Summary
Pathogen infection leads to the activation of defense signaling networks in plants. To study these networks and the relationships between their components, we introduced various defense mutations into acd6-1, a constitutive gain-of-function Arabidopsis mutant that is highly disease resistant. acd6-1 plants show spontaneous cell death, reduced stature, and accumulate high levels of camalexin (an anti-fungal compound) and salicylic acid (SA, a signal molecule). Disruption of several defense genes revealed that in acd6-1, SA levels/signaling was positively correlated with the degree of disease resistance and defense gene expression. SA also modulates the severity of cell death. However, camalexin accumulation in acd6-1 is largely unaffected by reducing SA levels. In addition, acd6-1 shows ethylene- and jasmonic acid-mediated signaling that is antagonized and therefore masked by the presence of SA. Mutant analysis revealed a new relationship between the signaling components NPR1 and PAD4 and also indicated that multiple defense pathways were required for acd6-1-conferred phenotypes. In addition, our data confirmed that the size of acd6-1 was inversely correlated with SA levels/signaling. We exploited this unique feature of acd6-1 to identify two genes disrupted in acd6-1 suppressor (sup) mutants: one encodes a known SA biosynthetic component (SID2) and the other encodes an uncharacterized putative metalloprotease (At5g20660). Taken together, acd6-1 is a powerful tool not only for dissecting defense regulatory networks but also for discovering novel defense genes.
Keywords: salicylic acid, suppressor, disease resistance, signal transduction, Pseudomonas syringae, camalexin
Introduction
Pathogen infection evokes sophisticated responses in plants. Plants have evolved surveillance systems that detect various pathogen-derived molecules and subsequently activate defense responses. In the absence of this recognition and/or preformed defenses, plants are highly susceptible to pathogen attacks and disease ensues (Flor, 1971; Gomez-Gomez and Boller, 2002; Jones and Dangl, 2006; Zipfel, 2008). An array of genes works together to detect pathogens, transduce defense signaling, and activate defense responses. Therefore, it is critical to identify such defense genes, understand their mechanisms of action, and delineate the defense signaling networks.
Salicylic acid (SA), a small phenolic compound, is a key signaling molecule for plant disease resistance. Accumulation of SA is induced by pathogen attacks and other stress conditions (Schenk et al., 2000; Uknes et al., 1992). Exogenous application of SA or SA analogs such as benzo (1,2,3) thiadiazole-7-carothioic acid and 2,6-dichloroisonicotinic acid induces enhanced disease resistance in plants (Gorlach et al., 1996; Lawton et al., 1996; Metraux et al., 1991; White, 1979). In addition, mutations that reduce SA accumulation or block SA signaling lead to compromised defense responses. At least three types of SA regulators have been described. The type I regulators include enzymes involved in SA biosynthesis. SA INDUCTION-DEFICIENT 2 (SID2) converts chorismate to isochorismate for SA biosynthesis (Wildermuth et al., 2001). Although alternative SA biosynthetic pathways have been proposed (Verberne et al., 1999), the highly reduced SA levels in the sid2 mutants suggests that SID2 is part of the major pathway for SA biosynthesis. The type II regulators affect SA accumulation, but may not be biosynthetic enzymes. Examples of such SA regulators include ACCELERATED CELL DEATH 6 (ACD6), AGD2-LIKE DEFENSE 1 (ALD1), ENHANCED DISEASE SUSCEPTBILITY 1 (EDS1), PHYTOALEXIN DEFICIENT 4 (PAD4), and SID1/EDS5 (Falk et al., 1999; Jirage et al., 1999; Lu et al., 2003; Nawrath et al., 2002; Song et al., 2004). Although how these proteins act is not fully understood, it is conceivable that they affect the availability of SA precursors, the activities of enzymes involved in SA biosynthesis, and/or SA catabolism. The type III regulators transduce defense signaling downstream of SA. NONEXPRESSOR OF PR GENES 1 (NPR1) is critical for SA-mediated defense signaling (Cao et al., 1997; Ryals et al., 1997; Shah et al., 1997). There are also NPR1-independent SA signaling pathways, some of which have not yet been molecularly identified (Bowling et al., 1997;Desveaux et al., 2004; Rate and Greenberg, 2001; Shah et al., 2001). The identification of many SA regulators suggests that there are multiple pathways feeding into the regulation of SA signaling.
In contrast to plants with compromised defense, there are mutants that display constitutive defense, often associated with cell death (also called a “lesion mimic phenotype”) and elevated SA accumulation (for review, see (Lorrain et al., 2003)). Interestingly, the phenotypes of such mutants are often differentially affected by mutations in various SA regulators (Clarke et al., 2000; Jirage et al., 2001; Zhang et al., 2003a). Observations from these studies support the view that SA-mediated signaling is important for plant defense and cell death formation and also suggest that some SA regulators likely act in parallel pathways to independently affect SA-mediated responses. Moreover, some lesion mimic mutants have been used to dissect interactions between SA and two other defense signaling pathways mediated by ethylene (ET) or jasmonic acid (JA) (Clarke et al., 2000; Kachroo et al., 2003; Kachroo et al., 2001; Reymond and Farmer, 1998). Some studies indicate that ET and JA can either synergistically or antagonistically affect SA signaling, and that such intricate interactions fine-tune plant defense responses (reviewed in (Feys and Parker, 2000; Kunkel and Brooks, 2002)).
We previously characterized a type II SA regulator ACD6 in Arabidopsis. ACD6 is a plasma membrane protein with a cytoplasmic ankyrin repeat motif (Lu et al., 2005; Lu et al., 2003). ACD6 and SA-derived signals interact mutually to amplify defense responses in Arabidopsis. acd6-1 is a gain-of-function mutant caused by a leucine to phenylalanine substitution in the transmembrane domain of ACD6. The hallmarks of acd6-1 include extreme dwarfism, punctate cell death, accumulation of high levels of SA and camalexin (an anti-fungal metabolite) (Schuhegger et al., 2006), and constitutive resistance to Pseudomonas syringae and Hyaloperonospora parasitica (Lu et al., 2003; Rate et al., 1999; Song et al., 2004). Therefore, dissecting signaling pathways that regulate acd6-1-conferred phenotypes will give insight into the mechanisms of plant disease resistance and cell death formation.
We showed previously that some acd6-1-conferred phenotypes are partially dependent on SA regulators, such as PAD4, ALD1, and NPR1. In addition, depletion of SA by salicylate hydroxylase (NahG), encoded by a bacterial-originated transgene (Gaffney et al., 1993), completely suppressed acd6-1-conferred phenotypes (Lu et al., 2003; Rate et al., 1999; Song et al., 2004). Based on these observations, we hypothesized that SA is required for acd6-1-conferred phenotypes. However, some SA regulators may also affect other defense signals and NahG can have SA-independent effects (Heck et al., 2003; van Wees and Glazebrook, 2003). Therefore, there are still many open questions. Do acd6-1-conferred phenotypes truly require SA? If so, how do different SA regulators interact with each other to affect acd6-1-conferred phenotypes and defense signaling? Are SA-independent pathways also involved in regulating acd6-1-conferred phenotypes?
To begin answering these questions, we performed a genetic analysis by introducing mutations causing defects in various defense pathways into the acd6-1 background. Our study suggests that the defense signaling networks are complex, involving multiple factors that may act either independently in different pathways or together in the same pathway. Increased SA signaling antagonizes ET and JA signaling in the acd6-1 background, but genetically blocking ET or JA signaling has no detectable effect on acd6-1-conferred phenotypes. Importantly, we show that acd6-1-conferred defense phenotypes indeed are largely dependent on SA. The finding that the small size of acd6-1 is largely SA-dependent enabled us to employ a facile genetic screen to isolate acd6-1 suppressor (sup) mutants that affect SA levels and/or signaling. Among the SUP genes cloned, SUP1 is SID2 and SUP6 encodes an uncharacterized putative metalloprotease (At5g20660). SUP6 is important for basal resistance in otherwise wild-type Arabidopsis, highlighting the utility of acd6-1 for uncovering novel defense components as well as the novel features of the defense networks.
Results
Multiple regulators affect SA accumulation in acd6-1
We sought to determine whether several known SA regulators affect acd6-1-conferred phenotypes and possibly show genetic interactions. Therefore, we compared the phenotypes of newly constructed acd6-1eds5-1, acd6-1sid2-1 and acd6-1npr1-1pad4-1 mutants to the respective single and previously constructed double mutant parents. We first determined effects of mutations in these SA regulators on the levels of SA and its glucoside conjugate SAG.
Similar to disruption of the PAD4 gene (Figure 1a and (Lu et al., 2003)), an EDS5 mutation also partially suppressed both SA and SAG accumulation in acd6-1. In contrast, acd6-1npr1-1 accumulated three-fold more free SA than acd6-1, although total SA levels were comparable in these two genotypes. This effect of npr1-1 on SA accumulation was previously observed in other constitutive defense mutants, and suggests that NPR1 participates in negative feedback regulation of SA (Clarke et al., 2000; Delaney et al., 1995; Zhang et al., 2003b; Zhou et al., 1998). The high production of SA in acd6-1npr1-1 was largely PAD4-dependent (Figure 1a, compare SA levels in acd6-1npr1-1 and acd6-1npr1-1pad4-1). Additionally, acd6-1npr1-1pad4-1 produced lower SA levels than acd6-1pad4-1 (Figure 1a), suggesting NPR1 can positively regulate SA in the absence of PAD4. Finally, of all the double and triple mutants analyzed, acd6-1sid2-1 had the lowest overall levels of SA. However, the SA and SAG levels were still slightly higher in acd6-1sid2-1 than sid2-1, indicating a small SID2-independent biosynthetic source of SA is produced in acd6-1 (Figure 1a). These data indicate that all three types of SA regulators contribute to SA accumulation, and that SID2 is the major contributor.
Figure 1.
Effects of various mutations on SA accumulation, disease resistance, and defense gene expression in acd6-1. (a) SA quantification. SA was extracted from the indicated plants at 20 days of age and analyzed by HPLC. P< 0.01 (n=3). (b) Bacterial growth. The 5th to 7th leaves of each plant were infected with P. syringae maculicola (Pma) ES4326 DG3 (OD600 = 0.0001) for 3 days before bacterial measurement. P < 0.05 (n=6). (c) Defense gene expression. Total RNA was extracted from each plant and subject to Northern blot analysis with the indicated probes. The blots for ALD1 and PAD4 were exposed overnight and those for PR1, PDF1.2, and EF1a were exposed for 1 hour. No PR1 expression was detected in the acd6-1sid2-1 mutant in an overnight exposed film (data not shown). Similarly low levels of PDF1.2 expression were detected in Col, eds5-1, sid2-1, npr1-1, pad4-1, and acd6-1 in an overnight exposed film (data not shown). For (a) and (b), statistical differences among the samples were labeled with different letters. These experiments were repeated two times with similar results.
Multiple regulators affect disease resistance and defense signaling in acd6-1
To examine whether changes in SA levels and/or signaling were correlated with acd6-1-conferred disease resistance, we infected our double and triple mutant plants with P. syringae pv. maculicola ES4326 strain PmaDG3. The resistance of acd6-1eds5-1 and acd6-1sid2-1 was positively correlated with the SA levels in these plants, similar to what we previously found in acd6-1pad4-1 ((Lu et al., 2003) and Figure 1b). The npr1-1 mutation was previously shown to partially suppress disease resistance in acd6-1 (Rate et al., 1999). The presence of pad4-1 did not significantly enhance the susceptibility of the acd6-1npr1-1 mutant. Additionally, the fact that acd6-1sid2-1, acd6-1npr1-1, and acd6-1npr1-1pad4-1 were significantly more resistant than the single mutants sid2-1, npr1-1 and pad4-1 suggests that the small amount of SA, additional SA regulators and/or an SA-independent pathway in these plants are responsible for the residual disease resistance (Figure 1b).
To further study how defenses are affected by the SA regulators in acd6-1, we examined the expression of several defense-related genes in these plants. PR1 transcript levels are usually highly correlated with SA-mediated signaling (Glazebrook et al., 1997). We previously found that npr1-1 and pad4-1 partially suppressed PR1 expression in acd6-1 (Rate et al., 1999). Consistent with the observations on SA levels, the presence of both npr1-1 and pad4-1 further suppressed PR1 transcript levels in acd6-1pad4-1npr1-1 (Figure 1c). Compared with that in acd6-1, expression of PR1 was also much reduced in acd6-1eds5-1, but was completely abolished in acd6-1sid2-1. Since acd6-1sid2-1 had the lowest total SA levels (4.4 µg/g fresh weight) among all the plants in the acd6-1 background that were tested in this study (Table S1), our results suggest that a threshold level of SA is necessary to induce PR1 expression. ALD1 and PAD4 are type II SA regulators whose transcript levels are highly induced in acd6-1 and in wild-type plants after P. syringae infection or SA (or SA agonist) treatment (Jirage et al., 1999; Song et al., 2004). Compared with that of PR1, expression of ALD1 and PAD4 was not appreciably affected in eds5-1 and sid2-1, but was drastically reduced in acd6-1npr1-1pad4-1. One possible explanation for these results is that expression of these two genes can be induced by low SA levels and such a high sensitivity to SA is NPR1- and PAD4-dependent. In addition, an NPR1- and PAD4-independent pathway is also attributable to the residual expression of both ALD1 and PAD4 in acd6-1npr1-1pad4-1.
Taken together, these data indicate that acd6-1-conferred constitutive defenses were largely SA-dependent. Both PAD4-/NPR1-dependent and –independent pathways are required for acd6-1 disease resistance and defense gene expression. In addition, a SID2-independent pathway is also activated in the acd6-1 background and contributes to its constitutive defense.
Accumulation of camalexin is largely SID2-independent
To test whether SA regulators affect camalexin accumulation in acd6-1, we measured camalexin levels in the plants used in Figures 1. As shown in Figure 2, most mutants, except acd6-1sid2-1, did not significantly alter camalexin accumulation in acd6-1. Compared with that of the SA levels (27-fold reduction), the reduction of camalexin (<2-fold) caused by sid2-1 was quite small in acd6-1 (Table S1). Therefore, such a decrease in camalexin likely reflects an indirect role of SID2 in affecting camalexin accumulation in acd6-1.
Figure 2.
Camalexin accumulation in acd6-1 is SA-independent. Same samples used in Figure 1 were quantified for camalexin by HPLC analysis. Col, eds5-1, sid2-1, npr1-1, and pad4-1 did not accumulate detectable camalexin (not shown). P < 0.001 (n=3). Statistical differences among the samples were labeled with different letters. These experiments were repeated three times with similar results.
To further investigate how camalexin production is regulated in acd6-1, we made a double mutant between acd6-1 and pad3-1, a mutant defective in pathogen-induced camalexin accumulation (Glazebrook and Ausubel, 1994; Zhou et al., 1999). Whereas the SA levels were only slightly decreased, camalexin accumulation was greatly reduced in acd6-1pad3-1 compared with acd6-1. However, the rosette size and bacterial growth remained similar in acd6-1pad3-1 and acd6-1 (Table 1). Taken together, these data suggest that camalexin accumulation in acd6-1 is largely independent of SA and can be uncoupled from plant size, bacterial disease resistance, and SA accumulation.
Table 1.
Effect of PAD3, ETR1, and JAR1 on acd6-1-conferred phenotypes. 20-day-old plants were harvested for camalexin (n=3) and/or SA (n=3) analyses by HPLC and size measurement (n=15). For bacterial growth, the 5th to 7th leaves of the indicated plants were infected with PmaDG3 (OD600 = 0.0001). Three days after infection, leaf discs were taken for quantifying bacterial growth. n: sample size. Each data represents the average result of n samples ± standard error. N/A: data not available.
| Genotype | Camalexin (µg/gFW) n=3 |
Free SA (µg/gFW) n=3 |
Total SA (µg/gFW) n=3 |
Rosette diameter (cm) n=15 |
Bacterial growth (×1 04 cfu /leaf disc) n=6 |
|---|---|---|---|---|---|
| acd6-1 | 98.1 ± 3.9* | 6.4 ± 0.3 | 148.1 ± 5.2 | 0.8 ± 0.1 | 2.9 ± 1.4 |
| acd6-1pad3-1 | 3.0 ± 0.5 | 3.9 ± 0.3 | 121.5 ± 5.0 | 0.8 ± 0.1 | 1.7 ± 0.8 |
| acd6-1etr1-1 | N/A | 5.5 ± 0.7 | 139.6 ± 12.7 | 0.8 ± 0.1 | 6.6 ± 2.9 |
| acd6-1jar1-1 | N/A | 4.8 ± 0.1 | 130.8 ± 3.2 | 1.0 ± 0.1 | 3.6 ± 1.0 |
indicates statistical difference between one sample and other samples in the same column with a P value < 0.001. FW is fresh weight.
SA regulators modulate cell death in acd6-1
One of the hallmarks of acd6-1 is spontaneous cell death patches on its leaves. A close examination of acd6-1 leaves revealed three categories of cell death: single foci, intermediate clusters (2–20 cells), and very large clusters (>20 cells) (Figure 3). Cell death usually starts with single cells and spreads to neighboring cells to different extents. The cell death pattern of acd6-1 suggests a loss of control of both cell death initiation and spreading. We previously showed that the cell number in the acd6-1 leaf was similar to that in wild type, although cell size of acd6-1 was much smaller (Vanacker et al., 2001). Thus, in order to quantify the degree of cell death in the acd6-1 mutants, we measured the number of dead cells on quarter leaf basis. Figure 3a shows that all double or triple mutants abolished or reduced the very large clusters of dead cells in acd6-1. acd6-1pad4-1 harbored a similar number of single cell death foci as acd6-1, possibly due to the relatively high levels of SA in this mutant. These observations suggest that high SA levels stimulate both initiation and spreading of cell death. However, since SA treatment of wild type does not induce cell death, a second signal(s) from acd6-1 that perhaps is SA-independent is required to act with SA in regulating these processes. Interestingly, although the number of single death foci was much reduced, some very large clusters of dead cells were found in acd6-1sid2-1, often adjacent to the vascular tissue (Figure 3b). It is possible that the low SA levels in acd6-1sid2-1 not only lead to reduced cell death initiation, but also render the plant more sensitized to the signal that controls death spreading.
Figure 3.
Effect of various SA-regulatory mutations on cell death formation in acd6-1. (a) Cell death quantification of trypan blue-stained leaves. The number of dead cells was counted from a quarter region of a leaf (n=5) at the same position in each genotype and data were plotted in a logarithmic scale. Three classes of cell death (single foci, 2–20 cell clusters, and >20 cell clusters) were recorded. Col, npr1-1, pad4-1, eds5-1, and sid2-1 did not have cell death (not shown). (b) Pictures of leaf tissue after trypan blue staining. These experiments were repeated two times with similar results.
ET and JA pathways are dispensable for acd6-1-conferred dwarfism and defense
To determine whether SA-independent pathways affect acd6-1-conferred phenotypes, we examined defense signaling mediated by ET and JA, which are known to cooperate with or antagonize SA during defense responses, depending on the types of pathogens and plants tested (Feys and Parker, 2000; Kunkel and Brooks, 2002). Figure 1c shows that expression of PDF1.2, a marker for activation of ET and JA pathways (Penninckx et al., 1998; Reymond and Farmer, 1998), was highly induced as the SA levels were decreased in acd6-1pad4-1, acd6-1eds5-1, and acd6-1sid2-1, relative to acd6-1. In addition, acd6-1npr1-1 expressed much more PDF1.2 transcripts than acd6-1, whose PDF1.2 expression was only detectable with a longer exposure and comparable to that in wild type (Figure 1c and data not shown). In addition, PDF1.2 expression was highest in acd6-1npr1-1pad4-1. The expression pattern of PDF1.2 was opposite to that of PR1, a marker of the SA signaling pathway. These data clearly demonstrate the antagonistic effect of SA on ET and JA: the reduction in SA levels and/or signaling resulted in the induction of the ET and JA signaling that leads to PDF1.2 expression in acd6-1.
To test if disruption of ET and JA signaling interferes with acd6-1-conferred phenotypes, we crossed acd6-1 to ethylene response 1-1 (etr1-1), a mutant defective in ET reception (Chang et al., 1993), or jasmonic acid resistant 1-1 (jar1-1), a mutant defective in JA signaling (Staswick et al., 1998). The double mutants, acd6-1etr1-1 and acd6-1jar1-1, were indistinguishable from acd6-1 in SA accumulation, rosette size, and resistance to P. syringae (Table 1). These results indicate that disruption of the JA and ET-mediated signaling does not affect acd6-1-conferred dwarfism and defense.
acd6-1-conferred dwarfism is largely SA-dependent
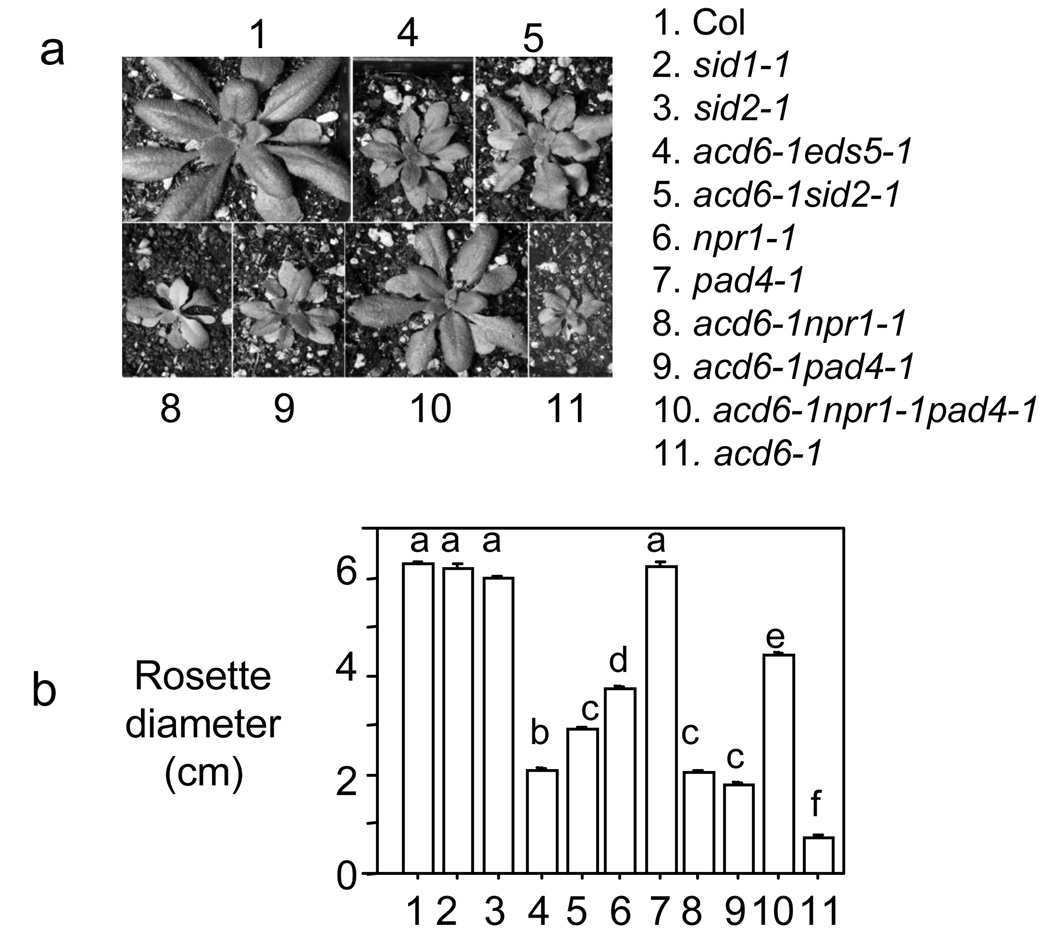
An obvious phenotypic difference among these mutants was the plant size, which appeared to be inversely correlated with SA levels and/or signaling (Figure 4 and Table S1). The rosette sizes of acd6-1, acd6-1pad4-1, acd6-1eds5-1 and acd6-1sid2-1 increased as the levels of SA in these plants decreased. The acd6-1sid2-1 plants differed in their appearance from other double mutants, as the leaves had a wavy appearance. As reported previously, blocking SA signaling with the npr1-1 mutation also slightly suppressed acd6-1 dwarfism and resulted in the bleaching of some leaves (Rate et al., 1999). Consistent with its low SA levels, the triple mutant acd6-1npr1-1pad4-1 was much larger than either acd6-1npr1-1 or acd6-1pad4-1. Interestingly, although it had about four times more total SA levels than acd6-1sid2-1, acd6-1npr1-1pad4-1 was much larger than acd6-1sid2-1. It is possible that residual SA in acd6-1sid2-1 affects plant size due to the activities of NPR1 and/or PAD4. Alternatively, SA-independent signaling in acd6-1sid2-1 may also affect the stature of acd6-1.
Figure 4.
Effect of various SA-regulatory and novel mutations on acd6-1-conferred dwarfism. (a) Pictures and (b) rosette diameters of 20-day old plants of the indicated genotypes. Statistical differences with a P<0.0001 among the samples (n>10) were labeled with different letters in (b). These experiments were repeated two times with similar results.
A suppressor of acd6-1-conferred dwarfism identifies a novel defense component
Since mutations that reduce acd6-1-conferred dwarfism showed increased disease susceptibility, we used T-DNA insertional mutagenesis to identify suppressors of acd6-1 with increased stature. Mutants that appear to be completely suppressed might carry mutations in the ACD6 gene (Lu et al., 2005). Therefore, we focused on mutations that conferred partial suppression. Compared with acd6-1, partial sup mutants were easily identified by the naked eye (Figure 5a). As a proof of concept, we characterized two of the 20 sup mutants, sup1-1 and sup6-1, which we have obtained to date.
Figure 5.
Identification of sup1-1 as an allele of SID2 in acd6-1 mutant screen. (a) Mutant screen for acd6-1 suppressors. Circles indicate a known suppressor, acd6-1pad4-1 (left), and novel suppressors (right) that were visually distinguishable from the densely planted acd6-1 20 days after being sown. (b) Disease symptoms. The 5th to 7th leaves of 20-day-old plants were infected with PmaDG3 (OD600 = 0.0001) for 3 days before being photographed. (c) Bacterial growth. Data of bacterial growth represented the average of six samples (n=6, P < 0.05). Statistical differences among the samples were labeled with different letters. These experiments were repeated twice with similar results.
The wavy rosette phenotype of acd6-1sup1-1 mutant strongly resembled acd6-1sid2-1 plants. Therefore, we sought to amplify the SID2 gene by PCR, but failed to detect the expected band in sup1-1, indicating that a T-DNA fragment likely inserted in the SID2 gene. To further test whether the mutation in SID2 was responsible for sup1-1-conferred phenotypes, we crossed sup1-1 to sid2-1 and infected the F1 plants with PmaDG3. We found that the sup1-1 × sid2-1 F1 plants showed similar susceptibility as sup1-1 and sid2-1 (Figure 5b and 5c). These data strongly suggest that sup1-1 is allelic to SID2.
The F2 progeny of a cross between acd6-1sup6-1 and Col showed 80 resistant: 25 plants that were sensitive to the herbicide glufosinate (conferred by the T-DNA), suggesting a single T-DNA insertion in the genome (X2=0.051, P> 0.8211). sup6-1 has a T-DNA insertion in the eighth exon of At5g20660, a single copy gene that encodes a protein with a predicted N-terminal peptidase domain commonly found in metalloproteases and a C-terminal transmembrane domain (Figure 6a). In silico analysis of gene expression profile with publicly available microarray database (Genevesitgator, (Zimmermann et al., 2004)) indicated that SUP6 was constitutively expressed during Arabidopsis development and in different organs and was not much affected under various conditions that lead to induction of numerous defense-related genes (data not shown). Proteins with high sequence similarity to SUP6 were found in many plants, as determined by the analysis of pBLAST data (http://blast.ncbi.nlm.nih.gov/Blast.cgi). However, the only experimentally characterized SUP6 homologue was a 24-kd vacuolar protein (VP24), which was enriched in the anthocyanin-containing vacuoles of cultured sweet potato (Ipomoea batatas) cells (Nozue et al., 1997; Xu et al., 2001). VP24 is derived from a larger precursor peptide that shares 50% identity to SUP6. The physiological functions of VP24 and its precursor are not well understood.
Figure 6.
Identification of SUP6 and characterization of its effects on the size of acd6-1. (a) SUP6 gene structure and positions of the two mutant alleles. Filled boxes indicate exons and lines indicate introns and untranslated regions. Open triangles indicate the T-DNA insertion sites in the two sup6 alleles. (b) RT-PCR analysis of SUP6 expression. The positions of the three sets of primers were illustrated in (a). (c) Picture of 20-day old plants. (d) Rosette diameters of plants shown in (c). At least 20 individual plants were measured for the rosette diameter. Asterisks indicate the significant difference between the two double mutants and acd6-1 (P<0.0001). Experiments for (b), (c), and (d) were repeated two times and similar results were obtained.
A second allele, sup6-2 (salk_072469), also harbors a T-DNA insertion in the eighth exon of SUP6. Both mutant alleles abolished expression of the SUP6 gene and possibly led to truncated sup6 transcripts before the T-DNA insertion sites (Figure 6b). Like sup6-1, sup6-2 also suppressed acd6-1 dwarfism (Figure 6c and 6d). Therefore, the suppression of acd6-1 dwarfism was caused by the mutations in the SUP6 gene.
As a secondary screen to test the effects of sup6 mutations on defense, we measured SA levels in acd6-1sup6-1 and acd6-1sup6-2. Both mutants caused a reduction of the total SA levels in acd6-1 (Figure 7a). In addition, both PR1 expression and cell death formation were much reduced in the acd6-1sup6 mutants (Figure 7b and c). To assess disease resistance, we infected both sup6 alleles (in the absence of the acd6-1 mutation) with PmaDG3 and found more bacterial growth in the mutants compared with the wild-type control (Figure 7d). Together, these data suggest that SUP6 is likely a type II SA regulator important for basal defense responses in Arabidopsis.
Figure 7.
Characterization of the defense phenotypes of acd6-1sup6 and sup6 mutants. (a) SA quantification. (b) PR1 expression by Northern blot analysis. (c) Cell death assay by trypan blue staining. (d) Bacterial growth. The 5th to 7th leaves of 20-day old plants were infected with PmaDG3 (OD600 = 0.0001) and assayed for bacterial growth at the indicated times (n=6). Statistical difference was observed between the two sup6 alleles and Col at day 2 and day 3 post infection with a P value < 0.1. These experiments were repeated twice with similar results.
Discussion
Pathogen infection induces multifaceted defense responses in plants, including activation of signaling pathways, accumulation of antimicrobial compounds, and promotion of cell death. The Arabidopsis mutant acd6-1 constitutively exhibits these defense responses in the absence of pathogens. Therefore, dissecting signaling pathways activated in acd6-1 and required for acd6-1-conferred phenotypes will help in the determination of disease resistance mechanisms. From the genetic analysis described here, we have gained a better understanding of the factors regulating acd6-1-conferred phenotypes and came to the following conclusions: 1) Defense responses with respect to SA accumulation, disease resistance, and expression of some defense genes are positively correlated in acd6-1. The small size of acd6-1, however, is inversely related to the strength of defense signaling; 2) SA is neither necessary nor sufficient for camalexin accumulation in acd6-1; 3) the severity of cell death in acd6-1 is affected by SA and another signal(s); 4) ET and JA mediated signaling are not required for acd6-1-conferred dwarfism and resistance; 5) suppression of acd6-1-conferred dwarfism can be used to dissect the interactions among SA regulators and to identify novel defense components.
Multiple components are activated to regulate SA-mediated defense in acd6-1
Our studies showed that acd6-1-conferred disease resistance and defense gene expression are largely SA-dependent and require the activities of multiple SA regulators (Lu et al., 2003; Rate et al., 1999; Song et al., 2004). The residual SA levels in acd6-1sid2-1 suggest that whereas SID2 mediates the synthesis of the majority of SA, a SID2-independent pathway is activated in acd6-1, which contributes to the partial induction of many defenses in acd6-1 (Figure S1). Hence, the acd6-1sid2-1 double mutant will provide a useful tool for studies of SID2-independent SA synthesis.
Interestingly, nahG had a greater impact in lowering SA levels in acd6-1 (Vanacker et al., 2001) than sid2-1 and also suppressed acd6-1-conferred phenotypes to a much greater extent (Rate et al., 1999). Differences in acd6-1sid2-1 and acd6-1nahG phenotypes are likely due to the fact that ACD6-1 transcript levels are undetectable in acd6-1nahG, whereas acd6-1sid2-1 plants still express ACD6-1 (our unpublished data).
Our studies also indicate that at least three type II SA regulators, ALD1, PAD4, and EDS5, are required for acd6-1-conferred phenotypes (this study and (Song et al., 2004)). However, compared with sid2-1, disease resistance and defense gene expression were not as greatly affected by mutations in these genes. This suggests that each of these type II SA regulators may only represent one branch of the networks regulating the full accumulation of SA. Consistent with this notion, no single mutant affecting SA levels tested so far completely suppressed SA accumulation and constitutive defense in acd6-1. It is conceivable that additional known and yet-to-be-discovered type II SA regulators also contribute to the regulation of acd6-1-conferred phenotypes (Figure S1). However, although a mutation in the NON-RACE-SPECIFIC DISEASE RESISTANCE gene compromises pathogen-induced SA accumulation (Shapiro and Zhang, 2001), the acd6-1ndr1-1 mutant did not have altered defenses and plant size, compared with acd6-1 (our unpublished observations). This suggests that only a subset of SA regulators is required for acd6-1-conferred phenotypes.
Phenotypic analysis of the triple mutant acd6-1npr1-1pad4-1 revealed potentially novel roles of the type III regulator NPR1 and the type II regulator PAD4 in defense signaling. Mutations in both NPR1 and PAD4 additively affected SA levels, defense responses, and plant size in the acd6-1 background. This suggests a positive role for NPR1 in affecting SA accumulation, in addition to NPR1’s ability to negatively regulate SA as well as positively transduce SA signaling. The negative function of NPR1 may require PAD4. When PAD4 is mutated, the negative role of NPR1 is abolished but its positive role is not affected. Therefore, the acd6-1pad4-1 mutant should still have relatively high levels of SA, which is corroborated by our results (Figure 1a and (Lu et al., 2003)). However, when both PAD4 and NPR1 are mutated, both the negative and positive effects of NPR1 are eliminated, leading to much reduced SA and defense levels. PAD4 activity or a component upstream of PAD4 may be the direct target of NPR1, forming a negative feedback loop with NPR1 to regulate SA levels (Figure S1). An interesting open question is how additional SA regulators interact with each other to form the complex defense signaling networks.
SID2-independent defenses are activated in acd6-1
Disruption of SID2 in acd6-1 did not completely suppress several acd6-1-conferred phenotypes. Since acd6-1sid2-1 plants have slightly higher SA levels than sid2-1, the phenotypes of acd6-1sid2-1 plants could result from the residual SA or SA-independent signaling. The strongest indication of a SID2-and SA-independent pathway being activated is the high accumulation of camalexin in acd6-1sid2-1 (Figure 2). Camalexin induction during pathogen infection is known to be SID2-independent (Nawrath and Metraux, 1999). Importantly, camalexin levels are unaffected by SA treatment of wild type (Zhou et al., 1998). Our data are consistent with ACD6 regulating SA levels/signaling and camalexin accumulation (Lu et al., 2003) through separate pathways.
Analysis of PDF1.2 expression suggests that ET/JA signaling has the potential to be highly activated in acd6-1 plants. However, this only occurs when SA levels/signaling is blocked, consistent with the known antagonizing effect of SA on ET/JA signaling (reviewed in (Feys and Parker, 2000; Kunkel and Brooks, 2002)). ET/JA signaling does not significantly impact the size, bacterial disease resistance, or SA accumulation in acd6-1, indicating that these two pathways are dispensable for acd6-1-conferred phenotypes. Possibly, activation of ET/JA signaling results from the cell death conferred by acd6-1.
acd6-1 is a useful tool to identify novel defense genes
In addition to its usefulness in studying the interactions among the known defense components, acd6-1 can be further employed to identify additional defense genes. The potential of the suppressor genetics was validated by the isolation of the known SA regulator SID2 and the novel defense component SUP6. A high conservation between SUP6 and the precursor peptide of the vacuolar protein VP24 suggests that SUP6 is a potential vacuolar protease and possibly plays a role in plant defense and/or cell death as some reported vacuolar proteases do (Hara-Nishimura et al., 2005). We have presented evidence to show that SUP6 regulates basal defense in Arabidopsis. Additional studies are necessary to fully discern how SUP6 acts to regulate plant defense and to place SUP6 in the complex defense signaling networks. Further characterization of SUP6 and additional novel SUP proteins will facilitate an understanding of the fundamental mechanisms of plant defense responses, expand our knowledge of the defense regulatory networks, and eventually provide molecular targets for genetic engineering to produce crops with enhanced disease resistance.
Experimental procedures
Plant Materials
Plants used in this report were in the Columbia (Col) background. The mutants acd6-1, acd6-1npr1-1 and acd6-1pad4-1, etr1-1, jar1-1, npr1-1, pad3-1, pad4-1, eds5-1, sid2-1 were described previously (Chang et al., 1993; Lu et al., 2003; Nawrath et al., 2002; Rate et al., 1999; ; Song et al., 2004; Staswick et al., 1998; Thomma et al., 1999). Double mutants (acd6-1eds5-1, acd6-1sid2-1, acd6-1etr-1, acd6-1jar-1, and acd6-1pad3-1) were constructed by crossing acd6-1 to each corresponding single mutant and screening for the respective double mutant from each F2 population using relevant derived cleaved amplified polymorphic sequence (dCAPS) markers. The triple mutant acd6-1npr1-1pad4-1 was made by crossing acd6-1npr1-1 to acd6-1pad4-1 and screening for homozygous F2ants using dCAPS markers for acd6-1, npr1-1 and pad4-1, respectively. Primers for all dCAPS markers used in this study were listed in Table S2.
To detect the mutation in the SID2 gene in sup1-1, we used the primer set sup1-1(sid2) (Table S2) and expect a 836-bp band in wild type, but no amplification in sup1-1. sup6-1 is a suppressor identified from acd6-1 suppressor screen (see below). sup6-2 (salk_072496) was ordered from the Arabidopsis Biological Resource Center. The double mutant acd6-1sup6-2 was made by crossing acd6-1 to sup6-2 and homozygous F2 plants were selected by using the dCAPS markers for acd6-1 and the primer sets for the sup6-2 mutant (Table S2).
Bacterial culture and infection
Pseudomonas syringae pv. maculicola (Pma) DG3 is a derivative of PmaES4326 (Guttman and Greenberg, 2001). For the bacterial growth assay, the fifth to seventh leaves of 20-day old plants grown in growth chambers with 16h light/8h dark were infected with PmaDG3 for 3 days before leaf discs were taken for measurement of the number of bacteria. Bacterial culturing, infection, and growth analysis were performed as described previously (Greenberg et al., 2000; Lu et al., 2003).
Cell Death Staining and cell death quantitation
Fifth to seventh leaves of each genotype were collected and stained with trypan blue as described (Rate et al., 1999) and were examined using a Stemi SV 1.1 stereomicroscope (Zeiss, Inc., Germany). To quantify cell death, dead cell foci (single dead cell, 2–20 dead cell cluster, and over 20-dead cell cluster) were counted in an area equivalent to a quarter region of a whole leaf on the tip area. Pictures of stained leaves were taken with an AxioCam MRc5 camera (Zeiss, Inc., Germany).
RNA analysis
Extraction of total RNA and Northern blot analysis were performed as previously described (Lu et al., 2003). The DNA fragment specific to each gene was amplified by PCR from the genomic DNA, confirmed by sequencing, and labeled with 32P-dCTP as a probe for Northern blot analysis. For RT-PCR, one microgram of the total RNA extracted from each sample was reverse transcribed with the First Strand cDNA Synthesis kit (Fermentas International, Glen Burnie, MD) according to the manufacturer’s instructions. PCR reactions were carried out with gene specific primers and samples were taken at 25, 30, and 35 cycles. All primers used for RNA analysis were listed in Table S2.
SA and Camalexin Measurement
SA and camalexin were extracted and analyzed by HPLC as previously described (Lu et al., 2003; Song et al., 2004).
Screen T-DNA insertion mutagenized acd6-1
acd6-1 plants (T0) were transformed with Agrobacteria carrying the pAOV1.3 vector, which harbors the BAR gene to confer resistance to herbicide glufosinate (Mylne and Botella, 1998). The T1 seeds collected from the primary transformation were planted on soil and selected for T1 transgenic plants by spraying Liberty®, a glufosinate containing product (Bayer Crop Science, VA). T2 seeds from each 500 T1 plants were collected in a pool and a total of 70 pools containing about 35,000 lines were harvested. At least 5,000 T2 seeds from each pool were planted for selection for transgenic plants resistant to glufosinate and visually screened for acd6-1sups with a larger size. acd6-1sups were crossed back to acd6-1 to confirm the suppression phenotypes and also to Col to separate a sup mutation from acd6-1. For acd6-1sups with a confirmed larger size, we performed TAIL-PCR (Liu et al., 1995) to identify the junction region between a T-DNA insertion and an Arabidopsis chromosome. Twenty putative acd6-1sups were isolated from the screen for further analysis.
Supplementary Material
Acknowledgements
We thank Drs. Mauricio Bustos, Ho Won Jung, Jiyoung Lee, and Stephen Miller for critical reading of this manuscript and useful discussions. We thank members of the Arabidopsis thaliana Research Initiative at the University of Maryland (ATRIUM) for suggestions and inputs into this study. We thank Susan Ferrari at University of Chicago for initial help with generating suppressor mutants. We thank Dr. Joy Bergelson at UC for sharing the usage of her HPLC instrument and Dr. Charles Bieberich at UMBC for sharing his dissecting microscope with us. We thank Tim Ford for taking pictures for this publication. This work was supported by startup funds from UMBC to H. L; National Science Foundation (IOB-0450207) and National Institutes of Health (R01 GM54292) grants to J.T.G. S.S. was supported by Minority Access to Research Career (funded by NIGMS/NIH), Meyerhoff (funded by Meyerhoff and NSF), and the Provost Undergraduate Research award at UMBC.
Footnotes
Accession number: At5g20660 (SUP6)
Contributor Information
Hua Lu, Email: hualu@umbc.edu.
Sasan Salimian, Email: sasan1@umbc.edu.
Emily Gamelin, Email: emilygamelin@gmail.com.
Guoying Wang, Email: gywang@umbc.edu.
Jennifer Fedorowski, Email: fedjenn1@umbc.edu.
William LaCourse, Email: lacourse@umbc.edu.
Jean T. Greenberg, Email: jgreenbe@midway.uchicago.edu.
References
- Bowling SA, Clarke JD, Liu Y, Klessig DF, Dong X. The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1- independent resistance. Plant Cell. 1997;9:1573–1584. doi: 10.1105/tpc.9.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell. 1997;88:57–63. doi: 10.1016/s0092-8674(00)81858-9. [DOI] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- Clarke JD, Volko SM, Ledford H, Ausubel FM, Dong X. Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis. Plant Cell. 2000;12:2175–2190. doi: 10.1105/tpc.12.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney TP, Friedrich L, Ryals JA. Arabidopsis signal transduction mutant defective in chemically and biologically induced disease resistance. Proc. Natl. Acad. Sci. USA. 1995;92:6602–6606. doi: 10.1073/pnas.92.14.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desveaux D, Subramaniam R, Despres C, Mess JN, Levesque C, Fobert PR, Dangl JL, Brisson N. A "Whirly" transcription factor is required for salicylic acid-dependent disease resistance in Arabidopsis. Dev. Cell. 2004;6:229–240. doi: 10.1016/s1534-5807(04)00028-0. [DOI] [PubMed] [Google Scholar]
- Falk A, Feys BJ, Frost LN, Jones JD, Daniels MJ, Parker JE. EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc. Natl. Acad. Sci. USA. 1999;96:3292–3297. doi: 10.1073/pnas.96.6.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys BJ, Parker JE. Interplay of signaling pathways in plant disease resistance. Trends Genet. 2000;16:449–455. doi: 10.1016/s0168-9525(00)02107-7. [DOI] [PubMed] [Google Scholar]
- Flor HH. Current status of the gene-for-gene concept. Annu. Rev. of Phytopathol. 1971;9:275–296. [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J. Requirement of salicylic acid for the induction systemic acquired resistance. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Ausubel FM. Isolation of phytoalexin-deficient mutants of Arabidopsis thaliana and characterization of their interactions with bacterial pathogens. Proc. Natl. Acad. Sci. USA. 1994;91:8955–8959. doi: 10.1073/pnas.91.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J, Zook M, Mert F, Kagan I, Rogers EE, Crute IR, Holub EB, Hammerschmidt R, Ausubel FM. Phytoalexin-deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics. 1997;146:381–392. doi: 10.1093/genetics/146.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gomez L, Boller T. Flagellin perception: a paradigm for innate immunity. Trends Plant Sci. 2002;7:251–256. doi: 10.1016/s1360-1385(02)02261-6. [DOI] [PubMed] [Google Scholar]
- Gorlach J, Volrath S, Knauf-Beiter G, Hengy G, Beckhove U, Kogel KH, Oostendorp M, Staub T, Ward E, Kessmann H, Ryals J. Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell. 1996;8:629–643. doi: 10.1105/tpc.8.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg JT, Silverman FP, Liang H. Uncoupling salicylic acid-dependent cell death and defense-related responses from disease resistance in the Arabidopsis mutant acd5. Genetics. 2000;156:341–350. doi: 10.1093/genetics/156.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman DS, Greenberg JT. Functional analysis of type III effectors AvrRpt2 and AvrRpm1 of Pseudomonas syringae with the use of a single copy genomic integration system. Mol. Plant Microbe Interact. 2001;14:145–155. doi: 10.1094/MPMI.2001.14.2.145. [DOI] [PubMed] [Google Scholar]
- Hara-Nishimura I, Hatsugai N, Nakaune S, Kuroyanagi M, Nishimura M. Vacuolar processing enzyme: an executor of plant cell death. Curr. Opin. Plant Biol. 2005;8:404–408. doi: 10.1016/j.pbi.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Heck S, Grau T, Buchala A, Metraux JP, Nawrath C. Genetic evidence that expression of NahG modifies defence pathways independent of salicylic acid biosynthesis in the Arabidopsis-Pseudomonas syringae pv. tomato interaction. Plant J. 2003;36:342–352. doi: 10.1046/j.1365-313x.2003.01881.x. [DOI] [PubMed] [Google Scholar]
- Jirage D, Tootle TL, Reuber TL, Frost LN, Feys BJ, Parker JE, Ausubel FM, Glazebrook J. Arabidopsis thaliana PAD4 encodes a lipase-like gene that is important for salicylic acid signaling. Proc. Natl. Acad. Sci. USA. 1999;96:13583–13588. doi: 10.1073/pnas.96.23.13583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirage D, Zhou N, Cooper B, Clarke JD, Dong X, Glazebrook J. Constitutive salicylic acid-dependent signaling in cpr1 and cpr6 mutants requires PAD4. Plant J. 2001;26:395–407. doi: 10.1046/j.1365-313x.2001.2641040.x. [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Kachroo A, Lapchyk L, Fukushige H, Hildebrand D, Klessig D, Kachroo P. Plastidial fatty acid signaling modulates salicylic acid- and jasmonic acid-mediated defense pathways in the Arabidopsis ssi2 mutant. Plant Cell. 2003;15:2952–2965. doi: 10.1105/tpc.017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo P, Shanklin J, Shah J, Whittle E, Klessig D. A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc Natl Acad Sci USA. 2001;98:9448–9453. doi: 10.1073/pnas.151258398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel BN, Brooks DM. Cross talk between signaling pathways in pathogen defense. Curr. Opin. Plant Biol. 2002;5:325–331. doi: 10.1016/s1369-5266(02)00275-3. [DOI] [PubMed] [Google Scholar]
- Lawton KA, Friedrich L, Hunt M, Weymann K, Delaney T, Kessmann H, Staub T, Ryals J. Benzothiadiazole induces disease resistance in Arabidopsis by activation of the systemic acquired resistance signal transduction pathway. Plant J. 1996;10:71–82. doi: 10.1046/j.1365-313x.1996.10010071.x. [DOI] [PubMed] [Google Scholar]
- Liu YG, Mitsukawa N, Oosumi T, Whittier RF. Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 1995;8:457–463. doi: 10.1046/j.1365-313x.1995.08030457.x. [DOI] [PubMed] [Google Scholar]
- Lorrain S, Vailleau F, Balague C, Roby D. Lesion mimic mutants: keys for deciphering cell death and defense pathways in plants? Trends Plant Sci. 2003;8:263–271. doi: 10.1016/S1360-1385(03)00108-0. [DOI] [PubMed] [Google Scholar]
- Lu H, Liu Y, Greenberg JT. Structure-function analysis of the plasma membrane- localized Arabidopsis defense component ACD6. Plant J. 2005;44:798–809. doi: 10.1111/j.1365-313X.2005.02567.x. [DOI] [PubMed] [Google Scholar]
- Lu H, Rate DN, Song JT, Greenberg JT. ACD6, a novel ankyrin protein, is a regulator and an effector of salicylic acid signaling in the Arabidopsis defense response. Plant Cell. 2003;15:2408–2420. doi: 10.1105/tpc.015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metraux JP, Ahl-Goy P, Staub T, Speich J, Steinemann A, Ryals J, Ward E. Induced resistance in cucumber in response to 2,6-dichloroisonicotinic acid and pathogens Dordrecht . The Netherlands: Kluwer Academic Publishers; 1991. [Google Scholar]
- Mylne J, Botella JR. Binary Vectors for Sense and Antisense Expression of Arabidopsis ESTs. Plant Mol. Biol. Rep. 1998;16:257–262. [Google Scholar]
- Nawrath C, Heck S, Parinthawong N, Metraux JP. EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell. 2002;14:275–286. doi: 10.1105/tpc.010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C, Metraux JP. Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell. 1999;11:1393–1404. doi: 10.1105/tpc.11.8.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozue M, Yamada K, Nakamura T, Kubo H, Kondo M, Nishimura M. Expression of a vacuolar protein (VP24) in anthocyanin-producing cells of sweet potato in suspension culture. Plant Physiol. 1997;115:1065–1072. doi: 10.1104/pp.115.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx IA, Thomma BP, Buchala A, JP M, Broekaert WF. Concomitant Activation of Jasmonate and Ethylene Response Pathways Is Required for Induction of a Plant Defensin Gene in Arabidopsis. Plant Cell. 1998;10:2103–2114. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rate DN, Cuenca JV, Bowman GR, Guttman DS, Greenberg JT. The gain-of-function Arabidopsis acd6 mutant reveals novel regulation and function of the salicylic acid signaling pathway in controlling cell death, defenses, and cell growth. Plant Cell. 1999;11:1695–1708. doi: 10.1105/tpc.11.9.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rate DN, Greenberg JT. The Arabidopsis aberrant growth and death2 mutant shows resistance to Pseudomonas syringae and reveals a role for NPR1 in suppressing hypersensitive cell death. Plant J. 2001;27:203–211. doi: 10.1046/j.0960-7412.2001.1075umedoc.x. [DOI] [PubMed] [Google Scholar]
- Reymond P, Farmer EE. Jasmonate and salicylate as global signals for defense gene expression. Curr. Opin. Plant Biol. 1998;1:404–411. doi: 10.1016/s1369-5266(98)80264-1. [DOI] [PubMed] [Google Scholar]
- Ryals J, Weymann K, Lawton K, Friedrich L, Ellis D, Steiner HY, Johnson J, Delaney TP, Jesse T, Vos P, Uknes S. The Arabidopsis NIM1 protein shows homology to the mammalian transcription factor inhibitor I kappa B. Plant Cell. 1997;9:425–439. doi: 10.1105/tpc.9.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, Manners JM. Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc. Natl. Acad. Sci. USA. 2000;97:11655–11660. doi: 10.1073/pnas.97.21.11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhegger R, Rauhut T, Glawischnig E. Regulatory variability of camalexin biosynthesis. J. Plant Physiol. 2006 doi: 10.1016/j.jplph.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Shah J, Kachroo P, Nandi A, Klessig DF. A recessive mutation in the Arabidopsis SSI2 gene confers SA- and NPR1- independent expression of PR genes and resistance against bacterial and oomycete pathogens. Plant J. 2001;25:563–574. doi: 10.1046/j.1365-313x.2001.00992.x. [DOI] [PubMed] [Google Scholar]
- Shah J, Tsui F, Klessig DF. Characterization of a salicylic acid-insensitive mutant (sai1) of Arabidopsis thaliana, identified in a selective screen utilizing the SA- inducible expression of the tms2 gene. Mol. Plant-Microbe Interact. 1997;10:69–78. doi: 10.1094/MPMI.1997.10.1.69. [DOI] [PubMed] [Google Scholar]
- Shapiro AD, Zhang C. The role of NDR1 in avirulence gene-directed signaling and control of programmed cell death in Arabidopsis. Plant Physiol. 2001;127:1089–1101. [PMC free article] [PubMed] [Google Scholar]
- Song J, Lu H, McDowell J, Greenberg J. A key role for ALD1 in activation of local and systemic defenses in Arabidopsis. Plant J. 2004;40:200–212. doi: 10.1111/j.1365-313X.2004.02200.x. [DOI] [PubMed] [Google Scholar]
- Staswick PE, Yuen GY, Lehman CC. Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J. 1998;15:747–754. doi: 10.1046/j.1365-313x.1998.00265.x. [DOI] [PubMed] [Google Scholar]
- Thomma BP, Nelissen I, Eggermont K, Broekaert WF. Deficiency in phytoalexin production causes enhanced susceptibility of Arabidopsis thaliana to the fungus Alternaria brassicicola. Plant J. 1999;19:163–171. doi: 10.1046/j.1365-313x.1999.00513.x. [DOI] [PubMed] [Google Scholar]
- Uknes S, Mauch-Mani B, Moyer M, Potter S, Williams S, Dincher S, Chandler D, Slusarenko A, Ward E, Ryals J. Acquired resistance in Arabidopsis. Plant Cell. 1992;4:645–656. doi: 10.1105/tpc.4.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wees SC, Glazebrook J. Loss of non-host resistance of Arabidopsis NahG to Pseudomonas syringae pv. phaseolicola is due to degradation products of salicylic acid. Plant J. 2003;33:733–742. doi: 10.1046/j.1365-313x.2003.01665.x. [DOI] [PubMed] [Google Scholar]
- Vanacker H, Lu H, Rate DN, Greenberg JT. A role for salicylic acid and NPR1 in regulating cell growth in Arabidopsis. Plant J. 2001;28:209–216. doi: 10.1046/j.1365-313x.2001.01158.x. [DOI] [PubMed] [Google Scholar]
- Verberne M, Budi Muljono A, R V. Salicylic acid biosynthesis. In: K L, M H, PJJ H, editors. Biochemistry and Molecular Biology of Plant Hormones. London: Elsevier; 1999. pp. 295–312. [Google Scholar]
- White RF. Acetylsalicylic acid (aspirin) induces resistance to tobacco mosaic virus in tobacco. Virology. 1979;99:410–412. doi: 10.1016/0042-6822(79)90019-9. [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;414:562–565. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- Xu W, Shioiri H, Kojima M, Nozue M. Primary structure and expression of a 24-kD vacuolar protein (VP24) precursor in anthocyanin-producing cells of sweet potato in suspension culture. Plant Physiol. 2001;125:447–455. doi: 10.1104/pp.125.1.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Goritschnig S, Dong X, Li X. A gain-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1-1, constitutive 1. Plant Cell. 2003a;15:2636–2646. doi: 10.1105/tpc.015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tessaro MJ, Lassner M, Li X. Knockout analysis of Arabidopsis transcription factors TGA2, TGA5, and TGA6 reveals their redundant and essential roles in systemic acquired resistance. Plant Cell. 2003b;15:2647–2653. doi: 10.1105/tpc.014894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Tootle T, Glazebrook J. PAD3, a gene required for camalexin biosynthesis, encodes a putative cytochrome P450 monoxygenase. Plant Cell. 1999;11:2419–2428. doi: 10.1105/tpc.11.12.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Tootle TL, Tsui F, Klessig DF, Glazebrook J. PAD4 functions upstream from salicylic acid to control defense responses in Arabidopsis. Plant Cell. 1998;10:1021–1030. doi: 10.1105/tpc.10.6.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C. Pattern-recognition receptors in plant innate immunity. Curr. Opin. Immunol. 2008;20:10–16. doi: 10.1016/j.coi.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W. GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 2004;136:2621–2632. doi: 10.1104/pp.104.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.