Abstract
Positron emission tomography (PET)/computed tomography (CT) imaging is frequently requested in Oncology. Radiologists and nuclear medicine physicians are often asked to perform a panel of imaging examinations as part of the initial staging or follow-up of cancer patients. Medical imaging must therefore integrate polyvalent skills enabling imaging specialists to understand and interpret all types of images. In this context, PET imaging combined with non-enhanced CT, and diagnostic quality contrast-enhanced CT scan and optimisation of CT settings, is part of this multidisciplinary approach requiring the specific skills of a radiologist and a nuclear medicine physician. This approach must therefore be conducted in both directions: radiologists and nuclear medicine physicians should both know how to correlate PET and CT images, while preserving the specificities of each discipline. Radiologists need to be aware of several aspects of PET imaging: PET technology, the examination procedure and injection of iodinated contrast agent for high quality diagnostic CT, ideally followed by double interpretation of CT images, PET images and fused images. Radiologists should be familiar with PET imaging, as this procedure may be associated with several pitfalls and artefacts that need interpretation by a trained specialist. The authors analyse the examination technique of PET combined with non-enhanced and/or contrast-enhanced CT and the proposals for optimal interpretation of normal or pathological PET/CT fusion images.
Keywords: PET/CT, Oncology, Image interpretation, Contrast-enhanced CT
Introduction
Hybrid positron emission tomography (PET)/computed tomography (CT) machines constitute major progress in the global management of cancer patients for the initial diagnosis, evaluation of treatment and prognosis, and for surveillance throughout the course of the disease and possible relapse[1,2]. The exact position for PET/CT imaging in the diagnostic or therapeutic strategy according to the disease concerned has not yet been clearly defined[3]. For example, although PET/CT imaging has become mandatory in the initial assessment of lymphoma or lung nodules, its place is much less clearly defined for other indications such as prostate cancer in which tracers other than glucose should be used[4].
Recent American guidelines based on an intersociety dialogue between the American College of Radiology, the Society of Nuclear Medicine and the Society of Computed Body Tomography and Magnetic Resonance were published in July 2005[5]. These guidelines, although only a preliminary draft, consider all aspects of the interdisciplinary approach, the modifications and adaptations required by the use of these hybrid machines, and the consequences for both radiologists and nuclear medicine physicians. It is specified that both specialists must work in conjunction, simultaneously if possible, on the same images in order to provide results that would be more relevant than if only PET, or only CT, had been performed on the same patient. In a way, this approach tries to do more with less, i.e. provide more diagnostic information with fewer examinations[6].
Consequently, in order to improve patient management, nuclear medicine is currently encouraged to perform high quality diagnostic imaging[7]. Image fusion has a major diagnostic value, allowing detection and localisation of hypermetabolic or fluorodeoxyglucose (FDG)-avid lesions, improving the quality of PET imaging, without inducing any additional artefacts. This technique offers a considerable gain of time by allowing two examinations to be performed in a single session[6,][7].
What the radiologist needs to know about PET requires some effort in comprehension, as the basic principles of nuclear medicine are applied to a new radioactive element, fluorine-18, associated with new concepts combining standard nuclear medicine in terms of whole body scintigraphy and the distribution of 18F-labelled glucose in the body. These nuclear medicine issues are then matched with computed tomography with or without contrast administration, thus linking the two imaging procedures, nuclear medicine and radiology.
Radiologists should therefore be familiar with the following:
Basic principles of the PET technique
Concepts of radioprotection
Problems related to iodinated contrast agent injection in PET settings
Interpretation of PET/CT image fusion.
Basic principles of the PET technique
PET is a lengthy examination (2 h) performed in a patient who has been fasting for at least 4–6 h, requiring 1 h of rest after injection of [18F]FDG. The patient's blood glucose must be close to normal to avoid problems of competition between fluorine-labelled glucose and circulating blood glucose. Glycaemia is measured prior to examination and protocols must take into account whether glycaemia in diabetic patients is controlled or uncontrolled.
The FDG injection must be performed by highly qualified and well-trained personnel able to work as rapidly as possible to avoid irradiation of paramedical personnel during the injection. This injection mode is therefore rigorously defined and complex (setting up of an infusion, injection behind a lead screen, tungsten protection of radioactive syringes, long IV lines with power injectors to avoid close contact with the patient and controlled radioactive waste management).
The patient is then kept at a distance, under video surveillance, as it is recommended to avoid contact with an irradiating patient except when absolutely necessary. Prescribed drugs (usual treatments, anxiolytics, etc.) and oral contrast agents must be administered prior to the injection of FDG.
In the conventional PET/CT imaging procedure, a non-enhanced CT scan is performed at the beginning of the examination, on free breathing and at low amperage dose. This CT scan is designed to provide an attenuation map in order to correct PET images, and precise anatomical localisation of the FDG-avid areas observed on PET scintigraphy. The attenuation map is a distribution map of attenuation coefficients measured by CT and designed to obtain more legible PET images, thereby allowing PET and CT image fusion.
In terms of protocol acquisition, it is difficult to obtain high quality CT in such a PET setting, for two major reasons. One is free breathing, and some centres propose a breath hold during CT acquisition, in order to minimise movement artefacts on pulmonary CT. This solution does not solve the issue of poor imaging in pulmonary bases due to alveolar condensation related to the duration of the resting position. Second is the position of the patient's arms which are placed either up or down, thus potentially causing artefacts in the area (lungs or cervical area) where they are superimposed.
Concepts of radioprotection
Concepts of radioprotection are essential for all workers in controlled radioactive zones, especially in the “hot” laboratory, the injection room and in close contact with injected irradiating patients.
Publications have shown that the measurements provide a total effective dose of about 14 µSv per day (i.e. about 5.5 µSv per examination under normal examination conditions)[8]. This dose corresponds to the standards of conventional nuclear medicine, i.e. about 12 µSv in a context of intense activity[8]. The International Commission on Radiological Protection (ICRP) recommends that the occupational exposure limit for workers should not exceed an effective dose of 20 mSv per year averaged over 5 years, with no more than 50 mSv in any single year.
Personnel radioprotection issues are satisfactorily resolved by application of clinical protocols in the context of validated procedures designed to ensure low-risk or no-risk activities. A diagnostic quality CT scan with correct contrast agent bolus injection during breath-holding can be performed when the entire examination sequence has been perfectly defined, in compliance with strict procedures and when each member of the team is attentive to his/her role[9].
The practical rule applied in radioprotection is the law of the inverse square of the distance, which means that the irradiation dose is lower the greater the distance from the patient. Recommendations in the literature generally consist of observing a distance of 2 m between the patient and medical personnel at all times, which is not respected when installing the patient in the machine (close contact). The patient must be installed rapidly, without necessarily trying to achieve perfect alignment except when explicitly requested by the physician in charge of the imaging procedure. In this situation, it must be remembered that technicians may receive irradiation doses higher than the acceptable daily limit.
It is also for this reason that PET/CT examination cannot be performed in very young children, as it is impossible to remain at their side throughout the examination (which takes about 20–40 min depending on the child's age). Parents can sometimes provide a limited contribution[10].
When optimal radioprotection conditions are observed, the combination of CT scan with intravenous and oral iodinated contrast agent constitutes a valuable aid to interpretation of PET/CT based on a single examination. This contributes to the patient's comfort, as the patient only has to undergo one examination and it also optimises use of the PET/CT room by combining two investigations.
Problems related to iodinated contrast agent injection
As seen above, iodinated contrast agent injection raises problems of radioprotection. The iodinated contrast agent injection IV line is extended to allow remote injection. The PET/CT room has to be equipped with a remote-controlled power injector. This injection is performed separately from the FDG injection.
A history of allergy, or even simple intolerance to the iodinated contrast agent may contraindicate the PET/CT examination, as vomiting can contaminate the machine, causing cleaning difficulties related to radioprotection and delaying subsequent examinations, as it takes at least one half-life of the product (about 2 h) for half of the radioactivity to decay. However, vomitus is not highly radioactive and therefore only slightly contaminant compared to urine, which it is particularly irradiating.
The patient's urine after FDG injection contains a very high radioactive concentration and every precaution must be taken to ensure that no trace of urine contaminates the patient or the machine. Once again, strict procedures have been defined and must be observed in order to prevent situations associated with a risk of urinary contamination. Plastic protection shields may be used to protect the gantry.
It is therefore difficult to examine incontinent patients by PET/CT imaging, even with the use of appropriate protective pads, which also become heavily contaminated, raising radioactive waste disposal problems. If the pads are left in place during the examination, they can induce artefactual uptake that could interfere with interpretation of adjacent organs. Bladder catheterisation is sometimes proposed in cases of incontinence.
If the patient is not well after iodinated contrast agent injection, and as it is recommended to avoid contact with the patient, it makes it difficult to administer antihistamines or corticosteroids.
In an emergency situation, however, the personnel can obviously take care of the patient, but the potentially irradiated personnel may not be able or willing to receive any supplementary doses and may need to avoid any potentially irradiating activity until their exposure level has returned to acceptable limits.
The limits fixed are still quite high, and they are never reached; but sometime in the future, ICRP may lower the maximal admissible dose in accordance with the ALARA principle and PET/CT settings will need to be prepared for such an eventuality.
Exposure data are managed by the medical physics departments and the radiocompetent physicist, who analyse the doses potentially received and evaluate exposure to the body and hands in the different posts. In France, issues regarding dosimetry and monitoring of workers in controlled areas are conducted under the supervision of the institution's occupational health physician according to regulatory measurements and authorisations given by national government-related entities (IRSN)[11].
The indications for contrast-enhanced CT scans must therefore be clearly defined by taking into account the benefit/risk factors. In practice, the nuclear medicine physician takes the decision to perform this examination in compliance with radioprotection guidelines.
Interpretation of PET/CT image fusion
Non-isotropic attenuation requires matching attenuation coefficients of the various organs studied in order to obtain appropriate imaging. An attenuation coefficient map is provided by a non-enhanced thoraco-abdomino-pelvic CT without breath-holding. This CT scan is used to correct PET scintigraphic images in order to improve the quality of the raw images[12].
Tumour FDG uptake is correlated with cell proliferation. The standard uptake value (SUV), a semi-quantitative measure of uptake, can be determined at any point of the PET image according to the following formula: SUV = FDG concentration (kBq/ml)/injected activity (MBq)/patient's weight (kg). This parameter may have a possible role in helping to discriminate a malignant lesion (high SUV) versus a benign inflammatory uptake (low SUV). However, this index is only semi-quantitative, as it depends on numerous parameters related mainly to patient status, product pharmacokinetics and machine settings.
In spite of SUV value modifications, significant artefacts have not been induced by oral or IV contrast media administration[13–15]. This is an important result as it accommodates the principle of fusion images with contrast-enhanced PET/CT, associated with high quality diagnostic CT.
In this context, interpretation of contrast enhanced PET/CT fusion images requires a multidisciplinary approach, as each discipline contributes its specific skills to ensure optimal image analysis. The ideal situation would be a simultaneous collaborative reading by two imaging specialists (radiology/nuclear medicine), but this can be difficult to organise in terms of the availability and workload in each discipline. Multidisciplinary staff meetings may be organised to discuss and decide on imaging interpretations. This also impacts health economics as more time is necessary to allocate for image interpretation involving different specialties.
Physiological uptake (brain, myocardium, liver and spleen, gastrointestinal and urinary tracts) are always present with various intensities, and variants (head and neck, pulmonary hili, mediastinum, muscles, brown fat, peri-articular regions, testes, endometrium, nipples, vessels, thyroid, thymus) are sometimes visible (Fig. 1)[16,17].
Figure 1.
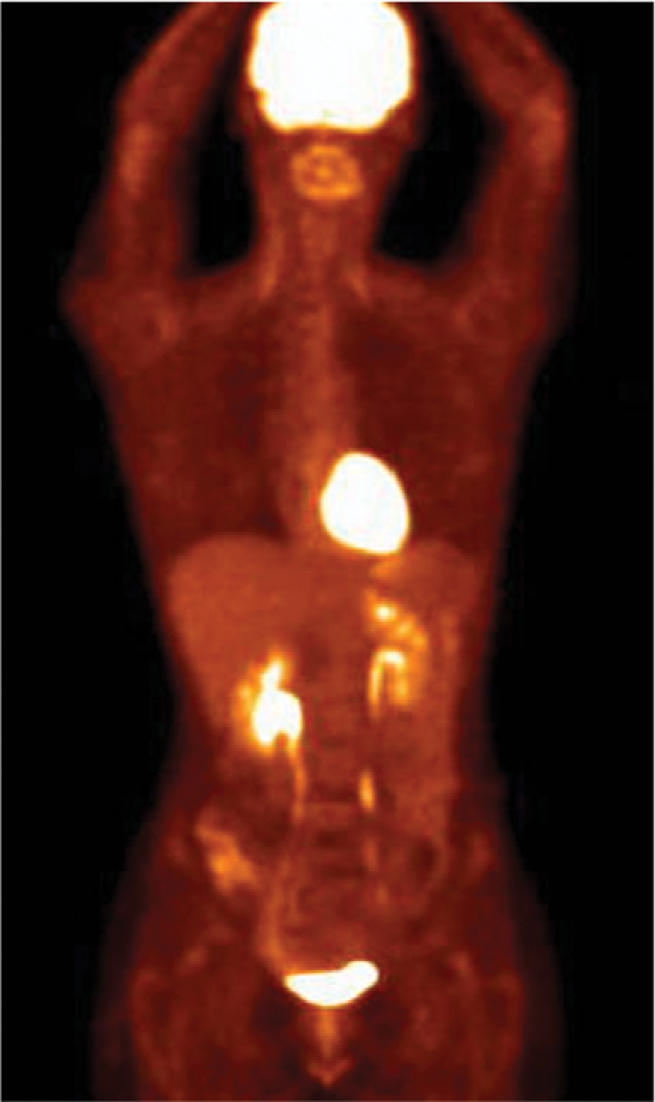
Physiological uptake (brain, myocardium, liver and spleen, gastrointestinal and urinary tracts).
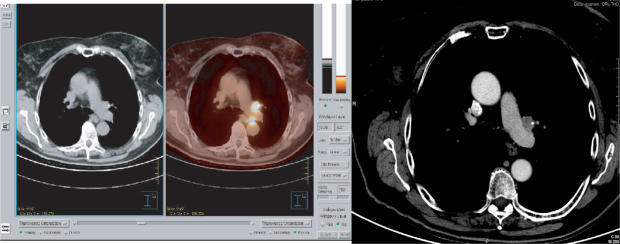
True artefacts (post-treatment effects (chemotherapy, post-radiation, post-operative), injection or biopsy sites, scars, fibrosis, catheters, metal implants, respiratory movements) often generate false-positive images that may raise difficulties in image interpretation[18–20]. Non-enhanced CT reduces errors of interpretation by showing correct anatomical localisation or exogenous devices not seen on PET (Fig. 2)[21,22].
Figure 2.
Artefactual uptake of an oesophageal tube in a patient with head and neck cancer. PET image shows a foci of FDG uptake which may be misinterpreted. CT visualises the tube.
Difficulties related to artefacts can be partly resolved by simultaneous analysis of non-enhanced CT, fusion images and triangulation, but continue to raise considerable problems of interpretation. Radiologists should be familiar with these artefacts to avoid considering artefactual uptake as pathological. This is where a high quality contrast-enhanced CT differentiating vascularised structures and adenopathies may be performed to improve the diagnostic value of PET/CT (Fig. 3).
Figure 3.
Non-enhanced CT versus enhanced CT: visualisation of FDG-avid necrotic nodes in lymphoma. These nodes are better delineated with contrast-enhanced CT.
In addition to artefacts, some discordance may be found between PET and CT related to the size of lesions and to the lack of specificity of both techniques. Lesions smaller than 5–7 mm may be missed by PET, especially if they are located in a high uptake background. Similarly, some confusion may arise when inflammatory and/or benign lesions present with strong FDG uptake. Even with adequate CT reading, it may appear difficult or impossible to determine whether the area of uptake should be considered potentially malignant or not.
Conclusion
PET/CT image fusion in nuclear medicine tends to adjust the concept of PET/low quality CT to that of PET/high quality CT when necessary, requiring the skills of a radiologist specialised in cancer imaging for diagnostic CT interpretation. If performed in routine settings, the resulting PET/contrast-enhanced CT imaging requires optimal interpretation by both disciplines involved in order to render all the diagnostic information contained in this new powerful imaging modality in oncology.
References
- 1.Czernin J, Allen-Auerbach M, Schelbert HR. Improvements in cancer staging with PET/CT: literature-based evidence as of September 2006. J Nucl Med. 2007;48:S78–88. [PubMed] [Google Scholar]
- 2.Francis IR, Brown RKJ, Avram AM. The clinical role of CT/PET in oncology: an update. Cancer Imaging. 2005;5:S68–75. doi: 10.1102/1470-7330.2005.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bybel B, Brunken RC, Shah S, Wu G, Turbiner E, Neumann DR. PET and PET/CT imaging: what clinicians need to know. Cleve Clin J Med. 2006;73:1075–87. doi: 10.3949/ccjm.73.12.1075. [DOI] [PubMed] [Google Scholar]
- 4.Hicks RJ, Ware RE, Lau EWF. PET/CT: will it change the way that we use CT in cancer imaging? Cancer Imaging. 2006;6:S69–79. doi: 10.1102/1470-7330.2006.9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman RE, Delbeke D, Guiberteau MJ, et al. Concurrent PET/CT with an integrated imaging system: intersociety dialogue from the joint working group of the American College of Radiology, the Society of Nuclear Medicine, and the Society of Computed Body Tomography and Magnetic Resonance. J Nucl Med. 2005;46:1225–1229. [PubMed] [Google Scholar]
- 6.Schöder H, Yeung HWD, Larson S. CT in PET/CT: essential features of interpretation. J Nucl Med. 2005;46:1249–51. [PubMed] [Google Scholar]
- 7.Kuehl H, Veit P, Rosenbaum SJ, Bockisch A, Antoch G. Can PET/CT replace separate diagnostic CT for cancer imaging? Optimizing CT protocols for imaging cancers of the chest and abdomen. J Nucl Med. 2007;48:S45–57. [PubMed] [Google Scholar]
- 8.Benatar NA, Cronin BF, O’Doherty MJ. Radiation dose rates from patients undergoing PET: implications for technologists and waiting areas. Eur J Nucl Med. 2000;27:583–9. doi: 10.1007/s002590050546. [DOI] [PubMed] [Google Scholar]
- 9.Beyer T, Antoch G, Bockisch A, Stattaus J. Optimized Intravenous contrast administration for diagnostic whole-body F18-FDG PET/CT. J Nucl Med. 2005;46:429–35. [PubMed] [Google Scholar]
- 10.Wegner EA, Barrington SF, Kingston JE, Robinson RO, Ferner RE, Taj M. The impact of PET scanning on management of paediatric patients. Eur J Nucl Med Mol Imaging. 2005;32:23–30. doi: 10.1007/s00259-004-1645-3. [DOI] [PubMed] [Google Scholar]
- 11.INRS. Institut national de recherché et de sécurité pour la prevention des accidents du travail et des maladies professionnelles. Médecine nucléaire Fiche ED 4239. 2006 http://www.inrs.fr.
- 12.Moretti JL, Weinmann P, Tamgac F, Rigo P. Imagerie fonctionnelle par positons en oncology nucléaire. Les valeurs standardisées de fixation (SUV) France: Springer-Verlag; 2004. ] pp. p. 19–21. [Google Scholar]
- 13.Yau YY, Chan WS, Tam YM, Vernon P, Wong S. Application of intravenous contrast in PET/CT: does it really introduce significant attenuation correction error? J Nucl Med. 2005;46:283–91. [PubMed] [Google Scholar]
- 14.Berthelsen AK, Holm S, Loft A, Klausen TL, Andersen F. PET/CT with intravenous contrast can be used for PET attenuation correction in cancer patients. Eur J Nucl Med Mol Imaging. 2005;32:1167–75. doi: 10.1007/s00259-005-1784-1. [DOI] [PubMed] [Google Scholar]
- 15.Mawlawi O, Erasmus JJ, Munden RF, Pan T, Knight AE. Quantifying the effect of IV contrast media on integrated PET/CT: clinical evaluation. AJR. 2006;186:308–19. doi: 10.2214/AJR.04.1740. [DOI] [PubMed] [Google Scholar]
- 16.Gorospe L, Raman S, Echeveste J, Avril N, Herrerao Y, Hernandez S. Whole-body PET/CT: spectrum of physiological variants, artefacts and interpretative pitfalls in cancer patients. Nucl Med Commun. 2005;26:671–87. doi: 10.1097/01.mnm.0000171779.65284.eb. [DOI] [PubMed] [Google Scholar]
- 17.Castellucci L, Nanni C, Farsad M, Alinari L, Zinzani L. Potential pitfalls of F18-FDG in a large series of patients evaluated for malignant lymphoma: prevalence and scan interpretation. Nucl Med Commun. 2005;26:689–94. doi: 10.1097/01.mnm.0000171781.11027.bb. [DOI] [PubMed] [Google Scholar]
- 18.Blake MA, Singh A, Setty BN, Slattery J, Kalra M. Pearls and pitfalls in interpretation of abdominal and pelvic PET-CT. RadioGraphics. 2006;26:1335–53. doi: 10.1148/rg.265055208. [DOI] [PubMed] [Google Scholar]
- 19.Sureshbabu W, Mawlawi O. PET/CT imaging artefacts. J Nucl Med Technol. 2005;33:156–61. [PubMed] [Google Scholar]
- 20.Abouzied MM, Crawford ES, Nabi HA. F18-FDG imaging: pitfalls and artefacts. J Nucl Med Technol. 2005;33:145–55. [PubMed] [Google Scholar]
- 21.Rodriguez-Vigil B, Gomez-Leon N, Pinilla I, Hernandez-Maraver D, Coya J. PET/CT in lymphoma: prospective study of enhanced full dose PET/CT versus unenhanced low-dose PET/CT. J Nucl Med. 2006;47:1643–8. [PubMed] [Google Scholar]
- 22.Antoch G, Freudenberg LS, Beyer T, et al. To enhance or not to enhance? F18-FDG and CT contrast agents in dual-modality F18-FDG PET/CT. J Nucl Med. 2004;45:S56–65. [PubMed] [Google Scholar]