Abstract
Persistent, chronic oxidative injury may play a mechanistic role in late radiation injury. Thus antioxidants may be useful as mitigators of radiation injury. The antioxidants deferiprone, genistein and apocynin were tested in a rat radiation nephropathy model that uses single-fraction total-body irradiation (TBI) followed by syngeneic bone marrow transplant. Deferiprone was added to the drinking water at 1.0 or 2.5 g/liter, starting 3 days after the TBI. Urinary bleomycin-detectable iron, which could enhance production of oxygen radicals, was reduced in the rats on deferiprone compared to untreated rats, but deferiprone did not mitigate radiation nephropathy. Genistein added to the chow at 750 mg/kg starting immediately after TBI did not mitigate radiation nephropathy. Apocynin added to the drinking water at 250 mg/liter immediately after TBI did not mitigate radiation nephropathy. Thus three different types of antioxidants, when used at doses consistent with an antioxidant effect, had no mitigation efficacy against radiation nephropathy.
INTRODUCTION
Reactive oxygen species (ROS) have been implicated in many tissue injuries and are the accepted mechanism for acute radiation effects on normal tissue. The hydroxyl radicals generated within milliseconds after irradiation are key to its genotoxic effects. It has been proposed that oxidative stress plays an additional, longer-term role in radiation injury at days, weeks or months after irradiation (1). This hypothesis could explain the benefit from existing treatments, such as angiotensin-converting enzyme inhibition or angiotensin receptor blockade (1), but also could point toward new treatments. This would be particularly important for late-responding tissues such as lung, brain and kidney.
Kuin et al. (2) reported a lack of benefit of the antioxidant n-acetyl-cysteine in murine radiation ne-phropathy. Mouse kidneys are, however, relatively resistant to the effects of radiation; confirmation in other species is thus important. In recent studies, we have used a rat model of radiation nephropathy to test for evidence of oxidative stress. Thus far, this search has yielded no evidence for increased oxidative stress at weeks to months after irradiation (3). Lack of evidence could reflect a subtle or hard-to-find occurrence of oxidative stress. We therefore tested three antioxidant compounds in this model.
METHODS
Rat Syngeneic Bone Marrow Transplant (BMT) Model
Total-body irradiation (TBI) regimens were used to cause radiation nephropathy (4). This radiation nephropathy is characterized by proteinuria, azotemia and progressive hypertension that leads to renal failure after a median time of 20 to 30 weeks. Renal failure (uremia) is the only significant cause of illness and death in this model. The studies were performed in syngeneic WAG/Rij/Cmcr rats that were bred and housed in a moderate-security barrier. The animals were free of Mycoplasma pulmonis, Pseudomonas and common rat viruses. No antibiotics or immunosuppressive drugs were used. The rats were maintained in the Biomedical Resource Center of the Medical College of Wisconsin, which is fully accredited by the American Association of Accreditation of Laboratory Animal Care. The animal protocols were approved by the Institutional Animal Care and Use Committee.
Seven- to 8-week-old rats received TBI in a single dose. Irradiation was done with a 300 kVp orthovoltage source with a half-value layer of 1.4 mm copper; the radiation dosimetry was described in detail by Cohen et al. (5). For irradiation, unanesthetized rats were immobilized in a specially constructed jig. Within 24 h after TBI, the rats received BMT from a syngeneic donor.
Animals were monitored daily in all experiments. Development of uremia was assessed for up to 26 weeks after TBI, and animals whose blood urea nitrogen (BUN) exceeded 120 mg/dl (i.e., those that were uremic) were euthanized. BUN, urine protein and urine creatinine were determined at specified intervals with commercial kits. Urine protein excretion was expressed as the ratio of urine protein to urine creatinine (UP/UC); this was done to account for the known urine-concentrating defect that occurs in renal injury and to normalize for animal size differences.
Antioxidant Interventions
The three compounds (an iron chelator, a soy isoflavone, and a substituted catechol) were assessed in mitigation regimens (6) in which therapy is started after irradiation but before the evidence of tissue injury. All therapy continued for the duration of the study. The different types of agents were used to enable a broader test of the hypothesis.
The iron chelator deferiprone (DFP) was tested because iron can act as a co-factor in the Haber-Weiss reaction to form hydroxyl radicals (7–9). DFP was obtained from Cormedix, Inc. (Summit, NJ); it was used at 1.0 and 2.5 g/liter in the drinking water. These doses were based on data for use in humans, gerbils and rats (10); at 1.0 and 2.5 g/liter in the drinking water, the daily dose was 50 or 125 mg/kg per day, respectively. Animals were placed on DFP-containing water 3 days after TBI.
The soy isoflavone genistein has antioxidant effects, possibly mediated via an estrogen receptor (11), and has radioprotective effects in mice (12, 13). Genistein was obtained from LC Laboratories (Woburn, MA), as 4′,5,7-trihydroxyisoflavone and was mixed into a soy-free diet at 750 mg/kg of diet. In rats, this dose will achieve serum levels of 0.2–3 µmol/liter (14, 15), levels that are high enough to have antioxidant effects (11, 16). Control rats in this part of the study were fed the soy-free diet. Animals were placed on the special diets immediately after TBI.
The substituted catechol apocynin, which is derived from the Picorhiza kurroa plant, has antioxidant effects and, in particular, inhibits NADPH oxidase activation (17). It also has anti-hypertensive activity and attenuates diabetic nephropathy (18, 19). It was obtained from Sigma-Aldrich (St Louis, MO) as 4′-hydroxy-3′methoxyacetophenone. A dose of 250 mg/liter in the drinking water was chosen based on doses reported to have an antioxidant effect (20–22). The apocynin was dissolved in 1% ethanol in water to enhance solubility; control rats in this part of the study were given 1% ethanol in their drinking water. Animals were started on the apocynin-containing water immediately after TBI.
Testing for Bleomycin-Detectable Iron
Urinary bleomycin detectable iron was measured as described by Evans and Halliwell (23); this assay is based on the formation of a bleomycin-iron complex, which reacts with DNA, resulting in its degradation. The DNA degradation products were measured by the thiobarbituric acid reaction. A standard curve was prepared fresh with each batch of the assay, using FeCl3 (Sigma, Steinheim, Germany) with iron concentrations ranging from 0.1–1000 µmol/liter. All reactions were carried out in disposable polypropylene tubes to avoid iron contamination, and all the reagent solutions except the bleomycin were treated overnight with 30 mg/ml Chelex (Bio-Rad, Hercules, CA) to remove any iron.
Statistical Analysis
Data are shown as geometric means with 95% confidence intervals. The Mann-Whitney tests were used for two-group comparisons. Statistical significance was set at P < 0.05.
RESULTS
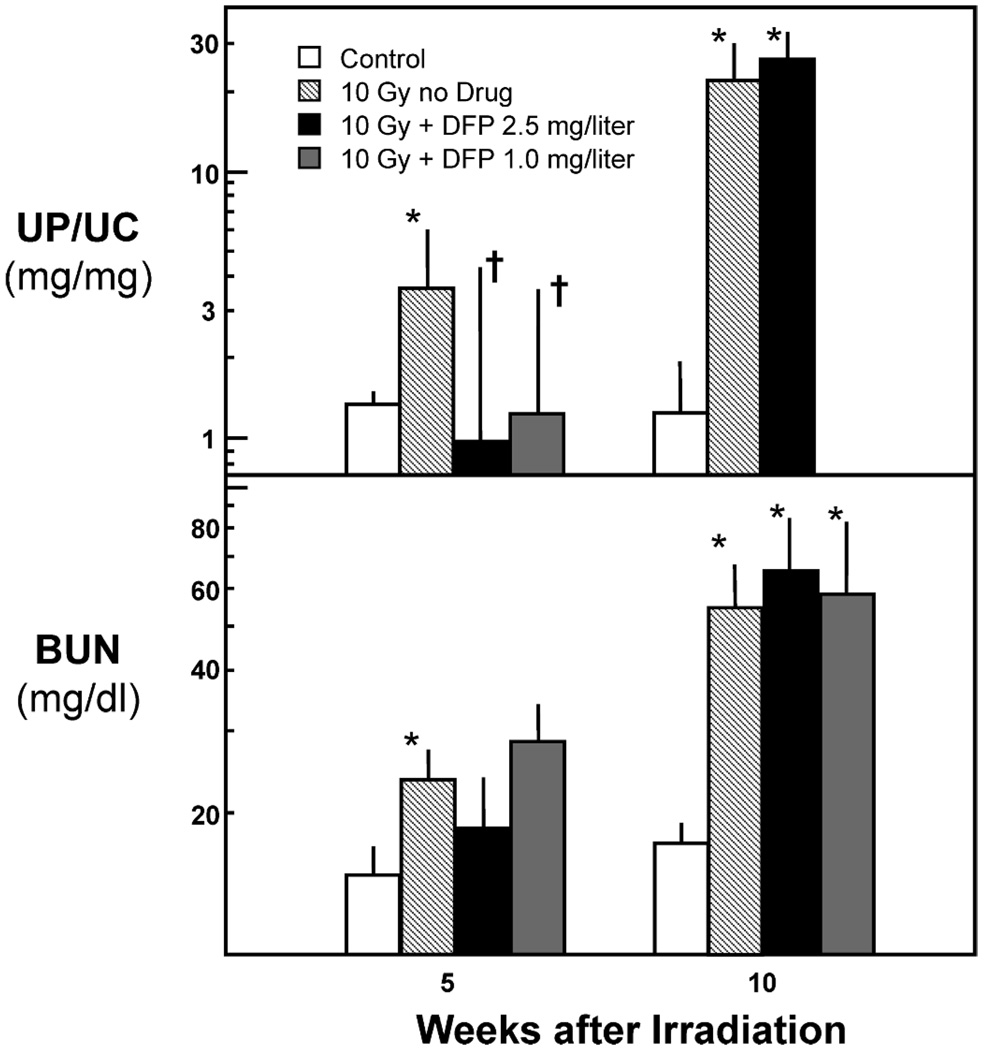
In the first DFP study, male animals were randomized to no DFP or TBI (n = 11), 10 Gy TBI (n = 11), and 10 Gy TBI plus DFP at 2.5 g/liter (n = 11). DFP appeared to mitigate radiation-induced proteinuria at 5 weeks after irradiation (P < 0.05) but not at 10 weeks (Fig. 1). There was no benefit of DFP on azotemia (as BUN) at either 5 or 10 weeks (Fig. 1). DFP did appear to exert a biological effect, as shown by its lowering of urinary bleomycin-detectable iron in irradiated rats from 77 (25–237) µmol per mmol creatine to 8 (4–17) µmol/mmol (P < 0.003). Note that TBI alone reduced bleomycin-detectable iron, because the level in age-matched normal animals was 280 (120–650) µmol/mmol (P = 0.03 compared to TBI alone). Because of a concern that DFP toxicity might be concealing a mitigating effect of DFP, the study was repeated with animals given 10 Gy TBI and randomized to DFP at 1.0 g/liter (n = 12) or to no drug (n = 6). At neither 5 nor 10 weeks after TBI did DFP at 1.0 g/liter have a mitigating effect on radiation-induced azotemia (Fig. 1). In neither study did DFP delay the development of uremia (data not shown).
FIG. 1.
Effect of deferiprone (DFP) at 1.0 or 2.5 g/liter in drinking water on proteinuria (as UP/UC, upper panel) and azotemia (as BUN, lower panel) after 10 Gy TBI plus BMT. Data are shown as geometric means with upper 95% confidence limits. The asterisks (*) indicate differences (P < 0.05) from unirradiated controls and daggers (†) differences (P < 0.05) from animals receiving TBI alone.
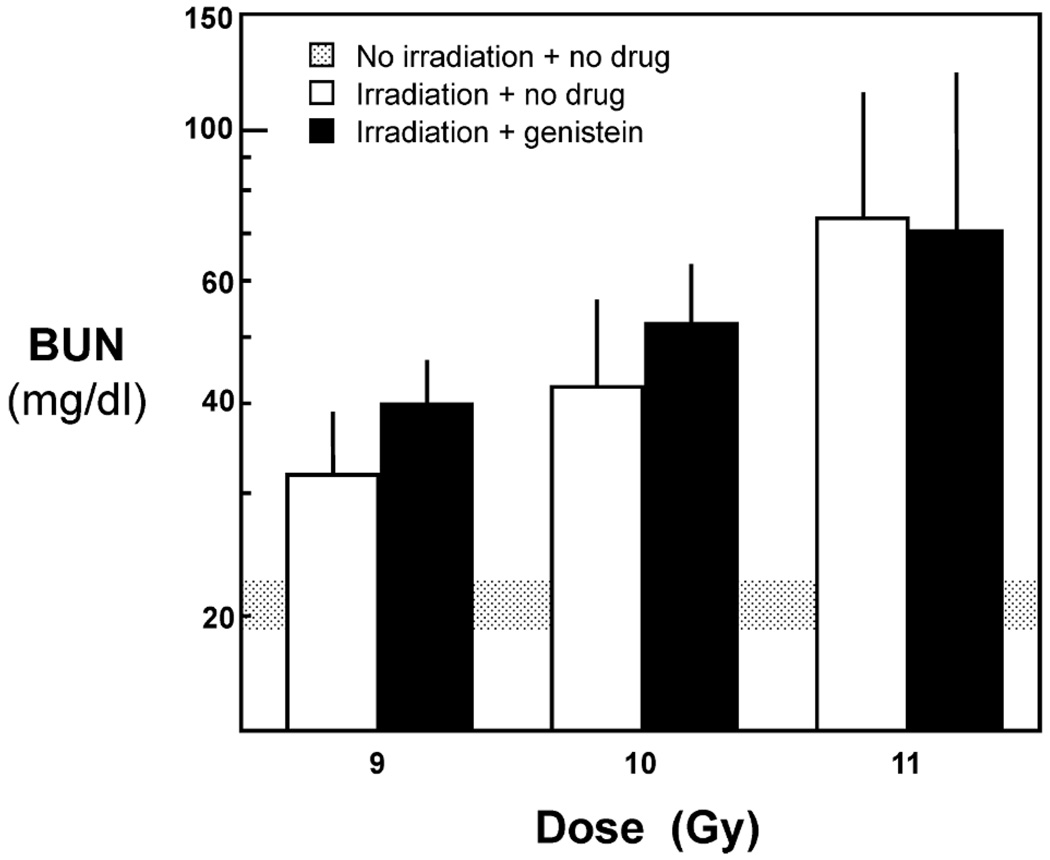
In the first genistein study, male rats were given TBI at 9, 10 or 11 Gy, with eight animals given TBI alone at each dose and eight placed on the genistein diet immediately after irradiation. Genistein did not mitigate radiation-induced azotemia (Fig. 2) or proteinuria (data not shown) after 13 weeks of treatment, and the study was terminated. To address the potential confounding by an effect of estrogen, an additional 16 female rats underwent 10 Gy TBI, of which eight were placed on genistein. In the female rats, radiation-induced proteinuria, radiation-induced azotemia, and the development of uremia were not mitigated by the genistein.
FIG. 2.
Effect of genistein given at 750 mg/kg in the diet on kidney function (as BUN) at 13 weeks after TBI plus BMT. Data are shown as geometric means with upper confidence limits.
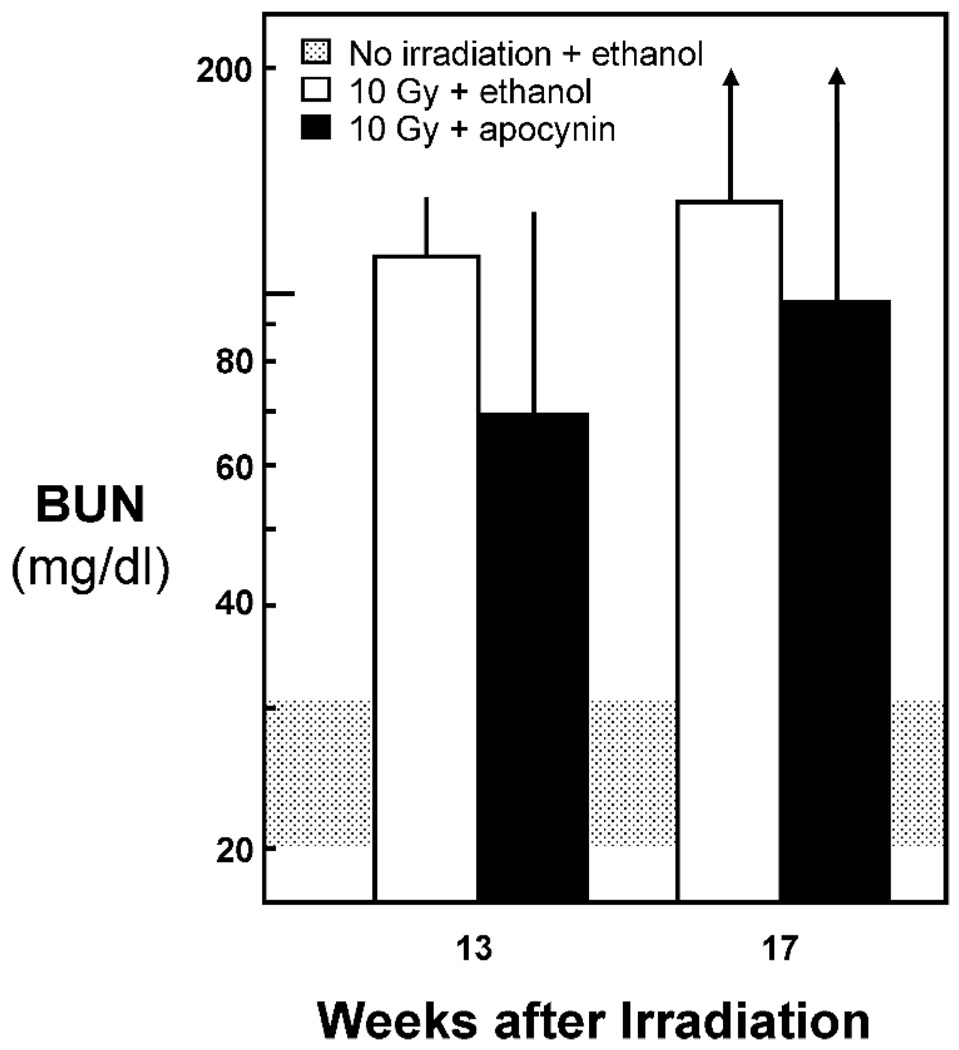
In the apocynin study, male animals were randomized to 1% ethanol in their drinking water and no TBI (n = 5), 10 Gy TBI alone (n = 5), and 10 Gy TBI plus apocynin in 1% ethanol (n = 5). Apocynin did not mitigate radiation-induced azotemia (Fig. 3) or proteinuria (data not shown) after 13 weeks of therapy and did not delay the development of uremia (data not shown). Despite the addition of 1% ethanol to the drinking water, the unirradiated rats had normal values for BUN and for UP/UC.
FIG. 3.
Effect of apocynin given at 250 mg/liter in the drinking water on kidney function (as BUN) at 13 weeks after 10 Gy TBI. Data are shown as geometric means with upper confidence limits.
DISCUSSION
These studies show no statistically significant mitigation of radiation nephropathy by acknowledged antioxidants used at doses that are consistent with an antioxidant effect. Their lack of benefit provides evidence against the hypothesis that there is ongoing chronic oxidative stress in radiation nephropathy.
We have shown previously that radiation nephropathy can be mitigated by antagonists of the renin-angiotensin system using the same effective radiation doses as used in the present studies (24, 25). Thus lack of benefit in the present study is occurring in a model in which radiation injuries can be mitigated by other agents.
Genistein was tested because of its known antioxidant activity (12, 13) and because a colleague3 had evidence for mitigation efficacy in a rodent model of radiation-induced lung injury. While no evidence for efficacy was found in the renal model, it is possible that genistein did exert an antioxidant effect but that competing actions nullified that benefit. Thus, at serum levels of genistein just below those used in this study, rats have a 50% reduction in adrenal hormone synthesis (26), and such an effect could antagonize an antioxidant benefit.
DFP was chosen because of the role of iron in the production of ROS. This mechanism explains the deleterious effect of iron overload in thalassemia, for which iron chelation, and specifically deferiprone, is used. Iron chelation reduces renal injury in other models, such as nephrotoxic serum nephritis and cisplatinum nephrotoxicity (7). The possibility of benefit of an iron chelator for radiation injury is further emphasized by the finding of iron deposition in some clinical cases of radiation nephropathy that occur after BMT (27) and by reports of increased iron in serum after BMT (28). We did find a reduction of urinary bleomycin-detectable iron by radiation alone in the present studies, which is not consistent with an iron-mediated pathogenesis of injury in radiation nephropathy.
Apocynin was tested because of speculation that the established benefit of antagonists of the rennin-angiotensin system (RAS) for mitigation of radiation nephropathy (24, 29) might be due to the pro-oxidant activity of angiotensin II (AII) (1). Since AII is reported to activate ROS production via NADPH oxidase (30), an NADPH oxidase antagonist such as apocynin is a logical agent for trying to block this effect (31). Its lack of efficacy in the present studies supports a lack of ROS excess in radiation nephropathy and is also consistent with the lack of RAS activation in this model. It is possible that a higher dose of apocynin could have had a beneficial effect. Paliege et al. (32) reported that apocynin at 2.5 mmol/liter in the drinking water did lower the urinary isoprostanes in hypertensive rats, but there was no reduction of the hypertension in that study.
Oxidative injury is reported to play a significant role in many forms of kidney disease, including diabetes, cisplatinum toxicity, and acute ischemic renal failure. However, antioxidants are not always beneficial (33, 34). In addition, many studies that show benefit of antioxidants have used them in a prevention mode (i.e., with the agent being given before the injury). Such prevention studies may inform us about acute injury but not about the subsequent mechanistic cascade that may lead to chronic organ failure.
These results do not exclude the possibility that there may be mitigation of radiation nephropathy by other antioxidants, but when taken together with the lack of direct evidence for chronic renal oxidative stress after irradiation (3), these results cast doubt on the hypothesis that chronic oxidative stress plays a major role in the pathogenesis of radiation nephropathy.
ACKNOWLEDGMENTS
These studies were supported by an NCI grant (CA24652) and an NIAID cooperative agreement (AI067734). Yvonne A. Morauski assisted with preparation of the manuscript. Marylou Mäder provided expert technical assistance.
Footnotes
A. Para, V. Calveley, A. Langan, I. Yeung, J. Van Dyk and R. P. Hill, Mitigation and treatment of radiation-induced lung damage by genistein. Presented at the 13th International Congress of Radiation Research, San Francisco, 2007.
REFERENCES
- 1.Zhao W, Diz DI, Robbins ME. Oxidative damage pathways in relation to normal tissue injury. Br. J. Radiol. 2007;80:S23–S31. doi: 10.1259/bjr/18237646. [DOI] [PubMed] [Google Scholar]
- 2.Kuin A, Kruse JJ, Stewart FA. Proteinuria and vascular changes after renal irradiation: The role of reactive oxygen species (ROS) and vascular endothelial growth factor (Vegf) Radiat. Res. 2003;159:174–181. doi: 10.1667/0033-7587(2003)159[0174:pavcar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Lenarczyk M, Cohen EP, Fish BL, Irving AA, Sharma M, Driscoll CD, Moulder JE. Chronic oxidative stress as a mechanism for radiation nephropathy. Radiat. Res. 2009;171:164–172. doi: 10.1667/RR1454.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moulder JE, Fish BL. Late toxicity of total body irradiation with bone marrow transplantation in a rat model. Int. J. Radiat. Oncol. Biol. Phys. 1989;16:1501–1509. doi: 10.1016/0360-3016(89)90955-3. [DOI] [PubMed] [Google Scholar]
- 5.Cohen EP, Fish BL, Sharma M, Li XA, Moulder JE. The role of the angiotensin II type-2 receptor in radiation nephropathy. Trans. Res. 2007;150:106–115. doi: 10.1016/j.trsl.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, Dynan WS, Fike JR, Grdina DJ, Zaharevitz D. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI workshop, December 3–4, 2003. Radiat. Res. 2004;162:711–728. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 7.Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV. In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int. 1998;53:394–401. doi: 10.1046/j.1523-1755.1998.00767.x. [DOI] [PubMed] [Google Scholar]
- 8.Wood JC, Otto-Duessel M, Gonzalez I, Aguilar MI, Shimada H, Nick H, Nelson M, Moats R. Deferasirox and deferiprone remove cardiac iron in the iron-overloaded gerbil. Transl. Res. 2006;148:272–280. doi: 10.1016/j.trsl.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das UN. Tumoricidal and anti-angiogenic actions of gamma-linolenic acid and its derivatives. Curr. Pharm. Biotechnol. 2006;7:457–466. doi: 10.2174/138920106779116892. [DOI] [PubMed] [Google Scholar]
- 10.Piga A, Roggero S, Vinciguerra T, Sacchetti L, Gallo V, Longo F. Deferiprone: New insight. Ann. NY Acad. Sci. 2005;1054:169–174. doi: 10.1196/annals.1345.019. [DOI] [PubMed] [Google Scholar]
- 11.Borrás C, Gambini J, Gómez-Cabrera MC, Sastre J, Pallardó FV, Mann GE, Viña, Genistein J. a soy isoflavone, up-regulates expression of antioxidant genes: involvement of estrogen receptors, ERK1/2, and NFκB. FASEB J. 2006;20:2136–2138. doi: 10.1096/fj.05-5522fje. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Mi MT. Genistein stimulates hematopoiesis and increases survival in irradiated mice. J. Radiat. Res. (Tokyo) 2005;46:425–433. doi: 10.1269/jrr.46.425. [DOI] [PubMed] [Google Scholar]
- 13.Davis TA, Clarke TK, Mog SR, Landauer MR. Subcutaneous administration of genistein prior to lethal irradiation supports multilineage, hematopoietic progenitor cell recovery and survival. Int. J. Radiat. Biol. 2007;83:141–151. doi: 10.1080/09553000601132642. [DOI] [PubMed] [Google Scholar]
- 14.Allred CD, Allred KF, Ju YH, Clausen LM, Doerge DR, Schantz SL, Korol DL, Wallig MA, Helferich WG. Dietary genistein results in larger MNU-induced, estrogen-dependent mammary tumors following ovariectomy of Sprague-Dawley rats. Carcinogenesis. 2004;25:211–218. doi: 10.1093/carcin/bgg198. [DOI] [PubMed] [Google Scholar]
- 15.Manjanatha MG, Shelton S, Bishop ME, Lyn-Cook LE, Aidoo A. Dietary effects of soy isoflavones daidzein and genistein on 7,12-dimethylbenz[a]anthracene-induced mammary mutagenesis and carcinogenesis in ovariectomized Big Blue® transgenic rats. Carcinogenesis. 2006;27:2555–2564. doi: 10.1093/carcin/bgl195. [DOI] [PubMed] [Google Scholar]
- 16.Mahn K, Borrás C, Knock GA, Taylor P, Khan IY, Sugden D, Poston L, Ward JP, Sharpe RM, Mann GE. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J. 2005;19:1755–1757. doi: 10.1096/fj.05-4008fje. [DOI] [PubMed] [Google Scholar]
- 17.Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am. J. Resp. Cell Mol. Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 18.Asaba K, Tojo A, Onozato ML, Goto A, Quinn MT, Fujita T, Wilcox CS. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int. 2005;67:1890–1898. doi: 10.1111/j.1523-1755.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 19.Bäumer AT, Krüger CA, Falkenberg J, Freyhaus HT, Rösen R, Fink K, Rosenkranz S. The NAD(P)H oxidase inhibitor apocynin improves endothelial NO/superoxide balance and lowers effectively blood pressure in spontaneously hypertensive rats: comparison to calcium channel blockade. Clin. Exp. Hypertens. 2007;29:287–299. doi: 10.1080/10641960701500398. [DOI] [PubMed] [Google Scholar]
- 20.Elmarakby AA, Loomis ED, Pollock JS, Pollock DM. NADPH oxidase inhibition attenuates oxidative stress but not hypertension produced by chronic ET-1. Hypertension. 2005;45:283–287. doi: 10.1161/01.HYP.0000153051.56460.6a. [DOI] [PubMed] [Google Scholar]
- 21.Virdis A, Neves MF, Amiri F, Touyz RM, Schiffrin EL. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J. Hypertens. 2004;22:535–542. doi: 10.1097/00004872-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Hu L, Zhang Y, Lim PS, Miao Y, Tan C, McKenzie KU, Schyvens CG, Whitworth JA. Apocynin but not L-arginine prevents and reverses dexamethasone-induced hypertension in the rat. Am. J. Hypertens. 2006;19:413–418. doi: 10.1016/j.amjhyper.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Evans PJ, Halliwell B. Measurement of iron and copper in biological systems: bleomycin and copper-phenanthroline assays. Methods Enzymol. 1994;233:82–92. doi: 10.1016/s0076-6879(94)33010-7. [DOI] [PubMed] [Google Scholar]
- 24.Moulder JE, Cohen EP. Future strategies for mitigation and treatment of chronic radiation-induced normal tissue injury. Semin. Radiat. Oncol. 2007;17:141–148. doi: 10.1016/j.semradonc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Cohen EP, Fish BL, Moulder JE. Mitigation of radiation nephropathy. J. Invest. Med. 2006;54(Suppl 2):S353. [Google Scholar]
- 26.Ohno S, Nakajima Y, Inoue K, Nakazawa H, Nakajin S. Genistein administration decreases serum corticosterone and testosterone levels in rats. Life Sci. 2003;74:733–742. doi: 10.1016/j.lfs.2003.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Cohen EP, Lawton CA, Moulder JE, Becker CG, Ash RC. Clinical course of late-onset bone marrow transplant nephropathy. Nephron. 1993;64:626–635. doi: 10.1159/000187412. [DOI] [PubMed] [Google Scholar]
- 28.Dürken M, Herrnring C, Finckh B, Nagel S, Nielsen P, Fischer R, Berger HM, Moison RMW, Pichlmeier U, Kohlschütter A. Impaired plasma antioxidative defense and increased nontransferrin-bound iron during high-dose chemotherapy and radiochemotherapy preceding bone marrow transplantation. Free Radic. Biol. Med. 2000;28:887–894. doi: 10.1016/s0891-5849(00)00174-x. [DOI] [PubMed] [Google Scholar]
- 29.Cohen EP, Fish BL, Moulder JE. The renin-angiotensin system in experimental radiation nephropathy. J. Lab. Clin. Med. 2002;139:251–257. doi: 10.1067/mlc.2002.122279. [DOI] [PubMed] [Google Scholar]
- 30.Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J. Am. Soc. Nephrol. 2007;18:2439–2446. doi: 10.1681/ASN.2007020149. [DOI] [PubMed] [Google Scholar]
- 31.Collins-Underwood JR, Zhao WL, Sharpe JG, Robbins ME. NADPH oxidase mediates radiation-induced oxidative stress in rat brain microvascular endothelial cells. Free Radic. Biol. Med. 2008;45:929–938. doi: 10.1016/j.freeradbiomed.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paliege A, Pasumarthy A, Mizel D, Yang T, Schnermann J, Bachmann S. Effect of apocynin treatment on renal expression of COX-2, NOS1, and renin in Wistar-Kyoto and spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R694–R700. doi: 10.1152/ajpregu.00219.2005. [DOI] [PubMed] [Google Scholar]
- 33.Bird JE, Milhoan K, Wilson CB, Young SG, Mundy CA, Parthasarathy S, Blantz RC. Ischemic acute renal failure and antioxidant therapy in the rat. The relation between glomerular and tubular dysfunction. J. Clin. Invest. 1988;81:1630–1638. doi: 10.1172/JCI113498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prabhakar S, Starnes J, Shi S, Lonis B, Tran R. Diabetic nephropathy is associated with oxidative stress and decreased renal nitric oxide production. J. Am. Soc. Nephrol. 2007;18:2945–2952. doi: 10.1681/ASN.2006080895. [DOI] [PubMed] [Google Scholar]