Abstract
Magnetic resonance (MR) imaging is increasingly being used in patients with gynaecological disorders due to its high contrast resolution compared to computed tomography (CT) and ultrasound. In women presenting with an adnexal mass, ultrasound remains the primary imaging modality in the detection and characterisation of such lesions. However, in recent years overwhelming evidence has accumulated for the use of MR imaging in patients with indeterminate adnexal masses particularly in younger women and where disease markers are unhelpful. In staging ovarian cancer and for evaluating therapeutic response MR imaging is as accurate as CT but CT remains the imaging modality of choice because it is more widely available and quicker. This article reviews that evidence and outlines a place for the use of MR imaging in ovarian cancer.
Keywords: MR imaging, ovarian cancer, adnexal masses
Introduction
Magnetic resonance (MR) imaging is increasingly being used in patients with gynaecological disorders due to its high contrast resolution compared to computed tomography (CT) and ultrasound (US). In recent years overwhelming evidence has accumulated for the use of MR imaging in patients with indeterminate adnexal masses particularly in younger women and where disease markers are unhelpful. This article reviews that evidence and outlines a place for the use of MR imaging in ovarian cancer.
Technique
A high field MR system with good gradients is required for optimal imaging in patients with suspected ovarian cancer in order to obtain high resolution images rapidly. Image acquisition is enhanced by the use of phased array coils that are compatible with parallel imaging techniques. These techniques use spatial information from the individual elements of a radiofrequency (RF) receiver coil array to increase imaging speed[1].
For characterisation of adnexal masses, images should be obtained in at least two planes to help determine the organ of origin of the mass. Both T1- and T2-weighted images are important for pelvic anatomy and in tissue characterisation. Small field of view high resolution images may be used to improve the delineation of small structures such as papillary projections. Fat suppressed sequences help distinguish fatty (Fig. 1) from haemorrhagic masses. Fat-suppressed chemical shift techniques are preferable to short tau inversion recovery (STIR) sequences. This is to avoid confusion between fat and haemorrhagic lesions, as haemorrhagic lesions may have the same T1 relaxation time as fat on the STIR images. Contrast medium enhanced fat suppressed T1-weighted images improve lesion characterisation by increasing the conspicuousness of nodules (Figs. 2 and 3) and septa in complex masses[2–5]. Contrast medium enhanced scans also increase detection of peritoneal and omental implants. Limited experience in small series with diffusion weighted imaging have shown that malignant and benign ovarian cystic fluid cannot be differentiated based on findings on echo planar diffusion weighted imaging (EPDWI) or apparent diffusion coefficient (ADC) value[6]. Therefore these sequences are not used routinely but only in a research setting.
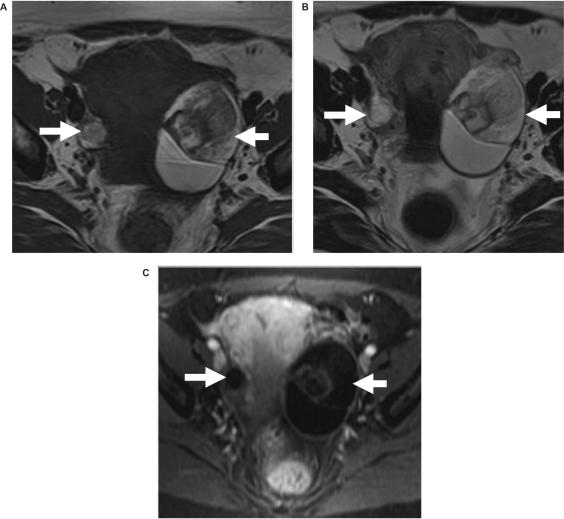
Figure 1.
Bilateral dermoids. Axial (a) T1-weighted, (b) T2-weighted, and (c) post-intravenous gadolinium enhanced fat suppressed T1-weighted images show bilateral pelvic mass (arrows) containing fat in keeping with dermoids. The fat can be seen as high signal intensity on both the T1- and T2-weighted scans and shows loss of signal on the fat suppressed image, i.e. similar signal intensity to intra-pelvic and subcutaneous fat.
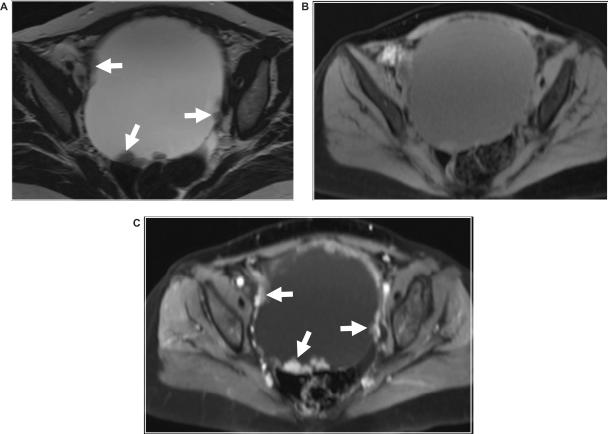
Figure 2.
Ovarian adenocarcinoma. Axial (a) T2-weighted and fat suppressed T1-weighted images (b) before and (c) after intravenous gadolinium enhancement shows a large predominantly cystic ovarian mass with nodular vegetation seen on its internal surface (arrows).
Figure 3.
Ovarian cystadenoma. (a) Sagittal T2-weighted images shows complex ovarian tumour with a low signal intensity nodule (arrows). Fat suppressed T1-weighted images (b) before and (c) after intravenous gadolinium enhancement shows that this nodule does not show any enhancement and therefore does not contain any vegetation. This case illustrates the importance of contrast medium enhancement in the evaluation of adnexal masses.
When MR imaging is used for staging ovarian cancer, the whole abdomen and pelvis should be imaged. Images are usually acquired in the axial plane. Coronal images are helpful to examine spread to the liver surface, diaphragm and pelvic sidewall. Sagittal sequences in the pelvis outline the relationship of the ovarian neoplasm to the uterus, bladder and rectum. Contrast enhanced fat suppressed T1-weighted images are essential in staging ovarian cancer as they improve tumour delineation, tumour characterisation, increase the conspicuousness of peritoneal deposits and facilitate detection of serosal infiltration. Anti-peristaltic agents should be used when imaging the abdomen and pelvis to reduce bowel motility artefacts.
Diagnosis/characterisation of pelvic masses
Role of MR imaging in the characterisation of adnexal lesions
Adnexal masses are a common clinical problem and imaging is often required for patients presenting with symptoms relating to the presence of an adnexal mass. However, of those undergoing surgery for a suspected mass less than 25% of the masses prove to be malignant[7]. For this reason, imaging is now often directed towards accurate characterisation of a mass. Establishing that it is benign may negate the need for surgery or alter the surgical approach. Ultrasound (abdominal, trans-vaginal, and Doppler), CT, MRI and radionuclide imaging have all been used in this way.
Studies comparing US and MRI have shown that contrast enhanced MRI is superior to US in characterising adnexal mass lesions[8–11]. Both techniques are highly sensitive, but MR imaging is more specific than ultrasound at identifying malignant masses. The greater specificity of MRI is due to its ability to identify correctly dermoid, endometriotic cysts, and fibroids which may appear malignant on US[8]. A recent meta-analysis evaluated the performance of combined gray-scale and Doppler US, CT, and non-enhanced or contrast material enhanced MR imaging after initial gray-scale US with indeterminate results. This showed that in women with an indeterminate ovarian mass at gray-scale US, MR imaging findings contributed to a change in probability of ovarian cancer in both pre- and post-menopausal women more than did CT or combined gray-scale and Doppler US results[12]. Furthermore, in a prospective multi-centre study of 143 patients with a pelvic mass, where diagnostic uncertainty existed, MRI improved diagnostic accuracy, when judged by an expert radiologist[13].
In practice, ultrasound and Doppler techniques are performed as the initial investigation for a clinically suspected adnexal mass. No other imaging is required if the mass has features clearly indicating it is benign or if the ultrasound findings, together with tumour marker and clinical findings strongly indicate malignancy. However, if the ultrasound features are equivocal or suspicious for malignancy but the patient is young or the CA125 is normal or minimally elevated, then the patient may need further evaluation in order to decide subspecialty referral. In such cases a risk of malignancy index (RMI) scoring can be used to predict the likelihood of a mass being malignant. The RMI score is based on menopausal status, ultrasound (US) findings, and serum CA125 and has previously been described and validated in the primary evaluation of women with adnexal masses and is widely used in selective referral of women from local cancer units to specialized cancer centres[14]. An RMI score of less than 25 has a 3% chance of malignancy and a RMI greater than 250 has a 75% chance of malignancy[14]. In a prospective study we investigated the value of further specialist imaging in 196 women referred to a teaching hospital with an adnexal mass with an RMI values of 25–1000[15]. Sensitivity and specificity for specialist US were 100% and 57%, for MRI 92% and 86%, respectively. Analysis of 123 patients with RMI of between 25 and 1000 managed sequentially with US and MRI provided a sensitivity of 94% and a specificity of 90%. We concluded that rather than using the traditional RMI threshold value of 250 to refer to a cancer centre, for patients with an RMI of between 25 and 1000, imaging by specialist US and MR imaging can increase the proportion of patients with cancer appropriately referred to a cancer centre, with no change in the proportion of patients with benign disease being managed in a local unit[15].
MRI features of malignant ovarian tumours
Findings suggestive of malignancy include the demonstration of solid masses, solid/cystic masses and the presence of papillary projections (vegetations) and thick septa in a cystic lesion (Figs. 2, 4 and 5). Secondary features of malignancy are: peritoneal, mesenteric or omental involvement, pelvic side wall invasion and lymphadenopathy. Analysis of the MR imaging features has shown that the characteristics most predictive of malignancy are vegetations in a cystic lesion (Fig. 2), presence of ascites, a maximal diameter greater than 6 cm, and necrosis in a solid lesion[16,17]. The finding of pelvic ascites, although suggestive of malignancy, can also be seen in ovarian torsion, pelvic inflammatory disease and benign ovarian fibroma and is therefore not specific[18]. Abdominal ascites alone or the finding of ascites anterior to the uterus is highly suggestive for malignant ascites[18,19].
Figure 4.
Bilateral borderline ovarian tumours. (a) Axial and (b) coronal T2-weighted images show bilateral complex ovarian tumour with a low signal intensity nodule (arrows). This shows enhancement on the (c) axial fat suppressed T1-weighted images after intravenous gadolinium.
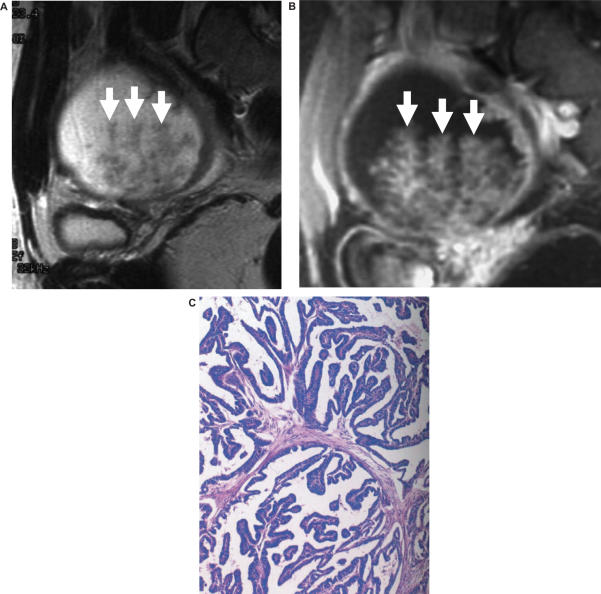
Figure 5.
Serous borderline ovarian tumours with papillary architecture. Sagittal (a) T2-weighted and (b) fat suppressed T1-weighted images after intravenous gadolinium shows a cystic mass with fronds of tissues. (c) Histopathology of the specimen showed that that this was a serous borderline ovarian tumour with papillary architecture.
MRI characteristics of ovarian tumours
MR imaging can distinguish several types of tissue and fluid from their signal intensity patterns. The signal intensities of a tumour depend upon the presence, type and extent of solid tumour and cystic components of the mass. Tumours with predominantly smooth muscle or fibrotic component, such as fibroma, fibrothecoma, cystadenofibroma, Brenner tumour and leiomyoma, have low to intermediate signal on T2-weighted images[20,21]. Predominantly or uniformly low signal intensity within a lesion is therefore a feature of benign tumours[16].
The signal intensity of the cystic component of an ovarian neoplasm may vary depending upon protein content of the fluid. Mucinous tumours and cystic lesions with haemorrhage and cellular debris often have high protein content. Consequently fat, haemorrhage and some mucin-containing lesions have high signal on T1-weighted images.
An advantage of MRI is its high sensitivity for detecting blood-related products. Characteristically, endometriomas show very high signal intensity on T1- and low signal on T2-weighted images. In a series of 86 endometrial cysts, 55 (64%) were reported to have low signal intensity on T2-weighted images[22]. This signal intensity pattern is believed to be caused by a magnetic susceptibility effect from the haemosiderin in old haemorrhage, densely concentrated fluid, or fibrosis. Other features of endometrioma include a thickened low signal intensity wall, and adhesion to adjacent structures. Consequently an endometrioma can be diagnosed with a high degree of specificity on MRI[22]. Other haemorrhagic cystic lesions such as simple cyst, corpus luteal cysts, benign and malignant ovarian neoplasm can have similar signal intensity characteristics. Under these circumstances the same morphological criteria are used to distinguish benign from malignant tumours as are applied for non-haemorrhagic cystic lesions.
Rarely endometriosis is associated with malignant ovarian tumours, particularly endometrioid and clear cell adenocarcinomas[23–25]; malignancy occurs in ovarian endometriosis in less than 1% of cases[23]. Conversely, the incidence of endometriosis in patients with ovarian cancer varies from 4% to 15%[23]. Endometriotic cysts with malignant transformation rarely show low signal intensity on T2-weighted images and usually have enhancing mural nodules (Fig. 6). Because the enhancement of mural nodules is often difficult to evaluate on conventional T1-weighted images, subtraction of the pre- and post-contrast enhanced imaging may be helpful.
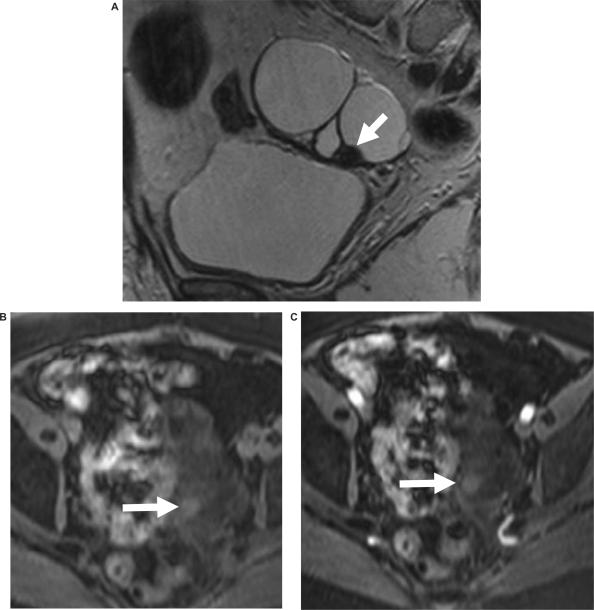
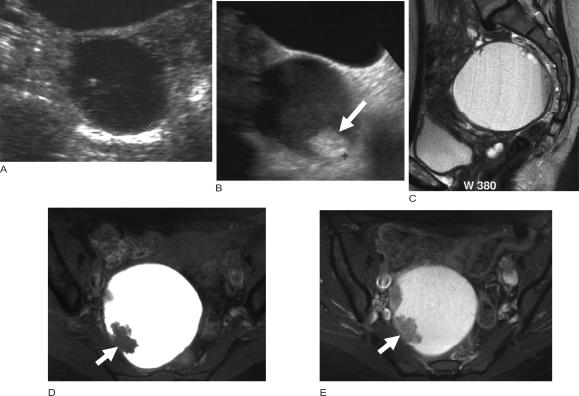
Figure 6.
Malignant change in an endometriotic cyst. (a) Ultrasound images from 8 years ago showed an endometriotic cyst which on serial follow up showed no change until the most recent ultrasound (b) which showed the development of the nodule in the wall of the cyst. MR imaging was performed; (c) sagittal T2-weighted image shows a cystic mass and axial fat suppressed T1-weighted images (d) before and (e) after intravenous gadolinium showing enhancing nodules from the malignant change within the endometriotic cyst.
The wide variety of histological types of ovarian cancer combined with the fact that many tumours show mixed cell types results in a wide range of appearances. Although different ovarian histological types often cannot be made on imaging, certain features may suggest a particular pathology.
Epithelial tumours
Benign epithelial tumours whether mucinous or serous cystadenomas appear on MRI as thin walled cystic lesions without any evidence of soft tissue components, irregular walls or papillary projections (Fig. 2)[19,26].
Malignant epithelial tumours have a more complex appearance with multiple features suggestive of the malignancy. Cyst-adenocarcinomas are usually larger, more complex multi-locular masses which contain soft tissue projections extending into the cystic spaces. Endometrioid tumours and clear cell carcinomas have a variable appearance on imaging ranging from entirely cystic masses to complex masses with solid and cystic components. Brenner tumours may appear as solid masses with or without calcification and their signal intensity pattern is similar to fibroma, thecoma or leiomyomas.
Borderline or tumours of low malignant potential are histologically characterised by epithelial anaplasia without stromal invasion. Most tumours are either serous or mucinous but other histological types are occasionally seen. Morphologically the features of these tumours are between those of the benign and their malignant counterpart (Figs. 4 and 5). In a comparison of borderline ovarian tumours with stage I cancers, the thickness of septations and size of solid components were larger in stage I cancers, However, neither feature allowed confident differentiation between borderline tumours and stage I cancers[27].
Germ cell tumours
Germ cell tumours include teratoma, dysgerminoma, endodermal sinus (yolk sac) tumours embryonal and choriocarcinomas. Ovarian cystic teratomas may contain sebum, hair, epithelium, calcium and other elements which give rise to their complex appearance. These characteristic features often permit a definitive diagnosis on MR imaging. Fat is of high signal on T1-weighted MR images and is most effectively distinguished from high signal intensity of blood-related products or proteinous material by frequency selective fat saturation with loss of signal similar to abdominal fat[28]. A fat–fluid interface is highly characteristic of a cystic teratoma.
Mixed germ cell tumours contain more than one germ cell component. The imaging features of mixed germ cell tumours are variable due to the diversity of their components. When a predominantly solid and heterogeneous ovarian tumour contains fatty areas or calcifications suggestive of a mature cystic teratoma or when a mature cystic teratoma contains an enhancing solid portion, a diagnosis of a mixed germ cell tumour is possible.
Malignancy associated with mature cystic teratoma is rare and occurs in 1–2% of cases[29,30]. Ovarian teratoma with malignant transformation appears as a fat-containing tumour with an enhancing, irregularly marginated solid component. The solid component tends to be relatively large and to show extensive transmural extension and direct invasion of neighbouring structures. The contrast enhancement of the Rokitansky protuberance should raise the possibility of malignant transformation[29,31]. The imaging findings of malignant transformation may be similar to those seen in mixed germ cell tumours. Elevated serum α-fetoprotein and human chorionic gonadotropin levels and younger age can help in the diagnosis of mixed germ cell tumours.
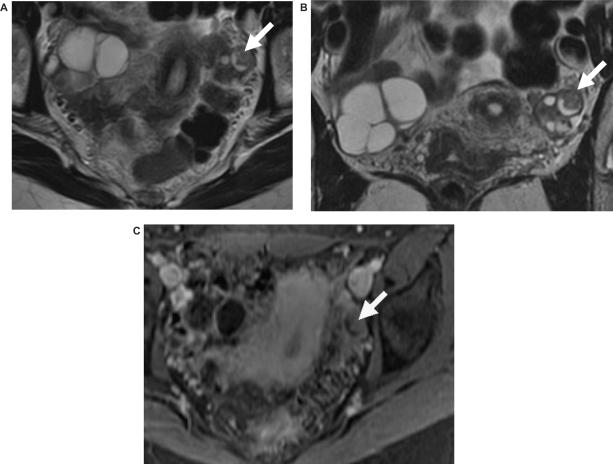
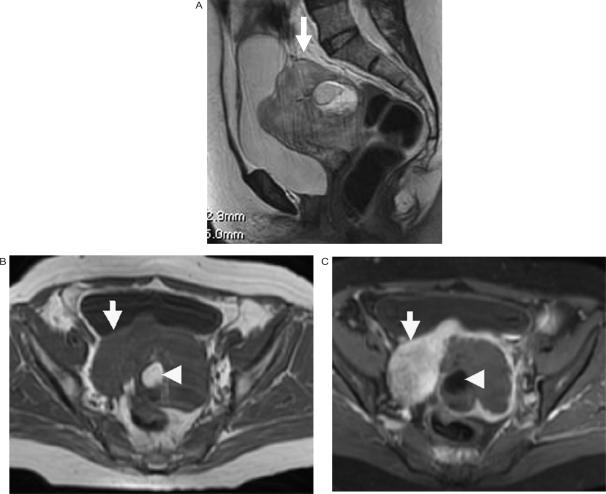
Figure 7.
Epithelial ovarian cancer with a co-existing dermoid. (a) Sagittal T2-weighted, (b) axial T1-weighted and (c) axial fat suppressed T1-weighted images after intravenous gadolinium enhancement showing the enhancing soft tissue component (arrow) of the ovarian cancer and with fat (arrowhead) from the dermoid seen centrally.
Dysgerminomas are malignant and appear as solid masses, which may contain cystic areas with haemorrhage or necrosis[32]. These tumours appear as soft tissue masses, occasionally showing areas of high or low signal intensity due to fresh blood, the breakdown products of haemoglobin or fluid. A characteristic finding in a dysgerminoma is fibrovascular septa within a solid mass best seen on contrast enhanced MR imaging[33]. The imaging appearances of endodermal sinus tumours are similar to those of dysgerminomas.
Sex cord–stromal cell tumours
Sex cord–stromal tumours of the ovary are rare ovarian neoplasms that arise from stromal cells and primitive sex cords in the ovary. They affect all age groups and account for most of the hormonally active ovarian tumours. Granulosa cell tumours are usually large multi-loculated cystic masses with variable solid portions and are associated with endometrial abnormalities[34,35]. Ovarian fibromas are well circumscribed solid tumours which demonstrate low signal intensity on both T1- and T2-weighted images, not unlike leiomyomas. Fibromas show minimal enhancement[36,37]. The multi-planar imaging allows differentiation of an ovarian fibroma from an exophytic leiomyoma or other tumours[20,21]. Sclerosing stromal tumours show typical early peripheral enhancement with centripetal progression[34]. Sertoli–Leydig cell tumours appear as well-defined, enhancing solid masses with variable-sized intra-tumoural cysts[34]. Steroid cell tumours show a heterogeneous solid mass with internal areas of intracellular lipid[34].
Ovarian metastases
Metastases to the ovary are usually bilateral and may be solid, partially solid and cystic, or occasionally as a multi-loculated cystic lesion. They are indistinguishable from primary ovarian tumours on imaging[38]. Krukenberg tumours are ovarian metastases with mucin filled signet ring cells. They are usually bilateral and solid and may have areas of haemorrhage and necrosis[37].
Defining extent of disease/staging
The need for imaging prior to a staging laparotomy depends on individual surgical teams. Imaging cannot replace surgical staging which remains the gold standard for staging ovarian cancer and is far superior to any imaging technique especially for the detection of peritoneal deposits. However, more surgical teams are using cross sectional imaging to plan surgery and to decide if optimal de-bulking is feasible or whether the patient may benefit from initial chemotherapy. In general CT is preferred over MR imaging for staging as it is more readily available and quicker.
Imaging features of spread are similar on MR and CT. As with CT, MRI can detect tumour involvement of many intra-abdominal and pelvic structures. These include: small and large bowel, urinary tract, peritoneum and mesentery, liver, lymph nodes, ascites.
The accuracy for the detection of peritoneal tumour deposits is dependent on their location, size and presence of ascites, which increases their conspicuousness. On MRI peritoneal metastases may appear as rounded, ‘cake-like’, stellate or ill-defined masses. These tumour deposits enhance following contrast medium injection and appear as soft tissue nodules along the peritoneum, or the whole of the peritoneal surface may be thickened. Peritoneal infiltration is best seen in the right subphrenic space, the greater omentum and pouch of Douglas.
MRI and CT have similar sensitivity for the detection of peritoneal deposits greater than 1 cm in diameter[26,39]. However, disease within the mesentery or implants on the wall of bowel loops and calcified deposits are better detected by CT[40]. An ‘omental cake’ can be identified as an infiltrating mass lying beneath the anterior abdominal wall and finger-like projections of tumour may be seen extending into the surrounding fat. Fat suppression sequences increase the conspicuousness of enhancing peritoneal tumour deposits and omental disease by suppressing the signal from hyperintense fat in the abdomen and pelvis[3]. The use of oral contrast medium such as supraparamagnetic iron oxide particles may be helpful in the detection of peritoneal metastases[41,42], as it lowers the signal intensity of the bowel contents on T1-weighting, whereas enhancing tumour deposits have a high signal intensity[41,42]. Nevertheless, contrast enhanced MRI has a high false positive rate in the detection of peritoneal tumour deposits due to enhancement of benign tissue such as granulation tissue and post-operative adhesions and also has a high false negative rate due to the difficulty in identifying small volume disease.
The spread of ovarian cancer into adjacent organs such as the uterus, sigmoid colon, bladder and rectum may be better appreciated on MRI than on CT using a combination of T1- and T2-weighted sequences[43]. Pelvic organ invasion by ovarian cancer may be difficult to diagnose accurately when the mass abuts adjacent structures. For example uterine invasion is particularly difficult to diagnose because the parametrial fat plane may be lost without necessarily invading the uterine serosa[44]. Indeed, in patients with large ovarian masses it may be difficult to ever identify the uterus, which may be partially or completely surrounded by tumour. Pelvic sidewall invasion should be suspected when the primary tumour lies within 3 mm of the pelvic sidewall or when the iliac vessels are surrounded or distorted by tumour[2]. Focal obliteration of the fat plane or tumour encasement of the bladder or recto-sigmoid is highly suspicious of involvement of these structures[45].
Accuracy of staging
Surgical staging is the gold standard for ovarian cancer but even so under-staging due to inadequate exploration at surgery is common, occurring in 30–40% of cases. The main reason for under-staging is that the pre-operative diagnosis is that of a benign tumour resulting in an inappropriate abdominal incision which precludes the detection of upper abdominal disease. The staging accuracy of CT/MRI ranges from 70 to 90%[49,50].
MR imaging and CT appear to be equivalent for staging abdomino-pelvic disease but the high cost and relatively long examination times of MRI precludes its use in most patients. Evaluation of disease within the pelvis is better demonstrated with MRI but within the abdomen both techniques under-stage small tumour implants[2]. The detection of enlarged retroperitoneal lymph nodes is comparable between CT and MRI. Thus at present CT is the recommended imaging modality of choice for staging ovarian cancer.
Tumour resectability
Aside from staging ovarian cancer, pre-treatment evaluation with CT or MR imaging may be helpful in identifying patients who are unlikely to undergo optimal cytoreduction or patients with irresectable disease. Criteria for non-resectability vary markedly form institution to institution and will depend on the aggressiveness of individual surgeons[51]. In general, however, optimal cytoredcution is not possible when the images show disease with tumour deposits larger than 2 cm in the porta hepatitis, disease in the inter segmental fissure of the liver, on the diaphragm, in the lesser sac, gastrosplenic ligament; presacral extra-peritoneal disease and lymph node enlargement at the level of the coeliac axis or above[2,52,53]. The accuracy of CT and MRI in predicting irresectability can be as high as 93–96% as both CT and MRI identify disease involvement at these sites extremely well[54].
Assessment of tumour response, residual disease, and detection of recurrent disease
Cross sectional imaging is used to detect persistent or recurrent ovarian cancer and to document tumour response to chemotherapy. In most hospitals this is routinely assessed with CT. The MR imaging findings in recurrent ovarian cancer are similar to those of CT, hence similar difficulties are encountered. The presence of ascites improves the detection of peritoneal disease and in the assessment of pre-treated disease. Comparisons of CT and MRI for restaging ovarian cancer are similar with sensitivity, specificity and accuracy for CT being 40–67%, 93–100% and 66–85%; the corresponding figures for MR imaging are 62–91%, 40–93% and 59–90%[41,47,55–60]. Certain areas are difficult to assess on CT, such as disease in the region of the vaginal vault, in the cul de sac and at the bladder base. These areas are better assessed with MR imaging. A retrospective study has shown that gadolinium-enhanced spoiled gradient-echo MR imaging depicts residual tumour in women with treated ovarian cancer, with an accuracy comparable to laparotomy and superior to serum CA125 values alone[61].
Conclusion
The role of MR imaging in the management of ovarian cancer continues to evolve. Ultrasound remains the primary imaging modality in the detection and characterisation of an ovarian mass prior to definitive diagnosis at surgery. MRI has been shown repeatedly to have far greater specificity in the diagnosis of malignancy but similar sensitivity. Therefore, MRI should be used for the characterisation of masses in those patients where the results of ultrasound are equivocal, particularly if the markers are normal, or in younger patients where conservative, rather than radical, surgery is contemplated.
Although, MRI is as accurate as CT in the evaluation of ovarian tumour spread, it is currently seldom used in routine clinical practice. CT remains the major imaging modality for patients with established ovarian cancer, both for staging prior to treatment and for evaluating therapeutic response.
References
- 1.Bammer R, Schoenberg SO. Current concepts and advances in clinical parallel magnetic resonance imaging. Top Magn Reson Imaging. 2004;15:129–58. doi: 10.1097/01.rmr.0000139666.23921.27. [DOI] [PubMed] [Google Scholar]
- 2.Forstner R, Hricak H, Occhipinti KA, Powell CB, Frankel SD, Stern JL. Ovarian cancer: staging with CT and MR imaging. Radiology. 1995;197:619–26. doi: 10.1148/radiology.197.3.7480729. [DOI] [PubMed] [Google Scholar]
- 3.Low RN, Sigeti JS., MR imaging of peritoneal disease. AJR. 1994;163:1131–40. doi: 10.2214/ajr.163.5.7976889. [DOI] [PubMed] [Google Scholar]
- 4.Ghossain MA, Buy JN, Ligneres C, et al. Epithelial tumors of the ovary: comparison of MR and CT findings. Radiology. 1991;181:863–70. doi: 10.1148/radiology.181.3.1947112. [DOI] [PubMed] [Google Scholar]
- 5.Thurnher S, Hodler J, Baer S, Marincek B, von Schulthess GK. Gadolinium-DOTA enhanced MR imaging of adnexal tumors. J Comput Assist Tomogr. 1990;14:939–49. doi: 10.1097/00004728-199011000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Katayama M, Masui T, Kobayashi S, et al. Diffusion-weighted echo planar imaging of ovarian tumors: is it useful to measure apparent diffusion coefficients? J Comput Assist Tomogr. 2002;26:250–6. doi: 10.1097/00004728-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Kinkel K, Hricak H, Lu Y, Tsuda K, Filly RA. US characterization of ovarian masses: a meta-analysis. Radiology. 2000;217:803–11. doi: 10.1148/radiology.217.3.r00dc20803. [DOI] [PubMed] [Google Scholar]
- 8.Sohaib SA, Mills TD, Sahdev A, et al. The role of magnetic resonance imaging and ultrasound in patients with adnexal masses. Clin Radiol. 2005;60:340–8. doi: 10.1016/j.crad.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita Y, Torashima M, Hatanaka Y, et al. Adnexal masses: accuracy of characterization with transvaginal US and precontrast and postcontrast MR imaging. Radiology. 1995;194:557–65. doi: 10.1148/radiology.194.2.7824738. [DOI] [PubMed] [Google Scholar]
- 10.Komatsu T, Konishi I, Mandai M, et al. Adnexal masses: transvaginal US and gadolinium-enhanced MR imaging assessment of intratumoral structure. Radiology. 1996;198:109–15. doi: 10.1148/radiology.198.1.8539360. [DOI] [PubMed] [Google Scholar]
- 11.Rieber A, Nussle K, Stohr I, et al. Preoperative diagnosis of ovarian tumors with MR imaging: comparison with transvaginal sonography, positron emission tomography, and histologic findings. AJR Am J Roentgenol. 2001;177:123–9. doi: 10.2214/ajr.177.1.1770123. [DOI] [PubMed] [Google Scholar]
- 12.Kinkel K, Lu Y, Mehdizade A, Pelte MF, Hricak H. Indeterminate ovarian mass at US: incremental value of second imaging test for characterization—meta-analysis and Bayesian analysis. Radiology. 2005;236:85–94. doi: 10.1148/radiol.2361041618. [DOI] [PubMed] [Google Scholar]
- 13.Engelen MJ, Bongaerts AH, Sluiter WJ, et al. Distinguishing benign and malignant pelvic masses: the value of different diagnostic methods in every day clinical practice. Eur J Obstet Gynecol Reprod Biol. 2006 doi: 10.1016/j.ejogrb.2006.10.004. (E-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 14.Davies AP, Jacobs I, Woolas R, Fish A, Oram D. The adnexal mass: benign or malignant? Evaluation of a risk of malignancy index. Br J Obstet Gynaecol. 1993;100:927–31. doi: 10.1111/j.1471-0528.1993.tb15109.x. [DOI] [PubMed] [Google Scholar]
- 15.Van Trappen PO, Rufford BD, Mills TD, et al. Differential diagnosis of adnexal masses: risk of malignancy index, ultrasonography, magnetic resonance imaging, and radioimmunoscintigraphy. Int J Gynecol Cancer. 2007;17:61–7. doi: 10.1111/j.1525-1438.2006.00753.x. [DOI] [PubMed] [Google Scholar]
- 16.Sohaib SA, Sahdev A, Van Trappen PO, Jacobs IJ, Reznek RH. Characterization of adnexal mass lesions on MR imaging. AJR Am J Roentgenol. 2003;180:1297–304. doi: 10.2214/ajr.180.5.1801297. [DOI] [PubMed] [Google Scholar]
- 17.Hricak H, Chen M, Coakley FV, et al. Complex adnexal masses: detection and characterization with MR imaging–multivariate analysis. Radiology. 2000;214:39–46. doi: 10.1148/radiology.214.1.r00ja3939. [DOI] [PubMed] [Google Scholar]
- 18.Forstner R, Hricak H, White S. CT and MRI of ovarian cancer (Review) Abdom Imaging. 1995;20:2–8. doi: 10.1007/BF00199633. [DOI] [PubMed] [Google Scholar]
- 19.Buy JN, Ghossain MA, Sciot C, et al. Epithelial tumors of the ovary: CT findings and correlation with US. Radiology. 1991;178:811–18. doi: 10.1148/radiology.178.3.1994423. [DOI] [PubMed] [Google Scholar]
- 20.Troiano RN, Lazzarini KM, Scoutt LM, Lange RC, Flynn SD, McCarthy S. Fibroma and fibrothecoma of the ovary: MR imaging findings. Radiology. 1997;204:795–8. doi: 10.1148/radiology.204.3.9280262. [DOI] [PubMed] [Google Scholar]
- 21.Outwater EK, Siegelman ES, Talerman A, Dunton C. Ovarian fibromas and cystadenofibromas: MRI features of the fibrous component. J Magn Reson Imaging. 1997;7:465–71. doi: 10.1002/jmri.1880070303. [DOI] [PubMed] [Google Scholar]
- 22.Togashi K, Nishimura K, Kimura I, et al. Endometrial cysts: diagnosis with MR imaging. Radiology. 1991;180:73–8. doi: 10.1148/radiology.180.1.2052726. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka YO, Yoshizako T, Nishida M, Yamaguchi M, Sugimura K, Itai Y. Ovarian carcinoma in patients with endometriosis: MR imaging findings. AJR Am J Roentgenol. 2000;175:1423–30. doi: 10.2214/ajr.175.5.1751423. [DOI] [PubMed] [Google Scholar]
- 24.Heaps JM, Nieberg RK, Berek JS. Malignant neoplasms arising in endometriosis. Obstet Gynecol. 1990;75:1023–8. [PubMed] [Google Scholar]
- 25.McMeekin DS, Burger RA, Manetta A, DiSaia P, Berman ML. Endometrioid adenocarcinoma of the ovary and its relationship to endometriosis. Gynecol Oncol. 1995;59:81–6. doi: 10.1006/gyno.1995.1271. [DOI] [PubMed] [Google Scholar]
- 26.Stevens SK, Hricak H, Stern JL. Ovarian lesions: detection and characterization with gadolinium- enhanced MR imaging at 1.5 T. Radiology. 1991;181:481–8. doi: 10.1148/radiology.181.2.1924792. [DOI] [PubMed] [Google Scholar]
- 27.deSouza NM, O'Neill R, McIndoe GA, Dina R, Soutter WP. Borderline tumors of the ovary: CT and MRI features and tumor markers in differentiation from stage I disease. AJR Am J Roentgenol. 2005;184:999–1003. doi: 10.2214/ajr.184.3.01840999. [DOI] [PubMed] [Google Scholar]
- 28.Stevens SK, Hricak H, Campos Z. Teratomas versus cystic hemorrhagic adnexal lesions: differentiation with proton-selective fat-saturation MR imaging. Radiology. 1993;186:481–8. doi: 10.1148/radiology.186.2.8421755. [DOI] [PubMed] [Google Scholar]
- 29.Rha SE, Byun JY, Jung SE, et al. Atypical CT and MRI manifestations of mature ovarian cystic teratomas. AJR Am J Roentgenol. 2004;183:743–50. doi: 10.2214/ajr.183.3.1830743. [DOI] [PubMed] [Google Scholar]
- 30.Comerci JT, Jr, Licciardi F, Bergh PA, Gregori C, Breen JL. Mature cystic teratoma: a clinicopathologic evaluation of 517 cases and review of the literature. Obstet Gynecol. 1994;84:22–8. [PubMed] [Google Scholar]
- 31.Kido A, Togashi K, Konishi I, et al. Dermoid cysts of the ovary with malignant transformation: MR appearance. AJR Am J Roentgenol. 1999;172:445–9. doi: 10.2214/ajr.172.2.9930800. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka YO, Kurosaki Y, Nishida M, et al. Ovarian dysgerminoma: MR and CT appearance. J Comput Assist Tomogr. 1994;18:443–8. [PubMed] [Google Scholar]
- 33.Kim SH, Kang SB. Ovarian dysgerminoma: color Doppler ultrasonographic findings and comparison with CT and MR imaging findings. J Ultrasound Med. 1995;14:843–8. doi: 10.7863/jum.1995.14.11.843. [DOI] [PubMed] [Google Scholar]
- 34.Jung SE, Rha SE, Lee JM, et al. CT and MRI findings of sex cord-stromal tumor of the ovary. AJR Am J Roentgenol. 2005;185:207–15. doi: 10.2214/ajr.185.1.01850207. [DOI] [PubMed] [Google Scholar]
- 35.MacSweeney JE, King DM. Computed tomography, diagnosis, staging and follow-up of pure granulosa cell tumour of the ovary. Clin Radiol. 1994;49:241–5. doi: 10.1016/s0009-9260(05)81848-3. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz RK, Levine D, Hatabu H, Edelman RR. Ovarian fibroma: findings by contrast-enhanced MRI. Abdom Imaging. 1997;22:535–7. doi: 10.1007/s002619900257. [DOI] [PubMed] [Google Scholar]
- 37.Stevens SK. The adnexa. In: Higgins CB, Hricak H, Helms CA, editors. Magnetic resonance imaging of the body. 2nd. New York: Raven Press; 1992. pp. p. 865–89. [Google Scholar]
- 38.Brown DL, Zou KH, Tempany CM, et al. Primary versus secondary ovarian malignancy: imaging findings of adnexal masses in the Radiology Diagnostic Oncology Group Study. Radiology. 2001;219:213–18. doi: 10.1148/radiology.219.1.r01ap28213. [DOI] [PubMed] [Google Scholar]
- 39.Outwater EK, Dunton CJ. Imaging of the ovary and adnexa: clinical issues and applications of MR imaging. Radiology. 1995;194:1–18. doi: 10.1148/radiology.194.1.7997533. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell DG, Hill MC, Hill S, Zaloudek C. Serous carcinoma of the ovary: CT identification of metastatic calcified implants. Radiology. 1986;158:649–52. doi: 10.1148/radiology.158.3.3945732. [DOI] [PubMed] [Google Scholar]
- 41.Low RN, Carter WD, Saleh F, Sigeti JS. Ovarian cancer: comparison of findings with perfluorocarbon-enhanced MR imaging, In-111-CYT-103 immunoscintigraphy, and CT. Radiology. 1995;195:391–400. doi: 10.1148/radiology.195.2.7724757. [DOI] [PubMed] [Google Scholar]
- 42.Payer LM, Stiglbauerg R, Kramer J, et al. Superparamagnetic particles as oral contrast medium in MRI of patients with treated ovarian cancer – comparison with plain MRI. Br J Radiol. 1993;66:415–19. doi: 10.1259/0007-1285-66-785-415. [DOI] [PubMed] [Google Scholar]
- 43.Potter ME, Partridge EE, Hatch KD, Soong SJ, Austin JM, Shingleton HM. Primary surgical therapy of ovarian cancer: how much and when. Gynecol Oncol. 1991;40:195–200. doi: 10.1016/0090-8258(90)90277-r. [DOI] [PubMed] [Google Scholar]
- 44.Walsh JW. CT of gynaecologic neoplasm. Radiol Clin North Am. 1992;30:826–30. [PubMed] [Google Scholar]
- 45.Coakley FV. Staging ovarian cancer: role of imaging. Radiol Clin North Am. 2002;40:609–36. doi: 10.1016/s0033-8389(01)00012-4. [DOI] [PubMed] [Google Scholar]
- 46.Fleischer AC. Transabdominal and transvaginal sonography of ovarian masses. Clin Obstet Gynaecol. 1991;34:433. doi: 10.1097/00003081-199106000-00027. [DOI] [PubMed] [Google Scholar]
- 47.Sanders RC, McNeil BJ, Finberg HJ, et al. A prospective study of computed tomography and ultrasound in the detection and staging of pelvic masses. Radiology. 1983;146:439–42. doi: 10.1148/radiology.146.2.6849090. [DOI] [PubMed] [Google Scholar]
- 48.Shiels RA, Peel KR, MacDonald HN, et al. A prospective trial of CT in staging of ovarian malignancy. Br J Obstet Gynaecol. 1985;92:407–12. doi: 10.1111/j.1471-0528.1985.tb01116.x. [DOI] [PubMed] [Google Scholar]
- 49.Johnson RJ, Blackledge G, Eddleston B, Crowther D. Abdomino-pelvic computed tomography in the management of ovarian carcinoma. Radiology. 1983;146:447–52. doi: 10.1148/radiology.146.2.6849092. [DOI] [PubMed] [Google Scholar]
- 50.Blaquiere RM, Husband JE. Conventional radiology and computed tomography in ovarian cancer: discussion paper. J R Soc Med. 1983;76:574–9. doi: 10.1177/014107688307600710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Axtell AE, Lee MH, Bristow RE, et al. Multi-institutional reciprocal validation study of computed tomography predictors of suboptimal primary cytoreduction in patients with advanced ovarian cancer. J Clin Oncol. 2007;25:384–9. doi: 10.1200/JCO.2006.07.7800. [DOI] [PubMed] [Google Scholar]
- 52.Meyer JI, Kennedy AW, Friedman R, Ayoub A, Zepp RC. Ovarian carcinoma: value of CT in predicting success of debulking surgery. AJR Am J Roentgenol. 1995;165:875–8. doi: 10.2214/ajr.165.4.7676985. [DOI] [PubMed] [Google Scholar]
- 53.Nelson BE, Rosenfield AT, Schwartz PE. Preoperative abdominopelvic computed tomographic prediction of optimal cytoreduction in epithelial ovarian carcinoma. J Clin Oncol. 1993;11:166–72. doi: 10.1200/JCO.1993.11.1.166. [DOI] [PubMed] [Google Scholar]
- 54.Semelka RC, Lawrence PH, Shoenut JP, et al. Primary ovarian cancer: prospective comparison of contrast-enhanced CT and pre-and postcontrast, fat-suppressed MR imaging, with histologic correlation. J Magn Reson Imaging. 1993;3:99–106. doi: 10.1002/jmri.1880030117. [DOI] [PubMed] [Google Scholar]
- 55.Prayer L, Kainz C, Kramer J, et al. CT and MR accuracy in the detection of tumor recurrence in patients treated for ovarian cancer. J Comput Assist Tomogr. 1993;17:626–32. doi: 10.1097/00004728-199307000-00021. [DOI] [PubMed] [Google Scholar]
- 56.Goldhirsch A, Triller JK, Greiner R, Dreher E, Davis BW. Computed tomography prior to second-look operation in advanced ovarian cancer. Obstet Gynecol. 1983;62:630–4. [PubMed] [Google Scholar]
- 57.Moskovic E, Fernando I, Blake P, Parsons C. Lymphography – current role in oncology. Br J Radiol. 1991;64:422–7. doi: 10.1259/0007-1285-64-761-422. [DOI] [PubMed] [Google Scholar]
- 58.Silverman PM, Osborne M, Dunnick NR, Bandy LC. CT prior to second-look operation in ovarian cancer. AJR Am J Roentgenol. 1988;150:829–32. doi: 10.2214/ajr.150.4.829. [DOI] [PubMed] [Google Scholar]
- 59.Forstner R, Hricak H, Powell CB, Azizi L, Frankel SB, Stern JL. Ovarian cancer recurrence: value of MR imaging. Radiology. 1995;196:715–20. doi: 10.1148/radiology.196.3.7644634. [DOI] [PubMed] [Google Scholar]
- 60.Low RN, Saleh F, Song SY, et al. Treated ovarian cancer: comparison of MR imaging with serum CA-125 level and physical examination – a longitudinal study. Radiology. 1999;211:519–28. doi: 10.1148/radiology.211.2.r99ma24519. [DOI] [PubMed] [Google Scholar]
- 61.Low RN, Duggan B, Barone RM, Saleh F, Song SY. Treated ovarian cancer: MR imaging, laparotomy reassessment, and serum CA-125 values compared with clinical outcome at 1 year. Radiology. 2005;235:918–26. doi: 10.1148/radiol.2353040447. [DOI] [PubMed] [Google Scholar]