Abstract
Light and dopamine regulate many physiological functions in the vertebrate retina. Light exposure decreases cyclic AMP formation in photoreceptor cells. Dopamine D4 receptor (D4R) activation promotes light adaptation and suppresses the light-sensitive pool of cyclic AMP in photoreceptor cells. The key signaling pathways involved in regulating cyclic AMP in photoreceptor cells have not been identified. In the present study, we show that the light- and D4R-signaling pathways converge on the type 1 Ca2+/calmodulin-stimulated adenylyl cyclase (AC1) to regulate cyclic AMP synthesis in photoreceptor cells. In addition, we present evidence that D4R activation tonically regulates the expression of AC1 in photoreceptors. In retinas of mice with targeted deletion of the gene (Adcy1) encoding AC1, cyclic AMP levels and Ca2+/calmodulin-stimulated adenylyl cyclase activity are markedly reduced, and cyclic AMP accumulation is unaffected by either light or D4R activation. Similarly, in mice with disruption of the gene (Drd4) encoding D4R, cyclic AMP levels in the dark-adapted retina are significantly lower compared to wild-type retina and are unresponsive to light. These changes in Drd4-/- mice were accompanied by significantly lower Adcy1 mRNA levels in photoreceptor cells and lower Ca2+/calmodulin-stimulated adenylyl cyclase activity in retinal membranes compared with wild-type controls. Reduced levels of Adcy1 mRNA were also observed in retinas of wild-type mice treated chronically with a D4R antagonist, L-745,870. Thus, activation of D4R is required for normal expression of AC1 and for the regulation of its catalytic activity by light. These observations illustrate a novel mechanism for cross-talk between dopamine and photic signaling pathways regulating cyclic AMP in photoreceptor cells.
Keywords: dopamine, photoreceptors, adenylyl cyclase, cyclic AMP, retina, calmodulin
Introduction
Dopamine is a neuromodulator that influences many physiological functions in the retina (reviewed by (Witkovsky 2004). It enhances cone input signals and decreases rod input signals, uncouples horizontal cell and AII amacrine cell gap junction networks, enhances contrast sensitivity, and modulates light- and dark-adaptation. Also, it has trophic actions within the retina, affecting circadian rhythms, retinal development, and ocular growth (Witkovsky 2004). Activation of dopamine receptors, particularly the dopamine D4 receptor (D4R) subtype from the D2 family of receptors, suppresses the light-sensitive pool of cyclic AMP in dark-adapted mouse photoreceptors (Cohen and Blazynski 1990;Cohen et al. 1992). Cyclic AMP levels in photoreceptor cells are highest in darkness and reduced by light exposure. Although it is known that light and D4R activation regulate the same pool of cyclic AMP, they appear to do so by different mechanisms. Nir et al. (2002) showed that the effect of light is not directly dependent on D4R activation; however, in mice lacking D4Rs (Drd4-/- mice), cyclic AMP levels were significantly lower in darkness and unresponsive to light when compared with wild-type controls. These observations suggested that D4R activation may exert a trophic regulatory influence on components of the cyclic AMP signaling system.
Cyclic AMP is synthesized by a large family of adenylyl cyclase isoforms (Hanoune and Defer 2001). In chicken cone photoreceptor cells, membrane potential regulates cyclic AMP synthesis in a Ca2+-dependent manner (Iuvone et al. 1991). Light exposure and dopamine receptor activation both decrease intracellular Ca2+ and cyclic AMP levels in photoreceptor cells (Krizaj and Copenhagen 2002;Thoreson et al. 2002;Ivanova et al. 2008). The type 1 adenylyl cyclase, AC1, is activated by Ca2+ through the Ca2+ binding protein calmodulin (CaM) and is uniquely expressed in neural tissues, including the retina (Xia et al. 1993). Therefore, we examined the potential role of AC1 in mediating the light- and D4R-evoked reductions in cAMP, and investigated which components of the cyclic AMP signaling system are aberrant when D4R signaling is disrupted.
Materials and methods
Experimental Procedures
Animals
All procedures conformed to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Emory University's Institutional Animal Care and Use Committee. Drd4-/- mice, lacking dopamine D4 receptors (Rubinstein et al. 1997), Adcy1-/- mice, lacking the type 1 adenylyl cyclase (Wu et al. 1995), and normal control [wild-type (WT)] mice, all on a C57BL/6J background, were studied. Mice were genotyped by polymerase chain reaction (PCR) analysis of genomic DNA. Mice were housed on a 12 h light/12 h dark cycle with lights provided by cool white fluorescent tubes. Mice were sacrificed by cervical dislocation. All samples were collected in the morning, unless otherwise specified, immediately frozen on dry ice, and stored at -80°C.
Ex Vivo Retinal Incubations
Mice were dark adapted overnight. Retinas were dissected and placed in cold, oxygenated Earle's salt solution (115 mM NaCl, 3 mM KCl, 1.8 mM MgSO4, 0.9 mM NaH2PO4, 26 mM NaHCO3, 5.5 mM glucose) at pH 7.4. To assess the effects of light on cyclic AMP accumulation, individual retinas where transferred to 6-well plates containing Earle's salt solution supplemented with 1 mM 3-isobutyl-1-methylxanthine (Sigma), 10 mM glutamate, and test drug or vehicle. Exogenous glutamate inhibits synaptic transmission to second order neurons in the retina, isolating the photoreceptor response to light in this assay (Cohen et al., 1992). The retinas were incubated in darkness at 37 °C in an atmosphere of 95% O2/5% CO2 for 5 minutes in a water bath. Subsequently, retinas were incubated in darkness or light for 10 additional minutes. To access the effects of D4R activation, retinas were incubated with or without PD168,077, a D4R-specific agonist (Glase et al. 1997), for 15 minutes in darkness. Retinas were immediately frozen on dry ice and stored at -80 °C until assayed. Cyclic AMP levels (pmol/mg protein) were measured by radioimmunoassay (RIA, Perkin Elmer, Waltham, Massachusetts 02451, USA).
Adenylyl Cyclase Assay
Partially purified membranes were prepared by homogenizing whole mouse retina in TME buffer containing 10 mM Tris-maleate, pH 7.5, 1 mM MgSO4, 1.2 mM EGTA, 0.5 mM DTT and Protease Inhibitor Cocktail III (EMD Biosciences, La Jolla, CA, USA). Homogenates were centrifuged at 20,000 × g for 20 min at 4°C. The resulting pellet was washed three times and resuspended in TME buffer. The protein content of the crude membrane preparation was determined (Lowry et al. 1951), using bovine serum albumin as a standard. Adenylyl cyclase activity was assayed by assessing the conversion of [α-32P] ATP to [32P]cyclic AMP using the method of (Salomon et al. 1974) with minor modifications. The final reaction mixture contained 80 mM Tris maleate, pH 7.5, 10 mM GTP, 1 mM DTT, 5 mM MgSO4, 0.5 mM cyclic AMP, 0.2 mM EGTA, 5 mM phosphocreatine, 0.1 mg/ml creatine phosphokinase, 4 units/ml adenosine deaminase, 50 μM ATP, [α32P]ATP (0.5 μCi/tube), with or without 195 μM CaCl2, and 120 nM CaM as indicated. Free [Ca2+], with 195 μM CaCl2 added, and was calculated to be 2 μM using WEBMAXCLITE v1.15 (http://www.stanford.edu/%7Ecpatton/webmaxc/webmaxclite115.htm). The reaction was initiated by the addition of membranes (20-30 μg protein) in a final reaction volume of 250 μl and samples were incubated at 37°C for 10 min. The reaction was stopped by addition of 750 μl of 10% TCA containing [3H]cyclic AMP (~4000 dpm) for recovery. After centrifugation, cyclic AMP was extracted by employing sequential chromatography on columns of Dowex AG50-W4 cation exchange resin and neutral alumina. The resulting elute (2.5 ml) was mixed with scintillation fluid (14 ml) and counted in a liquid scintillation counter using dual channel counting, one channel measuring [3H] for recovery of internal standard and the other channel measuring [32P]-labeled product.
Acute and Sub-chronic Drug Treatment
A selective dopamine D4 receptor antagonist, L-745,870 (Patel et al. 1997), was administered for 1 or 6 days by subcutaneous injection (1mg/kg, s.c.) thirty minutes before light onset in the morning (ZT 23.5). Control animals were injected with a vehicle, 0.3% carboxymethylcellulose (Sigma-Aldrich, St. Louis, MO, USA). The rationale for injecting drug before light onset was to block D4Rs prior to the burst of dopamine release that occurs at light onset (Nir et al. 2000). On the first or sixth day, retinas were dissected and processed for analysis of Adcy1 mRNA expression. In a separate experiment, mice were injected with L-745,870 or vehicle for six days as described above, but retinas were not dissected until the morning of the seventh day to allow for drug elimination.
Western Blotting
Retinas were homogenized in 50 mM Tris-HCl, 50 mM NaCl, 0.1 mM PMSF, 0.5% Triton X-100, pH 7.4 and were incubated at 4°C for 30 min with mixing. Homogenates were centrifuged at 20,000 X g for 30 min at 4°C. Pilot studies demonstrated that this procedure solubilized all detectable CaM protein (data not shown). 50 μg of supernatant protein was denatured by boiling for 5 min and was separated on a 10% Bis-Tris Criterion XT precast gel (BioRad Laboratories). The proteins on the gel were transferred to PVDF membrane using a semi-dry method. CaM was detected using a mouse monoclonal IgG1 antibody (05-173, Upstate, Lake Placid, NY, 1:500) plus horseradish peroxidase-labeled goat anti-mouse IgG (1:5,000, sc-2005, Santa Cruz Biotechnology, Santa Cruz, CA) with detection by enhanced chemiluminescence (Amersham Biosciences, Buckinghamshire, UK). The α-CaM antibody recognizes a C-terminal amino acid sequence that is 100 % conserved in CaM 1, 2 and 3 isoforms.
RNA Isolation and Quantitative Real-time PCR
Total RNA was extracted by a silica filter-binding method using the RNeasy kit (Qiagen Inc., Valencia, CA). Reverse transcription (RT) was performed on total RNA (2 μg) preparations using Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA) and oligo-dT or random primers (Invitrogen); oligo-dT was used in assays where mRNA expression was normalized with hypoxanthine phosphoribosyltransferase (Hprt) mRNA or glyceraldehyde-3-phosphate dehydrogenase (Gapdh) mRNA and random primers were used for normalization to 18S rRNA.
Reactions were performed in 25 μl total volume with 2μl cDNA, 1X SYBR Green PCR Master mix (Bio-Rad, Hercules, CA, USA) and 300 nM intron-spanning gene specific forward and reverse primers in a Bio-Rad iCycler (Bio-Rad). The PCR was heated at 95°C for 2 min, followed by 40 cycles of denaturing, annealing and extension at 95°C, 53° - 57°C, and 72°C for 30 seconds each, respectively. The quantification of transcript level was performed by comparing the threshold cycle for amplification of the unknown to those of six concentrations of standard cDNAs for each respective gene. Each sample was assayed in duplicate.
Laser Capture Microdissection
Whole mouse eyes were embedded in OCT (Tissue-Tek, Torrance, CA), frozen on dry ice, and stored at -80°C. Frozen tissues were cut at 8 μm thickness and mounted on uncharged glass slides (VWR Scientific). The frozen sections were thawed for 30 sec and fixed in 75% ethanol for 30 sec followed by a wash in RNase-free water for 30 sec. The sections were stained with HistoGene (Arcturus Engineering, Mountain View, CA) staining solutions for 15 seconds followed by a wash with RNase-free water for 30 sec. The sections were dehydrated in graded ethanol solutions (75%, 30 sec; 95%, 30 sec; 100%, 30 sec) and cleared in xylene (5 min). After air-drying for 5 min, the slides were kept in a vacuum desiccator for a minimum of 30 min.
Laser capture microdissection (LCM) was performed by lifting the outer nuclear layer and inner segments of photoreceptor cells, the inner nuclear layer, and the ganglion cell layer individually onto HS-CapSure non-contact LCM films using a PixCell IIe LCM system (Arcturus Engineering). In this technique, a thermoplastic polymer membrane is placed above the tissue section. The cells of interest are visually located by light microscopy and a focused, low energy infrared laser is used to soften the film, allowing it to bond to the cells on the surface of the tissue section. The film absorbs the infrared energy, preventing damage to DNA, RNA, or protein in the underlying cells (Bonner et al. 1997;Parlato et al. 2002). Removal of the film lifts the desired cells from the tissue section without damaging other cells. When dissecting the photoreceptor cell layer (inner segments and outer nuclear layer) or the inner nuclear layer, we leave untouched the cells abutting the outer plexiform layer to avoid cross contamination. Outer segments are not captured because mRNA levels are highest in the photoreceptor soma and inner segments, and to avoid contamination from the adjacent retinal pigment epithelium. The specificity of this approach has been validated using cell type specific markers (Liu et al. 2004).
Total RNA was extracted from the captured cells using the PicoPure RNA Isolation Kit (Arcturus Engineering). Samples were reverse transcribed using random primers and subjected to real-time PCR analysis as described above.
Statistical analysis
Statistical comparison of multiple group data was performed using one-way or two-way analysis of variance (ANOVA) with Student-Newman-Keuls test where applicable. Comparisons of two groups were made with Students t-test.
Results
Identification of the adenylyl cyclase isoform regulated by light and D4R activation
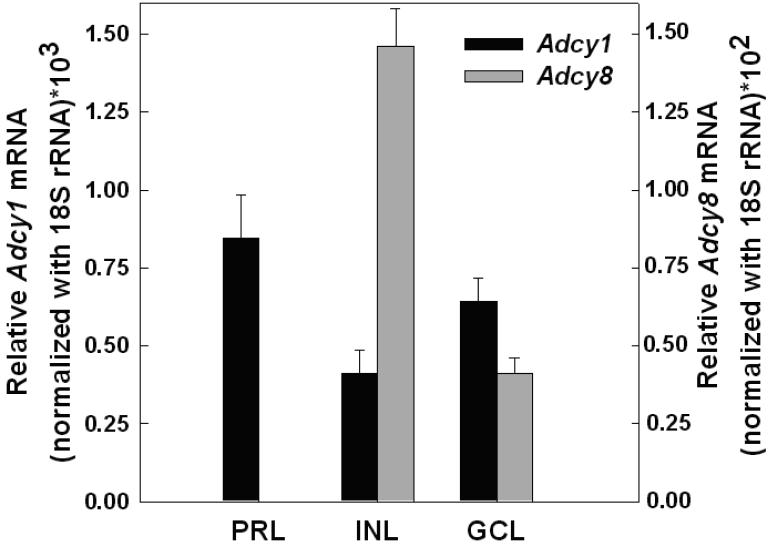
Light exposure and D4R activation decrease the same pool of cyclic AMP in mouse photoreceptor cells (Cohen and Blazynski 1990). Previous studies suggest that cyclic AMP in photoreceptor cells is regulated by a Ca2+-stimulated adenylyl cyclase (see Introduction). The primary Ca2+-stimulated isoforms are the type-1 and type-8 adenylyl cyclases, AC1 and AC8 respectively (Wang and Storm 2003). Therefore, we investigated the expression of these isoforms in mouse retina. Using LCM to isolate retinal cell layers, we determined that Adcy1 mRNA, which encodes AC1, was expressed in the photoreceptor layer, inner nuclear layer, and the ganglion cell layer (Fig.1). In contrast, Adcy8 mRNA was expressed in the inner nuclear and ganglion cell layers, but was undetectable in the photoreceptor layer. Therefore, in subsequent experiments, we focused our attention on AC1 as a putative cyclase isoform regulated by light and D4R in photoreceptor cells.
Figure 1. Expression of Adcy1 and Adcy8 transcripts in retinal layers isolated by laser capture microdissection (LCM).
The ganglion cell layer (GCL), inner nuclear layer (INL), and photoreceptor layer (PRL; inner segments and outer nuclear layer) were captured from frozen sections of retina as described in Material and methods. Adcy1 and Adcy8 mRNA levels, normalized to 18S rRNA, where determined by real-time RT-PCR. N= 4 retinas per group, except for Adcy8 GCL, where N= 3.
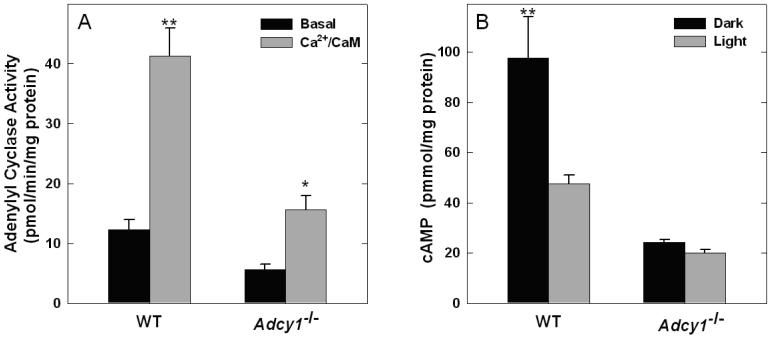
To assess the contribution of AC1 to retinal adenylyl cyclase activity, basal and Ca2+/calmodulin-stimulated enzyme activities were assessed in retinal membranes prepared from wild type and Adcy1-/- mice. In membranes prepared from WT retinas, Ca2+/CaM increased adenylyl cyclase activity ~ 5 fold (p<0.001) compared to basal conditions without Ca2+ and CaM in the reaction (Fig.2A). Both basal activity and Ca2+/CaM-stimulated activity were markedly reduced in retinal membranes from Adcy1-/- mice, indicating that AC1 is a major adenylyl cyclase isoform controlling cyclic AMP synthesis in the mouse retina. A small amount of Ca2+/CaM-stimulated activity was observed in Adcy1-/- retinal membranes (p=0.015), which probably reflects a contribution of AC8 from the inner retina. In order to investigate the role of AC1 in the photic regulation of cyclic AMP, the effect of light exposure on cyclic AMP accumulation was examined in retinas of wild type and Adcy1-/- mice incubated in vitro. The level of cyclic AMP accumulation was high in dark-adapted retinas from wild type mice and was significantly reduced by light exposure (p<0.001; Fig. 2B). In retinas of Adcy1-/- mice, cyclic AMP accumulation in darkness was markedly lower compared to wild type retinas (p<0.001) and light exposure had no significant effect (p = 0.714). A control experiment found no compensatory changes in the levels of transcripts encoding the related type 8 adenylyl cyclase isoform in retinas of mice deficient in AC1 ( WT: 1.39 ± 0.03; Adcy1-/-: 1.26 ± 0.06 Adcy8 mRNA / Hprt mRNA*10-2; N=6).
Figure 2. Adenylyl cyclase activity and cyclic AMP accumulation is low and unresponsive to light in mouse retinas lacking the type 1 adenylyl cyclase.
A. Adenylyl cyclase activity was measured in retinal membranes prepared from WT and Adcy1-/- mice in the presence or absence of Ca2+ and CaM, as described in Materials and methods. 2-factor ANOVA revealed significant effects of genotype (p<0.001), Ca2+/CaM (p<0.001), and a significant interaction of genotype and Ca2+/CaM (p=0.004). **p<0.001 vs. WT Basal, Adcy1-/- Basal, and Adcy1-/-Ca2+/CaM; *p=0.015 vs. Adcy1-/- Basal . B. Cyclic AMP accumulation was measured in retinal explants incubated in darkness for 15 min or in darkness (5 min) followed by light exposure (10 min), as described in Materials and methods. N= 6 per group; 2-factor ANOVA revealed significant effects of genotype (p<0.001, light conditions (p=0.004), and interaction of genotype and light conditions. **p<0.001 vs. all other groups.
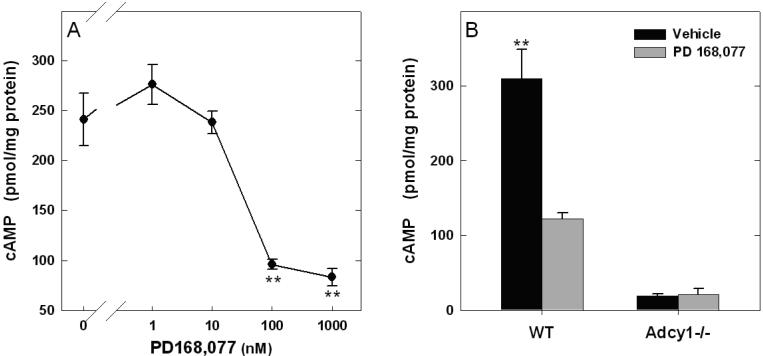
Further studies investigated whether AC1 is coupled to the D4R, measuring cyclic AMP accumulation in vehicle and D4R agonist (PD 168,077) treated retinas from WT, Adcy1-/-, and Drd4-/- mice. Incubation of dark-adapted WT retinas with PD 168,077 resulted in a concentration-dependent decrease of cyclic AMP accumulation, with an EC50 of 30nM (Fig. 3A). The effect of PD 168,077 was absent in Drd4-/- mouse retinas, confirming involvement of D4Rs (data not shown). Similarly, treatment with a supra-maximal concentration (3μM) of PD 168,077 in dark-adapted retinas of Adcy1-/- mice failed to decrease cyclic AMP accumulation (Fig. 3B; p = 0.92). In parallel with the previous light experiment (Fig. 2), the basal level of cyclic AMP accumulation in the Adcy1-/- retinas is significantly lower (p<0.001) compared with WT controls (Fig. 3B). Drd4 mRNA levels were unaffected in retinas of Adcy1-/- mice (WT: 5.89 ± 0.81; Adcy1-/-: 6.86 ± 1.23 Drd4 mRNA / Hprt mRNA*10-2; N=6). These data indicate that light and D4R activation control cyclic AMP production in mouse retina via AC1.
Figure 3. PD 168,077, a D4R agonist, inhibits cyclic AMP accumulation in WT but not Adcy1-/- retinas.
A. PD 168,077 inhibits cyclic AMP accumulation during a 15 min incubation with an EC50 value of 30 nM. **p<0.001 vs. control. B. In the presence of PD168077 (3μM) there is a significant reduction in cyclic AMP accumulation in WT, but not in Adcy1-/- retinas. N=4 / group; **p<0.001 vs. all other groups.
Expression of AC1 in Drd4-/- mouse retina
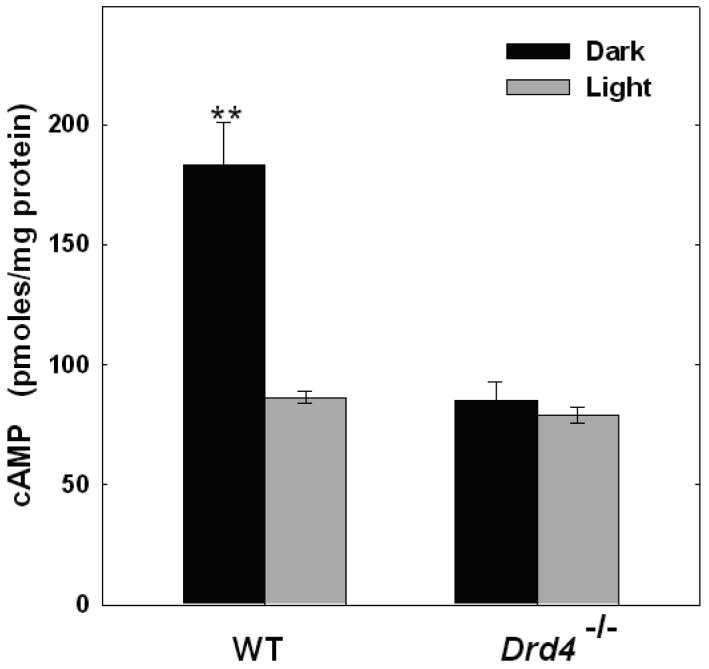
A previous study reported that cyclic AMP levels in dark-adapted retinas of Drd4-/- mice were low compared to WT controls and unresponsive to light (Nir et al., 2002). The present study confirms these observations; cyclic AMP accumulation in dark-adapted WT retinas was approximately twice that of dark-adapted retinas from Drd4-/- mice (p<0.001; Fig. 4) and, while light exposure significantly reduced cyclic AMP accumulation in WT retinas (p<0.001), it failed to do so in retinas of mice lacking D4Rs (Fig. 4). These observations led us to hypothesize that the expression of component(s) of the cyclic AMP signaling pathway regulated by light and by D4R was dysfunctional in retinas of Drd4-/- mice. To test this hypothesis, the activity of Ca2+/CaM-stimulated adenylyl cyclase, the transcript level of Adcy1, and the protein and transcript levels of calmodulin were examined.
Figure 4. Light decreases cyclic AMP accumulation in retinas of WT but not Drd4-/- mice.
Retinas were incubated in dark or light as described in Fig. 2B. N= 4-6; **p<0.001 vs. all other groups.
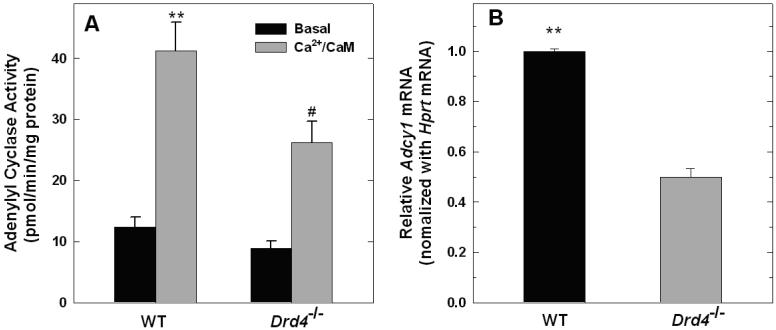
There was no significant difference in basal adenylyl cyclase activity between membranes prepared from wild-type and Drd4-/- mice (Fig. 5A; p=0.705). However, adenylyl cyclase activity in the presence of Ca2+/CaM was significantly lower in retinal membranes prepared from Drd4-/- mice compared to WT controls (p<0.001). In addition, quantitative real-time PCR revealed markedly lower levels of Adcy1 transcripts in Drd4-/- retinas than in WT samples (Fig. 5B; p<0.001).
Figure 5. Ca2+/CaM-stimulated adenylyl cyclase activity and Adcy1 mRNA levels in retinas of WT and Drd4-/- mice.
A. Ca2+/CaM stimulated adenylyl cyclase activity is significantly lower in the Drd4-/- retinal membranes compared to WT controls. N= 9 / group; **p<0.001 vs. all other groups.; #p<0.01 vs. Drd4-/- Basal. B. Adcy1 mRNA is significantly lower in retinas of Drd4-/- mice compared to WT controls. Adcy1 mRNA level was normalized to Hprt mRNA and expressed relative to WT. N= 6 / group; **p<0.001 vs. Drd4-/-.
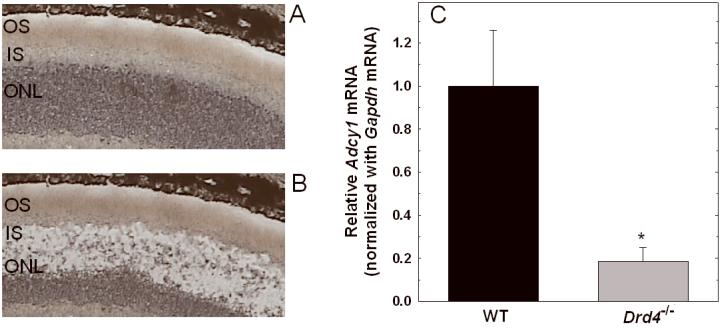
The aforementioned measurements were based on mRNA from whole retina. To determine if a difference in Adcy1 expression occurred in photoreceptors of the two genotypes, LCM was employed to isolate the photoreceptor layer. Figures 6A and B show a typical dissection of the photoreceptor inner segments and outer nuclear layer. RT-PCR analysis of the captured photoreceptors revealed that Adcy1 transcript levels were markedly lower in samples from the Drd4-/- mice compared to the corresponding WT samples (Fig. 6C; p=0.006).
Figure 6. Decreased expression of Adcy1 mRNA in photoreceptors of Drd4-/- mouse retina.
Photoreceptor inner segments and cell bodies were isolated by laser capture microdissection (LCM) as described in Methods. A. Representative retinal section before dissection. B. Retinal section after capture of inner segments and cell bodies. OS, outer segments; IS, inner segments, ONL, outer nuclear layer. C. Adcy1 mRNA was measured in captured IS/ONL from WT and Drd4-/- mice, normalized to Gapdh mRNA, and expressed relative to WT. N= 4 (WT) and 6 (Drd4-/-); *p=0.006 vs. WT mice.
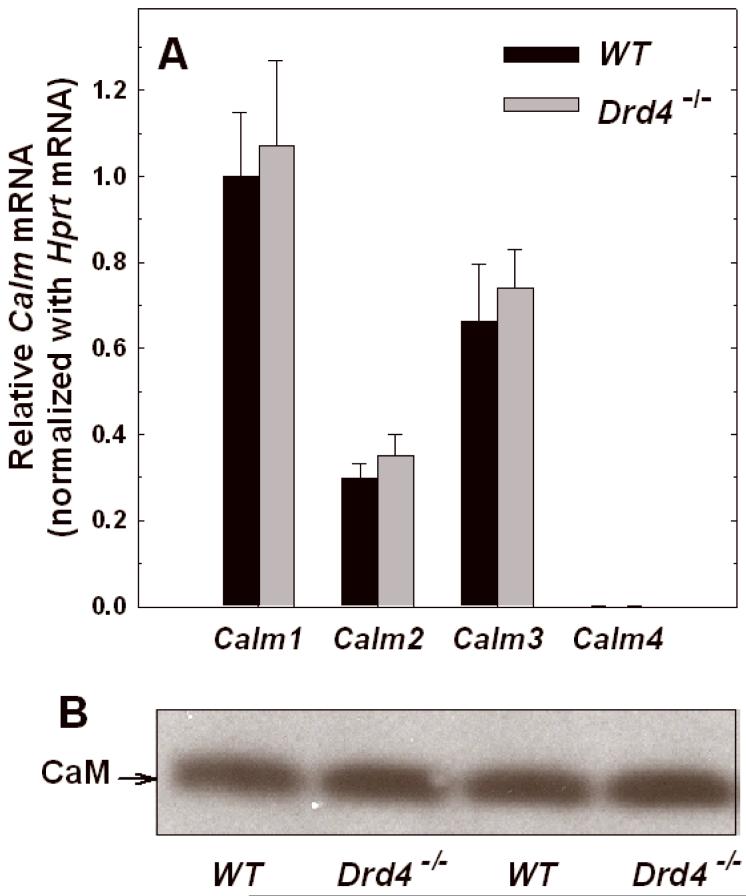
To further explore the mechanisms responsible for the decrease in cyclic AMP accumulation in retinas of D4R deficient mice, we studied the expression of CaM transcripts and protein. Four transcripts encode highly conserved CaM isoforms in mouse: Calm1, Calm2, Calm3, and Calm4 (Skinner et al. 1994;Hwang and Morasso 2003). Calm1-3 transcripts were found to be abundantly expressed in mouse retina (Fig. 7A). Calm4 mRNA was detectable, but relative abundance was <1% of Calm1 mRNA level. No differences in Calm1-4 transcript levels were observed between WT and Drd4-/- mouse retinas (Fig. 7A). Calm transcript expression was further explored in LCM isolated retinal layers, but no significant differences were observed between samples from WT and Drd4-/- mice (data not shown). Similarly, Western blot analysis of retinal homogenates, using an antibody that recognizes an amino acid sequence that is 100 % conserved in CaM 1, 2, and 3, revealed no difference between the WT and Drd4-/- mice (Fig. 7B). Thus, the dysfunction of Ca2+/CaM-stimulated adenylyl cyclase activity in Drd4-/- mouse retina appears to be due primarily to low levels of expression of the AC1 and not to changes in CaM expression.
Figure 7. Calmodulin expression in wild-type and Drd4-/- mouse retina.
A. Calm1-4 transcripts normalized to Hprt mRNA and expressed relative to WT Calm1 mRNA. The values are means ± SEM (N=6). B. Western blot analysis of calmodulin protein from the wild-type and Drd4-/- mouse retina. The blot shown is representative of an N of 6 from two separate experiments.
As a further control for compensatory changes, we examined the relative expression of transcripts encoding the five dopamine receptor subtypes in retinas of WT, Adcy1-/-, and Drd4-/- mice (Supplemental Fig. S1). Drd1, Drd2, Drd4, and Drd5 transcripts were observed in mouse retina; Drd3 mRNA expression was not detectable. Drd4 was the most highly expressed subtype. No significant differences were observed among genotypes, except for the absence of Drd4 mRNA in retinas of Drd4-/- mice.
Effect of D4R antagonism on Adcy1 expression
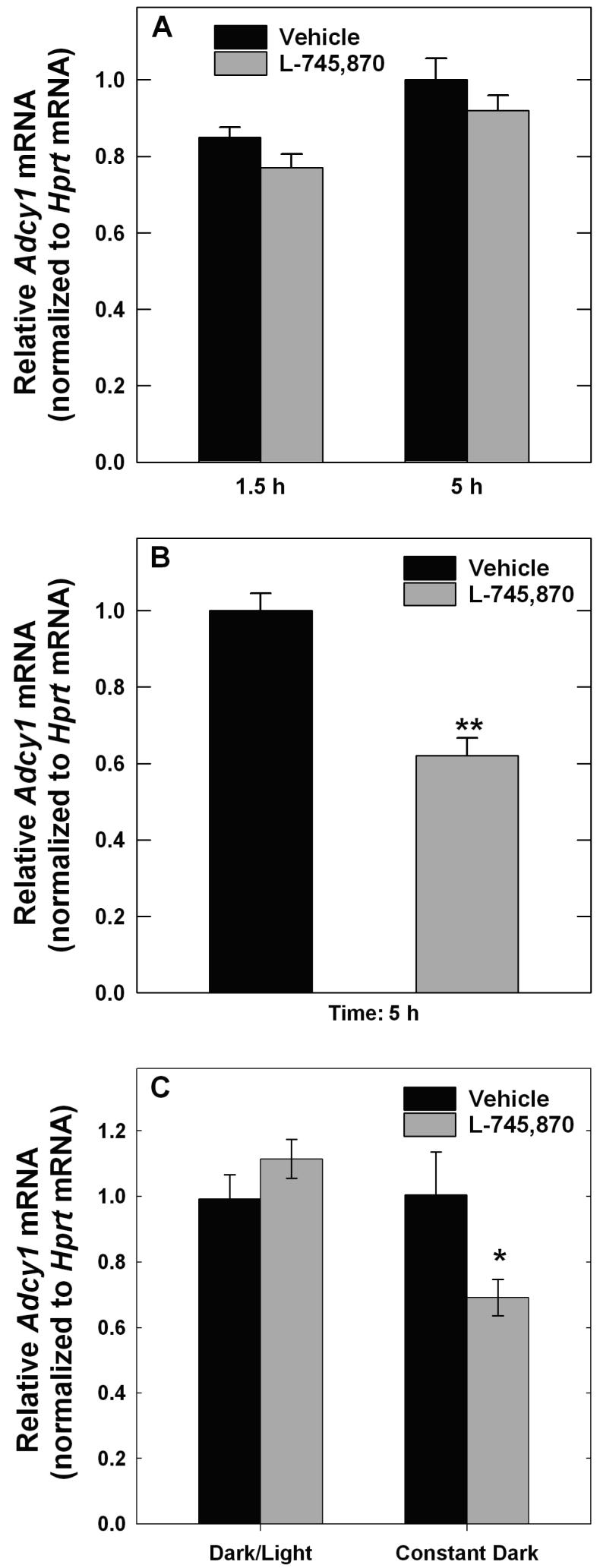
To investigate the role of D4R activation in the expression of Adcy1-/-, the effects of a D4R-selective antagonist, L-745,870 (Patel et al. 1997), were examined in WT mice. L-745,870 was administered 30 minutes prior to light onset in the morning to antagonize the surge of dopaminergic activity that occurs when dark-adapted retinas are first exposed to light in the morning (Nir et al. 2000). Following a single injection, L-745,870 had no significant effect on Adcy1 mRNA level measured 1.5 or 5 h after light onset (Fig. 8A). In contrast, retinas of mice treated with L-745,870 for 6 days had significantly lower levels of Adcy1 transcript compared to vehicle-treated controls (Fig. 8B; p<0.001). Recovery of Adcy1 mRNA levels following drug elimination for >29 h was examined in mice treated with L-745,870 for 6 days. In mice exposed to light in the morning for 4-5 hours to stimulate dopamine release, no difference was observed in Adcy1 transcript levels between vehicle and antagonist treated samples (Fig. 8C), indicative of recovery. However, in mice that were kept in darkness for the same period to prevent the morning increase of dopaminergic activity, Adcy1 mRNA levels remained lower in the L-745,870-treated mice than in the vehicle controls (Fig. 8C; p<0.05). These observations suggest that D4R activation tonically supports Adcy1 expression.
Figure 8. Effect of L-745,870 on Adcy1 transcript expression in WT mouse retina.
Mice were injected subcutaneously with L-745,870 (1 mg/kg body weight) or vehicle ~30 min before light onset in the morning. A. A single injection failed to significantly affect Adcy1 mRNA, measured 1.5 or 5 h after light onset. N= 6. B. Mice were injected with L745,870 or vehicle daily for 6 days and retinas dissected 5 h after light onset following the last injection. Adcy1 mRNA was significantly lower in L-745,879 treated mice compared to vehicle controls. Adcy1 mRNA was normalized to Hprt mRNA and expressed relative to Vehicle values from samples collected 5 h after light onset. N=8; **p<0.001 vs. Vehicle. C. Mice were injected for 6 days as in Figure 8B, but retinas were not dissected until the following day to allow for drug elimination. One set of subjects (Dark/Light) were exposed to light in the morning for 4-5 h to stimulate dopamine release, while the other set (Constant Darkness) remained in the dark for the same period of time. Light exposed samples showed recovery of Adcy1 mRNA levels, while those kept in darkness did not; N=6, *P=0.014 vs. Vehicle.
Discussion
Dopamine D4 receptors are highly expressed in retina relative to other central nervous system structures (Oak et al. 2000) and appear to be the only dopamine receptor subtype expressed by photoreceptors (Cohen et al. 1992). Activation of these receptors decreases a light-sensitive pool of cyclic AMP in mouse photoreceptors (Cohen and Blazynski 1990;Cohen et al. 1992;Nir et al. 2002). The absence of D4Rs has physiological consequences that manifest as impaired light adaptation and a decreased rate of dark adaptation following light exposure (Nir et al. 2002). Effects are observed in both the a-wave and b-wave of the electroretinogram, consistent with effects on synaptic transmission from the photoreceptors to inner retinal neurons. It is unknown at present if the dysfunctional cyclic AMP signaling is responsible for these deficits of adaptation in Drd4-/- mice, but recent studies have shown that the absence of D4Rs compromises light-evoked dephosphorylation of the PKA site of phosducin (Pozdeyev et al. 2008), a regulatory phosphoprotein that binds to transducin-βγ subunits (Lee et al. 1987).
A number of other photoreceptor functions are influenced by dopamine and/or cyclic AMP and could contribute to the physiological abnormalities observed in Drd4-/- mice. Dopamine, acting through D4Rs, inhibits Na+/K+-ATPase in rat rod outer segments (Shulman and Fox 1996). Dopamine increases the rate of rhodopsin dephosphorylation in frog retina (Udovichenko et al. 1998) and PKA has been shown to phosphorylate the γ-subunit of cGMP phosphodiesterase purified from bovine rod outer segments (Xu et al. 1998). Also, D4R activation regulates melatonin synthesis in photoreceptors by decreasing cyclic AMP formation (Iuvone 1986;Iuvone and Besharse 1986;Tosini and Dirden 2000;Zawilska and Nowak 1994;Zawilska et al. 1995). Dopamine and cyclic AMP affect rod and cone photoreceptor Ca2+ currents in salamander (Stella, Jr. and Thoreson 2000), photoreceptor retinomotor movements in Xenopus and fish (Besharse et al. 1982;Pierce and Besharse 1985;Hillman et al. 1995), and reset the phase of the circadian clock in Xenopus photoreceptors (Cahill and Besharse 1991;Hasegawa and Cahill 1999).
Previous observations that light-regulated cyclic AMP signaling is dysfunctional in retinas of Drd4-/- mice (Nir et al. 2002), confirmed in the present study, suggest that D4R activation regulates the expression of components of the cyclic AMP signaling system in photoreceptor cells. The major goal of this study was to identify which adenylyl cyclase(s) are regulated by light and the D4R, and to determine if expression of the cyclase or related signaling components is aberrant when D4R activation is disrupted. The results provide compelling support for the conclusions that light and dopamine acutely regulate the catalytic activity of AC1 in photoreceptor cells and that D4Rs tonically regulate the expression of the cyclase.
Cyclic AMP accumulation in dark-adapted retinas of Adcy1-/- mice is dramatically lower than that in wild type mice, indicating that AC1 contributes significantly to synthesis of the cyclic nucleotide under these conditions. In addition, the failure of either light or dopamine receptor activation to further reduce cyclic AMP accumulation in Adcy1-/- retinas indicates that the response to both stimuli is mediated by a reduction in AC1 activity. While we can not exclude the possibility that other adenylyl cyclases contribute to the light- and dopamine-regulated pool of cyclic AMP in photoreceptors, their contribution would seem to be small relative to that of AC1. The possibility must be considered that disruption of Adcy1 results in down-regulation of other cyclase genes, but this was not seen, at least for Adcy8. The inhibition of AC1 catalytic activity by light and dopamine likely involves the reduction in intracellular Ca2+ in photoreceptors elicited by both stimuli (Krizaj and Copenhagen 2002;Thoreson et al. 2002;Ivanova et al. 2008), decreasing CaM stimulation of the cyclase. The reduction of Ca2+ in response to light occurs secondary to decreased cGMP-gated currents, hyperpolarization of the plasma membrane, and closure of voltage-gated Ca2+ channels. The decrease in Ca2+ subsequent to D4R activation is less well understood, but may involve a Gi/G0-dependent inhibition of voltage-gated channels (Mei et al. 1995), dopamine receptor-mediated interactions of Ca2+ and Cl- channels (Thoreson et al. 2002), or modulation of Ca2+ channels by cyclic AMP-dependent phosphorylation (Stella, Jr. and Thoreson 2000). Inhibition of AC1 activity by D4R may also involve direct effects of Gi, Go, or transducin (Oak et al. 2000;Yamaguchi et al. 1997).
Disruption of Drd4 or subchronic antagonism of D4Rs with L-745,870 in wild type mice decreased the expression of Adcy1 mRNA. The reduced expression of Adcy1 in retinas of Drd4-/- mice was accompanied by lower Ca2+/CaM-stimulated adenylyl cyclase activity and a lower level of cyclic AMP accumulation in dark-adapted retinas compared to wild type controls. In addition, the photic control of cyclic AMP accumulation was absent in photoreceptors of mice lacking D4Rs, presumably due to the reduced expression of AC1. These results emphasize the importance of AC1 in the light-evoked reduction of cyclic AMP and suggest that D4R activation plays a significant role in regulating the expression of the cyclase.
The decreased expression of Adcy1 mRNA following L-745,870 treatment suggests that D4R receptor activation is required for the normal expression of the cyclase in photoreceptors. The observation that repeated but not acute treatment with the antagonist also suggests that receptor activation has a trophic influence on the expression of the cyclase. Moreover, Adcy1 expression level recovered 1 day after the last of the six injections of antagonist in mice exposed to light to stimulate dopamine release, but not in mice kept in darkness.
The mechanism whereby D4Rs regulate expression of the Ca2+/CaM-stimulated adenylyl cyclases is presently unknown. In pinealocytes, increasing cyclic AMP levels inhibits Adcy1 gene expression (Chan et al. 2001). The decrease of cyclic AMP levels in response to D4R activation in photoreceptors may elicit an increase in expression of the cyclase gene. However, this mechanism appears contradictory to the observation that both cAMP accumulation and Adcy1 expression are low in Drd4-/- retina. The D4R also contains SH3 binding domains (Oldenhof et al. 1998) and D4R stimulation activates Akt/nuclear factor kappa-B signaling, increases ERK1/2 phosphorylation, and induces cFOS (Zhen et al. 2001;Bitner et al. 2006). Thus, D4R activation in retina may recruit signaling cascades that regulate Adcy1 gene expression.
In summary, the present study demonstrates that the photic- and dopamine-signaling pathways in photoreceptor cells converge on AC1 to regulate cyclic AMP formation. D4R activation not only acutely inhibits cyclic AMP formation in retina, but also exerts a trophic influence on the expression of the type 1 Ca2+/CaM-stimulated adenylyl cyclase.
Supplementary Material
Table 1.
Real time PCR primers.
| Primer Name | Sequence | Accession Number |
|---|---|---|
| Adcy1 | F:5'-CACAGCAGGAACCAAGGCTAAGAA-3' R:5'-TGCCAACTCGGAGAACAAAGTCGT-3' |
AF053980 |
| Adcy8 | F:5'-TCCGCTTGGAGACAGAGAACCAAA-3' R:5'-GAATACTGACGTTCTCATAGCGATGG-3' |
NM_009623 |
| Calm1 | F:5'-AGTGTGATGGTTCATTGCTGTGCG-3' R:5'-TGATGGTGTGCTCAAGTCCACAGA-3' |
NM_009790 |
| Calm2 | F:5'-TGGAGTTGGTCCAATGAGGGAACA-3' R:5'-ACGCAGAGTTACAGCTCCACACTT-3' |
NM_007589 |
| Calm3 | F:5'-AAACCATCTGGCTGGCTTTCTGAG-3' R:5'-TTTACAGAACAGCGGGACATGGCT-3' |
NM_007590 |
| Calm4 | F:5'-TCCTTGACCAGAATGGTGATGGCT-3' R:5'-TTCAATGTGGAGGCGCACAAACTC-3' |
NM_020036 |
| Hprt | F:5'-TGGACAGGACTGAAAGACTTGCTC-3' R:5'- GCAGGTCAGCAAAGAACTTATAGCCC-3' |
BC004686 |
| 18S rRNA | F:5'-TAGAGTGTTCAAAGCAGGCCCGA-3' R:5'-ATGCTTTCGCTCTGGTCCGTCTT-3' |
X00686 |
| Gapdh | F:5'-CAGTATGACTCCACTCACGGCAAATTCAA R:5'-GTGATGGGTGTGAACACGAGAA |
NM_008084 |
Acknowledgements
The authors are grateful to Dr. Nikita Pozdeyev, Amy Visser, Jane Abey, and Jim Wessel for assistance with these experiments, and to David Grandy and Malcolm Lowe for providing breeders of the Drd4-/- mice. This study was supported by NIH grants R01EY004864 and R01EY014764 (PMI), T32EY007092 (CRJ), the Core Grant for Vision Research P30EY006360, and NSF Award # 0450303 (CRJ).
Abbreviations
- AC1
type 1 adenylyl cyclase
- AC8
type 8 adenylyl cyclase
- CaM
calmodulin
- D4R
dopamine D4 receptor
- WT
wild type
References
- Besharse JC, Dunis DA, Burnside B. Effects of cyclic adenosine 3',5'-monophosphate on photoreceptor disc shedding and retinomotor movement. Inhibition of shedding and stimulation of cone elongation. J. Gen. Physiol. 1982;79:775–790. doi: 10.1085/jgp.79.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitner RS, Nikkel AL, Otte S, Martino B, Barlow EH, Bhatia P, Stewart AO, Brioni JD, Decker MW, Moreland RB. Dopamine D4 receptor signaling in the rat paraventricular hypothalamic nucleus: Evidence of natural coupling involving immediate early gene induction and mitogen activated protein kinase phosphorylation. Neuropharmacology. 2006;50:521–531. doi: 10.1016/j.neuropharm.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Bonner RF, Emmert-Buck M, Cole K, Pohida T, Chuaqui R, Goldstein S, Liotta LA. Laser capture microdissection: molecular analysis of tissue. Science. 1997;278:1481–1483. doi: 10.1126/science.278.5342.1481. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Resetting the circadian clock in cultured Xenopus eyecups: Regulation of retinal melatonin rhythms by light and D2 dopamine receptors. J. Neurosci. 1991;11:2959–2971. doi: 10.1523/JNEUROSCI.11-10-02959.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan GC, Lernmark U, Xia Z, Storm DR. DNA elements of the type 1 adenylyl cyclase gene locus enhance reporter gene expression in neurons and pinealocytes. Eur. J. Neurosci. 2001;13:2054–2066. doi: 10.1046/j.0953-816x.2001.01578.x. [DOI] [PubMed] [Google Scholar]
- Cohen AI, Blazynski C. Dopamine and its agonists reduce a light-sensitive pool of cyclic AMP in mouse photoreceptors. Vis. Neurosci. 1990;4:43–52. doi: 10.1017/s0952523800002753. [DOI] [PubMed] [Google Scholar]
- Cohen AI, Todd RD, Harmon S, O'Malley KL. Photoreceptors of mouse retinas possess D4 receptors coupled to adenylate cyclase. Proc. Natl. Acad. Sci. USA. 1992;89:12093–12097. doi: 10.1073/pnas.89.24.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glase SA, Akunne HC, Georgic LM, Heffner TG, MacKenzie RG, Manley PJ, Pugsley TA, Wise LD. Substituted [(4-phenylpiperazinyl)-methyl]benzamides: selective dopamine D4 agonists. J. Med. Chem. 1997;40:1771–1772. doi: 10.1021/jm970021c. [DOI] [PubMed] [Google Scholar]
- Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annu. Rev. Pharmacol. Toxicol. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Cahill GM. A role for cyclic AMP in entrainment of the circadian oscillator in Xenopus retinal photoreceptors by dopamine but not by light. J. Neurochem. 1999;72:1812–1820. doi: 10.1046/j.1471-4159.1999.0721812.x. [DOI] [PubMed] [Google Scholar]
- Hillman DW, Lin D, Burnside B. Evidence for D4 receptor regulation of retinomotor movement in isolated teleost cone inner-outer segments. J. Neurochem. 1995;64:1326–1335. doi: 10.1046/j.1471-4159.1995.64031326.x. [DOI] [PubMed] [Google Scholar]
- Hwang M, Morasso MI. The Novel Murine Ca2+-binding Protein, Scarf, Is Differentially Expressed during Epidermal Differentiation. J. Biol. Chem. 2003;278:47827–47833. doi: 10.1074/jbc.M306561200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuvone PM. Evidence for a D2 dopamine receptor in frog retina that decreases cyclic AMP accumulation and serotonin N-acetyltransferase activity. Life Sci. 1986;38:331–342. doi: 10.1016/0024-3205(86)90080-9. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Besharse JC. Dopamine receptor-mediated inhibition of serotonin N-acetyltransferase activity in retina. Brain Res. 1986;369:168–176. doi: 10.1016/0006-8993(86)90525-1. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Gan J, Avendano G. K+-evoked depolarization stimulates cyclic AMP accumulation in photoreceptor-enriched retinal cell cultures: Role of calcium influx through dihydropyridine-sensitive calcium channels. J. Neurochem. 1991;57:615–621. doi: 10.1111/j.1471-4159.1991.tb03792.x. [DOI] [PubMed] [Google Scholar]
- Ivanova TN, Alonso-Gomez AL, Iuvone PM. Dopamine D4 receptors regulate intracellular calcium concentration in cultured chicken cone photoreceptor cells: Relationship to dopamine receptor-mediated inhibition of cAMP formation. Brain Res. 2008;1207:111–119. doi: 10.1016/j.brainres.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, Copenhagen DR. Calcium regulation in photoreceptors. Front Biosci. 2002;7:d2023–d2044. doi: 10.2741/a896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Lieberman BS, Lolley RN. A novel complex from bovine visual cells of a 33 000-dalton phosphoprotein the β- and Γ-transducin: purification and subunit structure. Biochem. 1987;26:3983–3990. doi: 10.1021/bi00387a036. [DOI] [PubMed] [Google Scholar]
- Liu C, Fukuhara C, Wessel JH, III, Iuvone PM, Tosini G. Localization of Aa-nat mRNA in the rat retina by fluorescence in situ hybridization and laser capture microdissection. Cell Tissue Res. 2004;315:197–201. doi: 10.1007/s00441-003-0822-1. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mei YA, Griffon N, Martres MP, Vaudry H, Schwartz J-C, Sokoloff P, Cazin L. Activation of dopamine D4 receptor inhibits an L-type calcium current in cerebellar granule cells. Neurosci. 1995;68:107–116. doi: 10.1016/0306-4522(95)00116-z. [DOI] [PubMed] [Google Scholar]
- Nir I, Haque R, Iuvone PM. Diurnal metabolism of dopamine in the mouse retina. Brain Res. 2000;870:118–125. doi: 10.1016/s0006-8993(00)02409-4. [DOI] [PubMed] [Google Scholar]
- Nir I, Harrison JM, Haque R, Low MJ, Grandy DK, Rubinstein M, Iuvone PM. Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors. J. Neurosci. 2002;22:2063–2073. doi: 10.1523/JNEUROSCI.22-06-02063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oak JN, Oldenhof J, Van Tol HH. The dopamine D(4) receptor: one decade of research. Eur. J. Pharmacol. 2000;405:303–327. doi: 10.1016/s0014-2999(00)00562-8. [DOI] [PubMed] [Google Scholar]
- Oldenhof J, Vickery R, Anafi M, Oak J, Ray A, Schoots O, Pawson T, Von Zastrow M, Van Tol HHM. SH3 binding domains in the dopamine D4 receptor. Biochemistry. 1998;37:15726–15736. doi: 10.1021/bi981634+. [DOI] [PubMed] [Google Scholar]
- Parlato R, Rosica A, Cuccurullo V, Mansi L, Macchia P, Owens JD, Mushinski JF, De Felice M, Bonner RF, Di Lauro R. A preservation method that allows recovery of intact RNA from tissues dissected by laser capture microdissection. Anal. Biochem. 2002;300:139–145. doi: 10.1006/abio.2001.5463. [DOI] [PubMed] [Google Scholar]
- Patel S, Freedman S, Chapman KL, Emms F, Fletcher AE, Knowles M, Marwood R, McAllister G, Myers J, Curtis N, Kulagowski JJ, Leeson PD, Ridgill M, Graham M, Matheson S, Rathbone D, Watt AP, Bristow LJ, Rupniak NM, Baskin E, Lynch JJ, Ragan CI. Biological profile of L-745,870, a selective antagonist with high affinity for the dopamine D4 receptor. J. Pharmacol. Exp. Ther. 1997;283:636–647. [PubMed] [Google Scholar]
- Pierce ME, Besharse JC. Circadian regulation of retinomotor movements. I. Interaction of melatonin and dopamine in the control of cone length. J. Gen. Physiol. 1985;86:671–689. doi: 10.1085/jgp.86.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozdeyev N, Tosini G, Li L, Ali F, Rozov S, Lee RH, Iuvone PM. Dopamine modulates diurnal and circadian rhythms of protein phosphorylation in photoreceptor cells of mouse retina. Eur. J. Neurosci. 2008;27:2691–2700. doi: 10.1111/j.1460-9568.2008.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M, Phillips TJ, Bunzow JR, Falzone TL, Dziewczapolski G, Zhang G, Fang Y, Larson JL, McDougall JA, Chester JA, Saez C, Pugsley TA, Gershanik O, Low MJ, Grandy DK. Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell. 1997;90:991–1001. doi: 10.1016/s0092-8674(00)80365-7. [DOI] [PubMed] [Google Scholar]
- Salomon Y, Londos C, Rodbell M. A highly sensitive adenylate cyclase assay. Anal. Biochem. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Shulman LM, Fox DA. Dopamine inhibits mammalian photoreceptor Na+,K+-ATPase activity via a selective effect on the α3 isozyme. Proc. Natl. Acad. Sci. USA. 1996;93:8034–8039. doi: 10.1073/pnas.93.15.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner TL, Kerns RT, Bender PK. Three different calmodulin-encoding cDNAs isolated by a modified 5'-RACE using degenerate oligodeoxyribonucleotides. Gene. 1994;151:247–251. doi: 10.1016/0378-1119(94)90665-3. [DOI] [PubMed] [Google Scholar]
- Stella SL, Jr., Thoreson WB. Differential modulation of rod and cone calcium currents in tiger salamander retina by D2 dopamine receptors and cAMP. Eur. J. Neurosci. 2000;12:3537–3548. doi: 10.1046/j.1460-9568.2000.00235.x. [DOI] [PubMed] [Google Scholar]
- Thoreson WB, Stella SL, Jr., Bryson EI, Clements J, Witkovsky P. D2-like dopamine receptors promote interactions between calcium and chloride channels that diminish rod synaptic transfer in the salamander retina. Vis. Neurosci. 2002;19:235–247. doi: 10.1017/s0952523802192017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G, Dirden JC. Dopamine inhibits melatonin release in the mammalian retina: in vitro evidence. Neurosci. Lett. 2000;286:119–122. doi: 10.1016/s0304-3940(00)01117-4. [DOI] [PubMed] [Google Scholar]
- Udovichenko IP, Newton AC, Williams DS. Regulation of the phosphorylation state of rhodopsin by dopamine. J. Biol. Chem. 1998;273:7181–7184. doi: 10.1074/jbc.273.13.7181. [DOI] [PubMed] [Google Scholar]
- Wang H, Storm DR. Calmodulin-regulated adenylyl cyclases: cross-talk and plasticity in the central nervous system. Mol. Pharmacol. 2003;63:463–468. doi: 10.1124/mol.63.3.463. [DOI] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Doc. Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Wu Z, Thomas SA, Villacres EC, Xia Z, Simmons ML, Chavkin C, Palmiter RD, Storm DR. Altered Behavior and Long-Term Potentiation in Type I Adenylyl Cyclase Mutant Mice. Proceedings of the National Academy of Sciences. 1995;92:220–224. doi: 10.1073/pnas.92.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Choi E-J, Wang F, Blazynski C, Storm DR. Type I calmodulin-sensitive adenylyl cyclase is neural specific. J. Neurochem. 1993;60:305–311. doi: 10.1111/j.1471-4159.1993.tb05852.x. [DOI] [PubMed] [Google Scholar]
- Xu LX, Tanaka Y, Bonderenko VA, Matsuura I, Matsumoto H, Yamazaki A, Hayashi F. Phosphorylation of the gamma subunit of the retinal photoreceptor cGMP phosphodiesterase by the cAMP-dependent protein kinase and its effect on the gamma subunit interaction with other proteins. Biochemistry. 1998;37:6205–6213. doi: 10.1021/bi973087i. [DOI] [PubMed] [Google Scholar]
- Yamaguchi I, Harmon SK, Todd RD, O'Malley KL. The rat D4 dopamine receptor couples to cone transducin (Galphat2) to inhibit forskolin-stimulated cAMP accumulation. J. Biol. Chem. 1997;272:16599–16602. doi: 10.1074/jbc.272.26.16599. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Derbiszewska T, Sek B, Nowak JZ. Dopamine-dependent cyclic AMP generating system in chick retina and its relation to melatonin biosynthesis. Neurochem. Int. 1995;27:535–543. doi: 10.1016/0197-0186(95)00033-5. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Nowak JZ. Does D4 dopamine receptor mediate the inhibitory effect of light on melatonin biosynthesis in chick retina. Neurosci. Lett. 1994;166:203–206. doi: 10.1016/0304-3940(94)90486-3. [DOI] [PubMed] [Google Scholar]
- Zhen X, Zhang J, Johnson GP, Friedman E. D(4) dopamine receptor differentially regulates Akt/nuclear factor-kappa b and extracellular signal-regulated kinase pathways in D(4)MN9D cells. Mol Pharmacol. 2001;60:857–864. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.