Abstract
Although B. bronchiseptica efficiently infects a wide range of mammalian hosts and efficiently spreads among them, it is rarely observed in humans. In contrast to the many other hosts of B. bronchiseptica, humans are host to the apparently specialized pathogen B. pertussis, the great majority having immunity due to vaccination, infection or both. Here we explore whether immunity to B. pertussis protects against B. bronchiseptica infection. In a murine model, either infection or vaccination with B. pertussis induced antibodies that recognized antigens of B. bronchiseptica and protected the lower respiratory tract of mice against three phylogenetically disparate strains of B. bronchiseptica that efficiently infect naïve animals. Furthermore, vaccination with purified B. pertussis-derived pertactin, filamentous hemagglutinin or the human acellular vaccine, Adacel, conferred similar protection against B. bronchiseptica challenge. These data indicate that individual immunity to B. pertussis affects B. bronchiseptica infection, and suggest that the high levels of herd immunity against B. pertussis in humans could explain the lack of observed B. bronchiseptica transmission. This could also explain the apparent association of B. bronchiseptica infections with an immunocompromised state.
Introduction
The emergence of new infectious diseases from zoonotic sources may be constrained by at least three limiting steps. First, a pathogen must have the opportunity, observed when organisms “spillover” from their natural host to humans. Second, it must have the ability to colonize/infect an individual and to expand in numbers there. Third, it must successfully spread to new human hosts and establish a successful chain of transmission [1], [2]. It is important to consider the factors that can affect each of these steps in order to understand the risk of newly emerging diseases. Successful transmission requires shedding of the pathogen from an infected individual and contact with susceptible individuals. In the simplest case of a novel infectious agent, all of a new host population are often considered to be susceptible until they are infected, recover and may then be immune, at least for a time. However, the susceptibility of individual hosts to a novel agent may be affected by various factors, including the resident flora and immunity to it. Since pathogen control strategies such as vaccination can change the immune status of individuals, and the human population, it is important to understand how that might affect their susceptibility to not just that pathogen, but to other closely related pathogens that are likely to spillover from animal sources.
The classical bordetellae are closely related gram-negative bacterial species which colonize the respiratory tracts of a variety of mammalian hosts [3]. Bordetella bronchiseptica causes a chronic respiratory infection that can persist for the life of the animal [3], [4]. This pathogen has been isolated from a diverse range of mammalian hosts and is associated with kennel cough in dogs, snuffles in rabbits and atrophic rhinitis in pigs but may be present without symptoms in a majority of these and other animals [3]–[5]. The individual lineages of B. bronchiseptica do not appear to be host-specific, since very closely related strains have been shown to efficiently infect a wide range of mammals, including humans [5]–[16]. Although dogs and other animals are common hosts for a wide variety of lineages of B. bronchiseptica, one specialized lineage of Bordetella has adapted to occupy humans as its only apparent ecological niche; B. pertussis is prevalent worldwide as a highly infectious pathogen that causes the acute disease whooping cough [3]. Although infections are cleared by host immunity, B. pertussis persists within human populations by reinfecting previously infected or vaccinated individuals [3], [17]. We have suggested that the very high prevalence of antibodies to B. pertussis, detectable in greater than 90% of individuals, may reflect immunity that affects the emergence of closely related pathogens [17].
Other classical bordetellae retain the ability to infect humans, but only B. parapertussis appears to successfully occupy humans as a primary ecological niche [3], [18]. Since immune-mediated competition would be expected to exclude one or the other from a shared host population, we hypothesized, and recently showed, that B. parapertussis avoids cross-immunity via the expression of an O-antigen not shared by B. pertussis which prevents the binding of antibodies induced by the latter to shared antigens on the bacterial surface [13], [15], [19]–[21]. This appears to have allowed B. parapertussis to invade a human population in which B. pertussis was already endemic.
Animals in very close contact with humans, such as dogs and cats, are known to be infected with a very wide range of different B. bronchiseptica strains, which are highly infectious and spread amongst them rapidly. Interestingly, a similarly broad set of B. bronchiseptica strains have been occasionally isolated from humans, indicating they retain the ability to infect humans, but they generally are not observed to spread between humans [5], [12]. The observations that B. bronchiseptica can spillover to humans from animals, and can infect and cause disease, particularly in immunocompromised individuals, led us to explore how the infectious process may be affected by immunity to the resident pathogen, B. pertussis.
Here we quantified the effects of B. pertussis-induced immunity on the ability of B. bronchiseptica to successfully infect mice [22]–[24]. We show that B. pertussis infection- and vaccine-induced immunity protects against B. bronchiseptica colonization in the lower respiratory tract (LRT). This protection, defined as a significant decrease in bacterial load in the LRT of an immunized host, appears to be mediated by cross reacting antibodies which recognize shared antigens. Immunization with the B. pertussis-derived antigens in the acellular vaccine, Adacel, or the individual proteins pertactin or filamentous hemagglutinin, was sufficient to induce protective immunity to B. bronchiseptica. Thus B. pertussis vaccination- or infection-induced immunity can protect the LRT of mice against B. bronchiseptica. Together with our previous demonstration that B. parapertussis avoids B. pertussis-induced immunity, these data may explain, in whole or in part, why B. bronchiseptica is observed to spillover to humans from other animals in which it causes epidemics, but is generally associated with disease only in immunocompromised humans.
Materials and Methods
Bacterial Growth
Bordetella pertussis strain 536, a streptomycin resistant derivative of Tohama I [25], B. bronchiseptica strain RB50 [26], and B. bronchiseptica strain RB50G, a gentamicin-resistant derivative of RB50 [27], have been previously described. Human isolates of B. bronchiseptica, strains A345 (a.k.a. B2493 and GA96-01) and M0149 (a.k.a. D444 and B2494), were received from the CDC and multi-locus sequence typed as previously described [5]. All strains were maintained on Bordet-Gengou (BG) Agar (Difco, Sparks, MD) with 10% sheep's blood (Hema Resources, Aurora, OR) with 20 µg/ml of streptomycin or gentamicin. Bacteria were grown overnight at 37°C in Stainer-Scholte broth [28]–[30] to mid-exponential phase and diluted in phosphate buffered saline (PBS, Omnipur, Gibbstown, NJ) to the indicated concentration.
Animal Care and Housing
4 to 6 week old C57BL/6, µMT and RAG2 −/− mice were obtained from Jackson Laboratories (Bar Harbor, ME). IgA −/− mice were obtained from Dr. Innocent Mbawuike [31]. C3 −/− mice were obtained from Dr. Rick Wetsel [32]. Mice lacking Fcγ Receptor I, II, and III (FcγR−/−) were obtained from Taconic (Hudson, NY). All mice were maintained and bred in Pennsylvania State University approved housing facilities and were closely monitored in accordance with institutional policies and the Institutional Animal Care and Use Committee (IACUC) regulations (IACUC approval number for breeding: 31180 and for experiments: 31297).
Inoculation and Vaccination of Mice
For inoculation, mice were lightly sedated by a flow of 5% isoflurane in oxygen and a 50 µl inoculum containing 5×105 CFU was pipetted gently onto the external nares. This method of inhalation inoculation reliably distributes the bacteria throughout the respiratory tract [22]. For vaccination, the mice were intraperitoneally (i.p.) injected with 1×108 heat killed B. pertussis in 1 ml of PBS [33], 40 µg of purified pertactin 1 [34] or 5 µg of filamentous hemagglutinin (Sigma, St. Louis, MO) in 200 µL of PBS with Imject Alum (Pierce, Rockford, IL), or subcutaneously injected with 0.5 ml of 1∶5 dilution of the 5-component human vaccine, Adacel (aP) (Sanofi-Pasteur, Swiftwater, PA) in PBS with Imject Alum on Day 0 and Day 14. Mice were euthanized by CO2 inhalation and nasal cavities, trachea and lungs were excised, homogenized and serially diluted in PBS. Aliquots were then plated on BG Agar with appropriate antibiotics and the resultant colonies were counted two days later.
Antibiotic Treatment and Depletion of Immune Factors
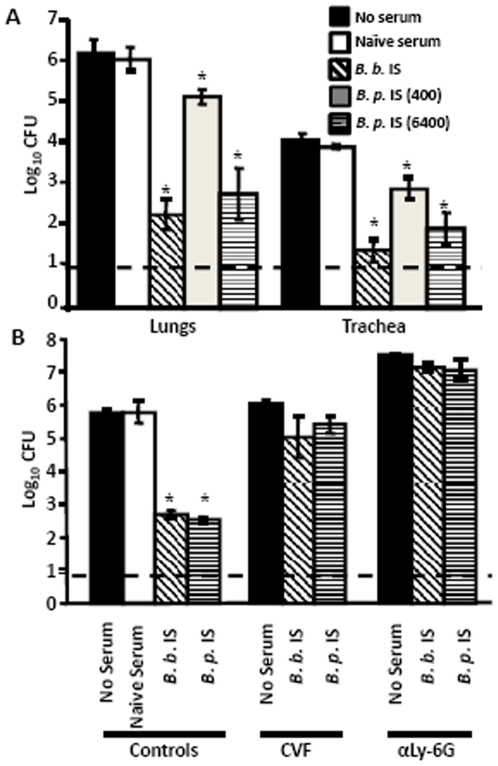
For all reinfection experiments, 1% gentamicin (Sigma, St. Louis, MO) was administered in the drinking water for 3 days beginning on day 23 post-B. pertussis inoculation [35]. Mice were then given untreated water for 2 days prior to challenge with RB50G. Previous studies have shown that gentimicin treatment does not hinder B. bronchiseptica strain RB50G colonization of the murine respiratory tract [35]. Neutrophils were depleted by i.p. injection of 1 mg of mAb from the hybridoma RB6-8C5 (αLy-6G) 48 hours prior to bacterial inoculation [33]. Depletion of 95% of blood neutrophils was confirmed via CBC differential count. Complement was depleted by two i.p. injections of 5 units of Cobra Venom Factor (CVF, Sigma, St. Louis, MO) 24 and 22 hours prior to inoculation [36].
Generation and Passive Transfer of Immune Serum
Convalescent mice were generated by inoculating mice with the indicated bacteria and allowing the mice to recover for 28 days [33]. Pooled serum was collected via post-mortem cardiac puncture from wild type convalescent or naive mice. To induce a higher titer of B. pertussis-specific antibodies, B. pertussis-inoculated mice were allowed to convalesce for 28 days followed by a second challenge with B. pertussis and subsequent cardiac puncture 3 days post secondary inoculation. To generate complement deficient serum, sera were heat-inactivated at 65°C for 30 minutes prior to passive transfer. Passive transfer experiments used 200 µL of serum i.p. injected at the time of inoculation [23], [33].
Western Blots Analysis
Westerns Blots were performed on lysates of 1×106 CFU of B. pertussis, or B. bronchiseptica in 10 µL Laemmli sample buffer (Bio-Rad, Hercules, CA). Lysates were run on 7% SDS-PAGE gels under denaturing conditions and then transferred to PVDF membranes. The membranes were then probed overnight with a 1∶100 dilution of serum collected from a naïve mouse or a mouse inoculated with a single dose of B. pertussis for 28 days (5×105 CFU in 50 ul) and a 1∶10,000 dilution of goat anti-mouse Ig (H+L) HRP-conjugated (Southern Biotech, Birmingham, AL) was used as the detector antibody. The membrane was visualized with ECL Western Blotting detection reagents (Amersham Biosciences, Piscataway, NJ).
Statistical Analysis
Student's two-tailed t-test was used to determine statistical significance between experimental groups. P-values of≤0.05 were considered significant. Error bars represent SEM.
Results
Immunity to B. pertussis protects against B. bronchiseptica challenge in the LRT
To test the hypothesis that B. pertussis-induced immunity protects against B. bronchiseptica colonization of the LRT, wild type mice were inoculated with live B. pertussis or vaccinated with heat-killed B. pertussis and allowed to recover for at least 28 days. By this time point, bacterial numbers in the respiratory tract were reduced to approximately 102 or fewer CFU and a strong immune response had been induced [23]. B. pertussis-vaccinated, -previously infected, or naïve mice were gentamicin treated to clear any remnant bacteria and then challenged with a gentamicin resistant strain of B. bronchiseptica and the bacterial load was enumerated on Days 3, 7, 10 and 14 post-challenge. Approximately 104 CFU in the trachea and 106 CFU in the lungs were recovered on both Days 3 and 7 post-challenge in naïve mice (Figure 1). However, only approximately 1% (102 CFU) and 0.05% (103 CFU) B. bronchiseptica were recovered from the trachea and lungs by Day 3 post-challenge in immunized mice as compared to naïve animals (Figure 1). By Day 7 post-challenge, previously infected mice retained approximately 102 CFU of B. bronchiseptica in the trachea, and 103 CFU of B. bronchiseptica in the lungs, and carried these low loads of B. bronchiseptica for approximately 2 weeks, until bacteria were undetectable on Day 14 post-challenge. Vaccinated mice cleared B. bronchiseptica from the trachea and lungs by Day 7 post-inoculation (Figure 1). These data support the hypothesis that immunity to B. pertussis protects against a B. bronchiseptica challenge in the LRT. In the nasal cavities, B. pertussis immunized mice have similar colonization kinetics to naïve mice (Figure 1). Although immunity to B. pertussis has limited effects on B. bronchiseptica colonization in the nasal cavity, the rapid and significant reduction of B. bronchiseptica numbers in the LRT of B. pertussis immunized hosts minimized the inflammation and pathology which would have further led to disease symptoms and transmission. Because a very large and significant effect of prior exposure to B. pertussis on the colonization of B. bronchiseptica was observed on Day 3 post-challenge, subsequent experiments were carried out at this timepoint.
Figure 1. B. bronchiseptica numbers in the respiratory tract of naïve and B. pertussis immune mice over time.
Groups of 3 to 4 C57BL/6 mice were left uninoculated (♦), inoculated with 5×105 CFU of B. pertussis (▪), or vaccinated with 2 i.p. injections of 108 heat killed B. pertussis on Days 0 and 14 (▴). On Day 21, mice were gentimicin-treated for 3 days. Mice were then challenged with a gentimicinR strain of B. bronchiseptica on Day 28 and sacrificed at the indicated day post-challenge for quantification of bacterial load in nasal cavity (A), trachea (B) and the lungs (C). Bacterial numbers are represented as the mean Log10 CFU+/−SEM. Dashed line represents the lower limit of detection. * indicates statistical difference (P value<0.05) between naïve and treated groups.
B. pertussis-induced immunity requires B cells, but not IgA, for protection against B. bronchiseptica challenge in the lungs
Since immunization with B. pertussis led to protection against B. bronchiseptica, we sought to determine if this was mediated by the adaptive immune response. B. pertussis-immunized RAG−/− mice were unable to reduce B. bronchiseptica numbers, indicating a role for adaptive immunity in protection (data not shown). We hypothesized that B. pertussis-induced antibodies could recognize B. bronchiseptica antigens and mediate the clearance of this pathogen. Supporting this hypothesis, there was no difference in bacterial load in the trachea or the lungs between immunized or naïve µMT mice on Day 3 post B. bronchiseptica challenge (Figure 2). These data indicate that B cells, and the antibodies they produce [23], are required for cross protection.
Figure 2. B. bronchiseptica numbers in the LRT of immunized B-cell and IgA deficient mice.
Naïve (black bars), B. pertussis-vaccinated (white bars), or B. pertussis-convalescent (hatched bars) C57BL/6, µMT and IgA−/− mice were dissected Day 3 post gentimicinR B. bronchiseptica challenge. Bacterial numbers in the trachea (A) and lungs (B) are represented as the mean Log10 CFU+/−SEM. Dashed line indicates the lower limit of detection. * indicates statistical difference (P value<0.05) between naïve and treated groups. ‡ indicates statistical difference (P value<0.05) between mutant mouse and similarly treated wild type mouse groups.
We further hypothesized that the major mucosal antibody, IgA, would be the primary protective antibody [35]. IgA−/− mice were vaccinated or infected with B. pertussis and allowed to convalesce for 28 days. The treated or naïve mice were then challenged with B. bronchiseptica and dissected Day 3 post-inoculation. The bacterial load in the lungs of naïve IgA−/− mice was not significantly different from naive wild type mice (Figure 2). Consistent with previous findings, immunized IgA−/− mice were not protected against B. bronchiseptica challenge in the trachea [35]. Immunization reduced B. bronchiseptica numbers in the lungs of IgA−/− mice by greater than 95%, although immunization reduced bacterial numbers by >99.9% in wild type mice, relative to naïve mice. Thus, IgA is required for B. pertussis-immune-mediated protection against B. bronchiseptica in the trachea, but is not as important in the lungs.
B. pertussis-induced sera require complement and neutrophils to reduce B. bronchiseptica colonization
To determine if B. pertussis-induced sera can clear B. bronchiseptica upon passive transfer into naïve mice, wild type mice were inoculated with B. bronchiseptica and immediately i.p. injected with 200 µL of naïve, B. bronchiseptica- or B. pertussis-induced immune serum. The mice were then dissected on Day 3 post-inoculation for bacterial enumeration. Compared to untreated mice, naïve serum had no effect on bacterial numbers (Figure 3A). In contrast, B. pertussis-immune serum (titer ∼400) reduced bacterial numbers in the lungs and trachea by greater than 85% (Figure 3A). B. pertussis-immune serum with a titer of 6400, a titer similar to that of B. bronchiseptica-induced immune serum, reduced B. bronchiseptica numbers in both organs by>99.9% relative to that of naïve serum treated mice, an effect similar to that of B. bronchiseptica-immune serum (Figure 3A). Together, these data suggest that B. pertussis-induced sera are able to efficiently reduce numbers of B. bronchiseptica in the LRT in a dose-dependent manner. Naïve serum, while containing other components such as albumin and complement, had no effect on bacterial numbers, suggesting B. pertusis-specific antibodies mediate the reduction of B. bronchiseptica colonization.
Figure 3. Effect of Complement and Neutrophils on B. pertussis serum antibody-mediated clearance of B. bronchiseptica.
(A) Wild type mice infected with B. bronchiseptica were untreated (black bars) or i.p. injected with naïve serum (white bars), B. bronchiseptica-immune serum (hatched bars), B. pertussis-immune serum titer 400 (gray bars), or B. pertussis-immune serum titer 6400 (horizontal lined bars) and then dissected Day 3 post-inoculation. (B) Wild type mice were complement (CVF) or neutrophil (αLy-6G) depleted, then inoculated with B. bronchiseptica, i.p. injected with the indicated serum and then dissected on Day 2 post-inoculation. B.b. indicates B. bronchiseptica-immune serum; B.p. indicates B. pertussis-immune serum titer 6400. Bacterial numbers are represented as the mean Log10 CFU+/−SEM. Dashed line indicates the lower limit of detection. * indicates P value of <0.05.
Our previous work showed that antibodies induced by B. bronchiseptica infection clear this bacterium from the lungs of mice via a complement and neutrophil-dependent mechanism [37], [38]. Thus, we hypothesized that B. pertussis-induced antibodies would also required the complement cascade and/or neutrophils in order to clear B. bronchiseptica. To test this hypothesis, mice were CVF treated to deplete complement or were treated with αLy-6G monoclonal antibody to deplete neutrophils prior to inoculation with B. bronchiseptica. While B. pertussis-immune serum reduced B. bronchiseptica numbers in untreated mice, it did not significantly reduce bacterial numbers in the lungs of CVF or αLy-6G treated mice, indicating that both complement and neutrophils are required (Figure 3B). In addition, all αLy-6G treated mice were moribund by Day 2 post-inoculation, indicating that B. pertussis-specific sera did not protect against the rapid virulence of B. bronchiseptica in animals lacking neutrophils.
To determine if complement and Fcγ Receptors are required for B. pertussis-immunization induced protection of the LRT against B. bronchiseptica, C3−/− mice, which lack the enzyme required for both the classical and the alternative complement cascades, and FcγR−/− mice, which lack Fcγ Receptors (I, II, and III) specific for the Fc region of IgG antibodies, were immunized with B. pertussis and then challenged 28 days later with B. bronchiseptica. In both C3−/− and FcγR−/− mice that were previously vaccinated or infected with B. pertussis, the B. bronchiseptica bacterial load in the lungs was significantly decreased by Day 3 post-inoculation when compared to naïve mice (Figure 4), indicating that either vaccination or infection can confer protection in the absence of either C3 or FcγRs. In the trachea, B. pertussis immunization reduced bacterial numbers significantly in FcγR−/− mice, but not significantly in C3−/− mice, relative to naïve mice (Figure 4). Together these results are consistent with our previous findings that adoptively transferred immune serum conferred protection against B. bronchiseptica that was dependent on both complement and FcγRs (Figure 3)[33], but infection-induced protection was much less dependent on either (Figure 4)[39].
Figure 4. Clearance of B. bronchiseptica from the LRT in B. pertussis-immunized C3−/− and FcγR−/− mice.
Naïve (black bars), B. pertussis-vaccinated (white bars), or B. pertussis-convalescent (hatched bars) mice were dissected Day 3 post gentimicinR B. bronchiseptica inoculation. Bacterial numbers in the (A) trachea and (B) lungs are represented as the mean Log10 CFU+/−SEM. Dashed line indicates the lower limit of detection. * indicates statistical difference (P value<0.05) between naïve and treated mouse. ‡ indicates statistical difference (P value<0.05) between mutant and similarly treated wild type mice.
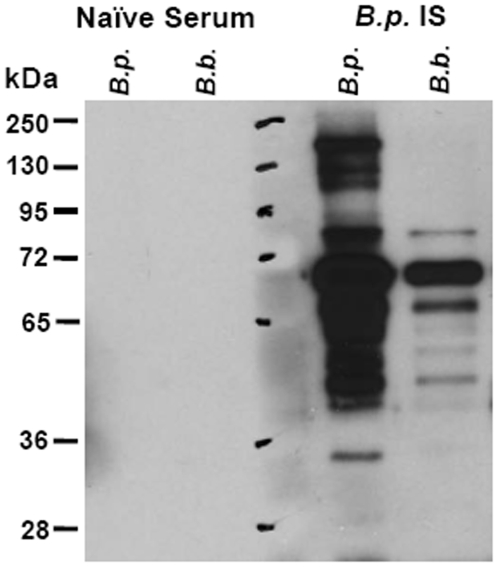
B. pertussis-induced sera recognize antigens on B. bronchiseptica
Since passive immunization with B. pertussis-induced sera can control B. bronchiseptica infection in the LRT, we examined recognition of B. bronchiseptica antigens by B. pertussis-infection-induced serum. Western Blot analysis of B. pertussis and B. bronchiseptica lysates probed with B. pertussis-infection-induced serum showed that B. pertussis-infection-induced serum antibodies were cross reactive with B. bronchiseptica antigens (Figure 5). The size of one of the cross-reactive bands at approximately 65-72 KDa suggested that the band was pertactin, an antigenic protein known to induce protective immunity [24], [34], [40]. Several other B. bronchiseptica antigens appeared to be cross-reactive to B. pertussis-infection-induced serum. There was little to no recognition of these lysates when probed with naïve serum at the same dilution.
Figure 5. Analysis of cross-reacting antigens between B. pertussis and B. bronchiseptica.
Lysates of B. pertussis (B.p.) or B. bronchiseptica (B.b.) were loaded in the indicated wells and separated by SDS-PAGE gel. Proteins were transferred to PVDF membrane, and then probed with naïve serum or B. pertussis-infection induced immune serum (B.p. IS).
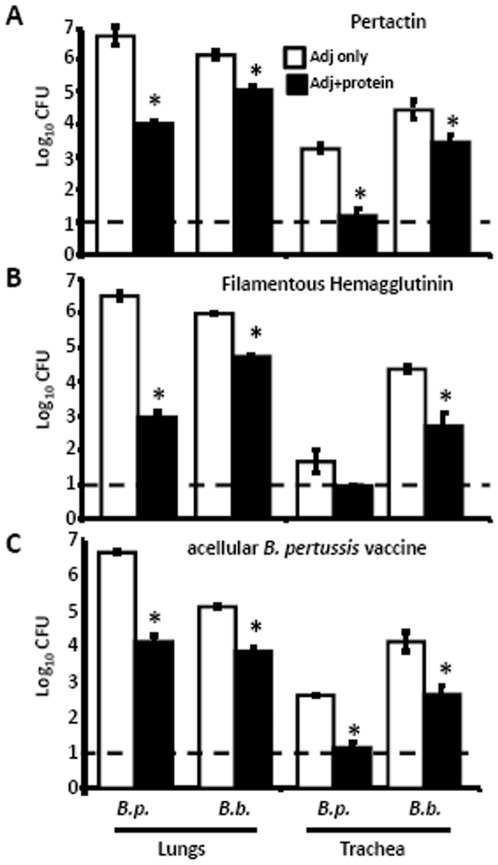
Immunization with B. pertussis-derived antigens is protective against B. bronchiseptica challenge
Since pertactin and filamentous hemagglutinin are expressed by both B. pertussis and B. bronchiseptica and are included in some current acellular vaccines, we went on to determine if immunity to pertactin or filamentous hemagglutinin contributed to B. pertussis-induced immunity to B. bronchiseptica. Wild type mice were immunized with purified B. pertussis-derived pertactin, filamentous hemagglutinin, or aP vaccine, which contains these two shared antigens. These mice were then challenged with B. pertussis or B. bronchiseptica and dissected 3 days later to quantify bacterial numbers. Immunization reduced numbers of B. pertussis by>99%, relative to adjuvant-treated mice, (Figure 6A, B and C), consistent with previous findings [34]. Immunization with these B. pertussis antigens reduced B. bronchiseptica numbers by>90% in the trachea and lungs, as compared to numbers in adjuvant-treated mice (Figure 6). These data indicate that immunization with B. pertussis-derived antigens induces an immune response which protects the trachea and lungs of mice against either B. pertussis or B. bronchiseptica infection.
Figure 6. Effect of vaccination with B. pertussis-derived antigens on B. bronchiseptica colonization in the LRT.
Groups of 3–4 C57BL/6 mice were vaccinated with (A) 40 µg PRN, (B) 5 µg FHA or (C) 0.5 mL of 1∶5 dilution of Adacel in PBS and Imject Alum on Days 0 and 14. Adjuvant-only control (white bars) and protein with adjuvant (black bars) vaccinated mice were challenged on Day 28 with B. pertussis (B. p.) or B. bronchiseptica (B. b.) and dissected Day 3 post-challenge. Bacterial numbers are represented as the mean Log10 CFU+/−SEM. Dashed line indicates the lower limit of detection. * indicates statistical difference (P value of <0.05) between adjuvant only and protein with adjuvant treated groups.
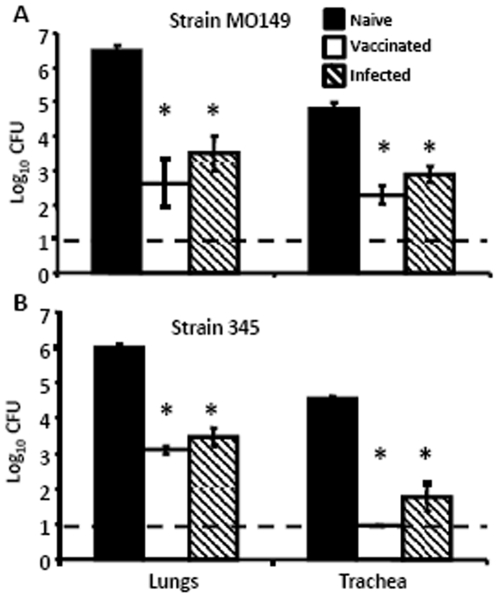
B. pertussis-induced immunity protects against human isolates of B. bronchiseptica
To determine if B. pertussis-induced immunity is sufficient to protect against recent human clinical isolates of B. bronchiseptica, wild type mice were immunized with B. pertussis and challenged with two B. bronchiseptica Complex IV isolates [5], strain M0149 and strain 345. These strains, which are divergent from the prototypical B. bronchiseptica Complex I strain, RB50, are more closely related to B. pertussis and are both from human-associated lineages [5]. Since the normal 5×105 CFU dose was lethal to naïve mice, strain 345 was delivered in inocula of 2×105 CFU. These bacteria grew to 106 in the lungs and 105 in the trachea of naïve mice (Figure 7). In contrast, B. pertussis-immunization reduced the numbers of both by greater than 99.9% in the lungs and trachea (Figure 7A and B) by Day 3 post-challenge; indicating that immunity to B. pertussis is sufficient to control B. bronchiseptica strains recently isolated from humans.
Figure 7. Human isolated, Complex IV B. bronchiseptica colonization of B. pertussis-immune mice.
Groups of 4 naïve (black bars), B. pertussis-vaccinated (white bars) or B. pertussis-convalescent (hatched bars) C57BL/6 mice were challenged with B. bronchiseptica strain (A) M0149 or (B) 345 and dissected Day 3 post-challenge. Bacterial numbers are represented as the mean Log10 CFU+/−SEM. Dashed line indicates the lower limit of detection. * indicates statistical difference (P value of <0.05) between naïve and treated groups.
Discussion
B. parapertussis avoids B. pertussis-induced immunity, a critical aspect of its ability to transmit efficiently to be successful in largely B. pertussis-immune human populations [19], [41]–[47] (X. Zhang, M. E. Rodríguez, E. T. Harvill, unpublished data). Although many B. bronchiseptica lineages have broad host ranges, infect large proportions of the animals in greatest contact with humans [3], [4], and are known to spillover from their animal hosts to humans [12], B. bronchiseptica strains are not observed to transmit amongst humans. Based on these observations we explored the possibility that B. bronchiseptica may be limited in its ability to infect humans because it cannot avoid B. pertussis-induced immunity. If B. bronchiseptica cannot avoid B. pertussis-induced immunity, then well-established ecological theory predicts that it cannot coexist with the human pathogen, explaining many of the observations described above.
Immunity induced by B. pertussis infection or vaccination significantly reduced B. bronchiseptica numbers in the LRT of mice (Figure 1). Although the mechanism of protection in the trachea and lungs appeared to be substantially similar, the major mucosal antibody, IgA, was not required for B. pertussis-induced protection against B. bronchiseptica in the lungs, but was important in the trachea (Figure 2). This is consistent with our recent observations that IgA is required for efficient control of B. bronchiseptica in the trachea but not the lungs [48]. Immune serum recognized B. bronchiseptica antigens and mediated protection that was dependent on complement and neutrophils, but immunization-induced immunity did not appear to require either complement or FcγRs (Figures 3, 4, and 5). Immunization with purified pertactin or filamentous hemagglutinin derived from B. pertussis also conferred protection against B. bronchiseptica (Figure 6). Together, these data indicate that B. pertussis-induced immunity provides protection against B. bronchiseptica in the LRT of mice.
In developed countries, vaccination against B. pertussis begins at a very early age. In certain populations, the seroprevalence of B. pertussis-specific antibodies exceeds 90% [49]. If the ability to colonize the LRT in large numbers and cause inflammation and disease symptoms (coughing) are important to B. bronchiseptica transmission, as they are to the other bordetellae, then these measures of immunity to B. pertussis are likely to limit the infectiousness of B. bronchiseptica in humans [17]. This “herd immunity” would not protect individuals in whom immunity had waned, or those that are immunodeficient, but the high prevalence of B. pertussis-immunity in their surroundings would dampen opportunities for transmission by decreasing the number of susceptible individuals. This would explain why B. bronchiseptica infections are generally associated with an animal source and why chains of transmission are generally not established [12]. Furthermore, this theoretical framework also explains the observed association of B. bronchiseptica disease in humans with an immunocompromised state [7]–[12], [50], [51].
Our data show that B. pertussis immunity does not protect the nasal cavities of mice from B. bronchiseptica colonization (Figure 1). It is possible, and consistent with our model, that B. bronchiseptica may be present in the nasal cavity of humans. However, it would be hard to imagine how either the bacterium or its immunological effects on B. pertussis epidemiology, and serological monitoring, could go unnoticed. Extensive samplings of nasophayngeal swabs and testing of serum reactivity that have revealed B. pertussis, B. parapertussis and even B. holmesii have not revealed B. bronchiseptica [3], [12], [42]–[44], [52]–[55], even though it is easier to culture. Alternatively, it is possible that the nasal cavity of humans is not conducive to Bordetella infection, possibly due to differences in physiology, immunology or other competitive flora. This could explain why B. pertussis appears to have lost the ability to persist in the nasal cavity of mice, while every B. bronchiseptica strain examined retains this ability [23], [56] (A.M. Buboltz, T.L. Nicholson, L.S. Weyrich, E.T. Harvill, unpublished data). However, since human Bordetella infections are highly contagious via the cough-induced respiratory droplets, the ability of B. pertussis-induced immunity to limit B. bronchiseptica to the nasal cavity should be sufficient to prevent its efficient transmission.
There are likely to be multiple factors that limit the success of an invading pathogen in a new host. Although spillover opportunity and individual host specificity are often considered, more often overlooked are the possible effects of resident flora on the success of the invader. Our data show that immunity to B. pertussis can have a substantial effect on B. bronchiseptica infection. Although a high level of individual immunity may prevent many initial infections or diseases, the variable immunity within human populations is more likely to allow occasional infections while having a more dramatic effect via “herd immunity” that inhibits the establishment of a successful chain of transmission. This model is consistent with much of the existing experimental and clinical data, including sporadic infections often associated with an immunocompromised state [8]–[10], [12], [50], [51]. It also leads to the prediction that B. bronchiseptica infections in humans may occur much more commonly than are reported, and improved surveillance may test this prediction. We can further predict that changes in the B. pertussis vaccination- or infection-induced immune status of human populations will affect the observed rate of zoonotic B. bronchiseptica disease and, more importantly, their opportunity to establish chains of infection to emerge as new infectious agents.
Acknowledgments
We would like to thank Dr. Innocent Mbawuike for the kind donation of IgA−/− mice, and Dr. Rick Wetsel for the kind donation of C3−/− mice. We would like to thank Dr. Fritz Mooi for the kind gift of purified pertactin 1. We would like to thank Dr. Gary Huffnagle for the donation of materials. We would also like to thank Dr. Daniel Wolfe and Dr. Anne Buboltz for the critical reading of this manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by NIH grant GM083113 (E.T.H.) and PSU College of Agriculture Graduate Student grant (E.M.G.). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Greger M. The human/animal interface: emergence and resurgence of zoonotic infectious diseases. Crit Rev Microbiol. 2007;33:243–299. doi: 10.1080/10408410701647594. [DOI] [PubMed] [Google Scholar]
- 2.Parrish CR, Holmes EC, Morens DM, Park EC, Burke DS, et al. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol Mol Biol Rev. 2008;72:457–470. doi: 10.1128/MMBR.00004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodnow RA. Biology of Bordetella bronchiseptica. Microbiol Rev. 1980;44:722–738. doi: 10.1128/mr.44.4.722-738.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diavatopoulos DA, Cummings CA, Schouls LM, Brinig MM, Relman DA, et al. Bordetella pertussis, the causative agent of whooping cough, evolved from a distinct, human-associated lineage of B. bronchiseptica. PLoS Pathog. 2005;1:e45. doi: 10.1371/journal.ppat.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumler AJ, Tsolis RM, Ficht TA, Adams LG. Evolution of host adaptation in Salmonella enterica. Infect Immun. 1998;66:4579–4587. doi: 10.1128/iai.66.10.4579-4587.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amador C, Chiner E, Calpe JL, Ortiz de la Table V, Martinez C, et al. Pneumonia due to Bordetella bronchiseptica in a patient with AIDS. Rev Infect Dis. 1991;13:771–772. doi: 10.1093/clinids/13.4.771. [DOI] [PubMed] [Google Scholar]
- 8.Belen O, Campos JM, Cogen PH, Jantausch BA. Postsurgical meningitis caused by Bordetella bronchiseptica. Pediatr Infect Dis J. 2003;22:380–381. [PubMed] [Google Scholar]
- 9.Gueirard P, Weber C, Le Coustumier A, Guiso N. Human Bordetella bronchiseptica infection related to contact with infected animals: persistence of bacteria in host. J Clin Microbiol. 1995;33:2002–2006. doi: 10.1128/jcm.33.8.2002-2006.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorenzo-Pajuelo B, Villanueva JL, Rodriguez-Cuesta J, Vergara-Irigaray N, Bernabeu-Wittel M, et al. Cavitary pneumonia in an AIDS patient caused by an unusual Bordetella bronchiseptica variant producing reduced amounts of pertactin and other major antigens. J Clin Microbiol. 2002;40:3146–3154. doi: 10.1128/JCM.40.9.3146-3154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodard DR, Cone LA, Fostvedt K. Bordetella bronchiseptica infection in patients with AIDS. Clin Infect Dis. 1995;20:193–194. doi: 10.1093/clinids/20.1.193. [DOI] [PubMed] [Google Scholar]
- 12.Woolfrey BF, Moody JA. Human infections associated with Bordetella bronchiseptica. Clin Microbiol Rev. 1991;4:243–255. doi: 10.1128/cmr.4.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lysenko ES, Ratner AJ, Nelson AL, Weiser JN. The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces. PLoS Pathog. 2005;1:e1. doi: 10.1371/journal.ppat.0010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan MW. Cross-species infections and their analysis. Annu Rev Microbiol. 2002;56:539–565. doi: 10.1146/annurev.micro.56.012302.161110. [DOI] [PubMed] [Google Scholar]
- 15.Casadevall A, Pirofski LA. Host-pathogen interactions: basic concepts of microbial commensalism, colonization, infection, and disease. Infect Immun. 2000;68:6511–6518. doi: 10.1128/iai.68.12.6511-6518.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuiken T, Holmes EC, McCauley J, Rimmelzwaan GF, Williams CS, et al. Host species barriers to influenza virus infections. Science. 2006;312:394–397. doi: 10.1126/science.1122818. [DOI] [PubMed] [Google Scholar]
- 17.Bjornstad ON, Harvill ET. Evolution and emergence of Bordetella in humans. Trends Microbiol. 2005;13:355–359. doi: 10.1016/j.tim.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe M, Nagai M. Whooping cough due to Bordetella parapertussis: an unresolved problem. Expert Rev Anti Infect Ther. 2004;2:447–454. doi: 10.1586/14787210.2.3.447. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe DN, Goebel EM, Bjornstad ON, Restif O, Harvill ET. The O Antigen Enables Bordetella parapertussis To Avoid Bordetella pertussis-Induced Immunity. Infect Immun. 2007;75:4972–4979. doi: 10.1128/IAI.00763-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerlach G, von Wintzingerode F, Middendorf B, Gross R. Evolutionary trends in the genus Bordetella. Microbes Infect. 2001;3:61–72. doi: 10.1016/s1286-4579(00)01353-8. [DOI] [PubMed] [Google Scholar]
- 21.Restif O, Grenfell BT. Integrating life history and cross-immunity into the evolutionary dynamics of pathogens. Proc Biol Sci. 2006;273:409–416. doi: 10.1098/rspb.2005.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvill ET, Cotter PA, Miller JF. Pregenomic comparative analysis between bordetella bronchiseptica RB50 and Bordetella pertussis tohama I in murine models of respiratory tract infection. Infect Immun. 1999;67:6109–6118. doi: 10.1128/iai.67.11.6109-6118.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirimanjeswara GS, Mann PB, Harvill ET. Role of antibodies in immunity to Bordetella infections. Infect Immun. 2003;71:1719–1724. doi: 10.1128/IAI.71.4.1719-1724.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Berg BM, David S, Beekhuizen H, Mooi FR, van Furth R. Protection and humoral immune responses against Bordetella pertussis infection in mice immunized with acellular or cellular pertussis immunogens. Vaccine. 2000;19:1118–1128. doi: 10.1016/s0264-410x(00)00329-7. [DOI] [PubMed] [Google Scholar]
- 25.Stibitz S, Yang MS. Subcellular localization and immunological detection of proteins encoded by the vir locus of Bordetella pertussis. J Bacteriol. 1991;173:4288–4296. doi: 10.1128/jb.173.14.4288-4296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cotter PA, Miller JF. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect Immun. 1994;62:3381–3390. doi: 10.1128/iai.62.8.3381-3390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cotter PA, Yuk MH, Mattoo S, Akerley BJ, Boschwitz J, et al. Filamentous Hemagglutinin of Bordetella bronchiseptica Is Required for Efficient Establishment of Tracheal Colonization. Infect Immun. 1998;66:5921–5929. doi: 10.1128/iai.66.12.5921-5929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imaizumi A, Suzuki Y, Ono S, Sato H, Sato Y. Heptakis(2,6-O-dimethyl)beta-cyclodextrin: a novel growth stimulant for Bordetella pertussis phase I. J Clin Microbiol. 1983;17:781–786. doi: 10.1128/jcm.17.5.781-786.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stainer DW, Scholte MJ. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970;63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- 30.von Koenig CH, Tacken A, Finger H. Use of supplemented Stainer-Scholte broth for the isolation of Bordetella pertussis from clinical material. J Clin Microbiol. 1988;26:2558–2560. doi: 10.1128/jcm.26.12.2558-2560.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Pacheco S, Acuna CL, Switzer KC, Wang Y, et al. Immunoglobulin A-deficient mice exhibit altered T helper 1-type immune responses but retain mucosal immunity to influenza virus. Immunology. 2002;105:286–294. doi: 10.1046/j.0019-2805.2001.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Circolo A, Garnier G, Fukuda W, Wang X, Hidvegi T, et al. Genetic disruption of the murine complement C3 promoter region generates deficient mice with extrahepatic expression of C3 mRNA. Immunopharmacology. 1999;42:135–149. doi: 10.1016/s0162-3109(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 33.Kirimanjeswara GS, Mann PB, Pilione M, Kennett MJ, Harvill ET. The complex mechanism of antibody-mediated clearance of Bordetella from the lungs requires TLR4. J Immunol. 2005;175:7504–7511. doi: 10.4049/jimmunol.175.11.7504. [DOI] [PubMed] [Google Scholar]
- 34.Hijnen M. The Bordetella pertussis protein Pertactin: Role in Immunity and Immune Evasion: Eijkman Winkler Institute. 2006. 200
- 35.Wolfe DN, Kirimanjeswara GS, Goebel EM, Harvill ET. Comparative role of immunoglobulin A in protective immunity against the Bordetellae. Infect Immun. 2007;75:4416–4422. doi: 10.1128/IAI.00412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapiro S, Beenhouwer DO, Feldmesser M, Taborda C, Carroll MC, et al. Immunoglobulin G monoclonal antibodies to Cryptococcus neoformans protect mice deficient in complement component C3. Infect Immun. 2002;70:2598–2604. doi: 10.1128/IAI.70.5.2598-2604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pishko EJ, Kirimanjeswara GS, Pilione MR, Gopinathan L, Kennett MJ, et al. Antibody-mediated bacterial clearance from the lower respiratory tract of mice requires complement component C3. Eur J Immunol. 2004;34:184–193. doi: 10.1002/eji.200324234. [DOI] [PubMed] [Google Scholar]
- 38.Wolfe DN, Kirimanjeswara GS, Harvill ET. Clearance of Bordetella parapertussis from the lower respiratory tract requires humoral and cellular immunity. Infect Immun. 2005;73:6508–6513. doi: 10.1128/IAI.73.10.6508-6513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gopinathan L, Kirimanjeswara GS, Wolfe DN, Kelley ML, Harvill ET. Different mechanisms of vaccine-induced and infection-induced immunity to Bordetella bronchiseptica. Microbes Infect. 2007;9:442–448. doi: 10.1016/j.micinf.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Poolman JT, Hallander HO. Acellular pertussis vaccines and the role of pertactin and fimbriae. Expert Rev Vaccines. 2007;6:47–56. doi: 10.1586/14760584.6.1.47. [DOI] [PubMed] [Google Scholar]
- 41.David S, van Furth R, Mooi FR. Efficacies of whole cell and acellular pertussis vaccines against Bordetella parapertussis in a mouse model. Vaccine. 2004;22:1892. doi: 10.1016/j.vaccine.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 42.He Q, Viljanen MK, Arvilommi H, Aittanen B, Mertsola J. Whooping cough caused by Bordetella pertussis and Bordetella parapertussis in an immunized population. JAMA. 1998;280:635–637. doi: 10.1001/jama.280.7.635. [DOI] [PubMed] [Google Scholar]
- 43.Heininger USK, Christenson P, Cherry JD. Evidence of efficacy of the Lederle/Takeda acellular pertussis component diphtheria and tetanus toxoids and pertussis vaccine but not the Lederle whole-cell component diphtheria and tetanus toxoids and pertussis vaccine against Bordetella parapertussis infection. Clin Infect Dis. 1998;28:602–604. doi: 10.1086/515154. [DOI] [PubMed] [Google Scholar]
- 44.Liese JG, Renner C, Stojanov S, Belohradsky BH, The Munich Vaccine Study G. Clinical and epidemiological picture of B pertussis and B parapertussis infections after introduction of acellular pertussis vaccines. Arch Dis Child. 2003;88:684–687. doi: 10.1136/adc.88.8.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stehr KCJ, Heininger U, Schmitt-Grohe S, uberall M, Laussucq S, Eckhardt T, Meyer M, Engelhardt R, Christenson P. A comparative efficacy trial in Germany in infants who received either the Lederle/Takeda acellular pertussis component DTP (DTaP) vaccine, the Lederle whole-cell component DTP vaccine, or DT vaccine. Pediatrics. 1998;101:1–11. doi: 10.1542/peds.101.1.1. [DOI] [PubMed] [Google Scholar]
- 46.Mastrantonio P, Stefanelli P, Giulano M, Herrera Rojas Y, Ciofi degli Atti M, Anemona A, Tozzi AE. Bordetella parapertussis infection in children: epidemiology, clinical symptoms, and molecular characteristics of isolates. J Clin Microbiol. 1998;36:999–1002. doi: 10.1128/jcm.36.4.999-1002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willems RJ, Kamerbeek J, Geuijen CA, Top J, Gielen H, Gaastra W, Mooi FR. The efficacy of a whole cell pertussis vaccine and fimbriae against Bordetella pertussis and Bordetella parapertussis infections in a respiratory mouse model. Vaccine. 1998;16:410–416. doi: 10.1016/s0264-410x(97)80919-x. [DOI] [PubMed] [Google Scholar]
- 48.Wolfe DN, Kirimanjeswara GS, Goebel EM, Harvill ET. Comparative Role of Immunoglobulin A in Protective Immunity against the Bordetellae. Infect Immun. 2007;75:4416–4422. doi: 10.1128/IAI.00412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maixnerova M. The 2001 serological survey in the Czech Republic–pertussis. Cent Eur J Public Health. 2003;11(Suppl):S17–22. [PubMed] [Google Scholar]
- 50.De Jesus-Berrios Y, Gonzalez S, Sante M. Respiratory pathogens in bronchoalveolar lavage in a Puerto Rican population infected with the human immunodeficiency virus. P R Health Sci J. 2005;24:197–202. [PubMed] [Google Scholar]
- 51.Llombart M, Chiner E, Senent C. [Necrotizing pneumonia due to Bordetella bronchiseptica in an immunocompetent woman]. Arch Bronconeumol. 2006;42:255–256. doi: 10.1016/s1579-2129(06)60457-6. [DOI] [PubMed] [Google Scholar]
- 52.Yih WK, Silva EA, Ida J, Harrington N, Lett SM, et al. Bordetella holmesii-like organisms isolated from Massachusetts patients with pertussis-like symptoms. Emerg Infect Dis. 1999;5:441–443. doi: 10.3201/eid0503.990317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schlapfer G, Cherry JD, Heininger U, Uberall M, Schmitt-Grohe S, et al. Polymerase chain reaction identification of Bordetella pertussis infections in vaccinees and family members in a pertussis vaccine efficacy trial in Germany. Pediatr Infect Dis J. 1995;14:209–214. doi: 10.1097/00006454-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Bonhoeffer J, Bar G, Riffelmann M, Soler M, Heininger U. The role of Bordetella infections in patients with acute exacerbation of chronic bronchitis. Infection. 2005;33:13–17. doi: 10.1007/s15010-005-4004-9. [DOI] [PubMed] [Google Scholar]
- 55.Hallander HO, Gnarpe J, Gnarpe H, Olin P. Bordetella pertussis, Bordetella parapertussis, Mycoplasma pneumoniae, Chlamydia pneumoniae and persistent cough in children. Scand J Infect Dis. 1999;31:281–286. doi: 10.1080/00365549950163581. [DOI] [PubMed] [Google Scholar]
- 56.Buboltz AM, Nicholson TL, Parette MR, Hester SE, Parkhill J, et al. Replacement of adenylate cyclase toxin in a lineage of Bordetella bronchiseptica. J Bacteriol. 2008;190:5502–5511. doi: 10.1128/JB.00226-08. [DOI] [PMC free article] [PubMed] [Google Scholar]