Abstract
Pd(0)-catalyzed intermolecular arylation of sp3 C–H bonds has been achieved using PR3/ArI. This protocol can be used to arylate a variety of aliphatic carboxylic acid derivatives, including a number of bioactive drug molecules. The use of fluorinated aryl iodides also allows for the introduction of fluorine into a molecule of interest.
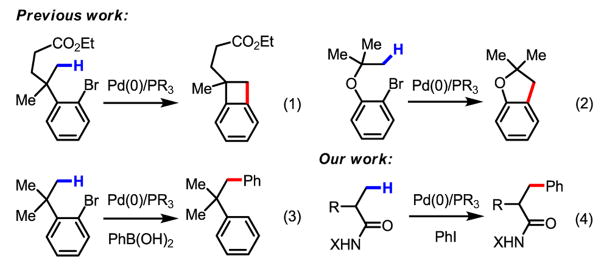
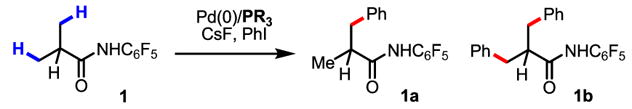
Palladium-catalyzed arylation of C–H bonds using Pd(0)/PAr3/ArI has attracted a great deal of attention during the past several decades.1–3 Compared to Pd(II)-catalyzed oxidative C–H functionalization processes, arylation using this type of catalytic system accommodates phosphine and NHC ligands and does not require a co-oxidant, both of which can be significant practical advantages. Despite pioneering efforts to apply this arylation reaction to sp3 C–H bonds, to date, only a few examples of alkyl C–H activation by an intramolecular ArPdBr species have been reported by Dyker and others (eq 1–3).4–5 Herein we report an example of Pd(0)-catalyzed intermolecular arylation of β-C–H bonds in a wide range of amide substrates (eq 4). The development of a simple and readily removable amide directing group was crucial for enabling facile C–H activation under our reaction conditions. Moreover, the use of CsF as the base allowed the substrate scope to be expanded to include amides that contain α-hydrogen atoms.
Previously, we have reported various auxiliary approaches for β-C–H functionalization of aliphatic acid derivatives using Pd(II)/Pd(IV) and Pd(II)/Pd(0) catalysis.6 In particular, the excellent observed reactivity of O-methyl hydroxamic acids for β-C–H activation prompted us to test whether intermolecular arylation of β-C–H bonds could be achieved using this substrate and an ArI/Pd(0)/PPh3 catalytic system. During our preliminary screening, however, we found that the N–H bond of the CONHOMe moiety readily underwent Buchwald–Hartwig amination with ArI. We hypothesized that by reducing the nucleophilicity of the amide N–H bond, we could suppress the amination pathway. To this end, we examined the effect of replacing the N–OMe group with a wide range of N–aryl groups (Table 1) and screened each of these substrates with a variety of different bases and ligands. Among the bases tested, only CsF was found to be effective for arylation with substrates that contained α-hydrogen atoms, although substrates that did not contain α-hydrogen atoms worked well with Cs2CO3 (see SI). With guidance from previous studies,1–4 we also screened an array of different phosphine ligands (Table 2). Overall, we found that the desired β-C–H arylation reaction proceed best using an CONH–C6F5 directing group, with Buchwald’s Cyclohexyl JohnPhos ligand and CsF as base. Notably, the use of Fu’s strategy for improving the stability of the phosphine ligands by forming the HBF4 salt7 allowed our reaction to be performed without using stringent air-sensitive techniques.
Table 1.
 | ||||
|---|---|---|---|---|
| X = OMe a 0% b 0% |
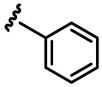 a 3% b 0% |
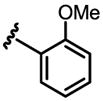 a 0% b 0% |
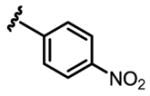 a 12% b 0% |
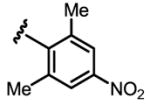 a 10% b 0% |
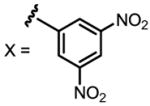 a 24% b 12% |
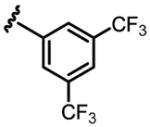 a 18% b 8% |
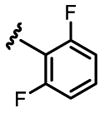 a 30% b 22% |
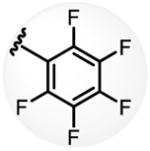 a 34% b 54% |
|
Conditions: 0.2 mmol of substrate, 10 mol% Pd(OAc)2, 20 mol% ligand, 3.0 equiv of CsF, 3.0 equiv of aryl iodide, 100 mg 3Å MS, 1 mL toluene, 100 °C, N2, 24 h.
Yield was determined by 1H NMR analysis of crude product using CH2Br2 as the internal standard.
Table 2.
Ligand Screeninga
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | PR3 | 1H NMR yield (%) | entry | PR3 | 1H NMR yield (%) | ||
| 1a | 1b | 1a | 1b | ||||
| 1 | PPh3 | 3 | 0 | 10 | PAd2nBu•HBF4 | 0 | 0 |
| 2 | P(p-Tol)3 | 2 | 0 | 11 | PCy2(o-Tol)•HBF4 | 0 | 0 |
| 3 | PiPr3•HBF4 | 36 | 18 | 12 | Cy-JohnPhos•HBF4 | 34 | 54 |
| 4 | PCy3•HBF4 | 42 | 20 | 13 | MePhos•HBF4 | 38 | 53 |
| 5 | PtBu3•HBF4 | 0 | 0 | 14 | DavePhos•HBF4 | 48 | 18 |
| 6 | PtBu2Me•HBF4 | 0 | 0 | 15 | SPhos•HBF4 | 11 | 0 |
| 7 | PCy2tBu•HBF4 | 8 | 0 | 16 | RuPhos•HBF4 | 5 | 0 |
| 8 | PPhEt2•HBF4 | 10 | 0 | 17 | XPhos•HBF4 | 0 | 0 |
| 9 | PPhHex2•HBF4 | 7 | 0 | ||||
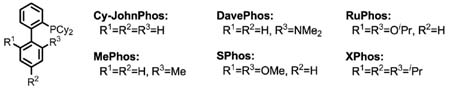 | |||||||
The reaction conditions are identical to those described in Table 1.
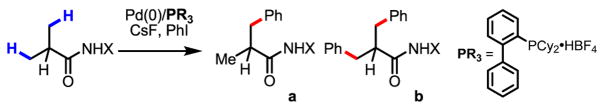
Using this newly developed protocol, a wide range of α-methylated carboxylic acids (after first being converted to the corresponding amides) were arylated in excellent yields. β-Arylation of amide 2, which contains a quaternary α-carbon atom, gave the mono-arylated product 2a in 78% yield. Amides with tertiary and secondary α-carbon atoms were also arylated in good yields (1a, 3a–6a), although the arylation of β-methylene C–H bonds in pentanoic acid derived substrate gives only trace amount of porduct. Since amides 1 and 3 are derived from abundant and cheap feedstock chemicals (propanoic and isopropanoic acid, respectively), this arylation process provides a potential method for installing these prevalent three- and four-carbon units onto advanced arene precursors in synthesis. Functional groups such as protected amines, ethers and ketones were tolerated (5a, 7a, 9a, 10a). The directing group in the product can be removed through either hydrolysis with 2 N KOH in ethylene glycol at 80 °C or treatment with MeI/Na2CO3 followed by 2 N HCl at 24 °C.
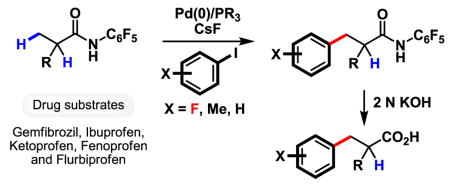
The potential of this new catalytic reaction for application in a host of different settings, including drug diversification was further demonstrated by the arylation of amides 7–11 derived from the drugs gemfibrozil, ibuprofen, ketoprofen, fenoprofen and flurbiprofen (Table 3). Strategically, the ability to selectively functionalize inert methyl groups in biologically active molecules is of great importance in drug discovery.
Table 3.
β-Arylation of Carboxylic Acid Amidesa
 | ||
|---|---|---|
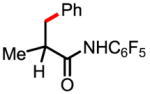 1a 30% mono 1b 53% di |
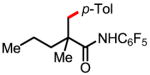 2a 78% mono trace of di |
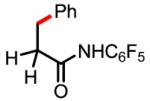 3a 58% |
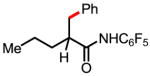 4a 80% |
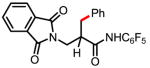 5a 64% |
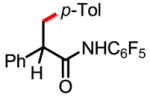 6a 84% |
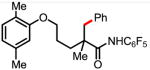 7a 60% mono 7b 22% di |
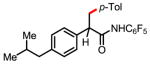 8a 80% |
|
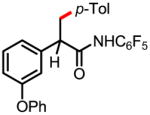 9a 68% |
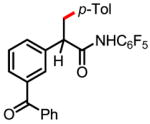 10a 70% |
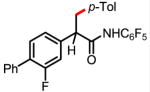 11a 72% |
The reaction conditions are the same as described in Table 1.
Considering the difficulty of introducing fluorine onto arenes, arylation of C–H bonds using fluorinated aryl iodides could be a useful alternative for the synthesis of fluorinated arenes in medicinal chemistry. To demonstrate this idea, a variety of fluorinated aryl iodides were shown to react with 6 (Table 4).
Table 4.
Arylation with Fluorinated ArIa
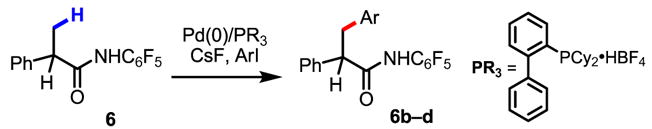 | ||
|---|---|---|
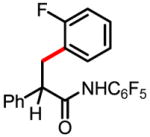 6b 68% |
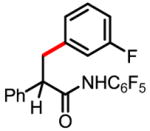 6c 72% |
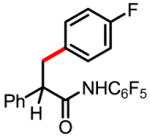 6d 66% |
The reaction conditions are identical to those described in Table 1.
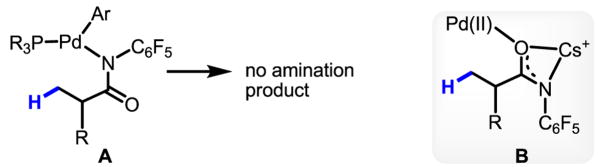
The mechanistic implications of this intermolecular C–H activation reaction merit discussion. The data in Table 1 show that an acidic N–H bond in the directing group is essential for reactivity. The fact that amination of the ArI is not observed in the presence of well-established ligands for amination is inconsistent with the involvement of a Pd–NCOAr species A (Fig. 1). We propose that the C–H activation precursor B bears a coordination mode analogous to that of the complex involved in ortho-C–H cleavage of phenyl acetic acid substrates by Pd(OAc)2 (Fig. 1).8 At this stage, the mechanism at play in the subsequent C–H cleavage step in our system has yet to be fully elucidated. For instance, it is unclear whether the phosphine ligand remains bound to the Pd(II) center during this process. Furthermore, the question of whether classical oxidative addition or proton abstraction by an external F− is the operative pathway also warrants further investigation (Fig. 1).
Figure 1.
Possible Coordination Modes
In summary, we have developed a novel protocol for intermolecular arylation of sp3 C–H bonds. This reaction provides a simple method for β-arylation of carboxylic acids. Studies to develop an enantioselective version of this reaction are currently underway in our laboratory.
Supplementary Material
Scheme 1.
Pd(0)-Catalyzed Arylation of C–H Bonds
Acknowledgments
We gratefully acknowledge The Scripps Research Institute, the National Institutes of Health (NIGMS, 1 R01 GM084019-02), Amgen and Eli Lilly for financial support. We thank the A. P. Sloan Foundation for a fellowship (J.-Q.Y.), and the National Science Foundation, the Department of Defense, and the Skaggs Oxford Scholarship program for predoctoral fellowships (K.M.E.).
Footnotes
Supporting Information Available: Experimental procedure and characterization of all new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For arylation of heterocycles see: Nakamura N, Tajima Y, Sakai K. Heterocycles. 1982;17:235.Akita Y, Ohta A. Heterocycles. 1982;19:329.Pivsa-Art S, Satoh T, Kawamura Y, Miura M, Nomura M. Bull Chem Soc Jpn. 1998;71:467.Park CH, Ryabova V, Seregin IV, Sromek AW, Gevorgyan V. Org Lett. 2004;6:1159. doi: 10.1021/ol049866q.Touré BB, Lane BS, Sames D. Org Lett. 2006;8:1979. doi: 10.1021/ol053021c.Seregin IV, Gevorgyan V. Chem Soc Rev. 2007;36:1173. doi: 10.1039/b606984n.
- 2.For pioneering work on Pd(0)-catalyzed arylation of arenes see: Hennings DD, Iwasa S, Rawal VH. J Org Chem. 1997;62:2. doi: 10.1021/jo961876k.Satoh T, Kawamura Y, Miura M, Nomura M. Angew Chem Int Ed. 1997;36:1740.
- 3.For Pd(0)-catalyzed intermolecular arylation of sp2 C–H bonds see: Kametani Y, Satoh T, Miura M, Nomura M. Tetrahedron Lett. 2000;41:2655.Lafrance M, Fagnou K. J Am Chem Soc. 2006;128:16496. doi: 10.1021/ja067144j.Chiong HA, Pham QN, Daugulis O. J Am Chem Soc. 2007;129:9879. doi: 10.1021/ja071845e.
- 4.(a) Dyker G. Angew Chem Int Ed. 1992;31:1023. [Google Scholar]; (b) Baudoin O, Herrbach A, Guéritte F. Angew Chem Int Ed. 2003;42:5736. doi: 10.1002/anie.200352461. [DOI] [PubMed] [Google Scholar]; (c) Zhao J, Campo M, Larock RC. Angew Chem Int Ed. 2005;44:1873. doi: 10.1002/anie.200462327. [DOI] [PubMed] [Google Scholar]; (d) Barder TE, Walker SD, Martinelli JR, Buchwald SL. J Am Chem Soc. 2005;127:4685. doi: 10.1021/ja042491j. [DOI] [PubMed] [Google Scholar]; (e) Ren H, Knochel P. Angew Chem Int Ed. 2006;45:3462. doi: 10.1002/anie.200600111. [DOI] [PubMed] [Google Scholar]; (f) Lafrance M, Gorelsky SI, Fagnou K. J Am Chem Soc. 2007;129:14570. doi: 10.1021/ja076588s. [DOI] [PubMed] [Google Scholar]; (g) Watanabe T, Oishi S, Fujii N, Ohno H. Org Lett. 2008;10:1759. doi: 10.1021/ol800425z. [DOI] [PubMed] [Google Scholar]
- 5.For an example of Cu(II)-catalyzed arylation of sp3 C–H bonds adjacent to a nitrogen atom with boronic acids see: Baslé O, Li CJ. Org Lett. 2008;10:3661. doi: 10.1021/ol8012588.
- 6.(a) Giri R, Liang J, Lei JG, Li JJ, Wang DH, Chen X, Naggar IC, Guo C, Foxman BM, Yu JQ. Angew Chem Int Ed. 2005;44:7420. doi: 10.1002/anie.200502767. [DOI] [PubMed] [Google Scholar]; (b) Wang DH, Wasa M, Giri R, Yu JQ. J Am Chem Soc. 2008;130:7190. doi: 10.1021/ja801355s. [DOI] [PubMed] [Google Scholar]
- 7.Netherton MR, Fu GC. Org Lett. 2001;3:4295. doi: 10.1021/ol016971g. [DOI] [PubMed] [Google Scholar]
- 8.For detailed discussion and evidence for cation-promoted Pd insertion into C–H bonds, see: Giri R, Yu JQ. J Am Chem Soc. 2008;130:14082. doi: 10.1021/ja8063827. and references therein.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.