Abstract
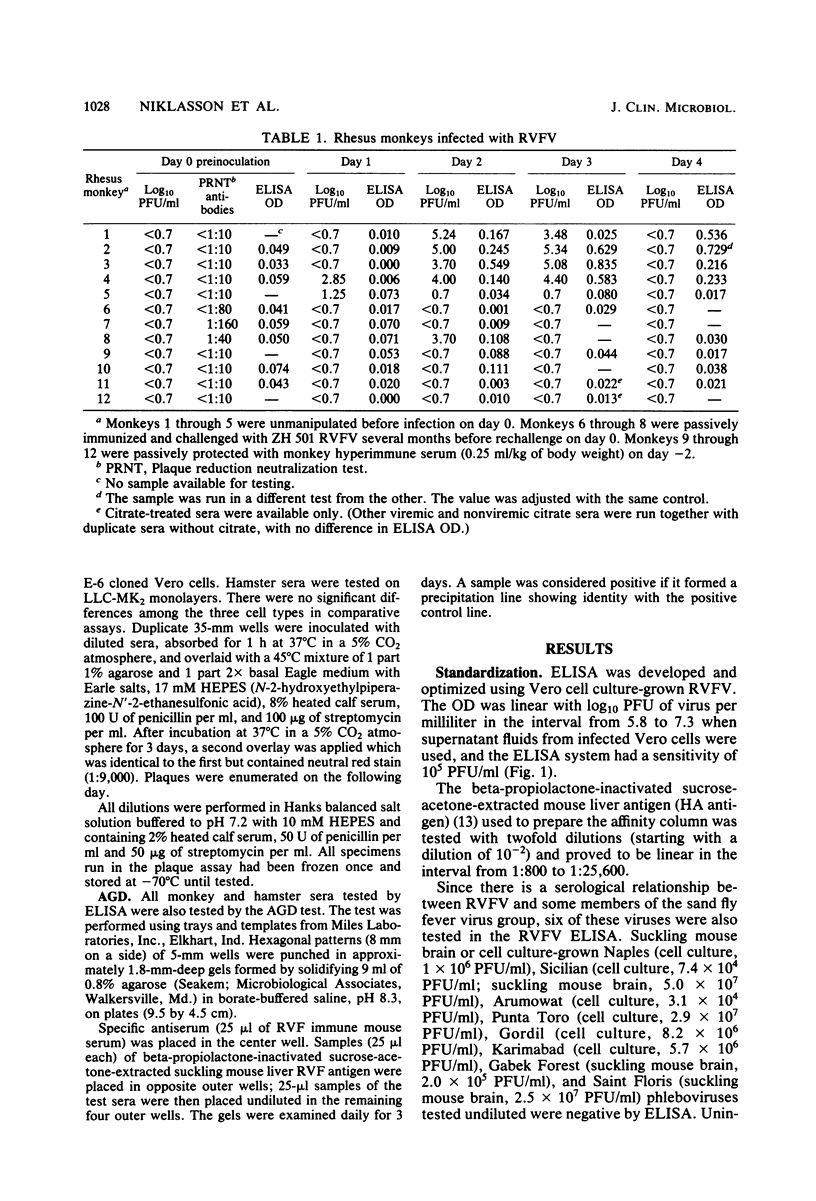
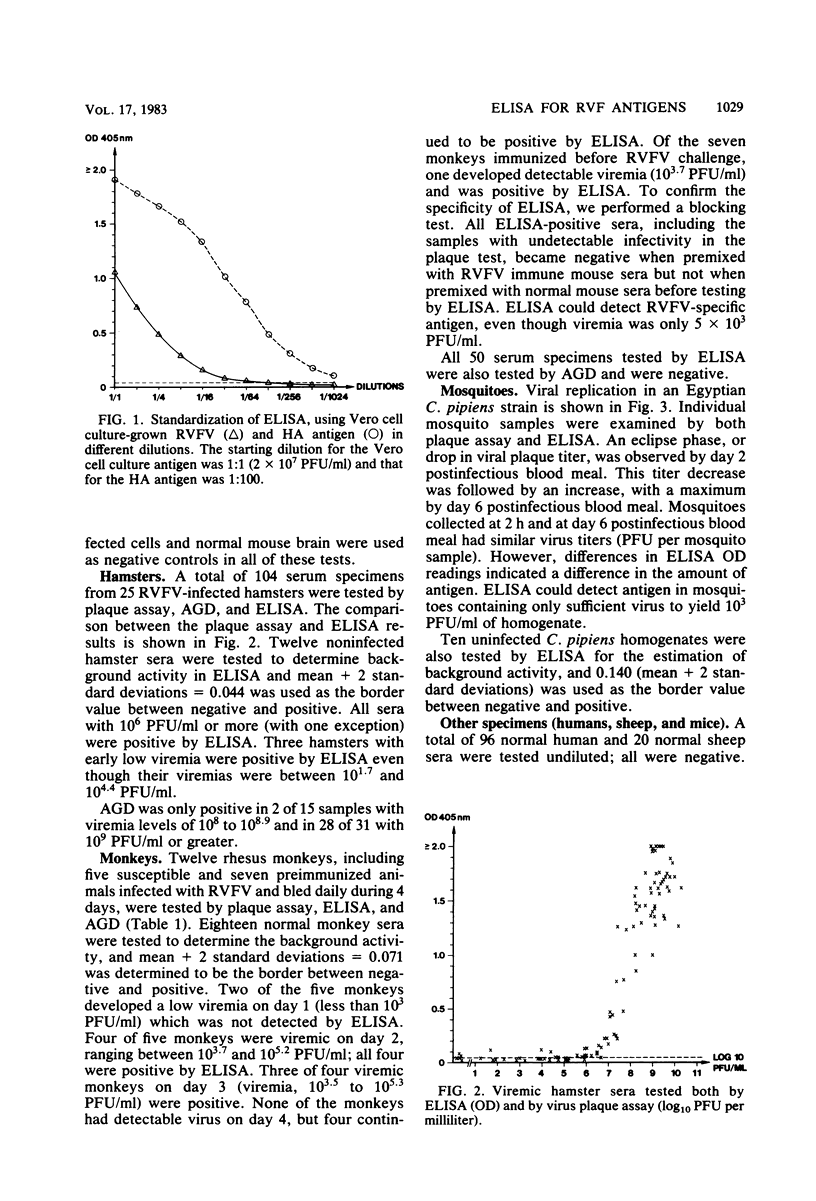
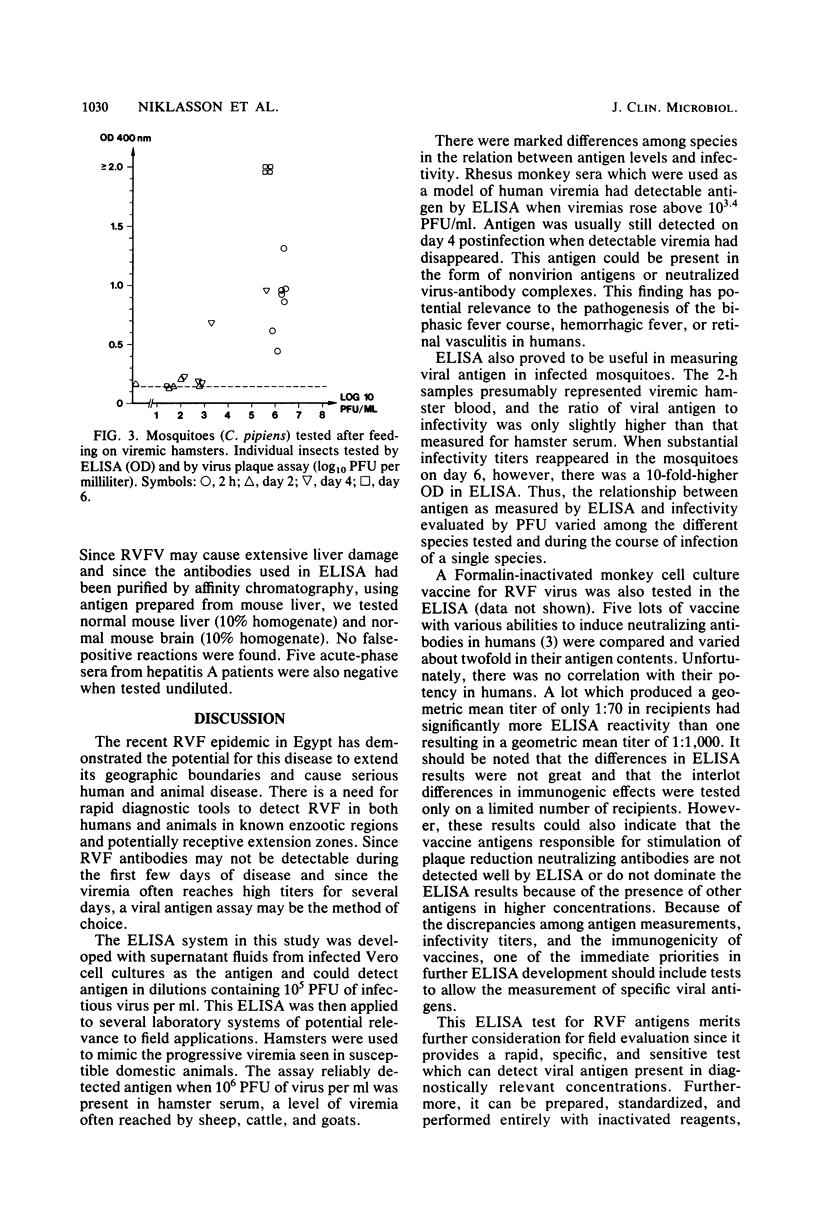
A double-antibody (sandwich) enzyme-linked immunosorbent assay (ELISA) was adapted to detect Rift Valley fever virus antigen. Antibodies were purified from hyperimmune mouse and rabbit sera by affinity chromatography, using CNBr-activated Sepharose 4B coupled to a beta-propiolactone-inactivated sucrose-acetone-extracted suckling mouse liver antigen. In the assay, antigen was captured by mouse antibody adsorbed to polystyrene plates and then detected by reacting sequentially with rabbit anti-Rift Valley fever virus antibody and swine anti-rabbit immunoglobulin G conjugated to alkaline phosphatase. ELISA proved to be useful in measuring viral antigen in different animal systems. However, great variation was found in the amount of antigen per PFU encountered in different circumstances. The ELISA system was optimized using supernatant fluids from infected Vero cell cultures and had a sensitivity of 10(5) PFU/ml. Hamsters develop progressive viremia, much as seen in susceptible domestic animals, such as lambs; ELISA could reliably detect 10(6) PFU/ml of viremic hamster serum. Rhesus monkeys with Rift Valley fever infection were positive by ELISA even when viremias were only 5 X 10(3) PFU/ml. ELISA also proved to be useful in measuring viral antigen in infected mosquitoes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard B. J., Botha M. J. An inactivated rift valley fever vaccine. J S Afr Vet Assoc. 1977 Mar;48(1):45–48. [PubMed] [Google Scholar]
- Hildreth S. W., Beaty B. J., Meegan J. M., Frazier C. L., Shope R. E. Detection of La Crosse arbovirus antigen in mosquito pools: application of chromogenic and fluorogenic enzyme immunoassay systems. J Clin Microbiol. 1982 May;15(5):879–884. doi: 10.1128/jcm.15.5.879-884.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M. E., Espmark J. A. Elimination of inter-species reactive anti-IgG antibodies by affinity chromatography. J Immunol Methods. 1978;21(3-4):285–293. doi: 10.1016/0022-1759(78)90155-2. [DOI] [PubMed] [Google Scholar]
- Laughlin L. W., Meegan J. M., Strausbaugh L. J., Morens D. M., Watten R. H. Epidemic Rift Valley fever in Egypt: observations of the spectrum of human illness. Trans R Soc Trop Med Hyg. 1979;73(6):630–633. doi: 10.1016/0035-9203(79)90006-3. [DOI] [PubMed] [Google Scholar]
- Meegan J. M. The Rift Valley fever epizootic in Egypt 1977-78. 1. Description of the epizzotic and virological studies. Trans R Soc Trop Med Hyg. 1979;73(6):618–623. doi: 10.1016/0035-9203(79)90004-x. [DOI] [PubMed] [Google Scholar]
- RANDALL R., GIBBS C. J., Jr, AULISIO C. G., BINN L. N., HARRISON V. R. The development of a formalin-killed Rift Valley fever virus vaccine for use in man. J Immunol. 1962 Nov;89:660–671. [PubMed] [Google Scholar]
- Swanepoel R., Manning B., Watt J. A. Fatal Rift Valley fever of man in Rhodesia. Cent Afr J Med. 1979 Jan;25(1):1–8. [PubMed] [Google Scholar]
- Voller A., Bartlett A., Bidwell D. E., Clark M. F., Adams A. N. The detection of viruses by enzyme-linked immunosorbent assay (ELISA). J Gen Virol. 1976 Oct;33(1):165–167. doi: 10.1099/0022-1317-33-1-165. [DOI] [PubMed] [Google Scholar]
- van Velden D. J., Meyer J. D., Olivier J., Gear J. H., McIntosh B. Rift Valley fever affecting humans in South Africa: a clinicopathological study. S Afr Med J. 1977 Jun 11;51(24):867–871. [PubMed] [Google Scholar]


