Abstract
Context
A role for the centrosome has been suggested in the pathology of major mental illnesses, especially schizophrenia (SZ).
Objectives
To show that pericentriolar material-1 protein (PCM1) forms a complex at the centrosome with Disrupted-In-Schizophrenia-1 (DISC1) and Bardet-Biedl syndrome-4 protein (BBS4), which provides a crucial pathway for cortical development associated with the pathology of SZ. To identify mutations in the PCM1 gene in a SZ population.
Design
Interaction of DISC1, PCM1, and BBS proteins was assessed by immunofluorescent staining and co-immunoprecipitation. Effects of PCM1, DISC1, and BBS on centrosomal functions and corticogenesis in vivo were tested by RNAi. PCM1 gene was examined by sequencing 39 exons and flanking splice sites.
Setting and Patients
Thirty-two probands with SZ from families that had excess allele sharing among affected individuals at 8p22, and 219 Caucasian controls.
Main Outcome Measures
Protein interaction and recruitment at the centrosome in cells; neuronal migration in the cerebral cortex; variant discovery in PCM1 in SZ patients.
Results
PCM1 forms a complex with DISC1 and BBS4 through discrete binding domains in each protein. DISC1 and BBS4 are required for targeting PCM1 and other cargo proteins, such as ninein, to the centrosome in a synergistic manner. In the developing cerebral cortex, suppression of PCM1 leads to neuronal migration defects, which are phenocopied by the suppression of either DISC1 or BBS4, and are exacerbated by the concomitant suppression of both. Furtheremore, a nonsense mutation that segregates with schizophrenia-spectrum psychosis is found in one family.
Conclusion
Our data further support for the role of centrosomal proteins in cortical development and suggest that perturbation of centrosomal function contributes to the development of mental diseases including SZ.
Introduction
Recent genetic studies have suggested that centrosomal dysfunction underlies risks for various neuro-psychiatric disorders, because variants in some genes that encode centrosomal proteins have been associated with schizophrenia (SZ) and bipolar disorder (BP).1–4 These genes include pericentriolar material-1 (PCM1) on chromosome 8p22,2 one of the reproducible linkage loci for SZ and BP,5–8 and Disrupted-In-Schizophrenia-1 (DISC1).3, 4 The centrosome plays a role in organizing microtubules, contributing to cell cycle progression, cell polarization, and ciliogenesis.9–12 Consequently, the centrosome is required for proper neurodevelopment, especially in the cerebral cortex.13–17
PCM1 is a component of centriolar satellites and acts as a scaffold to target several proteins to the centrosome in a dynein motor-dependent manner and regulate microtubular dynamics.18–20 PCM1 also interacts with Bardet-Biedl syndrome-4 protein (BBS4), which is encoded by one of the causative genes for Bardet-Biedl syndrome, an inherited disorder characterized by renal dysfunction, obesity, polydactyly, and diverse neuro-psychiatric symptoms.21–24 BBS is genetically heterogeneous, with 12 genes identified to date, but mutations in each of these genes lead to similar pathology in humans, suggesting that BBS proteins function through a common molecular pathway. Consistent with this notion, all BBS proteins investigated to date localize primarily at the centrosome and the basal body of ciliated cells, where they contribute to the maintenance of microtubular dynamics, as well as intracellular transport and ciliary function.25–30
We have reported previously that DISC1, a major susceptibility factor for SZ and BP, plays a crucial role at the centrosome,31, 32 while another group has reported consistently that DISC1 interacts with kendrin, a component of pericentiolar material.33 Consequently, DISC1 is required for neurite outgrowth and proper development of the cerebral cortex, such as neuronal migration and dendritic arborization.31 Therefore, we hypothesized that PCM1, DISC1, and the BBS proteins may interact and play a role in the centrosome and that such interactions might be relevant both to the DISC-associated neurodevelopmental functions and to the etiopathology of SZ.
Here we provide biological and genetic evidence that PCM1-DISC1-BBS proteins form a centrosomal pathway, potentially associating with major mental illnesses, such as SZ. These proteins form a complex at the centrosome through discrete binding domains. DISC1 and BBS4 act synergistically to recruit PCM1 and associated proteins to the centrosome. Disruption of the PCM1-DISC1-BBS4 pathway leads to profound defects in neuronal migration during cortical development. Finally, we report a pedigree in which a nonsense mutation in the PCM1 gene segregates with SZ-spectrum psychosis.
Methods
Plasmids and antibodies
All the deletion DISC1 and PCM1 expression constructs were made by PCR-based mutagenesis protocol.34 The deletion BBS4 expression constructs were made as described.21 PEGFP-F was purchased from BD Bioscience Clontech. Rabbit polyclonal antibodies against PCM1, ninein, BBS1, BBS4, and BBS8 antibody were prepared as described.20, 21, 25, 35 The following antibodies were also used: mouse monoclonal antibodies against β-tubulin and γ-tubulin (Sigma-Aldrich, St Louis, MO); mouse monoclonal antibodies against HA-tag and myc-tag (BAbCO, Berkeley, CA); rabbit polyclonal antibody against HA-tag (Clontech, Mountain View, CA); rabbit polyclonal antibody against myc-tag (Santa cruz, Santa cruz, CA); affinity-purified rabbit antiserum against green fluorescent protein (GFP; Molecular Probes, Eugene, OR); mouse monoclonal antibody against GFP (Nacalai tesque, Kyoto, Japan). The rabbit polyclonal anti-DISC1 antibody (D27) was a gift from Dr. Nicholas. J. Brandon (Wyeth Discovery Neuroscience, USA). Plasmids expressing interfering short hairpin RNA (shRNA)36 were generated to suppress endogenous DISC1, PCM1, and BBS4 protein expression. Their target sequences are as follows:
DISC1 RNAi, 5’-GGCAAACACTGTGAAGTGC-3’
PCM1 RNAi, 5’-TCAGCTTCGTGATTCTCAG-3’
BBS4 RNAi, 5’-GCAGCTATCAGCTGCCTAA-3’
.
A scrambled sequence without homology to any known mRNA was used to produce the control RNAi. The efficiency of all shRNAs was tested by the extent of suppression in endogenous target protein in rat PC12 cells by Western blotting.
Cell culture and transfection
HEK293 cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) with 10% fetal bovine serum (FBS) and 1% Penicillin-Streptomycin (PS). PC12 cells were maintained in DMEM with 10% FBS, 5% horse serum (HS), and 1% PS. Transfection of expression constructs or RNAi constructs was carried out with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) for PC12 cells and with PolyFect Transfection Reagent (Qiagen, Valencia, CA) for HEK293 cells. The molar ratio of pEGFP-F to RNAi plasmid(s) was 1:3 for the transfection. Rodent primary cortical neurons were prepared as described.37
Coimmunoprecipitation and cell extraction
Immunoprecipitation
cells were lysed in a RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM MgCl2, 5 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM ethylenediaminetetraacetic acid (EDTA), 1% Triton X-100, and protease inhibitor mixture (Roche, Basel, Switzerland). Pre-cleared supernatants (500 µg) from crude cell lysates by centrifugation at 14,000 × g for 10 min were incubated with primary antibodies (1 µg/ml rabbit polyclonal antibody against HA-tag or against myc-tag) for overnight, which was followed by the addition of TrueBlot™ Anti-Rabbit Ig IP Beads (eBioscience, San Diego, CA) (30 µl) or proteinG Plus/Protein A agarose (Calbiochem, Darmstadt, Germany) (30 µl) for 1 h. The immunoprecipitates were washed three times by a TBS-based buffer with 0.05% Tween-20 and analyzed with SDS-PAGE/Western blotting. In the stringent wash conditions, we added NaCl up to the final concentration at 500 mM. ProFoundTM Mammalian HA Tag IP/Co-IP Kit (Pierce, Rockford, IL) was used for immunoprecipitation in experiments of Figure 2A.
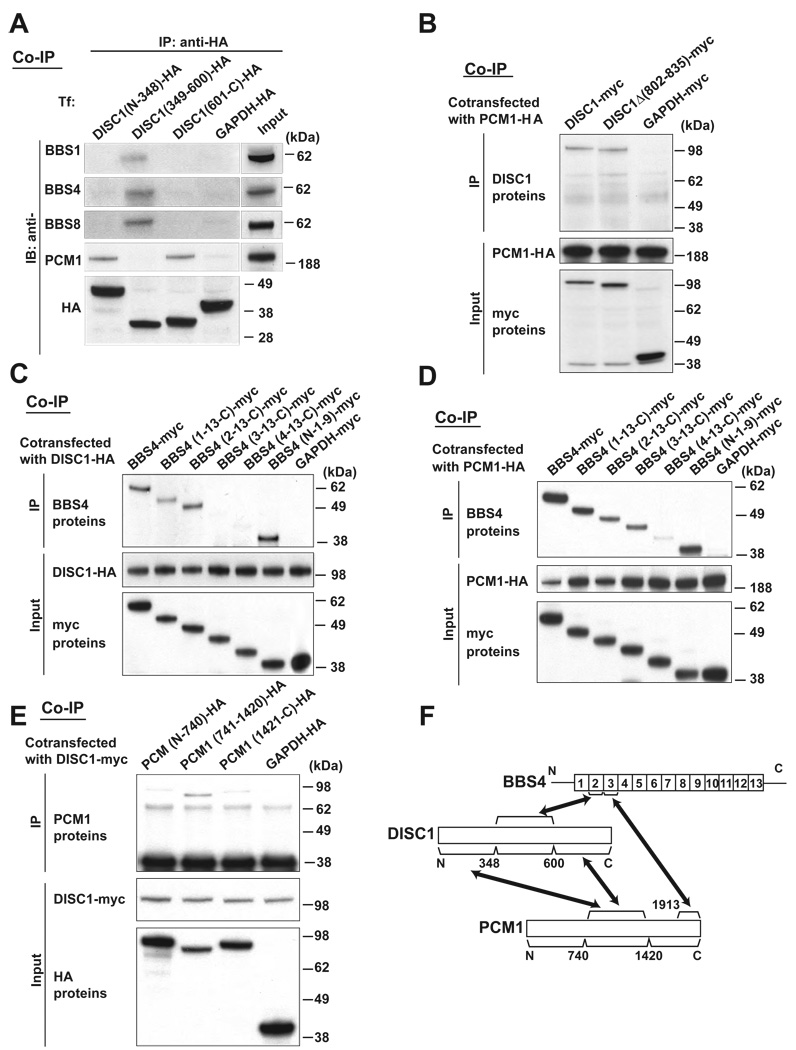
Figure 2. PCM1, DISC1, and BBS4 interact with each other through distinct binding domains.
(A) The middle portion of DISC1 (amino acids 349–600) is crucial for DISC1-BBS4 protein interaction. The N-terminal portion (amino acids 1–348) and the C-terminal portion (amino acids 601–854) of DISC1 are important for the DISC1-PCM1 binding. HA-tagged three DISC1 protein fragments [DISC1 (N-348), DISC1 (349–600), and DISC1 (601-C)] were expressed in HEK293 cells for co-immunoprecipitation with an anti-HA antibody. The middle portion of DISC1, but not the N- nor C-terminal DISC1, binds to each of BBS1, 4, and 8, whereas the N- and C-terminal DISC1 bind to PCM1 (upper panels). The inputs of each protein are shown at the right and bottom panels. IB indicates antibodies used for Western blotting.
(B) The C-terminus domain of DISC1 for interaction with PCM1 is distinct from the NDEL1 binding domain of DISC1. Deletion of DISC1-NDEL1 binding region [DISC1Δ(802–835)] had no effect on the interaction of DISC1 with PCM1. The inputs are shown in the middle and bottom panels.
(C) The second TPR motif of BBS4 is crucial for the BBS4-DISC1 interaction. A series of myc-tagged BBS4 truncation mutants were co-expressed with HA-tagged DISC1 in HEK293 cells for co-immunoprecipitation with an anti-HA antibody. Deletion of the N-terminal region in [BBS4 (1-13-C)] and further deletion of the first TRP motif [BBS4 (2-13-C)] does not affect the BBS4-DISC1 interaction. By contrast, BBS4 mutants with further deletion of the second TRP motif [BBS4 (3-13-C) and BBS4 (4-13-C)] did not bind with DISC1. BBS4 lacking the C-terminal region [BBS4 (N-1-9)] bind to DISC1. The inputs are also shown (middle and bottom panels).
(D) The third TPR motif of BBS4 is important for the BBS4-PCM1 interaction. Deletion of the third TPR motif [BBS4 (4-13-C)] dramatically weakened the interaction of myc-tagged BBS4 with HA-tagged PCM1. An anti-HA antibody was used for co-immunoprecipitation. The inputs are shown in the middle and bottom panels.
(E) The middle portion of PCM1 is important for DISC1-PCM1 interaction. Myc-tagged DISC1 was co-expressed with HA-tagged PCM1 protein fragments in HEK293 cells for co-immunoprecipitation with an anti-myc antibody. PCM1 (741–1420) has stronger binding affinity to DISC1 than PCM1 (N-740) and PCM1 (1421-C). The inputs of each protein are also shown in middle and bottom panels.
(F) Schematic of DISC1, BBS4, and PCM1 interaction shows that these proteins may interact with each other through distinct binding domains.
Cell extraction
cells were sonicated in ice-cold lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, and a protease inhibitor mixture). Extracted cells were mixed with SDS-PAGE loading buffer after protein concentrations were measured. Each protein sample (10 µg) was analyzed with SDS-PAGE followed by Western blotting.
Immunofluorescent staining
Cells were fixed with ice-cold methanol at −20°C 3 days after transfection. After blocking with 1.5% bovine serum albumin (BSA) and 0.5% normal goat serum in PBS, cells were treated with primary antibodies (dilution: γ-tubulin, 1:100; DISC1, 1:200; PCM1, 1:500; ninein, 1:500; BBS1, 1:300; BBS4, 1:500; BBS8, 1:500) for 1 h followed by the reaction with secondary antibodies conjugated to Rhodamine Red-X (dilution, 1:300) and Cy5 (dilution, 1:300) (Jackson ImmunoResearch, West Grove, PA) for 1 h. Hoechst 33258 (Molecular Probes) was used at 1:500 dilution for 3 min to visualize nuclei. Confocal microscopy (Zeiss LSM 510 Meta, Grottingem, Germany) was used for epifluorescent image collection. To obtain clearer images of cell morphology under methanol fixation, cells were co-transfected with RNAi constructs together with pEGFP-F, a membrane-attached isoform of GFP as a transfection marker. To quantify the distribution of PCM1 and ninein at the centrosome, a circle with 3-µm diameter was drawn centering on the γ-tubulin and defined as the area, including the centrosome. In all experimental groups, the immunointensity of PCM1 or ninein in the whole cell area versus centrosome area was quantified with Image J (http://rsb.info.nih.gov/ij/). The intensity ratio of the signal of more than 30 cells per group was analyzed in three independent experiments in a blinded manner. Statistical analyses were conducted with one-way ANOVA followed by post-hoc testing. Values depicted are means ± SEM.
In utero electroporation and immunohistochemistry
In utero electroporation was performed as described.31, 38 Validated shRNA plasmids in cell cultures (at a concentration of 4 µg/µl in 1–2 µl) were introduced directly into the ventricular zone by in utero electroporation of E15 embryos as reported.38 To confirm the specificity of the effects, dilution series of each RNAi plasmid in 1–2 µl were introduced, and their dose-correlated effects were confirmed. A GFP expression vector with CAG promoter was co-transfected with RNAi constructs at a concentration of 2 µg/µl. Coronal slices of the developing cerebral cortex were prepared at P0 as described.38 Briefly, the brains were fixed with 4% paraformaldehyde and sectioned with a cryostat at 20 µm on P0. Green fluorescent images were captured after immunofluorescent staining with an anti-GFP antibody (dilution, 1:500). Nuclei were labeled with propidium iodide (Molecular Probes). Slice images were acquired with a confocal microscope (Zeiss LSM510 and Olympus Optical FV300).
Quantitative bin analysis of brain slices
To quantify the pattern of migration, the numbers of GFP-positive cells in developmental cerebral cortex, including the ventricular zone (VZ), the subventricular zone (SVZ)/intermediate zone (IZ), and the cortical plate (CP) were counted from three independent sections. We quantified the RNAi effect on neuronal migration status by bin analysis, in which the developing cerebral cortex was divided into 10 equal spaces (10 bins) and the percentage of GFP-positive cells in each bin was determined. The numbers of neurons in each category from more than five independent experiments were counted in a blinded manner. Migration distance was defined as the relative distance of each cell migration (from the surface of the ventricle) to the radial thickness of the cerebral cortex where the cells are located. Image J (http://rsb.info.nih.gov/ij/) was used for the assay. Statistical analyses were conducted with one-way ANOVA followed by post-hoc testing. Values depicted are means ± SEM.
Sequence Analysis of PCM1 in SZ patients
We analyzed 32 unrelated schizophrenic patient DNAs, from families that have been reported previously to have excess allele sharing among affected individuals at 8p22,7 for exonic variations in PCM1. DNA samples were extracted according to standard protocol. Details about the clinical assessment of the samples are available in our previous study.7 We also screened 219 Caucasian control samples, matched for ethnicity to the SZ patients and evaluated for the absence of mental illnesses (DSM-IV criteria). For all the subjects (both controls and cases), we utilized two independent genotyping methods: first, we performed PCR and then bidirectional sequencing using BigDye-Primer v.1 and the ABI377 sequencer (Applied Biosystems, Foster City, CA); second, we screened by custom TaqMan SNP genotyping assays (Applied Biosystems).
Results
Interaction of PCM1, DISC1, and BBS proteins at the centrosome
To explore a possible functional relationship among PCM1, DISC1, and BBS proteins, we first tested whether these molecules could interact with each other. Exogenous protein interactions were tested by co-immunoprecipitation in HEK293 cells. HA-tagged PCM1 co-precipitated with myc-tagged DISC1, but not with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Figure 1A). HA-tagged DISC1 co-precipitated with all the BBS proteins we tested that were tagged with myc (BBS1, BBS2, BBS4, BBS5, BBS6, BBS7, and BBS8), but not with GAPDH (Figure 1B). This result suggests that DISC1 might be an important component in the BBS common pathway. Our previous study had already demonstrated an interaction of BBS4 and PCM1 proteins at the centrosome.21 Thus, we tested co-localization of DISC1, PCM1, BBS1, and BBS4 proteins at the centrosome in immature cortical neurons (Figure 1C). DISC1, BBS1, and BBS4 co-localized almost perfectly with γ-tubulin, an established centrosomal marker, whereas PCM1 localized as granular structures at and around the centrosome in a manner reminiscent of its distribution in fibroblasts and other cell types.19
Figure 1. PCM1, DISC1, and BBS proteins interact, localizing with γ-tubulin at the centrosome.
(A) PCM1 interacts with DISC1. HA-tagged PCM1 was co-expressed with myc-tagged DISC1 in HEK293 cells. Cell extracts were immunoprecipitated with an anti-HA antibody. Immunoprecipitates were analyzed by Western blotting with an anti-myc antibody (upper panel). The input of each protein is also shown (middle and bottom panels).
(B) BBS proteins interact with DISC1 in co-immunopecipitation in HEK293 cells. Myc-tagged BBS1, 2, 4, 5, 6, 7, and 8 all bind to HA-tagged DISC1. Consistent results were observed in both under low stringency (150mM NaCl) washing condition (upper panels) as well as high stringency (500mM NaCl) washing condition (lower panels). The input of each protein is shown in the middle and bottom panels.
(C) Localization of BBS1, BBS4, DISC1, and PCM1 in immature cortical neurons at 3 days in vitro (DIV3). Endogenous BBS1, BBS4, and DISC1 (red) are co-localized with γ-tubulin at the centrosome (arrowheads). Endogenous PCM1 (red) mainly occurs just adjacent to γ-tubulin with slight overlap with each other. Blue, nucleus; green, γ-tubulin. Scale bar, 10 µm.
PCM1, DISC1, and BBS4 interact with each other through distinct binding domains
To characterize DISC1 domains crucial for the interaction with PCM1 and BBS proteins, we expressed three HA-tagged DISC1 fragments in HEK293 cells. Endogenous BBS1, BBS4, and BBS8 co-precipitated commonly with the middle portion of DISC1 containing amino acids 349–600 [DISC1 (349–600)], but not the N-terminal [DISC1 (N-348)] nor the C-terminal DISC1 fragments [DISC1 (601-C)] (Figure 2A). By contrast, the N-terminal [DISC1 (N-348)] and C-terminal [DISC1 (601-C)] domains, distinct from the domain for BBS proteins, mediated the interaction between DISC1 and PCM1 (Figure 2A). The C-terminal domain of DISC1 for binding with PCM1 is distinct from the domain for NDEL1 binding, demonstrated by interaction of PCM1 to DISC1 lacking the NDEL1 binding site [DISC1Δ(802–835)] (Figure 2B).32 BBS4 is required for the recruitment of PCM1 to the centrosome.21 We therefore focused on BBS4 for further analysis of the DISC1-PCM1-BBS protein interaction. The BBS4 protein is composed of 13 tandem tetratricopeptide repeat (TPR) motifs, flanked with short N- and C-terminal sequences. Sequential deletion of BBS4 protein from the N-terminus indicated that HA-tagged DISC1 could interact with BBS4 protein that maintains the portion from the 2nd TPR to the C-terminus, but failed to bind to BBS4 once the 2nd TPR was lost (Figure 2C). In contrast, the same sequential deletion of BBS4 for testing interaction with PCM1 revealed that deletion of the 3rd TPR dramatically reduced the PCM1-BBS4 binding (Figure 2D). The domain of PCM1 for binding to DISC1 was tested by using three PCM1 fragments, indicating that the middle portion of PCM1 (amino acids 741–1420) was required for the PCM1-DISC1 interaction (Figure 2E), which, given that the C-terminal portion of PCM1 (amino acid 1913 to the C-terminus) is required for binding to BBS4,21 suggests that the PCM1-DISC1 interaction is discrete from the PCM1-BBS4 interaction. Overall, based on these pairwise binding data between the three proteins, we conclude that PCM1, DISC1, and BBS4 likely interact with each other through distinct binding domains (Figure 2F).
DISC1 and BBS4 act synergistically to influence recruitment of PCM1 and ninein to the centrosome
We reported previously that DISC1 plays a role in recruiting dynein motor proteins, such as dynein intermediate chain and dynactin p150glued to the centrosome.31 We also showed that BBS4 binds to p150glued that is required for recruiting PCM1 to the centrosome.21 Because PCM1 interacts with DISC1 and BBS4 through distinct domains, we hypothesized that DISC1 and BBS4 may act synergistically to recruit PCM1 to the centrosome. To test this idea, we used RNA interference (RNAi) against each of DISC1, BBS4, and PCM1 (Figure 3A) and examined the effects in PC12 cells (Figure 3B). Knockdown expression of DISC1 reduced accumulation of PCM1 to the centrosome. Consistent with our previous findings in HeLa cells,21 knockdown expression of BBS4 resulted in decreased enrichment of PCM1 to the centrosome. Of most importance, knockdown of both DISC1 and BBS4 had a significantly stronger influence on the distribution of PCM1 than either single knockdown, consistent with the hypothesis that DISC1 and BBS4 cooperate to regulate the recruitment of PCM1 to the centrosome. PCM1 plays a role in further recruiting other centrosomal proteins, such as ninein.18 We therefore tested whether DISC1 and BBS4 also influence PCM1-associated molecular recruitment to the centrosome in a synergistic manner, by examining the effects of RNAi on DISC1, BBS4, and PCM1 with respect to the localization of ninein (Figure 3C). Knockdown expression of either PCM1 or both DISC1 and BBS4 similarly reduced the amount of ninein at the centrosome in PC12 cells.
Figure 3. Synergistic effect of DISC1 and BBS4 on recruitment of PCM1 and ninein to the centrosome.
(A) Efficient suppression of DISC1, BBS4, and PCM1 by RNAi. RNAi to DISC1, BBS4, and PCM1 suppress 78, 65, and 78% of endogenous DISC1, BBS4, and PCM1 expression, respectively, in PC12 cells (top panels). RNAi to DISC1 or BBS4 does not affect the levels of endogenous PCM1 (middle panels). IB: antibodies used for Western blotting.
(B) Suppression of DISC1 and BBS4 reduces accumulation of PCM1 to the centrosome in PC12 cells in a synergistic manner. To quantify the accumulation, immunointensity of PCM1 in the centrosome area (white circle) relative to that in the whole cell region surrounded by green line was quantified. Bars represent averages of each group of cells in three independent and blinded experiments (***P<0.001, ** P<0.01, * P<0.05). Error bars represent SEM. Representative images are shown. Blue, nucleus; red, PCM1; green, pEGFP-F; white, γ-tubulin (also indicated by arrowheads). Scale bar, 10 µm.
(C) Accumulation of ninein at the centrosome is disturbed by synergistic application of DISC1 and BBS4 RNAi, or PCM1 RNAi. Although neither application of DISC1 RNAi nor BBS4 RNAi leads to a significant effect on ninein, the synergistic application of both RNAi reduces accumulation of ninein to the centrosome, resembling the phenotype in the presence of RNAi to PCM1. To quantify the accumulation, immunointensity of ninein in the centrosome area (white circle) relative to that in the whole cell region surrounded by the green line was quantified. Bars represent averages of each group of cells in three independent and blinded experiments (* P<0.005, ** P<0.0001). Error bars represent SEM. Representative images of PC12 cells are shown. Blue, nucleus; red, ninein; green, pEGFP-F; white, γ-tubulin (also indicated by arrowheads). Scale bar, 10 µm.
Knockdown expression of PCM1, DISC1, and BBS4 leads to neuronal migration defects in the developing cerebral cortex in vivo
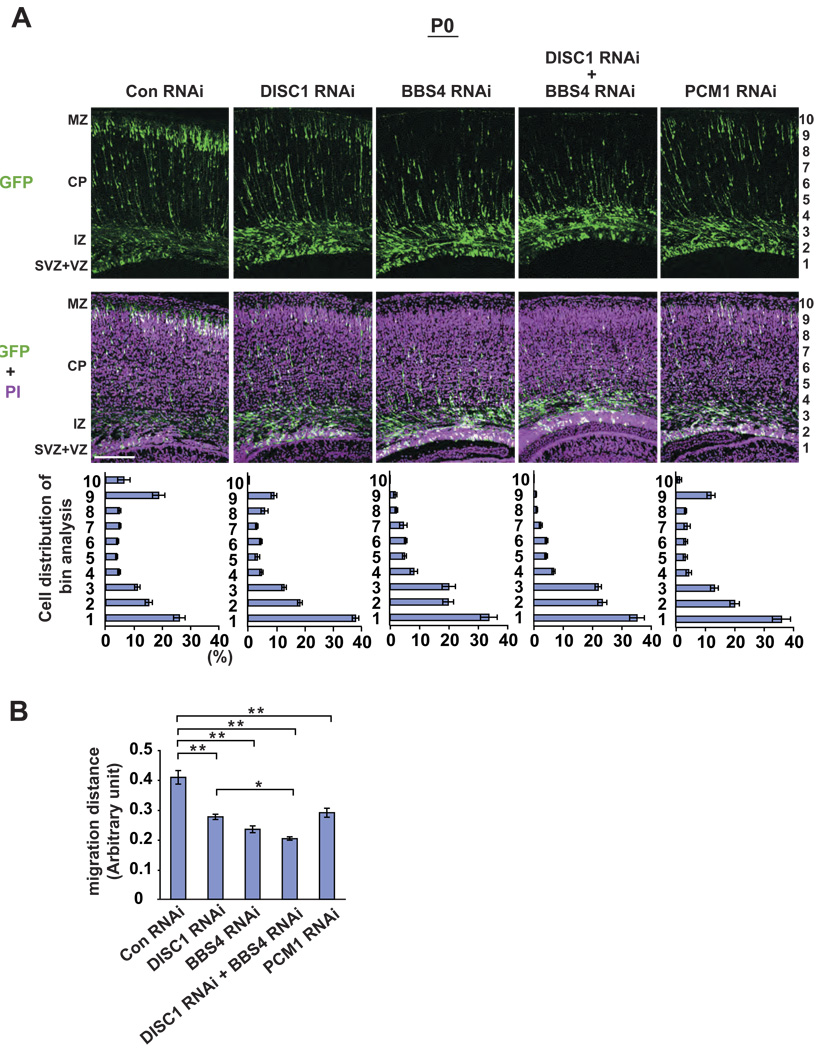
To evaluate the physiological relevance of our findings, we tested the influence of PCM1, DISC1, and BBS4 in vivo by suppressing their expression in the developing cerebral cortex by in utero gene transfer.31, 38 Embryos were electroporated with shRNA at embryonic day 15 (E15), and the effect of suppression was evaluated by immunohistochemistry, followed by a bin distribution analysis of neurons at postnatal day 0 (P0) (Figure 4A, B and Suppl. Figure 1). Brain slices electroporated with control RNAi (Con RNAi) together with a GFP marker showed that 25% of GFP-labeled cells completed migration through the cortical wall and formed the superficial layers of the cortex that corresponded to bins 9 and 10. By contrast, in brain slices electroporated with DISC1 RNAi, radial neuronal migration was significantly delayed, as reported previously.31 Suppression of either BBS4 or PCM1 phenocopied the DISC1 phenotype in neuronal migration. Importantly, concomitant suppression of both DISC1 and BBS4 led to significantly more severe impairment in migration, compared to that of DISC1 alone.
Figure 4. Knockdown of DISC1, BBS4, and PCM1 leads to neuronal migration defects in the developing cerebral cortex.
(A) RNAi constructs and GFP expression vectors were electroporated into the ventricular zone (VZ) at E15 and analyzed at P0. In brains with control RNAi (Con RNAi), 40% of GFP-labeled cells exited the VZ, and 25% of GFP-labeled cells completed migration and formed the superficial layers of the cortex that correspond to bins 9 and 10. By contrast, only less than 15% of GFP-positive cells reached the superficial layers in brain slices with DISC1 RNAi, BBS4 RNAi, or PCM1 RNAi, with the majority of GFP-positive cells remaining in the intermediate zone (IZ), subventricular zone (SVZ), and VZ. Green, cells co-transfected with GFP and RNAi constructs; purple, propidium iodide (PI). Scale bar: 100 µm.
(B) A migration distance is shown. Silencing of DISC1, BBS4, or PCM1 induces delayed radial migration (** P<0.0001). Silencing of both DISC1 and BBS4 expression leads to a more severe defect compared with that with either DISC1 RNAi or BBS4 RNAi. * P<0.05. Values are mean ± SEM.
A candidate pathogenic PCM1 mutation in a schizophrenia family
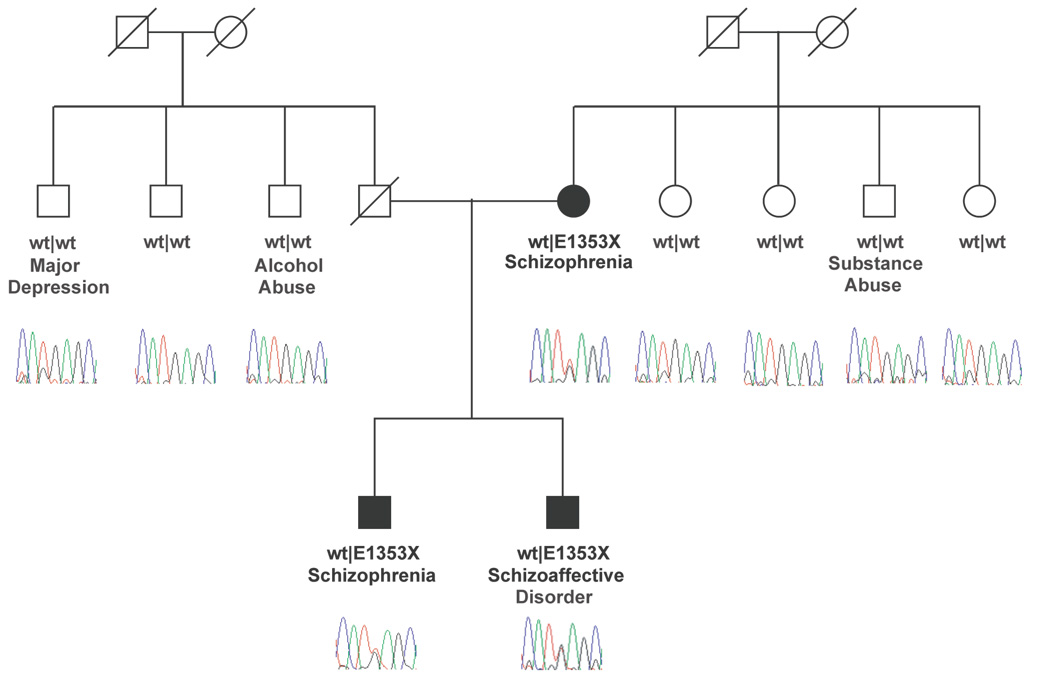
Our data have shown that DISC1 and BBS4 are necessary for targeting PCM1 to the centrosome, with concomitant targeting effects for ninein, and likely other molecules for their transport to the centrosome, in a PCM1-dependent manner. Consistent with this notion, a recent study reported association of PCM1 haplotypes with SZ and volumetric defects in the gray matter of the orbitofrontal cortex,2 although a causative mutation has not been found to date. We therefore examined the PCM1 gene for mutations in a SZ cohort by focusing on the coding region of the gene, since variations there would be less challenging to interpret. An emerging hypothesis in the field of SZ is that a portion of the genetic load may be contributed by rare, possibly strong, alleles.39 We therefore focused on testing primarily for rare alleles by performing direct bidirectional sequencing of the 39 exons and flanking splice sites in 32 probands.7 In addition to synonymous SNPs that are unlikely to affect the PCM1 transcript or protein, we found two previously known missense mutations in our cohort (Table 1). The first allele, SNP rs370429 (encoding a T1543I change) has been reported to be associated with SZ.2 Different from the data by Gurling et al2, we failed to find any association between this SNP and SZ, probably due to our small sample size. Likewise, for a second missense allele (rs412750; S159N at the amino acid level), we failed to detect allelic association, which is consistent with previous work.2 The genotypic frequency of this variant is significantly different in SZ patients (Fisher’s exact test P=0.01; Table 1). We think it unlikely that this represents a genotyping error inherent to the assay, since we saw no deviation from Hardy-Weinberg equilibrium (HWE) in the large control group (P=0.3481); nonetheless, to confirm this result, we re-genotyped all individuals from both cases and controls with a TaqMan assay. There was no genotyping error observed since the genotypes were attained in two different methods that had the same result. We found additional evidence of a relationship between PCM1 and SZ. In one individual, we found a heterozygous 4057G→T mutation that introduces a premature termination codon (E1353X) in exon 24, which leads to either truncation of the protein, eliminating 672 residues from the C-terminus, or, more likely, triggers the nonsense mediated decay by virtue of the introduction of a premature termination codon.40 This allele was not present in any of 219 ethnically matched controls, whereas segregation analysis showed that the E1353X allele was also present in the heterozygous state in the affected mother and the affected sibling of the proband, but not in the unaffected members of the maternal and paternal sibship (Figure 5). This result supports a possible role for this PCM1 loss of function allele for SZ in this family. Clearly, a mutation in a single family with a priori linkage to the 8p region is not sufficient to generalize the causal link between PCM1 loss of function and SZ. However, the combination of this result with the previous association of PCM1 with SZ2 and, importantly, the biochemical relationship of PCM1 to DISC1 as it pertains to key neurodevelopmental processes pose a compelling argument.
Table 1.
List of PCM1 variants in our cohort of SZ patients and controls.
| Genotype Frequency | ||||||||
|---|---|---|---|---|---|---|---|---|
| Exon | Amino Acid Change |
Nucleotide Change |
AA in SZ |
Aa in SZ |
aa in SZ |
AA in Control |
Aa in Control |
aa in Control |
| 5 | S159N | 476GT→AC/AT | 75 | 9.4 | 15.6 | 57.5 | 32.9 | 9.6 |
| (rs412750) | (24) | (3) | (5) | (126) | (72) | (21) | ||
| 24 | E1353X | 4057G→T | 96.9 | 3.1 | 0.0 | 100 | 0.0 | 0.0 |
| (31) | (1) | (0) | (219) | (0) | (0) | |||
| 28 | T1543I | 4628C→ 2192T | 93.8 | 6.2 | 0.0 | 92.2 | 7.8 | 0.0 |
| (rs370429) | (30) | (2) | (0) | (202) | (17) | (0) | ||
"A" corresponds to the major allele found in NM_006197
"a" corresponds to the minor allele found in NM_006197
There are no allelic association between rs412750 and schizophrenia (SZ) in this sample set, although the genotypic frequency of the S159N is significantly different in SZ patients (Fisher’s exact, P=0.01) compared to controls. The nonsense mutation (E1353X) was found in a single SZ patient and no controls. Numbers in parentheses are the counts (n) for each group. The distribution of genotypes for the controls for both rs412750 and rs370429 did not deviate significantly from Hardy-Weinberg equilibrium (HWE) (P=0.3481 and 0.9093 respectively).
Figure 5. A nonsense mutation in PCM1 in a family with SZ and schizoaffective disorder.
Mutation analysis of a Caucasian family JHU37007 shows a heterozygous 4057G→T mutation in exon 24 of PCM1, introducing a premature termination codon (E1353X); genotypes are shown below each individual, as are sequence traces. The psychiatric phenotype (if any) of each family member is also shown.
Comment
In the present study, we provide two lines of evidence that support a role for the centrosome in the pathology of SZ. Biological data indicate a centrosomal pathway that includes the PCM1, DISC1, and BBS proteins, playing a role in proper cortical development. Genetic data further confirm the notion that PCM1 is a risk factor for SZ, by providing a nonsense mutation that segregates with SZ-spectrum psychosis in a pedigree.
We found that DISC1 interacts with several BBS proteins (BBS1, BBS2, BBS4, BBS5, BBS6, BBS7, and BBS8) and that DISC1 may possess a common binding domain for at least BBS1, BBS4, and BBS8. Our data on the interaction of DISC1 with all BBS proteins tested suggest that DISC1 may regulate the common pathway involving BBS. We also found that PCM1, DISC1, and BBS4 interact with each other at least through “distinct” binding domains. As a future perspective, minimal binding domains for each protein interaction will be determined by a series of deletion mutants as well as full-length proteins that have specific deletion of identified binding domains.
Our data also suggest that DISC1 and BBS4 target PCM1 and the associated cargo protein ninein synergistically to the centrosome. It remains to be determined, however, whether these proteins interact directly and whether other centrosomal proteins such as centrin, and pericentrin are also regulated by the interaction of DISC1 with BBS4. One question is how either DISC1 RNAi alone or BBS4 RNAi alone shows minor effects on the accumulation of ninein to the centrosome, whereas such treatment affects the localization change of PCM1. This may be because accumulation of ninein to the centrosome is affected only when the levels of PCM1 at the centrosome fall below the threshold by the synergistic effects of both DISC1 and BBS4 RNAi.
Neuronal migration defects were observed when we knocked down DISC1, BBS4, or PCM1 in the developing cerebral cortex, which is consistent with the notion of the role of centrosome in corticogenesis. We believe that interpretation of the data should be viewed with caution, however, because the knockdown of these proteins may potentially affect their other cellular functions related to neuronal migration. For instance, DISC1 is a multifunctional protein localized at the centrosome, mitochondria, postsynaptic densities, and the nucleus.3 Future studies might address this issue by co-electroporation of RNAi and expression constructs of DISC1 in which co-expression of wild-type DISC1 rescues the phenotypes resulting from DISC1 RNAi, whereas mutant DISC1 selectively deficient in the binding domains for BBS4 or PCM1 may not rescue the pathology. That PCM1 knockdown has a weaker influence on migration defects than does co-knockdown of BBS4 and DISC1 might be explained by considering that knockdown of DISC1 and BBS4 may potentially affect their other cellular functions related to neuronal migration, whereas PCM1 has more restricted function associated with the centrosome.
In addition to DISC1 that presents the most compelling genetic argument for participation in SZ,3, 4 two centrosome-related genes, PCM1 and NDE1, have also been proposed as potential SZ susceptibility genes.1, 2 Nonetheless, it is difficult to identify the causal mutation(s), in part due to allelic heterogeneity. Here we have identified a bona fide loss of function mutation (a nonsense allele), which segregates with schizophrenia-spectrum psychosis. Given our functional data, we speculate that haploinsufficiency at the PCM1 locus will lead to compromised, but not abolished, PCM1-associated centrosomal functions, which can potentially lead to more subtle neurodevelopmental effects. Notably, patients with SZ who showed an association with PCM1 had gray matter deficits in the orbitofrontal cortex.2 Lesions in this brain region are likely to compromise mechanisms that support reward-related processes and motivated behaviors.41 These are consistent with the finding that families with evidence for linkage to 8p21-22 had significantly more affective deterioration, poorer outcome, more thought disorder and fewer depressive symptoms than did affected individuals from non-8p21-22-linked families.42 Taken together, we speculate that genetic variations of PCM1 are associated with a subtype of SZ that primarily displays negative symptoms, referred to commonly as deficit SZ.43 Nonetheless, chromosome locus of 8p21-22 has linked to both SZ and mood disorders.5–8 Thus, PCM1 may also be a risk factor for affective disorders by participating in some aspects of the pathophysiology of the diseases.
Our data also suggest that BBS genes might also be potential candidates contributing susceptibility alleles for major mental illnesses. Consistent with this notion, recent epidemiological findings have shown that BBS patients are at least twice as likely to develop SZ compared to the general population and, suffer from various psychiatric conditions at high prevalence (>30%).22, 23 Intriguingly, BBS and SZ also share other phenotypes, such as olfaction deficits, obesity, and type II diabetes mellitus.22, 23, 28, 44–46
Supplementary Material
Acknowledgments
We thank Yukiko Lema for preparation of the figures and manuscript and Drs. Pamela Talalay and David Valle for critical reading of this manuscript. We thank Dr. Dan Arking for his assistance in the statistical analysis. We appreciate the technical assistance of Dr. Norimasa Mitsuma, Dr. Jantje Gerdes, Ms. Sarah Elashvili, and Mr. Matthew B. Casio. We thank Dr. Phil Beales and Dr. Nicholas J. Brandon for BBS4 and DISC1 antibodies, respectively. We thank Drs. Raymond J. DePaulo, Timothy Moran, Solomon H. Snyder, Hongjun Song, Christopher A. Ross, Mikhail V. Pletnikov, and Kozo Kaibuchi for discussions. This work was supported by grants from U.S. Public Health Service Grant MH-69853 (AS), HD042601 and DK072301 (NK), and MH071473 (NC), as well as foundation grants from Stanley (AS, NC), NARSAD (AS, AK), S-R (AS). KN was supported by grants from the Japanese ministry and foundations of JSPS, Japan Brain, Tokyo Biochemical Research, and Brain Science.
References
- 1.Hennah W, Tomppo L, Hiekkalinna T, Palo OM, Kilpinen H, Ekelund J, Tuulio-Henriksson A, Silander K, Partonen T, Paunio T, Terwilliger JD, Lonnqvist J, Peltonen L. Families with the risk allele of DISC1 reveal a link between schizophrenia and another component of the same molecular pathway, NDE1. Hum Mol Genet. 2007;16(5):453–462. doi: 10.1093/hmg/ddl462. [DOI] [PubMed] [Google Scholar]
- 2.Gurling HM, Critchley H, Datta SR, McQuillin A, Blaveri E, Thirumalai S, Pimm J, Krasucki R, Kalsi G, Quested D, Lawrence J, Bass N, Choudhury K, Puri V, O'Daly O, Curtis D, Blackwood D, Muir W, Malhotra AK, Buchanan RW, Good CD, Frackowiak RS, Dolan RJ. Genetic association and brain morphology studies and the chromosome 8p22 pericentriolar material 1 (PCM1) gene in susceptibility to schizophrenia. Arch Gen Psychiatry. 2006;63(8):844–854. doi: 10.1001/archpsyc.63.8.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishizuka K, Paek M, Kamiya A, Sawa A. A review of Disrupted-In-Schizophrenia-1 (DISC1): neurodevelopment, cognition, and mental conditions. Biol Psychiatry. 2006;59(12):1189–1197. doi: 10.1016/j.biopsych.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 4.Porteous DJ, Thomson P, Brandon NJ, Millar JK. The genetics and biology of DISC1--an emerging role in psychosis and cognition. Biol Psychiatry. 2006;60(2):123–131. doi: 10.1016/j.biopsych.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, Williams NM, Schwab SG, Pulver AE, Faraone SV, Brzustowicz LM, Kaufmann CA, Garver DL, Gurling HM, Lindholm E, Coon H, Moises HW, Byerley W, Shaw SH, Mesen A, Sherrington R, O'Neill FA, Walsh D, Kendler KS, Ekelund J, Paunio T, Lonnqvist J, Peltonen L, O'Donovan MC, Owen MJ, Wildenauer DB, Maier W, Nestadt G, Blouin JL, Antonarakis SE, Mowry BJ, Silverman JM, Crowe RR, Cloninger CR, Tsuang MT, Malaspina D, Harkavy-Friedman JM, Svrakic DM, Bassett AS, Holcomb J, Kalsi G, McQuillin A, Brynjolfson J, Sigmundsson T, Petursson H, Jazin E, Zoega T, Helgason T. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet. 2003;73(1):34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu YF, McGrath JA, Thornquist MH, Wolyniec PS, Nestadt G, Swartz KL, Lasseter VK, Liang KY, Pulver AE. Genetic heterogeneity in schizophrenia II: conditional analyses of affected schizophrenia sibling pairs provide evidence for an interaction between markers on chromosome 8p and 14q. Mol Psychiatry. 2002;7(6):658–664. doi: 10.1038/sj.mp.4001045. [DOI] [PubMed] [Google Scholar]
- 7.Blouin JL, Dombroski BA, Nath SK, Lasseter VK, Wolyniec PS, Nestadt G, Thornquist M, Ullrich G, McGrath J, Kasch L, Lamacz M, Thomas MG, Gehrig C, Radhakrishna U, Snyder SE, Balk KG, Neufeld K, Swartz KL, DeMarchi N, Papadimitriou GN, Dikeos DG, Stefanis CN, Chakravarti A, Childs B, Housman DE, Kazazian HH, Antonarakis S, Pulver AE. Schizophrenia susceptibility loci on chromosomes 13q32 and 8p21. Nat Genet. 1998;20(1):70–73. doi: 10.1038/1734. [DOI] [PubMed] [Google Scholar]
- 8.Park N, Juo SH, Cheng R, Liu J, Loth JE, Lilliston B, Nee J, Grunn A, Kanyas K, Lerer B, Endicott J, Gilliam TC, Baron M. Linkage analysis of psychosis in bipolar pedigrees suggests novel putative loci for bipolar disorder and shared susceptibility with schizophrenia. Mol Psychiatry. 2004;9(12):1091–1099. doi: 10.1038/sj.mp.4001541. [DOI] [PubMed] [Google Scholar]
- 9.Badano JL, Teslovich TM, Katsanis N. The centrosome in human genetic disease. Nat Rev Genet. 2005;6(3):194–205. doi: 10.1038/nrg1557. [DOI] [PubMed] [Google Scholar]
- 10.Rieder CL, Faruki S, Khodjakov A. The centrosome in vertebrates: more than a microtubule-organizing center. Trends Cell Biol. 2001;11(10):413–419. doi: 10.1016/s0962-8924(01)02085-2. [DOI] [PubMed] [Google Scholar]
- 11.Bornens M. Centrosome composition and microtubule anchoring mechanisms. Curr Opin Cell Biol. 2002;14(1):25–34. doi: 10.1016/s0955-0674(01)00290-3. [DOI] [PubMed] [Google Scholar]
- 12.Doxsey S. Re-evaluating centrosome function. Nat Rev Mol Cell Biol. 2001;2(9):688–698. doi: 10.1038/35089575. [DOI] [PubMed] [Google Scholar]
- 13.Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell. 2007;128(1):29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Tsai LH, Gleeson JG. Nucleokinesis in neuronal migration. Neuron. 2005;46(3):383–388. doi: 10.1016/j.neuron.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Solecki DJ, Govek EE, Tomoda T, Hatten ME. Neuronal polarity in CNS development. Genes Dev. 2006;20(19):2639–2647. doi: 10.1101/gad.1462506. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Tsai LH, Wynshaw-Boris A. Life is a journey: a genetic look at neocortical development. Nat Rev Genet. 2002;3(5):342–355. doi: 10.1038/nrg799. [DOI] [PubMed] [Google Scholar]
- 17.Mochida GH, Walsh CA. Genetic basis of developmental malformations of the cerebral cortex. Arch Neurol. 2004;61(5):637–640. doi: 10.1001/archneur.61.5.637. [DOI] [PubMed] [Google Scholar]
- 18.Dammermann A, Merdes A. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J Cell Biol. 2002;159(2):255–266. doi: 10.1083/jcb.200204023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubo A, Tsukita S. Non-membranous granular organelle consisting of PCM-1: subcellular distribution and cell-cycle-dependent assembly/disassembly. J Cell Sci. 2003;116(Pt 5):919–928. doi: 10.1242/jcs.00282. [DOI] [PubMed] [Google Scholar]
- 20.Kubo A, Sasaki H, Yuba-Kubo A, Tsukita S, Shiina N. Centriolar satellites: molecular characterization, ATP-dependent movement toward centrioles and possible involvement in ciliogenesis. J Cell Biol. 1999;147(5):969–980. doi: 10.1083/jcb.147.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JC, Badano JL, Sibold S, Esmail MA, Hill J, Hoskins BE, Leitch CC, Venner K, Ansley SJ, Ross AJ, Leroux MR, Katsanis N, Beales PL. The Bardet-Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nat Genet. 2004;36(5):462–470. doi: 10.1038/ng1352. [DOI] [PubMed] [Google Scholar]
- 22.Moore SJ, Green JS, Fan Y, Bhogal AK, Dicks E, Fernandez BA, Stefanelli M, Murphy C, Cramer BC, Dean JC, Beales PL, Katsanis N, Bassett AS, Davidson WS, Parfrey PS. Clinical and genetic epidemiology of Bardet-Biedl syndrome in Newfoundland: a 22-year prospective, population-based, cohort study. Am J Med Genet A. 2005;132(4):352–360. doi: 10.1002/ajmg.a.30406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beales PL, Elcioglu N, Woolf AS, Parker D, Flinter FA. New criteria for improved diagnosis of Bardet-Biedl syndrome: results of a population survey. J Med Genet. 1999;36(6):437–446. [PMC free article] [PubMed] [Google Scholar]
- 24.Katsanis N. The oligogenic properties of Bardet-Biedl syndrome. Hum Mol Genet. 2004;13(Spec No 1):R65–R71. doi: 10.1093/hmg/ddh092. [DOI] [PubMed] [Google Scholar]
- 25.Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, Mah AK, Johnsen RC, Cavender JC, Lewis RA, Leroux MR, Beales PL, Katsanis N. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425(6958):628–633. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- 26.Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, Copp AJ, Eliot MM, Lupski JR, Kemp DT, Dollfus H, Tada M, Katsanis N, Forge A, Beales PL. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37(10):1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 27.Blacque OE, Reardon MJ, Li C, McCarthy J, Mahjoub MR, Ansley SJ, Badano JL, Mah AK, Beales PL, Davidson WS, Johnsen RC, Audeh M, Plasterk RH, Baillie DL, Katsanis N, Quarmby LM, Wicks SR, Leroux MR. Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev. 2004;18(13):1630–1642. doi: 10.1101/gad.1194004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulaga HM, Leitch CC, Eichers ER, Badano JL, Lesemann A, Hoskins BE, Lupski JR, Beales PL, Reed RR, Katsanis N. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet. 2004;36(9):994–998. doi: 10.1038/ng1418. [DOI] [PubMed] [Google Scholar]
- 29.Yen HJ, Tayeh MK, Mullins RF, Stone EM, Sheffield VC, Slusarski DC. Bardet-Biedl syndrome genes are important in retrograde intracellular trafficking and Kupffer's vesicle cilia function. Hum Mol Genet. 2006;15(5):667–677. doi: 10.1093/hmg/ddi468. [DOI] [PubMed] [Google Scholar]
- 30.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A Core Complex of BBS Proteins Cooperates with the GTPase Rab8 to Promote Ciliary Membrane Biogenesis. Cell. 2007;129(6):1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 31.Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y, Sawamura N, Park U, Kudo C, Okawa M, Ross CA, Hatten ME, Nakajima K, Sawa A. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol. 2005;7(12):1167–1178. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- 32.Kamiya A, Tomoda T, Chang J, Takaki M, Zhan C, Morita M, Cascio MB, Elashvili S, Koizumi H, Takanezawa Y, Dickerson F, Yolken R, Arai H, Sawa A. DISC1-NDEL1/NUDEL protein interaction, an essential component for neurite outgrowth, is modulated by genetic variations of DISC1. Hum Mol Genet. 2006;15(22):3313–3323. doi: 10.1093/hmg/ddl407. [DOI] [PubMed] [Google Scholar]
- 33.Miyoshi K, Asanuma M, Miyazaki I, Diaz-Corrales FJ, Katayama T, Tohyama M, Ogawa N. DISC1 localizes to the centrosome by binding to kendrin. Biochem Biophys Res Commun. 2004;317(4):1195–1199. doi: 10.1016/j.bbrc.2004.03.163. [DOI] [PubMed] [Google Scholar]
- 34.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa H, Kubo A, Tsukita S, Tsukita S. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat Cell Biol. 2005;7(5):517–524. doi: 10.1038/ncb1251. [DOI] [PubMed] [Google Scholar]
- 36.Yu JY, DeRuiter SL, Turner DL. RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci U S A. 2002;99(9):6047–6052. doi: 10.1073/pnas.092143499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawa A, Khan AA, Hester LD, Snyder SH. Glyceraldehyde-3-phosphate dehydrogenase: nuclear translocation participates in neuronal and nonneuronal cell death. Proc Natl Acad Sci U S A. 1997;94(21):11669–11674. doi: 10.1073/pnas.94.21.11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tabata H, Nakajima K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience. 2001;103(4):865–872. doi: 10.1016/s0306-4522(01)00016-1. [DOI] [PubMed] [Google Scholar]
- 39.McClellan JM, Susser E, King MC. Schizophrenia: a common disease caused by multiple rare alleles. Br J Psychiatry. 2007;190:194–199. doi: 10.1192/bjp.bp.106.025585. [DOI] [PubMed] [Google Scholar]
- 40.Hentze MW, Kulozik AE. A perfect message: RNA surveillance and nonsense-mediated decay. Cell. 1999;96(3):307–310. doi: 10.1016/s0092-8674(00)80542-5. [DOI] [PubMed] [Google Scholar]
- 41.Damasio AR, Van Hoesen GW, editors. Emotional disturbances associated with focal lesions of the limbic frontal lobe. New York: Guilford; 1983. [Google Scholar]
- 42.Kendler KS, Myers JM, O'Neill FA, Martin R, Murphy B, MacLean CJ, Walsh D, Straub RE. Clinical features of schizophrenia and linkage to chromosomes 5q, 6p, 8p, and 10p in the Irish Study of High-Density Schizophrenia Families. Am J Psychiatry. 2000;157(3):402–408. doi: 10.1176/appi.ajp.157.3.402. [DOI] [PubMed] [Google Scholar]
- 43.Carpenter WT, Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145(5):578–583. doi: 10.1176/ajp.145.5.578. [DOI] [PubMed] [Google Scholar]
- 44.Buckley PF. The clinical stigmata of aberrant neurodevelopment in schizophrenia. J Nerv Ment Dis. 1998;186(2):79–86. doi: 10.1097/00005053-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Iannaccone A, Mykytyn K, Persico AM, Searby CC, Baldi A, Jablonski MM, Sheffield VC. Clinical evidence of decreased olfaction in Bardet-Biedl syndrome caused by a deletion in the BBS4 gene. Am J Med Genet A. 2005;132(4):343–346. doi: 10.1002/ajmg.a.30512. [DOI] [PubMed] [Google Scholar]
- 46.Marder SR, Essock SM, Miller AL, Buchanan RW, Casey DE, Davis JM, Kane JM, Lieberman JA, Schooler NR, Covell N, Stroup S, Weissman EM, Wirshing DA, Hall CS, Pogach L, Pi-Sunyer X, Bigger JT, Jr, Friedman A, Kleinberg D, Yevich SJ, Davis B, Shon S. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161(8):1334–1349. doi: 10.1176/appi.ajp.161.8.1334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.