Abstract
Diagnosis of oesophageal cancer is usually made at endoscopic biopsy. Imaging is used to stage the tumour, assess response to therapy, and detect complications of therapy and recurrence. For therapy planning, differentiation of resectable (T1–T3, N0, localised N1) versus irresectable disease (T4, extensive N1, M1) is important. Endoscopic ultrasound (EUS) is the method of choice for diagnosing T1–T3 stages, and N0 versus N1, including endoscopic ultrasound (EUS)-guided fine-needle aspiration. Computed tomography (CT) or magnetic resonance imaging (MRI) are used to demonstrate infiltration of adjacent structures, distant lymphadenopathy and distant metastases, however, positron emission tomography (PET) and PET-CT are superior in this respect. If imaging suggests irresectable disease, histologic confirmation may be required in order not to prevent curative resection in false positive findings.
Keywords: Staging, CT, US, PET, TNM system, AJCC
Introduction
Oesophageal cancer accounts for approximately 10% of gastrointestinal malignancies. It usually involves elderly patients with a mean age between 50 and 60. Fifteen percent of tumours arise in the proximal third, 50% in the mid third and 35% in the distal third of the oesophagus. Histologically, there are two separate entities: squamous cell carcinoma typically presents in the proximal oesophagus. It is related to smoking and alcohol consumption. Adenocarcinoma is typically localised in the distal oesophagus, is not related to alcohol and smoking but rather develops in Barrett oesophagus, i.e. presence of gastric mucosa in the distal oesophagus, usually related to gastric reflux. The proportion of squamous cell carcinoma versus adenocarcinoma has changed recently. Adenocarcinoma was rare for decades, representing only 5% of tumours; its proportion has recently increased to 20% in some series.
As the oesophagus – different from other gastrointestinal organs – is not surrounded by a serosa, infiltration into adjacent mediastinal structures occurs early. Due to its rich lymphatic drainage, lymph node metastases and haematogenous metastases (liver, adrenal, lung, bone) occur early in the course of the disease. Long term survival is poor (less than 20%).
Clinical presentation of oesophageal cancer is usually either due to symptoms of narrowing of the lumen (dysphagia) or infiltration of adjacent structures or distant metastases. Early stage oesophageal cancer is usually asymptomatic and most patients present with advanced disease.
Staging system of oesophageal cancer
The TNM system and the AJCC classification[1] are used to describe the extent of the tumour (Tables 1 and 2). Oesophageal carcinoma in situ is limited to the epithelium (tunica mucosa) of the oesophageal surface without invading the submucosa (stage 0). Local tumour extent is described based on the layer of tissue of the oesophageal wall that is infiltrated by tumour cells. In carcinoma in situ (T0) there is no infiltration deep to the lamina propria, whereas infiltration of the lamina propria or tunica submucosa characterises T1, infiltration of the tunica muscularis propria is T2, infiltration of the tunica adventitia is T3 and infiltration of mediastinal structures is T4.
Table 1.
TNM staging of oesophageal cancer
| T | Primary tumour |
|---|---|
| T1 | Infiltration of lamina propria or tunica submucosa |
| T2 | Infiltration of tunica muscularis propria |
| T3 | Infiltration of tunica adventitia |
| T4 | Infiltration of mediastinal structures |
| N | Regional lymph nodes |
| N0 | No regional lymph nodes |
| N1 | Regional (mediastinal) lymph node metastases |
| M | Distant metastases |
| M0 | No distant metastases |
| M1a | Distant metastases |
aCervical or abdominal lymph node metastases may represent M1 depending on tumour location.
Table 2.
Stages of oesophageal cancer
| Stage | T (primary tumour) | N (regional lymph nodes) | M (distant metastases) |
|---|---|---|---|
| Stage I | T1 | N0 | M0 |
| Stage IIa | T2/3 | N0 | M0 |
| Stage IIb | T1/2 | N1 | M0 |
| Stage III | T3 | N1 | M0 |
| T4 | N0/1 | M0 | |
| Stage IV | T1–4 | N0–1 | M1 |
Regional lymph node metastases are diagnosed with involvement of supraclavicular, cervical and mediastinal lymph nodes in carcinoma of the cervical oesophagus, mediastinal, hilar and left gastric lymph nodes in upper and mid thoracic oesophageal carcinoma, and mediastinal, left gastric and upper abdominal lymph nodes in lower thoracic oesophageal tumours.
In tumours of the respective locations, all other lymph nodes are regarded as distant metastases.
Haematogenous metastases most frequently involve liver, lung, bone and adrenals. Differentiation is made between potentially resectable lesions (M1a) and irresectable lesions (M1b).
Based on local tumour extent and the presence or absence of lymph node and distant metastases, the stages of the tumour are defined and represent the basis for treatment planning.
Therapeutic strategies at different stages of oesophageal cancer
Stage 0
In carcinoma in situ (stage 0) tumours are usually small but may spread superficially and involve a large part of the oesophagus. Treatment for stage 0 oesophageal cancer involves surgical resection with wide margins. If there is no superficial spread, most stage 0 cancers can be removed endoscopically. The cure rate is greater than 90%.
Stage I
In stage I, the tumour invades the submucosa, but not into the muscularis and there are no lymph node or distant metastases. This is also called an early, superficial or localized cancer. Therapy of choice is radical resection (oesophagectomy). In patients who are deemed inoperable, combined radio-chemotherapy is used instead of surgery.
Stage II
Patients who have either infiltration of the adventitia (T3) but without lymph node metastases (stage IIa) or smaller tumours (T1–2) with regional lymph node metastases (stage IIb) can be treated with curative intent using either a primary surgical or a primary combined chemotherapy and radiation therapy approach. The American Society of Radiology has published guidelines for the treatment of stages I–III oesophageal cancer and currently recommends surgery alone as the best treatment for patients with stage II oesophageal cancer.
Stage III
Infiltration of adjacent structures (T4) or regional lymph nodes in the presence of a locally advanced tumour (T3) is a common situation at presentation of patients with oesophageal cancer. These patients may undergo radio-chemotherapy alone or before surgery (neoadjuvant radiochemotherapy).
Stage IV
In patients with distant metastases cure is usually not possible. Palliative therapy aims at reducing symptoms of dysphagia (oesophageal dilatation, stent placement, radiotherapy) and of infiltration of other structures by the primary tumour or metastases.
Imaging procedures in staging of oesophageal cancer
Imaging usually plays no role in detection of oesophageal cancer. The diagnosis is almost always made at endoscopy performed for dysphagia. Rarely, the tumour is diagnosed at endoscopy performed for other reasons.
Therefore, imaging is usually performed to stage the tumour, assess the effect of radiotherapy and chemotherapy, assist in surgical planning, detect complications of therapy and recurrent disease.
As discussed above treatment options mainly depend on the presence or absence of lymph node or distant metastases and infiltration of adjacent structures. Consequently, imaging procedures for tumour staging should ideally demonstrate or exclude:
infiltration of adjacent structures
metastatic involvement of mediastinal and other lymph nodes
distant metastases.
T staging
Fluoroscopy using oral contrast medium is used to demonstrate the degree of narrowing of the oesophageal lumen and assess potential perforation either spontaneously or after dilatation or stent placement. If aspiration or a fistula to the bronchial tree is a possibility, barium and hypertonic contrast media should be avoided and isotonic contrast (e.g. for intravenous application) should be given. Fluoroscopy is not appropriate to assess local tumour extent as the degree of narrowing does not predict infiltration of the oesophageal wall.
Cross-sectional imaging with computed tomography (CT) and magnetic resonance imaging (MRI) can demonstrate wall thickening but is not able to demonstrate the different layers of the oesophageal wall and, therefore, cannot differentiate between tumour stages T1, T2 and T3. Only if gross invasion of adjacent structures is demonstrated, can T4 be diagnosed.
Currently, the only imaging modality capable of demonstrating the wall layers is endoscopic ultrasound (EUS), which is, therefore, the method of choice for assessment of local tumour extent. In tumours extending beyond the oesophageal wall, the field of view of EUS may be too small and CT or MRI may be required. Also, if the lumen is too narrow to allow passage of the endoscope, CT and MRI may be the only option for analysis of local tumour extent.
Whereas frank infiltration of adjacent structures is easily detected at CT and MRI, it is usually difficult to exclude superficial invasion. If fat planes are preserved between the tumour and adjacent organs, infiltration can usually be excluded. Unfortunately, in many patients loss of body weight due to dysphagia results in poor visibility of fat planes.
Several signs have been used to describe the likelihood of infiltration:
aorta: direct contact between the tumour and more than 90° of the circumference of the aorta has been described as predicting invasion, whereas contact of less than 45° has been regarded as most likely excluding invasion. This sign, however, is unreliable. Also, deep invasion of the aortic wall is rare, whereas superficial invasion is resectable.
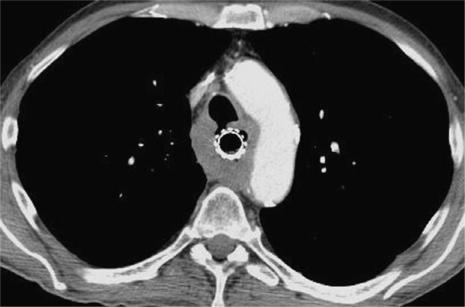
tracheobronchial tree: flattening and indentation of the membranous portion of the trachea and main bronchi are suspicious of invasion, only demonstration of tumour within the airway lumen is evidence of infiltration. Also, demonstration of a fistula between the tumour and the tracheobronchial tree confirms invasion, however, aspiration has to be excluded as the explanation for endobronchial contrast medium (Fig. 1).
pericardium: pericardial effusion is unreliable and minimal pericardial effusion does not preclude resection.
diaphragm: in cancer of the gastro-oesophageal junction, demonstration of fat planes, possibly using multiplanar imaging at MSCT or MRI, excludes invasion; its absence, however, is unreliable.
Figure 1.
CT scan at the level of the aortic arch demonstrating diffuse thickening of the oesophageal wall in histologically confirmed squamous cell carcinoma. There is a fistula to the trachea confirming T4 disease. A covered stent has been placed in the oesophagus to prevent aspiration.
Most of the signs are not specific enough to exclude potentially life-saving surgery. However, demonstration of the site of potential invasion and, thus, irresectable disease, is important for the planning of the operation. The surgeon will explore these sites early during the procedure to limit the extent of the operation and operation time if invasion of irresectable structures is present.
CT and MRI can also demonstrate the degree of dilatation of the lumen proximal to the tumour and suspect aspiration if bronchiolitis or consolidation in the dependent portions of the lung is demonstrated (in the supine position: the posterior segment of the right upper lobe, the apical segment of the right lower lobe).
N staging
To use lymph node size as a predictor of metastatic involvement has been shown to reach only moderate sensitivity and specificity (<70%, respectively). Obviously, small lymph nodes may be involved with cancer, whereas large lymph nodes may be due to benign lymphadenopathy particularly with ulceration of the tumour surface. Therefore, CT and MRI perform relatively poorly in N staging. The use of ultra-small particle iron oxide (USPIO) MR contrast agents, that are injected intravenously and lodge in normal lymphatic tissue but not in parts of the lymph nodes involved with tumour may in the future improve the N staging at MRI but has not been introduced into routine clinical practice.
Currently, two methods are available for N staging which do not rely exclusively on lymph node size. EUS can visualize regional lymph nodes and assess their size as well as echogenicity. Hypoechoic lymph nodes are likely involved with tumour, whereas hyper- or isoechoic lymph nodes are more likely to be benign. Furthermore, EUS-guided fine-needle aspiration (FNA) can be used to assess tumour infiltration of suspicious lymph nodes. However, again, EUS may be impossible in patients with major narrowing of the oesophageal lumen.
Positron emission tomography (PET) using [18F]fluorodeoxyglucose (18F-FDG-PET) can demonstrate increased glucose metabolism in normal size lymph nodes. Its limitations are due to potentially increased glucose uptake in benign inflammatory lymphadenopathy and the limited spatial resolution of PET which may prevent differentiation between the primary tumour and adjacent lymph node metastases. The latter problem is reduced – but not solved – by the combination of PET and CT (PET-CT). In cases of PET-positive lymph nodes, metastatic involvement should be confirmed histologically in order to prevent errors which would preclude a patient from potentially curative surgery.
M staging
EUS is usually not helpful for the detection of distant metastases due to its limited field of view. CT and MRI of chest and abdomen and bone scintigraphy are superior in this respect. PET and particularly PET-CT, however, is superior to these procedures and is recommended for staging of otherwise resectable oesophageal cancer.
Again, to avoid false positive results preventing curative surgery, potential metastases demonstrated at PET may require histological confirmation.
Conclusions for clinical practice
Oesophageal cancer is usually detected by symptoms such as dysphagia and confirmed with endoscopic biopsy. In this setting locally advanced or metastatic disease is likely. In most centres endoscopic ultrasound is performed to assess local tumour extent and in particular differentiate between resectable T3 and irresectable T4 tumours. Local lymph nodes are assessed with EUS and EUS-guided FNA. CT or MRI is used to demonstrate the extent of invasion into adjacent structures and distant metastases. It is also used in cases in which the lumen is too narrow for EUS and to assess potential complications (aspiration, fistula formation, etc.). In potentially resectable disease, PET or ideally PET-CT, should be used whenever available. Imaging findings that would prevent potentially curative surgery often require histologic confirmation either with endoscopic or percutaneous biopsy or with frozen section early during surgical exploration.
Reference
- 1.AJCC cancer staging manual. 6th ed. New York: Springer: 2002. Esophagus. In: American Joint Committee on Cancer; pp. 91–8. [Google Scholar]