22 | 8/6 | 5/5 | 7/7 | NS |
| Disease duration | NA | 11.5±8.6 | 14.0±12.8 | 8.9±8.0 | NS | |
| Height (m) | 1.68±0.10 | 1.66±0.11 | 1.69±0.09 | 1.71±0.08 | 1.67±0.08 | NS |
| Weight (kg) | 73.2±11.8 | 70.3±10.2 | 76.1±8.0 | 84.7±15.2 | 69.8±11.8 | 0.002 |
| Body mass index | 26.1±4.0 | 25.5±3.1 | 26.8±4.5 | 29.0±4.3 | 25.0±4.6 | 0.05 |
| Baseline systolic BP | 126.2±16.5 | 116.8±11.6 | 137.2±20.5 | 128.8±13.9 | 138.2±9.2 | <0.0001 |
| Baseline diastolic BP | 63.3±9.8 | 62.3±10.1 | 66.2±6.7 | 68.1±11.2 | 61.0±10.4 | NS |
| Hemoglobin A1C (%) | 6.3±1.2 | 5.4±0.4 | 7.3±1.1 | 6.9±0.7 | NA | |
| Glucose (mg/dL) | 95.4±45.3 | 81.3±16.6 | 134.1±85.7 | 116.1±43.8 | 82.1±9.7 | 0.0005 |
| Triglycerides (mg/dL) | 182.0±126.0 | 156.8±74.9 | 297.5±233.2 | 167.3±70.7 | 153.6±67.5 | 0.003 |
| DM neuropathy (yes) | 4 | 0 | 3 | 1 | NS | |
| DM retinopathy | 12 | 6 | 6 | |||
| Total brain volume (cm3) | 1460.6±131.5 | 1456.0±137.5 | 1481.9±115.5 | 1456.0±151.2 | 1456.7±126.1 | NS |
| Brain Tissue Volumes | ||||||
| GM volume % brain | 48.2±6.9 | 48.6±7.2 | 51.2±6.6 | 50.1±7.9 | 43.0±2.1 | 0.009 |
| WM volume % brain | 27.9±3.4 | 28.4±3.2 | 26.2±3.6 | 27.5±4.8 | 28.6±1.9 | NS |
| CSF volume % brain | 23.9±6.2 | 23.0±6.0 | 22.6±7.7 | 22.5±6.1 | 28.4±2.8 | 0.02 |
| WMHs volume % brain | 0.60±0.68 | 0.41±0.40 | 0.48±0.53 | 0.91±1.07 | 1.00±0.87 | 0.01 |
| Periventricular WMHs (sum) | 15.33±16.43 | 10.66±6.53 | 10.49±8.19 | 27.60±33.57 | 24.09±17.39 | 0.002 |
| Punctuate WMHs (sum) | 13.33±28.26 | 6.27±14.25 | 6.97±20.65 | 18.17±26.95 | 35.41±48.65 | 0.006 |
| Gait and Balance Measures | ||||||
| Gait Speed (m/s) | 1.09±0.17 | 1.13±0.18 | 1.00±0.11 | 0.96±0.16 | 1.12±0.18 | 0.03 |
| Anteroposterior range (y) (mm) | 45.0±23.2 | 41.2±19.7 | 43.5±21.4 | 46.3±20.5 | NS | |
| Anteroposterior (y) SD (mm) | 9.6±5.7 | 8.2±3.2 | 8.5±4.2 | 8.3±2.2 | NS | |
| Mediolateral range (x) (mm) | 28.0±15.2 | 27.7±13.2 | 29.3±13.0 | 33.6±18.7 | NS | |
| Mediolateral (x) SD (mm) | 4.3±2.1 | 4.1±1.9 | 4.9±2.5 | 4.8±1.3 | NS | |
Data are presented as mean ± SD; p denotes comparisons among groups.
BP, blood pressure;; DM, diabetes mellitus; GM, gray matter; WM, white matter; CSF, cerebrospinal fluid; WMHs, white matter hyperintensities.
NS- indicates that there is no difference between normotensive and diabetic-normotensive, and between hypertensive and diabetic-hypertensive subjects.
Protocol
Balance Assessment
Subjects were asked to sit with their legs elevated on a stool at a 90 degree angle above the force platform, and they were asked to stand up for 3 minutes with their eyes open. Subjects were instructed to place their feet up to ≈15 cm apart in the center of the force platform and to look straight ahead. Force displacement was continuously measured by the force platform (Type 5233A2, Kistler Instrument Corp., Amherst, NY) from the moment when their feet touch the ground in x, y, and z directions. Electrocardiogram, beat-to-beat blood pressure, and respiration were continuously monitored. Analog signals were recorded at 1000 Hz using Labview NIDAQ (National Instruments Data Acquisition System 64 Channel/100 Ks/s, Labview 6i, Austin, TX) on a Pentium Xeon 2 GHz dual processor computer.
MRI Sequences
High-resolution anatomical images were acquired in a GE 3 Tesla VHI scanner with quadrature head coil. The parameters are as following: 3D magnetization prepared rapid gradient echo (MP-RAGE) - T1 weighted; TE/TI/TR =3.1/600/7.8 ms, 3.0 mm slice thickness, 0 mm skip, 52 slices, bandwidth=62kHz, flip angle= 10°, 24 cm × 24 cm FOV; fluid-attenuation inversion recovery (FLAIR): TI/TE/TR = 2250/161/11000 ms, 5 mm slice thickness, 0 mm skip, 24 cm × 24 cm FOV, 256 × 160 matrix size; dual T2-weighted fast spin echo (FSE): TE = 25/117 ms, TR = 4000 ms, 5 mm slice thickness, 24 cm × 24 cm FOV, 256 × 256 matrix size; 3D-MR angiography (time of flight, TOF): TE/TR = 3.9/38 ms, flip angle of 25°,, 2 mm slice thickness, -1 mm skip, 20 cm × 20 cm FOV, 384 × 224 matrix size.
Image Analysis
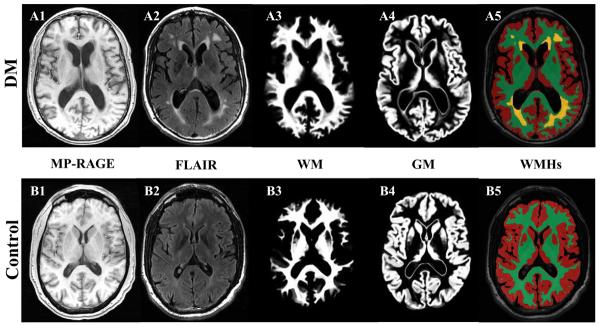
Periventricular WMHs present as hyperintense areas with >30% increase in signal intensity on T2-weighted images as compared with adjacent white matter. Focal lesions are well-defined areas of >2 mm with high signal characteristics [11]. FLAIR images were scored for WMHs using a semiquatitative scale from 0 to 3: 0 = no lesions; 1 = focal; 2 = beginning confluence; 3 = diffuse involvement of the entire region on each slice [11]. WMHs were graded on all slices in the frontal, temporal, parieto-occipital, cortical regions and the basal ganglia regions and quantified as a sum of lesions grade for each region, and for the whole brain. This clinical rating scale has shown good correlation with the WMHs volume (R2 =0.83, p<0.0001)[12]. The graders who scored (V.N.) and processed (P.Z.) images were blinded to the subject and group assignments. Segmentation of WMHs on FLAIR image was implemented by using the thresholding of hyperintense pixels and a region growing method that allowed an accurate WMHs detection without expertise, and it is programmed in Interactive Data Language (IDL, ITT Visual Information Solutions, Boulder CO). Figure 1 is an example of brain tissue and WMHs segmentation on axial MP-RAGE and FLAIR slices at the level of the ventricles, for a diabetic subject with hypertension (A) and a control subject (B). The brain volume was computed from the MP-RAGE image using Statistical Parametric Mapping software package (SPM, Wellcome Department of Imaging Neuroscience, University College London, UK). MP-RAGE image was segmented into white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF) by an inherently circular model in SPM involving spatial normalization and tissue classification. The MP-RAGE segmented image was registered on the FLAIR image by normalization module in SPM to compute the whole brain, GM, and WM volumes with the same resolution as WMHs, and to normalize WMHs for the total brain and WM volumes.
Figure 1.
Example of segmentation of gray matter (GM) and white matter (WM) on MP-RAGE (3D magnetization prepared rapid gradient echo) images and white matter hyperintensities (WMHs) segmentation on axial FLAIR (fluid attenuated inversion recovery) images slices at the level of the ventricles, for a diabetic subject with hypertension (A1-A5) and a control subject (B1-B5).
Partial volume effects may cause uncertainties in measuring the brain and WMHs volumes. We have estimated these uncertainties using a method published by Firbank et al, such that by creating a high resolution model of the brain and calculating these quantities twice. First calculating the exact values from the high resolution model, second, evaluating the same quantities using the experimental image parameters, in plane resolution, slice thickness and slice gaps. Implementing this method, we observed a mean uncertainty of ±2.4% in measuring these volumes [13].
Balance and Gait Assessment
Balance in the upright position is maintained through complex mechanisms that control interactions between musculoskeletal and somatosensory systems. Postural control was assessed from the center of pressure (COP) displacements using traditional static measures and stabilogram-diffusion analysis [14, 15]. Displacement of the COP has been used to assess postural sway and to demonstrate the impact of aging and age-related disorders on postural control [16, 17]. The traditional posturographic measures have been limited to summary statistics and have generally ignored the dynamic properties of the COP signals [18-20]. These parameters include the mean and maximum radius and range and the standard deviations in the mediolateral (x) and anteroposterior (y) directions and the swept area. Two parameters- anteroposterior range and the standard deviations have been associated with risk of falls. [16]
The stabilogram-diffusion analysis that enables extracting the dynamic information from COP signals [14] allows characterization of the postural sway dynamics in a physiologically meaningful way. This analysis provides dynamic measures of COP fluctuations in x and y directions: short-term positive correlation (mediolateral: Hxs; anteroposterior: Hys), long-term negative correlation (Hxl, Hyl), and fluctuation amplitude (RMSx, RMSy) and time scale (nx, ny) of transition from positive to negative correlations [21]. The COP signals behave as a positively correlated random walk over short-time scales characterized by a Hurst exponent (H). The parameter Hjs, called the scaling exponent, quantifies the correlation properties: if Hjs = 0.5, there is no correlation and the increments in displacement are statistically independent or random; if Hjs >0.5, there are positive correlations, wherein large increments are more likely to be followed by large increments (and vice versa for small increments); if Hjs <0.5, there are negative correlations, wherein large increments are more likely to be followed by small increments. The positive correlations in the COP signals suggest that the postural control system utilizes an open-loop control mechanism at small time scales (< 1 second). Negative correlations in the long-term region (time scales greater than ~2 seconds), indicate a close-loop controlled feedback. [14, 15, 22, 23]. The presence of negative correlations suggests that feedback mechanisms play an important role at large time scales and for large COP displacements, indicating a feedback control. The interpretation of positive correlations at small time scales has been debated, and it was suggested that they may reflect the sensory detection threshold, the time delay of sensory transmission and processing, the time delay due to the system's inertia, or a combination of all these factors [24-28].
Gait speed was measured during 12 minute walking in the hallway at normal walking speed.
Statistical Analysis
Descriptive statistics were used to summarize all variables. Demographic measures s were compared among the 4 study groups using MANOVA with multiple measures adjustments and Wilk's lambda post hoc tests. One-way analysis of variance and Fisher's exact test were used for non-repeated variables. Age has known effects on brain volumes and WMHs and body mass affects posturographic measures. Therefore, WMHs data and normalized to brain volumes were adjusted for age and posturographic measures and gait were adjusted for age and body mass index using a linear regression model prior to the analysis. The generalized mixed models were used to determine the relationships between age-adjusted WMHs and the traditional and dynamic posturographic measures. Models included posturographic measures as dependent variables and WMHs measures as model effects, group and brain regions were included as co-variants. The relationship between WMHs and gait speed were evaluated using same approach.
RESULTS
Characteristics of Study Cohort
A total of 76 subjects completed MRI, gait and balance assessments (Table 1). Because of the known associations between WMHs, hypertension and diabetes we also present separately demographic characteristics for 38 normotensive (NTN) controls, 14 diabetic-normotensive (DM-NTN), 10 diabetic-hypertensive (DM-HTN), and 14 nondiabetic-hypertensive (HTN) participants). Demographic factors were similar among the groups, except, as expected, for body weight (p=0.002), systolic blood pressure (p<0.0001), glucose (p=0.0005), lipid panels (p=0.03-0.003) that differed in the diseased groups compared to controls. History of smoking and alcohol consumption was not different. Whole brain and white matter volumes were not different among the groups. Participants with hypertension had greater normalized gray matter volume (p=0.009) and larger CSF volume (p=0.02). Periventricular WMHs were associated with higher baseline systolic blood pressure (p=0.02), and therefore, as expected, periventricular WMHs (p=0.002) and punctuate WMHs (p=0.006) were higher among DM-HTN and HTN participants. Gait speed was within normal range, but was slower in DM-HTN group (p=0.03), and was negatively correlated with BMI (p=0.001; R= -0.4) but was not significantly associated with age (p>0.2; R<0.02). Traditional posturographic measures were not different among the groups.
White Matter Hyperintensities and Dynamics of Postural Control
Table 2 summarizes traditional posturographic and dynamic balance measures for the entire cohort and their relationships to WMHs and brain volumes. WMHs were associated with certain dynamic balance parameters affecting the amplitude of postural sway and the transition time between short-term and long-term balance control during quiet standing with eyes open. Age-adjusted WMHs in fronto-temporal and parieto-occipital regions had distinct effects on dynamics of balance control. WMHs affected both amplitude and dynamics of postural sway, resulting in smaller and more random (less correlated) fluctuations. These effects were similar for both regions.
Table 2.
Posturographic measures and gait speed and the relationship to white matter hyperintensities
| Study Cohort | Sum. Continuous WMHs Fronto-Temporal | Sum Continuous WMHs Parieto-Occipital | Sum Punctuate WMHs Fronto-Temporal | Normalized CSF Volume Frontal | |
|---|---|---|---|---|---|
| Max Radius (mm) | 30.3±15.6 | Negative p=0.016, R=-0.21 | Positive p=0.01, R=+0.23 | ||
| Anteroposterior Direction (AP, y) | |||||
| AP Range (mm) | 44.3±21.6 | Negative p=0.045, R=-0.17 | Negative P=0.009, R=-0.22 | Negative p=0.03, R=-0.19 | |
| AP SD(mm) | 8.9±4.6 | Negative P=0.03, R=-0.22 | |||
| AP Short-term Amplitude (RMSy) | 6.8±4.6 | Negative P=0.05, R=-0.20 | |||
| AP-Long-term Correlations (Hyl) | 0.13±0.12 | ||||
| Mediolateral Direction (ML, x) | |||||
| ML Range (mm) | 29.2±15.2 | Positive p=0.02, R=+ 0.3 | |||
| ML Long-term Correlations (Hxl) | 0.15±0.11 | Positive p=0.0046, R=0.29 | Positive P=0.003, R=0.30 | Positive P=0.007, R=0.29 | |
| ML Short-term Amplitude (RMSx) | Negative P=0.0055, R=-0.27 | Negative P=0.005, R=-0.27 | Negative P=0.06, R=-0.21 | ||
| ML Crossover (nx) (sec) | 0.65±0.40 | Negative p=0.01, R=-0.23 | Negative P=0.05, R=-0.19 | ||
| Gait Speed | |||||
|---|---|---|---|---|---|
| Study Cohort | Normalized White matter Frontal | Normalized Gray matter Frontal | WMHs Sum & Volume | Normalized CSF Frontal | |
| Gait Speed (m/s) | 1.09±0.17 | Positive p=0.003, R=0.4 | Positive P=0.01, R=+0.3 | NS | |
In the fronto-temporal region, WMHs were associated with reduced anteroposterior range (p=0.045), reduced radius of motion (punctuate WMHs p=0.03), reduced amplitude of short-term fluctuations (RMSx, p=0.0006) and shorter transition time (nx1, p= 0.01).
In the parieto-occipital region, WMHs were also associated with reduced anteroposterior range (p=0.009) and standard deviation (p=0.03), smaller maximum radius (p=0.016), and reduced amplitude of short-term fluctuations (RMSx, p=0.005). In mediolateral direction, greater WMHs load was associated with less correlated long-term mediolateral fluctuations (Hxl: Normalized WMHs volume, p=0.03; periventricular WMHs fronto-temporal, p=0.0046 and parieto-occipital p=0.003; punctuate WMHs fronto-temporal, p=0.007). In the frontal region, increased CSF volume was associated with greater range of sway in the mediolateral direction (p=0.02), and greater maximal radius (p=0.01), but did not significantly affect body sway in the anteroposterior direction. We did not find association between WMHs volume and the whole brain GM, WM and CSF volumes.
White Matter Hyperintensities and Gait Speed
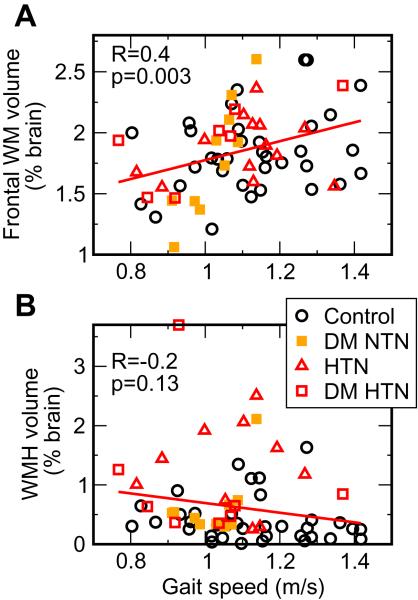
Body mass and height had significant effects on gait. Gait speed, adjusted for body mass index, was positively correlated to regional brain volumes (Figure 2A, B), WM volume in the frontal region (p=0.003, R=0.4), GM in the frontal region (p=0.01, R=0.3). WMHs measures (volumes, periventricular and punctuate sums) were not associated with gait speed (Figure 2B).
Figure 2.
Association of gait speed with (A) age-adjusted frontal WM volume and (B) white matter hyperintensities volume (WMHs).
DISCUSSION
This study demonstrated that diffuse periventricular hyperintensities and ischemic punctuate lesions affect the dynamics of postural control in older adults without a history of falls. WMHs were associated with reduced range of postural sway in anteroposterior direction and increased sway in mediolateral direction and more random fluctuations in both directions, suggesting negative effects on postural control feedback mechanisms. Increased WMHs load affected certain parameters of dynamic balance control, specifically those associated with long-term control of postural sway that required engagement of feedback mechanisms. These effects were significant for periventrical WMHs in both fronto-temporal and parieto-occipital regions. Gait speed was not affected by WMHs.
Subcortical WMHs were linked cross-sectionally with cognitive impairment [29, 30], and longitudinally with the rate of cognitive decline [31], in community-dwelling elders. Subjects with large subcortical WMHs have higher systolic blood pressure, more brain atrophy, reduced cerebral metabolism, and lower scores on tests of frontal lobe function than do age-matched controls [32],[33]. The strong relationship of WMHs with age [11], cognitive and motor slowing, and other risk factors for stroke suggest that they themselves may be manifestations of clinically important cerebrovascular disease [7], [12]. Cognitive decline, slow gait speed, and falls are common manifestations of subcortical frontal dysfunction and the atrophy that occurs with age-related disorders. A recent longitudinal study of 224 cognitively normal subjects older than 60 years [34] showed that an increase in the WMHs volume was associated with a concurrent decrease in white matter perfusion over an average of 5.8 years of follow-up. In addition, positron emission tomography studies showed that a significantly reduced frontal lobe metabolism was correlated with lower cognitive scores [35]. The LADIS (LeukoAraiosis and Disability) Study also demonstrated that older people with severe WMHs were at higher risk for functional decline and loss of independence in a short period of time due to cognitive deterioration [36, 37] These findings are most prominent in physically inactive individuals [38].
In a study of more than 700 community-dwelling participants from the Cardiovascular Health Study, WMHs were associated with worse performance on tests of balance using both clinical and dynamic posturography measures [8]. Among 1077 non-demented elderly men and women participating in the Rotterdam Scan Study [31], those with the most severe diffuse subcortical WMHs scored nearly 1 SD below the mean on tests of psychomotor speed. Whitman et al [39] compared the progression of WMHs in people with poor postural control and gait speed in healthy subjects. Subjects whose scores dropped more than 4 points on the Tinetti Performance Oriented Mobility Score over 4 years had a greater increase in WMHs volume and number of falls than those with normal scores. In a 10-year longitudinal study, Baloh and colleagues also reported a correlation between yearly changes in the Tinetti balance score and WMHs [40]. Because of the close proximity of frontal-subcortical circuits that control both motor and cognitive functions, it is not surprising that periventricular vascular lesions may simultaneously cause dysfunction in both systems. Therefore, regional brain metabolism may be exposed to fluctuations in perfusion pressure, compromising especially the periventricular watershed areas and regions with high metabolic demands.
The important findings of this study are that WMHs affect the dynamics of postural control in older people without a history of falls and that brain atrophy manifests as slower gait speed. In healthy people, long-term fluctuations of postural sway are negatively correlated, so that the sway in one direction is compensated by movement in the opposite direction, through engagement of compensatory feedback mechanisms. WMHs impair these mechanisms and sway fluctuations become more random, thereby affecting postural control during quiet standing. Abnormalities in postural sway in older adults are well documented, and research has linked greater amounts of postural sway to an increased risk of falling, a serious problem for older adults. Using multiscale entropy analysis, it was demonstrated that the postural sway dynamics of healthy young and healthy elderly subjects are more complex than that of elderly subjects with a history of falls [41]. Our study may support these findings by demonstrating that even in people without history of a falls, abnormalities in white matter pathways and regional brain atrophy affect dynamics of postural control and regional atrophy is associated with slower speed.
Some limitations to our approach may arise from fact that the study was cross-sectional and selected patients without a history of recurrent falls, and therefore the patients with the most severe lesion and balance abnormalities have not been evaluated. Our analysis were performed on data from the entire cohort, and therefore we cannot exclude that diabetes, hypertension or other risk factors may exert disease-specific effects on perfusion[12] and postural control. In contrast, WMHs are likely to be manifestations of misrovascular disease associated with these co-morbidities. This study was focused on people without a history of balance impairment and falls, and therefore we did not expect to find differences in traditional balance parameters among the groups.
This study addressed an important clinical question about the relationship between cerebromicrovascular disease and postural control in older people, and it provided evidence that WMHs on MRI affect dynamics of postural control during quiet standing. Continuous and punctuate WMHs affect long-term dynamics of postural control, which requires engagement of feedback mechanisms. Further prospective studies are needed to determine pathophysiology of WMHs and regional atrophy in older people with hypertension and diabetes and to differentiate their effects on mobility control. The effects of age-related WMHs and atrophy on gait and balance dynamics differ according to anatomical regions, and may contribute to mobility decline in the elderly.
Acknowledgments
This study was supported by an American Diabetes Association Grant to 1-03-CR-23 and 1-06-CR-25, NIH-NINDS 1R01-NS045745-01A2 to V. Novak, an NIH Older American Independence Center Grant 2P60 AG08812, and a General Clinical Research Center (GCRC) Grant MO1-RR01032.
We would like to acknowledge contributions of nurses from General Clinical Research Center; Sarah LaRose, BS, Laura DesRochers, BS from Division of Gerontology; Rob Marquise, BS, Fontini Kourtelidis, BS, and Susan LaRuche, BS, from Radiology Department, Beth Israel Deaconess Medical Center for their help with data acquisition.
References
- [1].van Swieten JC, van den Hout JH, van Ketel BA, Hijdra A, van Gijn J. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly. A morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain. 1991;114:761–74. doi: 10.1093/brain/114.2.761. [DOI] [PubMed] [Google Scholar]
- [2].Takao M, Koto A, Tanahashi N, Fukuuchi Y, Takagi M, Morinaga S. Pathologic findings of silent, small hyperintense foci in the basal ganglia and thalamus on MRI. Neurology. 1999;52:666–8. doi: 10.1212/wnl.52.3.666. [DOI] [PubMed] [Google Scholar]
- [3].Marstrand JR, Garde E, Rostrup E, Ring P, Rosenbaum S, Mortensen EL, Larsson HB. Cerebral perfusion and cerebrovascular reactivity are reduced in white matter hyperintensities. Stroke. 2002;34:972–6. doi: 10.1161/01.str.0000012808.81667.4b. [DOI] [PubMed] [Google Scholar]
- [4].Fazekas F, Schmidt R, Scheltens P. Pathophysiologic mechanisms in the development of age-related white matter changes of the brain. Dement Geriatr Cogn Disord. 1998;9:2–5. doi: 10.1159/000051182. [DOI] [PubMed] [Google Scholar]
- [5].Pantoni L, Garcia JH. Pathogenesis of leukoaraoisis: a review. Stroke. 1997;28:652–9. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- [6].Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224–32. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- [7].Desmond DW, Tatemichi TK, Myunghee P, Stern Y. Risk factors for cerebrovascular disease as correlate of cognitive function in a stroke-free cohort. Arch Neurol. 1993;50:162–6. doi: 10.1001/archneur.1993.00540020040015. [DOI] [PubMed] [Google Scholar]
- [8].Tell GS, Lefkowitz DS, Diehr P, Elster AD. Relationship between balance and abnormalities in cerebral magnetic resonance imaging in older adults. Arch Neurol. 1998;55:73–9. doi: 10.1001/archneur.55.1.73. [DOI] [PubMed] [Google Scholar]
- [9].Keymeulen B, Jacobs A, de Metx K, de Sadeleer C, Bossuyt A, Somers G. Regional cerebral hypoperfusion in long-term type 1 (insulin-dependent) diabetic patients: relation to hypoglycaemic event. Nucl Med Commun. 1995;16:10–6. doi: 10.1097/00006231-199501000-00005. [DOI] [PubMed] [Google Scholar]
- [10].Makimattila S, Malmberg-Ceder K, Hakkinen AM, Vuori K, Salonen O, Summanen P, Yki-Jarvinen H, Kaste M, Heikkinen S, Lundbom N, Roine RO. Brain metabolic alterations in patients with type 1 diabetes-hyperglycemia-induced injury. J Cereb Blood Flow Metab. 2004;24:1393–9. doi: 10.1097/01.WCB.0000143700.15489.B2. [DOI] [PubMed] [Google Scholar]
- [11].Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjogren M, Wallin A, Ader H, Leys D, Pantoni L, Pasquier F, Erkinjuntti T, Scheltens P. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–22. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- [12].Last D, Alsop DC, Abduljalil AM, Marquis RP, de Bazelaire C, Hu K, Cavallerano J, Novak V. Global and regional effects of type 2 diabetes mellitus on brain tissue volumes and cerebral vasoreactivity. Diabetes Care. 2007;30:1193–9. doi: 10.2337/dc06-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Firbank MJ, Coulthard A, Harrison RM, Williams ED. Partial volume effects in MRI studies of multiple sclerosis. Magn Reson Imaging. 1999;17:593–601. doi: 10.1016/s0730-725x(98)00210-0. [DOI] [PubMed] [Google Scholar]
- [14].Collins JJ, De Luca CJ. Open-loop and closed-loop control of posture: a random-walk analysis of center-of-pressure trajectories. Exp Brain Res. 1993;95:308–18. doi: 10.1007/BF00229788. [DOI] [PubMed] [Google Scholar]
- [15].Collins JJ, De Luca CJ. Random walking during quiet standing. Phys Rev Lett. 1994;73:764–7. doi: 10.1103/PhysRevLett.73.764. [DOI] [PubMed] [Google Scholar]
- [16].Laughton CA, Slavin M, Katdare K, Nolan L, Bean JF, Kerrigan DC, Phillips E, Lipsitz LA, Collins JJ. Aging, muscle activity, and balance control: physiologic changes associated with balance impairment. Gait Posture. 2003;18:101–8. doi: 10.1016/s0966-6362(02)00200-x. [DOI] [PubMed] [Google Scholar]
- [17].Norris JA, Marsh PM, Smith IJ, Kohut RI, Miller ME. Ability of static and statistical mechanics posturographic measures to distinguish between age and fall risk. Journal of Biomechanics. 2005;38:1263–72. doi: 10.1016/j.jbiomech.2004.06.014. [DOI] [PubMed] [Google Scholar]
- [18].Kirby RL, Price NA, MacLeod DA. The influence of foot position on standing balance. Journal of Biomechanics. 1987;20:423–7. doi: 10.1016/0021-9290(87)90049-2. [DOI] [PubMed] [Google Scholar]
- [19].Norré ME, Forrez G, Beckers A. Posturography measuring instability in vestibular dysfunction in the elderly. Age and Ageing. 1987;16:89–93. doi: 10.1093/ageing/16.1.89. [DOI] [PubMed] [Google Scholar]
- [20].Hasan SS, Lichtenstein MJ, Shiavi RG. Effect of loss of balance on biomechanics platform measures of sway: influence of stance and a method for adjustment. Journal of Biomechanics. 1990;23:783–9. doi: 10.1016/0021-9290(90)90025-x. [DOI] [PubMed] [Google Scholar]
- [21].Mandelbrot BB, Van Ness JW. Fractional Brownian motions, fractional noises and applications. SIAM Review. 1968;10:422–37. [Google Scholar]
- [22].Collins JJ, De Luca CJ. Upright, correlated random walks: A statistical-biomechanics approach to the human postural control system. Chaos. 1995;5:57–63. doi: 10.1063/1.166086. [DOI] [PubMed] [Google Scholar]
- [23].Saupe D. Algorithms for random fractals. In: Peitgen H-O, Saupe D, editors. The Science of Fractal Images. Springer-Verlag; New York: 1988. pp. 71–136. [Google Scholar]
- [24].Collins JJ, De Luca CJ. The effects of visual input on open-loop and closed-loop postural control mechanisms. Experimental Brain Research. 1995;103:151–63. doi: 10.1007/BF00241972. [DOI] [PubMed] [Google Scholar]
- [25].Collins JJ, De Luca CJ, Pavlik AE, Roy SH, Emley MS. The effects of spaceflight on open-loop and closed-loop postural control mechanisms: human neurovestibular studies on SLS-2. Experimental Brain Research. 1995;107:145–50. doi: 10.1007/BF00228026. [DOI] [PubMed] [Google Scholar]
- [26].Collins JJ, DeLuca CJ, Burrows A, Lipsitz LA. Age-related changes in open-loop and closed-loop postural control mechanisms. Experimental Brain Research. 1995;104:480–92. doi: 10.1007/BF00231982. [DOI] [PubMed] [Google Scholar]
- [27].Riley MA, Wong S, Mitra S, Turvey MT. Common effects of touch and vision on postural parameters. Experimental Brain Research. 1997;117:165–70. doi: 10.1007/s002210050211. [DOI] [PubMed] [Google Scholar]
- [28].Peterka RJ. Postural control model interpretation of stabilogram diffusion analysis. Biological Cybernetics. 2000;82:335–43. doi: 10.1007/s004220050587. [DOI] [PubMed] [Google Scholar]
- [29].Vermeer SE, Prins ND, den Heijer T, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;27:1215–22. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- [30].Vermeer SE, Hollander M, Van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: The Rotterdam scan study. Stroke. 2003;34:1126–9. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- [31].deGroot JC, de Leeuw FE, Ouderk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and subjective cognitive dysfunction: the Rotterdam Scan Study. Neurology. 2001;56:1539–41. doi: 10.1212/wnl.56.11.1539. [DOI] [PubMed] [Google Scholar]
- [32].Guralnik JM, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- [33].DeCarli Ch, Fletcher E, Ramey V, Harvey D, Jagust J. Anatomical Mapping of White Matter Hyperintensities (WMH) Exploring the Relationships Between Periventricular WMH, Deep WMH, and Total WMH Burden. Stroke. 2005;36:50–5. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Meyer JS, Rauch G, Rauch RA, Haque A. Risk factors for cerebral hypoperfusion, mild cognitive impairment, and dementia. Neurobiology of Aging. 2000;21:161–9. doi: 10.1016/s0197-4580(00)00136-6. [DOI] [PubMed] [Google Scholar]
- [35].DeCarli C, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–84. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- [36].Inzitari D, Simoni M, Pracucci G, Poggesi A, Basile AM, Chabriat H, Erkinjuntti T, Fazekas F, Ferro JM, Hennerici M, Langhorne P, O'Brien J, Barkhof F, Visser MC, Wahlund LO, Waldemar G, Wallin A, Pantoni L. Risk of rapid global functional decline in elderly patients with severe cerebral age-related white matter changes: the LADIS study. Arch Intern Med. 2007;167:81–8. doi: 10.1001/archinte.167.1.81. [DOI] [PubMed] [Google Scholar]
- [37].Jokinen H, Ryberg C, Kalska H, Ylikoski R, Rostrup E, Stegmann MB, Waldemar G, Madureira S, Ferro JM, van Straaten EC, Scheltens P, Barkhof F, Fazekas F, Schmidt R, Carlucci G, Pantoni L, Inzitari D, Erkinjuntti T. Corpus callosum atrophy is associated with mental slowing and executive deficits in subjects with age-related white matter hyperintensities: the LADIS Study. J Neurol Neurosurg Psychiatry. 2007;78:491–6. doi: 10.1136/jnnp.2006.096792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Baezner H, Blahak C, Poggesi A, Pantoni L, Inzitari D, Chabriat H, Erkinjuntti T, Fazekas F, Ferro JM, Langhorne P, O'Brien J, Scheltens P, Visser MC, Wahlund LO, Waldemar G, Wallin A, Hennerici MG. Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology. 2008;70:935–42. doi: 10.1212/01.wnl.0000305959.46197.e6. [DOI] [PubMed] [Google Scholar]
- [39].Whitman GT, Tang T. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology. 2001;57:990–4. doi: 10.1212/wnl.57.6.990. [DOI] [PubMed] [Google Scholar]
- [40].Baloh RW, Ying SH, Jacobson KM. A longitudinal study of gait and balance dysfunction in normal older people. Arch Neurol. 2003;60:835–9. doi: 10.1001/archneur.60.6.835. [DOI] [PubMed] [Google Scholar]
- [41].Costa M, Priplata AA, Lipsitz LA, Wu Z, Huang NE, Goldberger AL, Peng CK. Noise and poise: Enhancement of postural complexity in the elderly with a stochastic-resonance-based therapy. Europhys Lett. 2007;77:68008. doi: 10.1209/0295-5075/77/68008. [DOI] [PMC free article] [PubMed] [Google Scholar]