Abstract
Background
Although radiation therapy is a primary treatment for craniopharyngioma, it can exacerbate existing problems related to the tumor and pre-irradiation management. Survival is often marked by neurologic deficits, panhypopituitarism, diabetes insipidus, cognitive deficiencies and behavioral and social problems.
Procedure
The Achenbach Child Behavior Checklist (CBCL) was used to evaluate behavioral and social problems during the first five years of follow-up in 27 patients with craniopharyngioma treated with conformal radiation therapy.
Results
All group averages for the CBCL scales were within the age-typical range at pre-irradiation baseline. Extent of surgical resection was implicated in baseline differences for the Internalizing, Externalizing, Behavior Problem and Social scores. Significant longitudinal changes were found in Internalizing, Externalizing, Behavior Problem and School scores that correlated with tumor and treatment related factors.
Conclusions
The most common variables implicated in post-irradiation behavioral and social problems were CSF shunting, presence of an Ommaya reservoir, diabetes insipidus, and low pre-irradiation growth hormone levels.
Keywords: Craniopharyngioma, Brain Tumor, Child Behavior Checklist, Pediatric
INTRODUCTION
The morbidity associated with craniopharyngioma and its treatment is first among concerns for those who care for children with this locally aggressive brain tumor that is intimately associated with the hypothalamus-pituitary unit, optic apparatus and central cerebral vasculature [1-5]. These patients experience cognitive deficits that vary according to the treatment approach; those treated with radical surgery may be more likely to have acute and permanent deficits whereas late effects can be insidious in those treated with limited surgery and radiation therapy (RT) [1, 2, 4, 6-9]. Because survivorship after treatment for craniopharyngioma exceeds 85% [10], concerns about treatment-related morbidity have driven investigators to pursue treatment approaches that limit long-term effects [1, 2, 7, 11-13].
We showed that pre- and post-irradiation IQ in patients with craniopharyngioma treated with conformal radiation therapy (CRT) is directly correlated with the volume of irradiated brain and surgical factors including extent of resection, number of surgical procedures and surgically-induced diabetes insipidus [1]. Although these patients appeared to fare well in global cognitive abilities, we recently showed [14] they were more likely than other brain tumor patients to have changes in attention. This indicates that other measures are necessary to determine the impact of CRT and other treatments. Late effects extend beyond global cognitive abilities; to improve our understanding of the full patient experience and identify means for intervention, it is important to investigate behavioral outcomes and academic and social changes in these patients.
Most children with brain tumors develop cognitive late effects [3]. Psychological problems are common, and often entail behavioral or adjustment problems, academic failure or learning disabilities, as well as depression or anxiety [2, 3, 8, 11, 13-18]. Pedriera et al. report memory problems, academic decline, and frontal lobe disturbances in patients with craniopharyngioma [19]. They identified the following clinical predictors for problems: body mass index (BMI), endocrine status, and early psychological status [19]. In their study, patients experienced a reduced quality of life compared to age matched comparisons. RT is associated with cognitive late effects side effects including a decline in intellectual functioning, decreased attention and memory, and slower processing speed; most of these are influenced by the age and the dose spread over the volume receiving radiation [8].
Because of the sensitive location of craniopharyngioma, extensive surgery may result in damage, specifically diabetes insipidus and hypothalamic damage that can potentially lead to personality changes [8, 17, 20]. Hypothalamic damage can indicate frontal lobe disturbances that may result in emotional dysregulation presenting as anger and/or depression [20]. Low GH that may result from tumor or treatment leads to other health problems that may have psychological ramifications; these children tend to be much smaller which could lead to teasing and bullying [19].
In a prospective study, we evaluated patients with craniopharyngioma using the Achenbach Child Behavior Checklist (CBCL) before and serially after RT [21]. Using the CBCL, investigators have shown that internalizing and social difficulties are common among patients with craniopharyngioma citing tumor location, recurrence and multiple surgeries as variables affecting a poorer outcome [2, 22]. Our study investigated important demographic and clinical variables to evaluate their effect on pre- and post-irradiation functioning in craniopharyngioma survivors. The primary study goal was to investigate the quality of emotional and behavioral adjustment and explore demographic and clinical factors in order to identify factors most predictive of behavioral risk. While previous studies have utilized the CBCL to examine psychological effects, this study combines specific variables with five year longitudinal follow up to determine which clinical and demographic factors have predictive value.
PATIENTS AND METHODS
Patient Characteristics
The patients in this study were prospectively treated for craniopharyngioma on a Phase II trial of CRT conducted at St. Jude Children’s Research Hospital from 1997 – 2003. Criteria for study enrollment have been previously reported [1]. All children were between the ages of 1 and 18 years at the time of irradiation with no prior history of irradiation and adequate performance status (ECOG performance grade 0-2) [23]. The study included 27 patients with a median age of 7.4 + 4.16 years (range 3.2 – 17.6 years) at the time of irradiation. Patients were characterized according to specific clinical and treatment-related variables (Table I). The study was approved by the institutional review board and written informed consent was required.
Table I.
Patient Demographic and Clinical Characteristics (N= 27)
| Patient Demographic | N | % |
|---|---|---|
| Gender | ||
| Male | 11 | 40.7% |
| Female | 16 | 59.3% |
| Race | ||
| Caucasian | 19 | 70.4% |
| Non-Caucasian | 8 | 29.6% |
| Hydrocephalus at Diagnosis | ||
| Yes | 18 | 66.7% |
| No | 9 | 33.3% |
| CSF Shunting | ||
| Yes | 12 | 44.4% |
| No | 15 | 55.5% |
| Ommaya Reservoir for Cyst Drainage | ||
| Yes | 17 | 63.0% |
| No | 10 | 37.0% |
| Diabetes Insipidus | ||
| Yes | 17 | 63.0% |
| No | 10 | 37.0% |
| Duration of Symptoms prior to Diagnosis | ||
| ≤ 73 days | 14 | 51.9% |
| > 73 days | 13 | 48.1% |
| Peak Growth Hormone Level | ||
| Within Normal Range (>10 ng/ml) | 4 | 14.8% |
| Below Normal Range | 23 | 85.2% |
| Planning Target Volume (PTV) | ||
| ≤ 80.6 ml | 14 | 51.9% |
| > 80.6 ml | 13 | 48.1% |
| Extent of Resection | ||
| Minimal | 12 | 44.4% |
| Moderate | 7 | 25.9% |
| Extensive | 8 | 29.7% |
| Body Mass Index (BMI) | ||
| ≤ 1.03 (z score) | 14 | 51.9% |
| > 1.03 | 13 | 48.1% |
| Median | Range | |
| Age at Time of Irradiation (years) | 7.4 | 3.2-17.6 |
Abbreviations: CSF= cerebrospinal fluid; CRT=conformal radiation therapy
Clinical Variables
Extent of resection was defined as minimal, moderate and extensive as described in a prior publication [1]. Briefly, minimal was limited to exploration and decompression, biopsy, or both; moderate included microsurgical tumor dissection from the optic chiasm and carotid arteries with an attempt to preserve the infundibulum, hypothalamus and brain stem; and extensive included microsurgical dissection of the tumor including the hypothalamus, infundibulum or brainstem. Peak growth hormone levels prior to CRT were determined using arginine and carbidopa/levodopa. Hydrocephalus was categorized as present/not present based on neuroimaging evidence of ventricular enlargement at the time of diagnosis.
CRT
All patients received CRT administered over 6 to 7 weeks using conventional fractionation (1.8 Gy per day) with a prescribed dose of 54 Gy. The mean gross tumor volume (GTV) was 16.7 cc ± 16.8 cc (range 1.74 – 64.66 cc). RT was administered using conformal treatment methods. The clinical target volume (CTV) included a 10 mm margin surrounding the tumor to account for microscopic residual tumor. An additional margin of 3-5 mm was added to create the planning target volume (PTV). Detailed target volume definitions and treatment parameters have been reported previously [1].
Psychological Evaluation
Patients underwent serial neurocognitive and psychological assessments at baseline (before CRT) and at 6, 12, 24, 36, 48 and 60 months after the start of CRT. Of 147 possible evaluations, 127 were obtained. Among the 19 missing evaluations, 6 were not performed due to disease progression or death, 4 were not performed because a patient suffered a stroke and 9 were not performed due to noncompliance. The CBCL, a parent questionnaire that assesses a child’s level of behavioral and emotional adjustment, was included in the assessment battery [21]. This measure is comprised of both problem behavior and competence scales. The scales are standardized on a large sample representative of the US population with respect to socioeconomic status, ethnicity and geographic distribution. Age and gender based T-scores are derived for each scale with a mean of 50 and a standard deviation of 10. One-week test-retest reliabilities range from .70 to .92 for the competence scales and .82 to .95 for the problem scales [21].
For the problem scales, parents rate their child on 118 items using a three point scale (i.e., from 0= “not true” to 2= “very true or often true”). T-scores above 70 define the clinically impaired range [21]. For data reduction purposes, the composite scores (Internalizing, Externalizing, Behavior Problems) for the problem scales were used in statistical analyses. The Internalizing score is a composite score of the Withdrawn, Somatic and Anxious/Depressed scales; the Externalizing score is a composite of the Delinquent Behavior and Aggressive Behavior scales; and the Behavioral Problems score is a composite of the Social Problems, Thought Problems, Attention Problems and open-ended questions.
For the competence scales (Activities, Social, School), parents rate their child’s level and quality of involvement in activities, peer relationships and school. They also indicate whether their child is experiencing school problems or receiving special education services. T-scores below 30 define the clinically impaired range.
Statistical Design
A total of 127 evaluations were performed on 27 patients. The median follow-up at the time of this report was 36 months. We used linear mixed effects models with random intercept and random slope to investigate longitudinal change [24]. Separate models were created for each CBCL composite score. The intercept of the model represents an estimate of the baseline CBCL score, and the slope of the line indicates the score change per month (Supplemental Table I). The variables in Table I were included in univariate analyses conducted to investigate the impact of clinical and treatment-related variables on long-term behavioral outcomes. Continuous predictor variables were divided into two categories at the median value of the variable. All analyses were performed by the statistical coauthors using SAS software [24]
RESULTS
CBCL Problem Behavior Scales
CBCL Internalizing Score (Supplemental Table I)
For the CBCL Internalizing score, the baseline score for the group was within the average range. There was a significant difference in the baseline score based on surgical extent. Patients undergoing the most extensive resection exhibited the greatest amount of internalizing problems. All significant longitudinal changes were in the negative direction indicating a lower score or fewer problems over time. Those patients with a lower BMI at baseline, no diabetes insipidus, a longer duration of symptoms before diagnosis and no Ommaya reservoir demonstrated a significant reduction in internalizing problems over time.
CBCL Externalizing Score (Supplemental Table II)
For the CBCL Externalizing Score, the baseline score for the group was within the average range; however, there was a significant baseline difference in externalizing problems based on extent of surgical resection. There was a statistically significant improvement in the Externalizing score over time in patients who did not have severe growth hormone deficiency prior to CRT and those who did not require an Ommaya reservoir. There was also a statistically significant difference in the change in externalizing problems over time for those with and without pre-CRT growth hormone deficiency (Figure 1).
Figure 1.
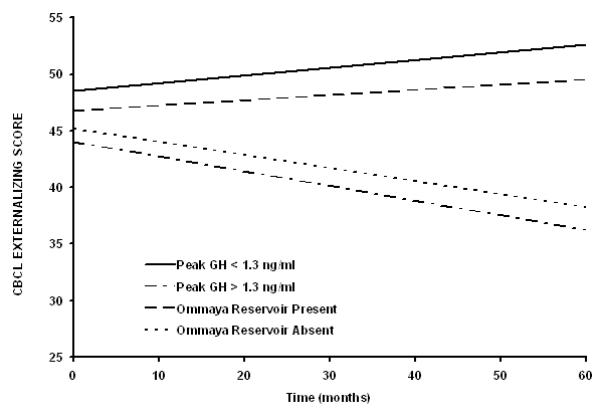
Patients who do not require an Ommaya reservoir for cyst drainage (P=0.0217) or who do not have severe growth hormone deficiency prior to conformal irradiation (P=0.0059) have improving CBCL Externalizing scores after radiation therapy. The differences between slopes for the subgroup curves are statistically significant (Peak GH, P=0.0054; Ommaya, P=0.0404).
CBCL Behavior Problems Score (Supplemental Table III)
For the CBCL Behavior Problems Score, the baseline score for the group was within the average range. When divided by clinical variables of interest, there was a significant baseline difference based on extent of surgical resection. All significant longitudinal changes were in the negative direction indicating a lower score or improvement over time. Those patients with no diabetes insipidus, higher pre-irradiation growth hormone level, lower BMI, no Ommaya reservoir and no CSF shunt demonstrated a significant reduction in behavior problems over time (Figure 2).
Figure 2.
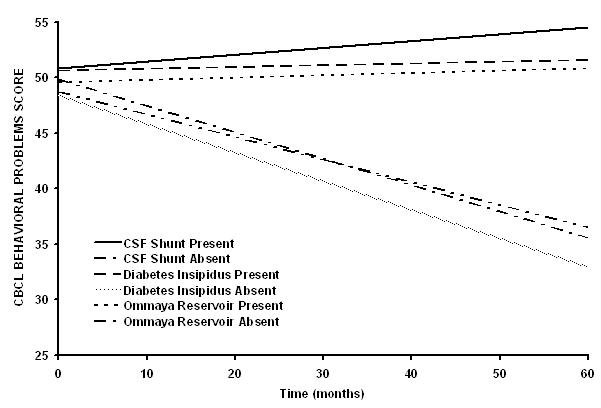
Patients who do not have diabetes insipidus (P=0.0150), CSF shunt (P=0.0134) or Ommaya reservoir for cyst drainage (P=0.0145) have improved CBCL Behavioral Problem scores after conformal irradiation. The differences between slopes for the subgroup curves are statistically significant (diabetes insipidus, P=0.0499; CSF shunt, P=0.0369; Ommaya, P=0.0477).
CBCL Competence Scores
CBCL Activities Score (Supplemental Table IV)
The baseline score for the group was within the average range. When divided by clinical variables of interest, there was a significant baseline difference in activities based on the planning target volume, with lower volume associated with a higher (better) score. White patients had higher baseline scores and there was a significant reduction over time in the number of activities for white children. There were no other significant longitudinal changes.
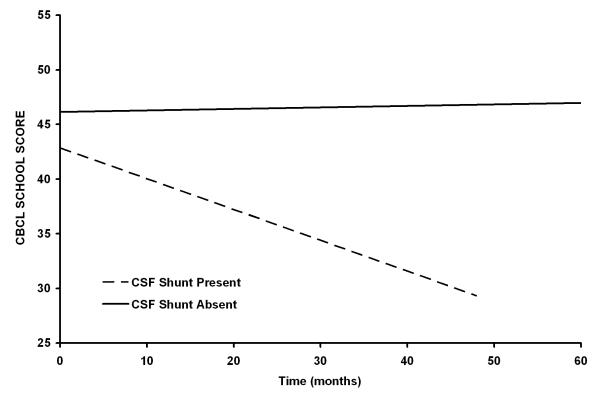
CBCL School Score (Supplemental Table V)
For the CBCL School score, the baseline score for the group was within the average range. There were no significant differences in school problems at baseline based on the clinical variables of interest; however, patients with a CSF shunt and an Ommaya reservoir showed a significant worsening of school performance over time (Figure 3).
Figure 3.
Patients who require CSF shunting (P=0.0091) have worsening CBCL School scores after conformal irradiation. The differences between slopes for the two curves are statistically significant (P=0.0319).
CBCL Social Score (Supplemental Table VI)
The baseline score for the group was within the average range. When divided by clinical variables of interest, there was a significant difference in social skills based on extent of surgical resection. There were no significant longitudinal changes in social problems over time.
Statistical Models of CBCL Scores and Proportion Clinically Impaired
Univariate models estimating baseline (pre-CRT) and longitudinal CBCL scores by clinical and treatment variables are presented (Supplemental Tables VII and VIII) to identify clinically significant pre-existing conditions, patients at risk for developing problems and the impact of specific variables. Based on actual subject data, the proportion of clinically impaired subjects over the time course of analysis was determined with the following average values: 3.3%, Internalizing Score; 2.5%, Externalizing Score; 3.3% Behavior Problems; 1.9%, Activities Score; 8.2%, School Score; 11.7%, Social Score.
DISCUSSION
This study demonstrates that demographic and clinical factors influence pre- and post-irradiation behavioral and social problems in children with craniopharyngioma. Many factors are related to the host, while others are related to tumor management.
Radical surgery or limited surgery and RT result in equivalent rates of long-term disease control because those who fail surgical management may be effectively salvaged with RT [9]. Controversies in the treatment of craniopharyngioma result in a spectrum of treatment approaches and varying consequences [1, 4, 8, 12, 25]. With exceptions for very young children and tumors that may be completely removed with limited morbidity, our preferred approach is limited surgery and RT [1]. Age is often considered the most important factor in selecting patients for surgery because morbidity after RT is age-related [1, 2, 8]. Our analysis of the effect of age was limited to categorical analysis of those older and younger than the median age of 7.4 years. Age did not affect the baseline or longitudinal scores.
Radical surgery has the potential to lead to hypothalamic damage and diabetes insipidus. Hypothalamic damage may be associated with personality changes [2, 9, 12] especially for patients with retrochiasmatic tumors. Extent of surgery was shown to affect Internalizing, Externalizing, Behavior Problems and Social scores. Damage to the hypothalamus has frequently been associated with a decreased attention span, short term memory problems and weight gain. These can lead to academic difficulties and social competence problems affecting these children [18]. Craniopharyngioma is often accompanied by weight gain which impacts patient self-esteem and social interaction [26]. Cancer survivors may be more inclined to depression from the chronic nature of their disease and increased rates of distorted body image [20].
Presence of an Ommaya reservoir may also indicate active cyst expansion with concomitant local or regional mass effect. Hydrocephalus that requires CSF shunting may impair sustained attention [27]. Shunt placement was found to be a predictor variable for the Behavior Problem and School scores.
Endocrine deficiencies are thought to be associated with memory impairment, confusion, and personality effects [9]. Children who are small for their age may be more prone to teasing. This is a possible explanation to growth hormone as a predictor of both Externalizing and Behavior Problem scores [11]. Endocrine levels predict future cognitive function [9]. GH levels tend to fall without treatment in the months immediately following RT because growth hormone replacement is not undertaken until the tumor has stabilized [1]. Steinhausen et al. showed that GH treatment in brain tumor patients improved CBCL scores [28].
Mulhern et al. found that 7% of the normal population reports at least one score in the borderline or clinically significant range on any category of the CBCL [3]. In the current study, patients with craniopharyngioma exhibited higher percentages of borderline or clinical scores per category in the School and Social scores. The CBCL is designed to detect clinically elevated behavioral or social problems. It does not necessarily look at the differences within the normal range of behavior and social categories [3]. It is important to note that while the change in the slope of the line representing scores from baseline to five years may be statistically significant, many of these children are not demonstrating clinically significant problems. This suggests that CRT may offer an advantage over conventional therapy with respect to behavioral adjustment [1, 7].
Limitations
This study utilized only the parent form of the CBCL; the accuracy of this form depends on the accuracy of the parent’s perception of the child. A more accurate representation of problems may be obtained from multiple informants using reports from parents, teachers, and patients. There are often discrepancies between parent and child report. It has been proposed this discrepancy may relate to the overprotective nature of a parent of a cancer survivor [29]. This relationship could result in a lack of information sharing or, more likely, a parent seeing problems that may not truly be present in their child. Additional limitations include the exclusive use of the CBCL for measuring behavioral and emotional adjustment, lack of a healthy comparison group and missing data. The CBCL is perhaps the best validated measure for addressing current study aims, our sample is similar to the normative population in demographic characteristics such that age and gender normed T-scores serve as a proxy for an independent control group, and statistics used in this study handle missing data well. The inclusion of measures assessing normal range behavior changes, as well as incorporation of multiple informants and multiple behavior rating scales is recommended for future studies.
Conclusions
This study adds further evidence to support the effects of tumor and pre-irradiation management on functional outcomes and demonstrates the sensitivity of the CBCL to common clinical factors. The predictor variables identified in this study may help to prepare families for potential behavioral, social or academic problems. Education regarding these findings will facilitate monitoring by parents and caregivers for emerging problems and early intervention to mitigate the severity of behavioral or social problems.
Supplementary Material
Acknowledgments
Funding: This work was supported in part by Cancer Center Support Grant CA21765 from the National Cancer Institute, by Research Project Grant RPG-99-252-01-CCE from the American Cancer Society and by the American Lebanese Syrian Associated Charities (ALSAC) and the Rhodes College Summer Plus Program.
REFERENCES
- 1.Merchant T, Kiehna E, Kun L, et al. Phase II trial of conformal radiation therapy for pediatric patients with craniopharyngioma and correlations of surgical factors and radiation dosimetry with change in cognitive function. Journal of Neurosurgery. 2006;104:94–102. doi: 10.3171/ped.2006.104.2.5. [DOI] [PubMed] [Google Scholar]
- 2.Sands S, Milner J, Goldberg J, et al. Quality of life and behavioral follow-up study of pediatric survivors of craniopharyngioma. J. Neurosurgery: Pediatrics. 2005;103:302–311. doi: 10.3171/ped.2005.103.4.0302. [DOI] [PubMed] [Google Scholar]
- 3.Mulhern R, Wasserman A, Friedman A, Fairclough D. Social competence and behavioral adjustment of children who are long-term survivors of cancer. Pediatrics. 1989;83(1):18–25. [PubMed] [Google Scholar]
- 4.Colangelo M, Ambrosio A, Ambrosio C. Neurological and behavioral sequelae following different approaches to craniopharyngioma. Child’s Nervous System. 1990;6:379–382. doi: 10.1007/BF00302222. [DOI] [PubMed] [Google Scholar]
- 5.Waber D, Pomeroy S, Chiverton A, et al. Everyday cognitive function after craniopharyngioma in childhood. Pediatric Neurology. 2006;34:13–19. doi: 10.1016/j.pediatrneurol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Brada M, Thomas D. Craniopharyngioma revisited. Int J Radiation Oncology Biol Phys. 1993;27:471–475. doi: 10.1016/0360-3016(93)90261-s. [DOI] [PubMed] [Google Scholar]
- 7.Habrand J, Saran F, Alapetite C, et al. Radiation therapy in the management of craniopharyngioma: current concepts and future developments. Journal of Pediatric Endocrinology and Metabolism. 2006;19:389–394. [PubMed] [Google Scholar]
- 8.Cavazutti V, Fischer E, Welch K. Neurological and psychophysiological sequelae following different treatments of craniopharyngioma in children. J Neurosurg. 1983;59:409–417. doi: 10.3171/jns.1983.59.3.0409. [DOI] [PubMed] [Google Scholar]
- 9.Merchant T. Craniopharyngioma radiotherapy: endocrine and cognitive effects. Journal of Pediatric Endocrinology and Metabolism. 2006;19:439–446. [PubMed] [Google Scholar]
- 10.Rajan B, Ashley S, Gorman C, et al. Craniopharyngioma--a long-term results following limited surgery and radiotherapy. Radiother Oncol. 1993;26(1):1–10. doi: 10.1016/0167-8140(93)90019-5. [DOI] [PubMed] [Google Scholar]
- 11.Anderson C, Wilkening F, Filley C, et al. Neurobehavioral outcome in pediatric craniopharyngioma. Pediatric Neurosurgery. 1997;26:255–260. doi: 10.1159/000121200. [DOI] [PubMed] [Google Scholar]
- 12.Scarzello G, Buzzaccarini M, Perilongo G, et al. Acute and late morbidity after limited resection and focal radiation therapy in craniopharyngiomas. Journal of Pediatric Endocrinology and Metabolism. 2006;19:399–405. [PubMed] [Google Scholar]
- 13.Mulhern R, Carpentieri S, Shema S, et al. Factors associated with social and behavioral problems among children recently diagnosed with brain cancer. J Pediatr Psychol. 1993;18(3):339–350. doi: 10.1093/jpepsy/18.3.339. [DOI] [PubMed] [Google Scholar]
- 14.Kiehna EN, Mulhern RK, Li C, et al. Changes in attentional performance of children and young adults with localized primary brain tumors after conformal radiation therapy. J Clin Oncol. 2006;24(33):5283–5290. doi: 10.1200/JCO.2005.03.8547. [DOI] [PubMed] [Google Scholar]
- 15.Mulhern R, Merchant T, Gajjar A, et al. Late neurocognitive sequelae in survivors of brain tumours in childhood. Oncology. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 16.Fossen A, Abrahamsen T, Storm-Mathisen I. Psychological outcome in children treated for brain tumor. Pediatr Hematol Oncol. 1998;15(6):479–488. doi: 10.3109/08880019809018309. [DOI] [PubMed] [Google Scholar]
- 17.Galatzer A, Nofar E, Beit-Halachmi N, et al. Intellectual and psychosocial functions of children, adolescents and young adults before and after operation for craniopharyngioma. Child: care, health, and development. 1981;7:307–316. doi: 10.1111/j.1365-2214.1981.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 18.Poretti A, Grotzer M, Ribi K, et al. Outcome of craniopharyngioma in children: long term complications and quality of life. Developmental medicine and child neurology. 2004;46(4):220–229. doi: 10.1017/s0012162204000374. [DOI] [PubMed] [Google Scholar]
- 19.Pedriera C, Stargatt R, Maroulis H, et al. Health related quality of life and psychological outcome in patients treated for craniopharyngioma in childhood. Journal of Pediatric Endocrinology & Metabolism. 2006;19:15–24. doi: 10.1515/jpem.2006.19.1.15. [DOI] [PubMed] [Google Scholar]
- 20.Riva D, Pantaleoni C, Devoti M, et al. Late neuropsychological and behavioral outcome of children surgically treated for craniopharyngioma. Child’s Nervous System. 1998;14:179–184. doi: 10.1007/s003810050207. [DOI] [PubMed] [Google Scholar]
- 21.Achenbach TM. Manual for the Child Behavior Checklist 4 – 18 and 1991 Profile. University of Vermont Department of Psychiatry; Burlington, VT: 1991. [Google Scholar]
- 22.Poggi G, Liscio M, Adduci A, et al. Psychological and adjustment problems due to acquired brain lesions in childhood: a comparison between post-traumatic patients and brain tumor survivors. Brain Injury. 2005;19(10):777–785. doi: 10.1080/0269905500110132. [DOI] [PubMed] [Google Scholar]
- 23.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 24.SAS Institute Inc . SAS/STAT user’s guide. version 9.1 SAS institute; Cary, NC: 2004. [Google Scholar]
- 25.Merchant T, Kiehna E, Sanford R, et al. Craniopharyngioma: the St Jude Children’s Research Hospital experience 1984 – 2001. Int J Radiation Oncology Biol. Phys. 2002;53(3):533–542. doi: 10.1016/s0360-3016(02)02799-2. [DOI] [PubMed] [Google Scholar]
- 26.Shiminski-Maher T. Patient/family preparation and education for complications and late sequelae of craniopharyngiomas. Pediatric Neurosurgery. 1994;21(suppl 1):114–119. doi: 10.1159/000120872. [DOI] [PubMed] [Google Scholar]
- 27.Merchant T, Kiehna E, Miles M, et al. Acute effects of irradiation on cognition: changes in attention on a computerized continuous performance test during radiotherapy in pediatric patients with localized primary brain tumors. Int. J. Radiation Oncology Biol. Phys. 2002;53(5):1271–1278. doi: 10.1016/s0360-3016(02)02828-6. [DOI] [PubMed] [Google Scholar]
- 28.Steinhausen HC, Door HG, Malin Z. Behavioral evaluation of GH treatment in short statured children and adolescents: findings from a pilot study. J. Endocrinological Investigation. 2002;25(4):351–6. doi: 10.1007/BF03344017. [DOI] [PubMed] [Google Scholar]
- 29.Carpentieri S, Meyer E, Delaney B, et al. Psychosocial and behavioral functioning among pediatric brain tumor survivors. Journal of Neuro-Oncology. 2003;63:279–287. doi: 10.1023/a:1024203323830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.