Abstract
Open heart surgery is a unique model to study the interplay between cellular injury, regulation of inflammatory responses and tissue repair. Stress-inducible heat shock protein 70-kDa (Hsp70) provides a molecular link between these events. In addition to molecular chaperoning, Hsp70 exerts modulatory effects on endothelial cells and leukocytes involved in inflammatory networks. Hsp70 residing in the intracellular compartment is part of an inhibitory feedback loop that acts on nuclear factor kappaB (NF-κB). In contrast, extracellular Hsp70 is recognized by multiple germline-encoded immune receptors, e.g., Toll-like receptor (TLR) 2, TLR4, LOX-1, CD91, CD94, CCR5 and CD40. Hsp70 is thereby able to enhance chemotaxis, phagocytosis and cytolytic activity of innate immune cells and stimulate antigen-specific responses. These apparent contradictory pro- and anti-inflammatory effects of endogenous Hsp70 in the context of cardiac surgery are still not fully understood. An all-embracing model of the compartmentalized effects of endogenous Hsp70 in the orchestration of inflammatory responses in cardiac surgery is proposed.
Keywords: Cardiac surgery, Heat-shock protein 70-kDa, Inflammation, Innate immunity, Nuclear Factor kappa B, Toll-like receptors
Introduction
Heat shock proteins (HSP) play a pivotal role in maintaining the homeostasis of cells and tissues in vertebrates. The members of the HSP superfamily are categorised based on their molecular weight (kD), including the small Hsp, Hsp40, Hsp60, Hsp70, Hsp90 and Hsp110. These subtypes are expressed in distinct compartments of the cell (e.g., nucleus, cytoplasm, endoplasmatic reticulum, mitochondria) and are associated with various structural proteins (e.g., actin, tubulin, procollagens) according to their diverse functions. For example, HSP are involved in the folding and translocation of proteins, regulation of apoptosis, stabilisation of the cytoskeleton and striated muscle fibers, quality control of collagen production and protein degradation (Benjamin and McMillan 1998). The best described function of HSP is molecular chaperoning: maintenance of the correct conformation of proteins throughout their lifespan, from newly synthesized polypeptide chains to (ir)reversible injury of denatured proteins during oxidative stress. The synthesis of HSP is regulated by heat shock transcription factor 1 (HSF1), which can be activated by noxious stimuli e.g., hyperthermia, ischemia, oxidative and cytokine-mediated stress. HSP thus render cells more resilient to increasing levels of cellular stress (Latchman 2001). The Hsp70 class of stress-induced proteins is the most studied subtype in cardiovascular and inflammatory disease. Experimental and clinical data suggest that inducible Hsp70 is a key component of endogenous pathways that limit the extent of myocardial damage in ischemia–reperfusion injury after cardiac surgery (Williams and Benjamin 2000; Lepore et al. 2001).
In addition, Hsp70 has immunoregulatory properties with both pro- and anti-inflammatory effects in a cross-species manner. Hsp70 residing in its native compartment (the cytosol) is able to hamper ongoing pro-inflammatory signaling cascades, thereby downregulating inflammatory activity. In contrast, extracellular Hsp70 displays potent immunostimulatory properties and has therefore been designated as a “chaperokine” (Asea 2007). The latter is in agreement with the “danger model” proposed by Matzinger (Matzinger 2002). This model states that endogenous HSP are released in the extracellular compartment upon cellular stress where they contribute to orchestrating inflammatory responses and tissue repair. In line with this, extracellular Hsp70 is recognized by a multitude of germline-encoded immune receptors, including Toll-like receptor (TLR) 2, TLR4, LOX-1, CD91, CD94, CCR5 and CD40. These ligand–receptor interactions of Hsp70 involve innate as well as adaptive immune cells, including monocytes, macrophages, dendritic cells, natural killer cells and T-lymphocytes (Pockley et al. 2008). Thus, whereas intracellular Hsp70 functions as an anti-inflammatory regulator, its extracellular action is mainly pro-inflammatory. The current challenge is to unite these apparent dichotomous immunoregulatory effects of Hsp70 on parenchymal and innate/adaptive immune cells. In this review, an all-embracing model for the regulation of inflammation and tissue repair by Hsp70 during the course of cardiac surgery is proposed.
Hsp70 and the myocardium in animal models
A certain degree of ischemic injury of cardiomyocytes, vascular smooth muscle cells, endothelial cells and connective tissue cells after open heart surgery is unavoidable. Aortic cross-clamping (ACC) and cardiopulmonary bypass (CPB) induce global ischemia of the myocardium. This affects the integrity of cytoskeletal and contractile proteins and could compromise cardiac function. The sequence of cellular events that take place during ischemia can be divided in consecutive stages ranging from reversible injury to irreversible injury and ultimately necrosis. In addition, reperfusion injury can be inflicted after restoration of the circulation due to changes in cellular metabolism and associated oxidative stress. Various approaches are employed in clinical practice to slow down the progression of ischemic injury (e.g., hyperkalemic cardioplegic solutions, buffers, hypothermia) with the intention to conserve intracellular energy stores and inhibit lethal cascades within the cytoplasm (Hearse 1998). The progression of cellular injury is prevented by the action of molecular chaperones, including the prototypic Hsp70 class expressed in the cytosol and nucleus. In addition to maintaining the integrity of cytosolic proteins and cytoskeleton (Latchman 2001), Hsp70 promotes cardiomyocyte survival by the conservation of antioxidants and inhibition of pro-apoptotic pathways including caspase- (Suzuki et al. 2002) and Fas-mediated death cascades (Zhao et al. 2007). The concept of heat shock preconditioning in order to enhance postischemic cardiac function was demonstrated in vivo for the first time with rats pretreated with hyperthermia 24 h prior to ischemic challenge. Preconditioning resulted in reduced levels of biomarkers of myocardial damage in addition to a faster recovery and improved contractile force of cardiac tissue (Currie et al. 1988). Soon thereafter, an inverse relation between the Hsp70 content of myocardial cells and infarct size was demonstrated (Hutter et al. 1994) and various transgenic approaches further established that Hsp70 plays a key role in myocardial protection in experimental models. For example, overexpression of inducible Hsp70 in cardiac tissue improved postischemic cardiac contractility, which inversely correlated with infarct size (Marber et al. 1995; Plumier et al. 1995). Similar results were also achieved in mice expressing a hsp70 transgene during brief ischemia (Trost et al. 1998), whereas it reduced mortality from myocardial infarction after prolonged ischemia (Hutter et al. 1996). In line with these results, hsp70 knockout mice did not benefit from preconditioning prior to ischemic challenge (Hampton et al. 2003). The expression of Hsp70 in cardiac tissue has also been manipulated by germline-independent techniques. For example, intracoronary infusion of hemagglutinating virus of Japan (HSV)–liposome–hsp70 constructs was applied in a rat model of prolonged cardioplegia and hypothermia. This Hsp70 overexpression resulted in enhanced recovery of postischemic endothelial (Jayakumar et al. 2000), mitochondrial and ventricular function (Jayakumar et al. 2001). Similarly, a rabbit model of ischemia–reperfusion injury showed that intramyocardial injection of recombinant adenovirus–human–hsp70 constructs resulted in a twofold reduction in infarct size compared to controls (Okubo et al. 2001). Thus, upregulation of Hsp70 in myocardial tissue in experimental models unambiguously improved salvage of cardiac tissue at risk of ischemic infarction and improved postischemic cardiac contractility.
Hsp70 and the human heart
Pioneering studies in cardiac surgery showed that even within a limited period of time, human myocardial tissue responds to ischemia by increasing the expression of Hsp70 class proteins. Myocardial samples showed upregulation of Hsp70 transcripts and protein at the end of coronary artery bypass grafting (Taggart et al. 1997). Studies that included patients undergoing open heart surgery demonstrated that Hsp70 levels were increased in roughly 40% of patients after ACC in either adults (Demidov et al. 1999) or children (Giannessi et al. 2003). Further analysis showed that this group predominantly involved patients with a longer period of preexistant disease or longer CPB time. Patients with higher preoperative myocardial Hsp70 contents showed reduced levels of biochemical markers of myocardial injury postoperatively (Demidov et al. 1999), whereas patients without perioperative upregulation of myocardial Hsp70 showed an almost twofold increase of biomarkers of myocardial injury after surgery (Giannessi et al. 2003). Ischemia–reperfusion injury is an important factor that contributes to the pathogenesis of postoperative atrial fibrillation (AF) (Archbold and Curzen 2003). Clinical studies showed a significant correlation with higher Hsp70 levels in atrial myocardial cells of patients with a postoperative sinus rhythm compared to subjects that developed AF after surgery (St Rammos et al. 2002), whereas serum Hsp70 levels did not differ between these groups (Mandal et al. 2005). In addition, the identification of a specific hsp70 gene polymorphism correlated with an increased risk of postoperative AF (Afzal et al. 2007). These combined results strengthen the concept that intracellular Hsp70 mediates protective effects against ischemic insults in the human myocardium, but also illustrate interindividual differences in pre- and postoperative levels of Hsp70. The latter correlates with cellular tolerance to oxidative stress (Boshoff et al. 2000) and is probably associated with the extent of preexistent (Rafiee et al. 2003) or perioperative ischemic stress (Schmitt et al. 2002), in addition to the hsp70 genotype (Storti et al. 2003). Importantly, the cardioprotective effects of Hsp70 appear to be age-independent (Bartling et al. 2003), underlining the important physiological role of this class of household proteins.
Kinetics of Hsp70 upregulation
The time course of Hsp70 induction influences the degree of functional Hsp70-associated chaperoning activity during periods of ischemia and reperfusion. Most animal models used a latent period of 24 h between preconditioning and ischemic challenge, which is less relevant for human cardiac surgery. A few studies specifically addressed the kinetics of Hsp70 upregulation, either in vitro or in vivo. In rat skeletal muscles, the increase of Hsp70 protein levels after heat shock occurred between 1–4 and 24–48 h in slow and fast muscles, respectively (Oishi et al. 2002). The temporal characteristics of Hsp70 upregulation in cardiac muscle appears to be intermediate between these patterns. Whole-body hyperthermia in a rat model induced Hsp70 upregulation after 3–72 h, whereas the beneficial effects of preconditioning on postischemic infarct size was maximal after 48–72 h (Yamashita et al. 1997). This bimodal expression pattern was also found in a study in which bovine aortic endothelial cells were challenged with heat shock with variable pre- and post-heating times. This showed that 1–2 h of pre-heating induced optimal Hsp70 upregulation directly post-heating, with peak levels at 5 and 12 h after the start of the experiment (Wang et al. 2003). These findings suggest that Hsp70 expression is a function of the duration and level of stress. In line with these results, accumulating myocardial levels of Hsp70 were found in the course of open heart surgery with a more than twofold increase after 2 h (Schmitt et al. 2002). Thus, the lag time between the onset of ischemia and upregulation of Hsp70 in human myocardial cells is probably similar to that in experimental models. This suggests that ischemic preconditioning prior to surgery may induce cardioprotective effects. Indeed, preconditioning by two short ischemia–reperfusion cycles before valve replacement surgery reduced myocardial ultrastructural damage and improved cardiac function postoperatively (Li et al. 1999). Together, these data demonstrate that Hsp70 is induced within a short period of time after initiation of an ischemic insult in human myocardial cells and that these de novo synthesized proteins are able to mediate clinically relevant effects.
Systemic inflammatory responses after on-pump cardiac surgery
Cardiac surgery with CPB represents a multifactorial model of systemic stress, associated with the acute onset of pro-inflammatory signaling cascades. This originates from, e.g., leukocyte contact activation in the extracorporeal circuit, initiation of tissue plasminogen activator and complement-mediated cascades (Rubens et al. 2005), tissue damage associated with thoracotomy (Prondzinsky et al. 2005), administration of anaesthetics and analgesics (Hambsch et al. 2002), perioperative leakage of endotoxins from the gut (Jansen et al. 1992) and activation of endothelial cells as a result of ischemia–reperfusion injury. The latter is associated with the enhanced release of endothelial adhesion molecules, including soluble intercellular and vascular cell adhesion molecule-1 (sICAM-1, sVCAM-1) and E-selectin (Kalawski et al. 1998). Activation of the complement system after initiation of cardiac surgery has been demonstrated by a decrease of plasma C3, C4, C5 and C1 inhibitor and increase of C3d and C5a levels. The alternative complement pathway via C3d appears to be particularly boosted by CPB, which can be explained by contact activation in the extracorporeal circuit (Tarnok et al. 1999). Neutrophils are early responders after an inflammatory insult, which are able to produce potent cytokines and chemokines including tumour necrosis factor (TNF)-α, interleukin (IL)-1, IL-6 and IL-8 (Adrie and Pinsky 2000). Upon activation, they also release reactive oxygen species and proteases that may damage the microcirculation, exacerbating local ischemia–reperfusion injury (Palatianos et al. 2004). Importantly, transcriptional profiles of circulating leukocytes after CPB-assisted (on-pump) cardiac surgery suggest that the inflammatory focus is localized in the peripheral tissues, not in the haematopoietic compartment (Tomic et al. 2005). Synergism between this multitude of pro-inflammatory stimuli can induce a systemic inflammatory response syndrome (SIRS), which can occur in up to 10% of patients after open heart surgery and is associated with a marked circulatory derangement (Cremer et al. 1996). The extent of immune reactivity changes appears to be related to the nature of the insult and concurrently with the clinical outcome. For example, trauma patients experienced a prolonged course of immune hyporesponsiveness compared to patients that underwent major surgery (Cavaillon et al. 2005). Thus, instead of a final common pathway that sets out a preprogrammed inflammatory course, systemic inflammation is more likely a customized multicellular response. This concept has also been demonstrated after on-pump cardiac surgery. The risk of developing multiorgan failure in SIRS was associated with a longer CPB and ACC time, in addition to higher postoperative levels of various pro-inflammatory cytokines (Sablotzki et al. 2002). Another study showed that a subgroup of patients that required prolonged mechanical ventilation after cardiac surgery underwent a longer ACC time and showed increased systemic levels of IL-6, IL-8, VCAM-1 and endotoxin (Rothenburger et al. 2003).
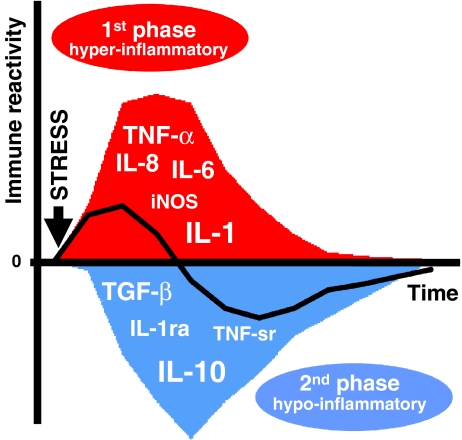
Importantly, the myriad of events in the course of systemic inflammation includes both pro- and anti-inflammatory reactivity. This has led to the concept that systemic inflammatory responses actually represent a dynamic inflammatory balance in which the initial hyper-inflammatory phase is followed by a hypo-inflammatory immune status (Fig. 1). Although the latter phase is associated with a reduced immune reactivity, also called “immune paralysis”, it is more appropriate to consider these changes as adaptation of innate and antigen-specific immune cells (Cavaillon et al. 2005). For the clinical management of individual patients with systemic inflammation, this means that temporal characteristics of both pro- and anti-inflammatory mediators must be taken into account for an accurate assessment of the inflammatory risk profile. Anti-inflammatory effects are mediated by, e.g., IL-10, transforming growth factor (TGF)-β, IL-4, plasma IL-1 receptor antagonist (IL-1ra) and soluble TNF receptors (TNF-sr; Rivera-Chavez et al. 2003; Millo et al. 2004). During on-pump cardiac surgery, increased IL-8 plasma levels were accompanied by elevated IL-10 levels at the end of CPB, followed by IL-1ra and TNF-sr peaks later on (McBride et al. 1996). Importantly, repeated cytokine measurements during on-pump cardiac surgery showed that anti-inflammatory cytokines are immediately released upon initiation of surgery (Hiesmayr et al. 1999; Nathan et al. 2000). Despite this rapid systemic release of anti-inflammatory mediators, the shift towards a net hypo-inflammatory state occurs nonetheless later on.
Fig. 1.
The inflammatory balance during cardiac surgery shifts from hyper-inflammatory (1st phase) to hypo-inflammatory (2nd phase). The latter phase has also been termed “immune paralysis” due to the reduced reactivity of both innate and adaptive immune cells. The diverse range of cytokines and enzymes released during the peri- and postoperative course contribute to this phenomenon. These proteins are classified as either pro-inflammatory, e.g., TNF-α, IL-1β, IL-6, IL-8 and inducible nitric oxide (iNOS), or anti-inflammatory, e.g., IL-10, TGF-β, IL-1 receptor antagonist (IL-1ra) and TNF soluble receptors (TNF-sr). In the early period, pro-inflammatory cytokines act synergistically to enhance systemic inflammatory responses (1st phase). Although the systemic release of anti-inflammatory mediators also occurs rapidly after the inciting stimulus (e.g., start of surgery), skewing of the net inflammatory balance to a hypo-inflammatory state is delayed until the postoperative course (2nd phase)
In contrast to the aforementioned complexity of involved cyto- and chemokine networks, the clinical management of deranged systemic inflammatory responses after on-pump cardiac surgery mainly relies on a single entity: corticosteroids (Chaney 2002). Prophylactic corticosteroids induced suppression of pro-inflammatory mediators (Jansen et al. 1991), release of reactive oxygen species in polymorphonuclear granulocytes (Bourbon et al. 2004) and enhanced anti-inflammatory cytokine production (El Azab et al. 2002; Bourbon et al. 2004). However, their effect on the clinical outcome after on-pump cardiac surgery remains controversial (Robertson-Malt et al. 2007). Some groups showed beneficial effects on postoperative organ function (Malagon et al. 2005; Halonen et al. 2007; Liakopoulos et al. 2007), whereas others found no significant differences (Fillinger et al. 2002; Yared et al. 2007) or even worsening of postoperative pulmonary function in the corticosteroid group (Morariu et al. 2005). Besides these controversial data on corticosteroids, the effectiveness and/or safety of novel anti-inflammatory candidates, e.g., aprotinin (Shaw et al. 2008), human recombinant-activated protein C (Marti-Carvajal et al. 2008) and anti-TNF-α therapy (Carlet et al. 2008) have been disappointing. These dissatisfactory results suggest a lack of insight into essential mechanisms of the multifactorial pathogenesis of systemic inflammation. The recently uncovered immunomodulatory effects of Hsp70 offer a new perspective on this subject.
Hsp70 is systemically released after cardiac surgery
Besides its function as a molecular chaperone in cardiac tissues, Hsp70 is also involved in the orchestration of inflammatory responses after cardiac surgery. These Hsp70-mediated immunomodulatory effects were predicted by the “danger model” originally described by Matzinger in 1994. This model states that endogenous substrates released in the course of cellular injury are able to activate antigen-presenting cells (APC). Through a diverse set of non-foreign danger signals, distressed tissues can thereby direct immune responses and tissue repair (Matzinger 2002). Extracellular Hsp70 may be a molecular link between myocardial injury and the pathogenesis of postoperative systemic inflammatory responses. In line with this concept, in vitro data show that necrotic but not apoptotic cell death resulted in abundant release of various heat shock proteins including Hsp70 (Basu et al. 2000). Furthermore, stressed and gradually dying cells markedly upregulated Hsp70, resulting in enhanced release after cell death (Saito et al. 2005). Others showed that Hsp70 can be released by stressed hematopoietic cells in exosomes generated from multivesicular bodies (Bausero et al. 2005) through a specialised secretory apparatus (Lancaster and Febbraio 2005). A similar pathway for Hsp70 in myocardial cells during oxidative stress (Gupta and Knowlton 2007) remains to be demonstrated.
Systemic release of Hsp70 in humans has been demonstrated after myocardial infarction (Dybdahl et al. 2005; Satoh et al. 2006) and coronary artery bypass grafting (Dybdahl et al. 2002). On-pump cardiac surgery was associated with higher postoperative levels of Hsp70 compared to off-pump surgery (Dybdahl et al. 2004; Szerafin et al. 2008). This difference may be explained by increased leukocyte and tissue damage or the increased complexity of surgical procedures in the on-pump group. Elevated systemic levels of Hsp70 have also been demonstrated in plasma during sepsis (Wheeler et al. 2005) and after major surgery (Kimura et al. 2004). Moreover, higher Hsp70 plasma levels correlated with worsened clinical outcome in various critical conditions (Wheeler et al. 2005; Ganter et al. 2006). In all these studies, Hsp70 levels showed only a transient increase after the insult, whereas the extent of systemic Hsp70 release correlated with the degree of cellular injury. Distinct from this pattern, pro-inflammatory cytokine levels remained elevated for prolonged periods. This is consistent with the concept that extracellular Hsp70 is a danger signal in the course of tissue injury that extinguishes after discontinuation of the inciting insult. Together, these data supplement the danger model with additional mechanisms by which Hsp70 could be able to fine tune immune responses in cellular stress.
Extracellular Hsp70 activates innate immune cells
Hsp70 shows major histocompatibility complex (MHC)-independent immune reactivity, indicating that this activity cannot solely be explained by chaperoning of immunogenic peptides. Indeed, extracellular Hsp70 whole protein or peptides specifically interact with a diverse range of transmembrane immune receptors. Many cell-surface structures involved in Hsp70-mediated signaling have been identified in the recent years, including TLR2, TLR4, LOX-1, CD91, CD94 (C-type lectin), CD40 and chemokine receptor CCR5. These transmembrane or membrane-associated immune receptors are differentially expressed on various cell types (Fig. 2). They belong to the family of pattern recognition receptors (TLR2, TLR4), are involved in the uptake of antigens by antigen-presenting cells (LOX-1, CD91) or mediate co-stimulatory signals (CCR5, CD40). Thus, Hsp70 may exert immunomodulatory effects on multiple levels in inflammatory networks. The potential pro- versus anti-inflammatory effects of this molecule may be dependent of, e.g., cell type, simultaneous signaling cascades, the level of intracellular oxidative stress and the (sub)cellular localisation of Hsp70. Tightly controlled experiments with highly purified Hsp70 are to be used to evaluate these signaling events, since recombinant Hsp70 is mainly produced by genetically engineered Escherichia coli strains. These protein preparations may be contaminated by bacterial cell wall contaminants with immunostimulatory potency, e.g., lipopolysaccharide (LPS) or lipoproteins (Gao and Tsan 2003). The LPS content can be assessed by the Limulus amebocyte lysate assay, whereas residual LPS activity is inhibited by, e.g., polymyxin B, lipid A or lipid IVa treatment (Tsan and Gao 2004). Alternatively, thermal denaturation or trypsin pretreatment can be performed to confirm loss of immunostimulatory activity of Hsp70 preparations (Rico et al. 2002). Another approach is to compare the signaling activity of Hsp70 peptides to irrelevant polypeptide sequences (Gross et al. 2003a).
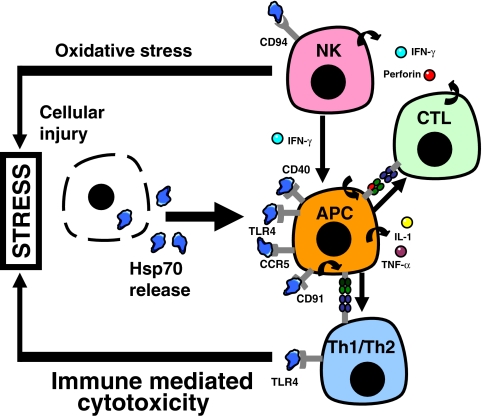
Fig. 2.
Differential expression of germline-encoded immune receptors that recognise extracellular Hsp70. This heterogenous group includes TLR2, TLR4, LOX-1, CD91, CD94, CCR5 and CD40 expressed on antigen-presenting cells (APC), natural killer (NK) cells, cytotoxic T cells (CTL) and T-helper cells (Th1/Th2). They communicate the “danger signal” provided by Hsp70 during in the course of cellular stress. Their integrated function is discussed in the text
The prototypic Toll-like receptor (TLR) family were the first receptors to be associated with Hsp70. The TLR family consists of multiple members, in which each subtype recognises specific ligands, e.g., TLR2 (triacylated lipoproteins), TLR4 (LPS), TLR5 (flagellin) and TLR9 (CpG DNA). Binding of TLR ligands and subsequent signaling cascades converge on transcription factors nuclear factor kappaB (NF-κB) and AP-1 (Takeda and Akira 2004). Activation of transcription factor NF-κB acts as a functional switch and is central to the initiation of inflammatory responses by innate cells, e.g., neutrophils, macrophages and dendritic cells (DC). TLR-mediated intracellular signaling cascades induce IκB kinase activity and degradation of I-κBα, the inhibitory subunit of NF-κB. This ultimately results in the translocation of active NF-κBp50/p65 heterodimers from the cytosol to the nucleus. The p65 subunits bind to promotor regions on the chromosome which enables the transcription of various pro-inflammatory molecules and enzymes, e.g., TNF-α, IL-1β, IL-6 and inducible nitric oxide synthase (iNOS; Lee and Kim 2007). Purified Hsp70 demonstrated similar cytokine-inducing effects on human monocytes, which could be abrogated by interfering with NF-κB transactivation. Importantly, the potency of Hsp70-induced cytokine production was similar to that of LPS (Asea et al. 2000). These effects were dependent on the co-expression of membrane-bound protein CD14 with TLR2 or TLR4 (Asea et al. 2002) and adaptor molecule MyD88. The latter protein binds to the cytoplasmic tail of ligand-activated TLR that contains the Toll/IL-1 receptor domain, which is pivotal for intracellular signaling (Vabulas et al. 2002). Moreover, expression of TLR2 and TLR4 showed synergistic effects on NF-κB promotor activity and IL-6 production in response to Hsp70. Functional effects of Hsp70 on dendritic cells were, e.g., enhanced synthesis of pro-inflammatory cytokines and upregulation of MHC class II and CD86 co-stimulatory molecules (Asea et al. 2002). In these experiments, interference by contaminating LPS was prevented by either boiling Hsp70 preparations (Vabulas et al. 2002) or polymyxin B pretreatment (Asea et al. 2002).
In concordance with the danger paradigm, these immunomodulatory effects were mainly exerted by the stress inducible form of Hsp70 (Bethke et al. 2002). Another study showed that Hsp70 purified from liver cell lysates induced secretion of IL-1β, TNF-α and IL-12 by murine macrophages (Basu et al. 2000). Similarly, neutralizing antibodies directed against Hsp70 abrogated the stimulatory effects of necrotic cell lysates on pro-inflammatory cytokine production by human monocytes (El Mezayen et al. 2007). Thus, Hsp70 released after cellular injury was highly effective in the priming of monocytes, macrophages and DC. Whether this is the result of infection, trauma or ischemia/reperfusion injury, extracellular Hsp70 could thereby participate in a positive feedback loop in inflammatory circuits (Quintana and Cohen 2005). This concept can be extended by cross-species interactions with microbial Hsp70, as various reports showed that non-self Hsp70 molecules are also recognized by TLR. In other words, endogenous Hsp70 released by infection-associated tissue damage and microbial Hsp70 may synergistically induce stimulatory effects on innate immune cells. For example, Mycobacterium tuberculosis-derived Hsp70 induced NF-κB activity in a human endothelial cell line through either TLR2 or TLR4 and stimulated production of pro-inflammatory cytokines (e.g., TNF-α, IL-6) by macrophages (Bulut et al. 2005). Others showed that Toxoplasma gondii Hsp70 induced upregulation of co-stimulatory molecules (e.g., CD40, CD80, CD86) on DC via TLR4-mediated signaling (Aosai et al. 2006). As mentioned earlier, microbial Hsp70 chaperoning of small amounts of LPS (Bausinger et al. 2002; Gao and Tsan 2003) or flagellin (Ye and Gan 2007) may contribute to such immunostimulatory effects. In any case, the capacity of Hsp70 to chaperone immunogenic microbial substrates adds up to its immunomodulatory properties.
In addition to phagocytic cells involved in antigen presentation, natural killer (NK) cells also demonstrated reactivity to extracellular Hsp70 (Multhoff et al. 1997). NK cells are a distinctive subset of innate cells, which are capable of producing cytokines (e.g., IFN-γ), exert direct cytotoxicity towards target cells (e.g., via perforin) and mediate antibody-dependent cellular cytotoxicity (Farag and Caligiuri 2006). Hsp70 binding to NK cells involved transmembrane protein CD94 which belongs to the C-type lectin class of NK receptors. More specifically, CD94 recognised a 14-mer sequence termed TKD from the C-terminal substrate-binding domain of Hsp70 (Gross et al. 2003a). This human-specific Hsp70 peptide stimulated NK cytolytic activity (Multhoff et al. 2001), enhanced upregulation of its receptor CD94 (Gross et al. 2003b) and NK cell chemotaxis (Gastpar et al. 2004). Through Hsp70-regulated NK activity, these cells can either directly participate in immune surveillance (Elsner et al. 2007) or serve as adjuvants in antigen-specific immune responses (Massa et al. 2005). Together, these findings showed that Hsp70 released in the extracellular space, derived from either host or pathogen, positively regulates activation of innate immune cells.
Extracellular Hsp70 enhances antigen-presentation
Antigen-specific T-cell-mediated immune responses are primed by APC, which provide T-cell receptor interactions via MHC/peptide complexes, in addition to costimulatory (signal 2) and polarizing signals (signal 3; Kalinski et al. 1999). As Hsp70 contains both protein chaperoning as well as immunomodulatory domains, this protein displays high potential for promoting antigen-specific responses. Pioneering experiments demonstrated that Hsp70/peptide complexes were able to induce MHC class I-restricted CD8+ T-cell responses (“cross-presentation”; Blachere et al. 1997). Since then, Hsp70 has been demonstrated to chaperone a large variety of immunogenic peptides with its peptide-binding pocket, e.g., tumor, viral, parasitic and other antigens (reviewed in Srivastava 2002). Cross-presentation of chaperoned peptides on MHC class I molecules requires a mechanism that transports these peptides across the lipid bilayer (Castellino et al. 2000). The α2-macroglobulin receptor CD91 has been proposed as the receptor for Hsp70 on macrophages and DC, mediating proximal events before the transport of extracellular peptides through the endosomal and cytosolic compartment (Basu et al. 2001). Another candidate transmembrane receptor for Hsp70 is the scavenger receptor LOX-1. It has been proposed that CD91 and LOX-1 are the principle Hsp70 receptors on macrophages and DC, respectively (Delneste et al. 2002). In addition to activation of CD8+ T-cell responses, enhanced MHC class II-dependent CD4+ T-cell reactivity through either endogenous (Mycko et al. 2004; Wang et al. 2006), or microbial (Tobian et al. 2004) Hsp70 was demonstrated. It is unclear whether similar endocytic receptors are involved in Hsp70-mediated presentation of exogenous peptides on MHC class II.
The identification of CD91 and LOX-1 as Hsp70 receptors complements data discussed earlier concerning the potent immunostimulatory Hsp70-mediated effects of necrotic cell lysates (Basu et al. 2000). However, other transmembrane proteins are more likely to mediate the potent adjuvant effects of Hsp70 on APC, shifting the immune balance away from tolerance (Millar et al. 2003). This could be provided by a combined action with TLR2 and/or TLR4 expressed on antigen-presenting cells as previously described (Asea et al. 2000, 2002; Vabulas et al. 2002). Peptide-loaded Hsp70 also stimulated intracellular signaling through CD40 (Becker et al. 2002), which may act synergistically with chemokine receptor CCR5 (Whittall et al. 2006). These data further set the outlines of a mechanistic framework for a “danger model” in cardiac surgery, with a central role for stress-induced Hsp70 that crossed the lipid bilayer (Dybdahl et al. 2002, 2004).
Intracellular Hsp70 disrupts NF-κB signaling
Whereas the effects of extracellular Hsp70 are integrated by transmembrane receptors, intracellular Hsp70 is able to directly intervene with inflammatory signaling pathways. A schematic overview of Hsp70 modulation of intra- and extracellular inflammatory pathways is represented in Fig. 3. In contrast to their extracellular counterparts, intracellular Hsp70 appears to act inhibitory on NF-κB promotor activity. This has been demonstrated with various cell types from different ancestry in vitro. For example, hsp70 mRNA-specific antisense oligonucleotides abrogated the inhibitory effects of heat shock preconditioning on LPS-induced NF-κB translocation in rat brain glial cells (Feinstein et al. 1996). Transfection of rat neonatal cardiomyocytes with an adenoviral hsp70 construct significantly reduced LPS-induced iNOS upregulation and the release of creatine kinase (Lau et al. 2000). Interestingly, intracellular signaling activity of NF-κB induced upregulation of HSF1 and Hsp70 (Hamilton et al. 2004), possibly due to NF-κB signaling-associated oxidative stress (Adrie and Pinsky 2000). Heat shock protein-mediated negative feedback on NF-κB activity could thereby represent an important immunoregulatory pathway (Ammirante et al. 2008). The intracellular anti-inflammatory effects of Hsp70 may involve inhibition of IκB kinase (IKK) activity by binding to its regulatory IKKγ subunit (Ran et al. 2004). In addition, Hsp70 may prevent degradation of I-κBα (Yoo et al. 2000; Weiss et al. 2007) or interfere with NF-κB p65 translocation from the cytosolic to the nuclear compartment (Tang et al. 2007). For example, Hsp70 may adhere to the active subunit of NF-κB after its dissociation from I-κBα whilst acting as molecular chaperone or prevent translocation to the nucleus by physically occluding nuclear pore complexes. Hsp70 could also influence more proximal signaling cascades. One study showed that the mitogen-activated protein kinases (MAPK) pathway, which includes extracellular signal-regulated kinase (ERK), p38 kinase and c-jun N-terminal kinases (JNK), was not affected by Hsp70 (Shi et al. 2006). In contrast, Hsp70 was demonstrated to bind and switch off tumor necrosis factor receptor-associated factor 6 (TRAF6; Chen et al. 2006), an important intermediate in TLR signaling and subsequent NF-κB activation (Lee and Kim 2007). The precise nature of the mechanisms involved in Hsp70 modulation on NF-κB activity remains to be elucidated. Furthermore, other members from the extensive HSP family could also contribute to this process (Park et al. 2003; Lee et al. 2005). Whereas our knowledge of this feature of Hsp70 is still in its infancy, it appears to have been exploited by intracellular pathogens. Transfection of virulent T. gondii strains with antisense hsp70 oligonucleotides increased NO synthesis and NF-κB translocation in host macrophages and also reduced the parasite burden in infected mice (Dobbin et al. 2002). In this scenario, protozoan Hsp70 could have interfered with NF-κB signaling at the nucleocytosolic interface in murine splenocytes, leading to an impaired host resistance. Thus, whether the inflammatory balance is shifted to pro- or anti-inflammatory responses most likely depends on the localisation of Hsp70 relative to the lipid bilayer.
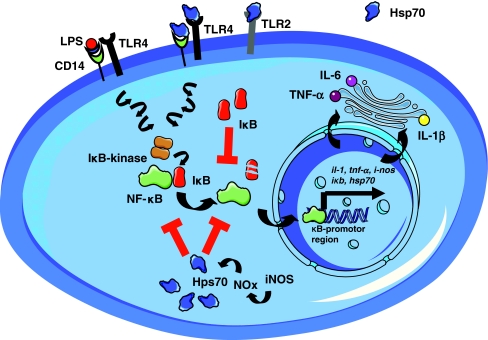
Fig. 3.
Interplay of the effects of extracellular (transmembrane, indirect) versus intracellular (direct) Hsp70 effects, which converge on transcription factor nuclear factor kappaB (NF-κB). Depending on the cell type and inflammatory context, stimulatory (receptor-mediated) or inhibitory (intracellular) Hsp70 effects may dominate
Immunomodulatory effects of Hsp70 in vivo
Hsp70 influences innate and adaptive immune responses in an intricate manner in vitro. Experimental models of organ dysfunction and/or systemic inflammation in vivo may provide less ambiguous clues about these immunomodulatory effects of Hsp70. HSF1 is an important molecular switch in the transactivation of Hsp70 gene expression (McMillan et al. 1998; Ahn et al. 2001). Hsf1 knockout mice not only failed to upregulate Hsp70 protein synthesis after heat shock, but also showed a markedly reduced survival rate in an experimental model of endotoxemia. This increased mortality correlated with significantly higher systemic levels of TNF-α (Xiao et al. 1999). These results suggest that the heat shock response is involved in the regulation of inflammatory cytokine production, which has been further evaluated in a TNF-α-induced model of systemic inflammation and intestinal organ injury. Heat shock preconditioning induced enhanced intestinal Hsp70 expression, reduced the release of pro-inflammatory mediators and diminished TNF-α-induced intestinal damage. However, these beneficial effects of heat shock were lost in hsp70 knockout mice (Van Molle et al. 2002). Similarly, in an experimental model of endotoxemia hsp70−/− mice showed elevated systemic levels of TNF-α and IL-6, in addition to increased lung injury and mortality rate. The damaged tissue showed enhanced NF-κB activity, demonstrated by prolonged p65 binding activity and gradual reduction of inhibitory I-κBα levels (Singleton and Wischmeyer 2006). In a different approach, targeted disruption of hsp70 transcripts in the ventrolateral medulla was used in rat model of endotoxemia to assess the protective role of Hsp70 on circulatory indices during systemic inflammation. Bilateral injection of an antisense hsp70 oligonucleotide abrogated the beneficial effects of heat shock preconditioning on circulatory failure, which was associated with modulation of NF-κB activity (Chan et al. 2004). Together, these results suggest that a normal boost of Hsp70 synthesis in the course of systemic inflammation reduces NF-κB activity at the intracellular level, inhibits the release of pro-inflammatory molecules and thereby prevents extensive parenchymal injury and fatality.
Importantly, this concept holds relevance for cardiac surgery and CPB-induced inflammatory responses. Administration of amino acid glutamine enhanced Hsp70 expression and reduced lung injury in the course of sepsis (Singleton et al. 2005). Similarly, preoperative glutamine administration in an experimental model of cardiopulmonary bypass enhanced Hsp70 expression in various tissues and reduced systemic levels of IL-6, IL-8 and NO species (Hayashi et al. 2002). Whereas these generally anti-inflammatory effects of Hsp70 are likely to be exerted in the intracellular compartment, there is only limited data on the effects of extracellular endogenous Hsp70 in vivo. In one approach, tail shock was applied to increase plasma Hsp70 levels in rats, followed by a subcutaneous bacterial challenge. Stressed animals showed elevated systemic Hsp70 levels and faster resolution of the local inflammatory response. Interestingly, this was associated with increased Hsp70 levels at the inflammatory site and potentiated local NO production (Campisi et al. 2003). This suggests that Hsp70 is not solely a global danger signal, but also provides site-specific directions to inflammatory cells. This is in agreement with the finding that Hsp70 is able to bind to endothelial cells (Theriault et al. 2005), thereby providing a potential homing signal for leukocytes during (systemic) inflammatory responses. The beneficial effects of preconditioning on local inflammation could be replicated by direct administration of exogenous Hsp70 (Campisi et al. 2003), which has been further evaluated in a rat model of endotoxemia. In this study, administration of mammalian Hsp70 before and after E. coli LPS injection decreased and increased mortality, respectively (Kustanova et al. 2006). The former may be explained by neutralization of circulating LPS and thereby limitation of pro-inflammatory signaling. The latter finding may be explained by synergistic effects of Hsp70 on TLR signaling, consistent with its function as a danger signal.
The first steps of the extrapolation of these results to humans have been realised by human genetics studies. The genes hsp70–1 (HSPA1A), hsp70–2 (HSPA1B) and hsp70-Hom (HSPA1L) are located on the MHC class III region on chromosome 6 (Ito et al. 1998). The first two genes encode for the stress inducible Hsp70 forms, the latter gene for its constitutively expressed counterpart (Singh et al. 2006). Association studies have exploited polymorphic loci within these genes to study their effect on clinical outcome. One study evaluated the correlation between Hsp70 polymorphisms and cytokine levels and outcome after major trauma. They showed that polymorphisms in the genes HSPA1B and HSPA1L correlated with higher IL-6 and prolonged elevations of TNF-α serum levels, whereas the latter also correlated with an increased risk of progression to multiple organ failure (Schroder et al. 2003). Another study assessed the risk of developing septic shock in the course of pneumonia. They showed that the presence of a specific HSPA1B genotype inferred a 3.5-fold risk to progress to this critical clinical condition (Waterer et al. 2003). Subsequent data suggest an indirect link between this HSPA1B polymorphism and the levels of LPS induced transcripts encoding for inducible Hsp70 in monocytes (Temple et al. 2004). Together, these data suggest that polymorphic loci in genes that encode for Hsp70 variants control the level of expression which subsequently affects modulation of systemic inflammatory responses and the clinical course after widespread tissue damage. However, others found no correlation between Hsp70 polymorphisms and outcome of critically ill patients (Bowers et al. 2006). Reported associations between polymorphisms and disease must be interpreted with caution due to inconsistent results (Hirschhorn et al. 2002; Lohmueller et al. 2003). Hence, additional clinical studies are needed to confirm the association between Hsp70 polymorphisms and the outcome of inflammatory disease.
Conclusions
The aforementioned data can be summarized in the following model. Cardiac surgery with cardiopulmonary bypass infers oxidative stress to myocardial tissue. This induces rapid Hsp70 upregulation which aids in the preservation of myocardial tissue and postoperative contractility of cardiac muscle. The level of induced Hsp70 in the cytosolic compartment depends on the duration and total level of cellular stress (Wang et al. 2003). Hence, increasing levels of ischemia–reperfusion injury result in elevated cytosolic Hsp70 concentrations. This also means that with increasing levels of stress, more Hsp70 will be released from injured or necrotic cells (Saito et al. 2005). Indeed, plasma levels of Hsp70 are associated with the extent of surgical stress (Dybdahl et al. 2004; Cavaillon et al. 2005; Szerafin et al. 2008). Ischemia–reperfusion injury and the imperative degree of tissue damage ensued from surgical intervention set off pro-inflammatory systemic responses (Cremer et al. 1996), among others mediated by extracellular endogenous Hsp70 (Dybdahl et al. 2002). As such, the extent of surgical stress determines the systemic pro-inflammatory input of extracellular Hsp70. This protein is a potent stimulator of NF-κB-mediated transcription of pro-inflammatory cytokines (e.g., TNF-α, IL-6) by leukocytes through the interaction with TLR2, TLR4, CD94 and other receptors (Asea et al. 2002; Vabulas et al. 2002; Gross et al. 2003a). Hsp70 thereby enhances phagocytosis, uptake of foreign antigens and expression of co-stimulatory molecules. These actions, in combination with chaperoning of immunogenic peptides by Hsp70, enhance antigen-specific responses by T cells (Millar et al. 2003; Wang et al. 2006).
Although this suggests that Hsp70 exerts predominantly pro-inflammatory effects, experimental models of systemic inflammation showed that abrogation of Hsp70 results in increased organ dysfunction and mortality (Xiao et al. 1999; Van Molle et al. 2002; Singleton and Wischmeyer 2006). Indeed, intracellular Hsp70 shows inhibitory activity on pro-inflammatory NF-κB signaling cascades, by either inhibiting IκB kinase, stabilisation of I-κBα or preventing NF-κB p65 translocation (Feinstein et al. 1996; Yoo et al. 2000; Ran et al. 2004; Tang et al. 2007; Weiss et al. 2007). The net effect on the inflammatory balance is dependent on the compartment in which Hsp70 exerts its dominant effects, which in turn depends on the extent of tissue injury. This is schematically represented in Fig. 4. In localized injury, e.g., focal infection, mild trauma or minor surgery, systemically released Hsp70 acts stimulatory on innate immune cells. These cells home to the inflammatory focus (Tomic et al. 2005) via upregulated endothelial adhesion molecules and increased local concentrations of Hsp70 (Campisi et al. 2003; Chase et al. 2007) bound to the plasma membrane (Arispe et al. 2004; Theriault et al. 2005; Vega et al. 2008). Through these mechanisms, Hsp70 is able to direct organ-specific (e.g., cardiac) inflammatory responses. In the absence of cytosolic stress in circulating inflammatory cells, there is little Hsp70-mediated suppression of NF-κB, and the net outcome is pro-inflammatory.
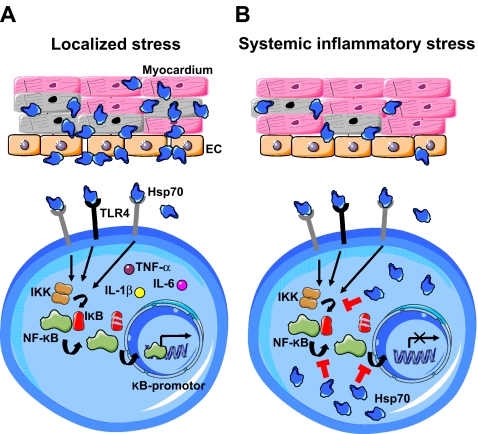
Fig. 4.
Model of the compartmentalized immunomodulatory effects of Hsp70 during localized versus systemic inflammatory stress. a In localized tissue damage (e.g., focal infection, mild trauma, minor surgery) Hsp70 is released in the extracellular space and circulation. Through recognition by transmembrane germline-encoded immune receptors (e.g., TLR4) on innate immune cells, cytosolic pro-inflammatory signaling cascades are ensued. This results in activation of IκB kinase (IKK), release of NF-κB from its inhibitory subunit (IκB) and transcription of pro-inflammatory products (e.g., TNF-α, IL-1β, IL-6). Furthermore, Hsp70 bound on the surface of endothelial cells (EC) in the inflammatory focus serves as a site-specific marker for circulating leukocytes. In this localized scenario, Hsp70 exerts mainly pro-inflammatory effects on the inflammatory balance. b In systemic inflammatory stress (e.g., on-pump cardiac surgery, sepsis) Hsp70 is not only released in the extracellular space, but also upregulated in the cytosol of leukocytes. In this intracellular compartment, Hsp70 is able to inhibit IKK activity, prevent degradation of I-κBα and interfere with translocation of NF-κB to the nucleus. These actions counteract TLR-mediated pro-inflammatory signaling and thereby prevent widespread, uncontrolled immune activation. As the latter is associated with circulatory failure and multi-organ dysfunction, the endogenous Hsp70 response thereby prevents the deleterious consequences of deranged systemic inflammation. In this systemic scenario, Hsp70 exerts mainly anti-inflammatory effects on the inflammatory balance through inhibition of pro-inflammatory signaling in leukocytes
In contrast, systemic stress (e.g., cardiopulmonary bypass, sepsis) ensues signaling by extracellular Hsp70 via transmembrane receptors that converge on NF-κB as well as the upregulation of intracellular Hsp70 in inflammatory cells (Oehler et al. 2001; Schroder et al. 2003; Temple et al. 2004). In this scenario, the intracellular inhibitory effects of Hsp70 on NF-κB signaling overrule pro-inflammatory signals mediated by innate immune receptors. This mechanism inhibits amplification of pro-inflammatory signaling cascades in deranged conditions of “whole body stress” and inflammatory dysregulation. This reduces the risk of deteriorating effects of systemic inflammation to take place (e.g., circulatory failure, organ dysfunction). This model could also explain the more pronounced effects of on-pump cardiac surgery on postoperative downregulation of immune reactivity, compared to off-pump surgery (Borgermann et al. 2007; Hadley et al. 2007). Enhanced stress-induced Hsp70 upregulation in inflammatory cells during CPB abrogates NF-κB signaling, thereby leading to immune hyporesponsiveness. Thus, this proposed model of the compartmentalized effects of Hsp70 during systemic inflammatory responses and tissue damage provides a fundamental basis for the unstable clinical course of patients after cardiac surgery. Future results from in vivo experimental models and clinical studies on the association between Hsp70 effects and modulation of systemic inflammation may be incorporated in this model.
Acknowledgements
B.J.P. is supported by Grants from the Dutch Organization for Scientific Research (NWO VIDI innovation grant), the 5th European Framework Grant ‘hsp for therapy’ and the Dutch Arthritis Foundation.
Abbreviations
- ACC
aortic cross-clamping
- APC
antigen-presenting cells
- CPB
cardiopulmonary bypass
- HSP
heat shock proteins
- IL-1
interleukin-1
- IFN-γ
interferon-gamma
- MHC
major histocompatibility complex
- NF-κB
nuclear factor kappa B
- SIRS
systemic inflammatory response syndrome
- TGF-β
transforming growth factor-beta
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor-alpha
References
- Adrie C, Pinsky MR. The inflammatory balance in human sepsis. Intensive Care Med. 2000;26:364–375. doi: 10.1007/s001340051169. [DOI] [PubMed] [Google Scholar]
- Afzal AR, Mandal K, Nyamweya S, Foteinos G, Poloniecki J, Camm AJ, Jahangiri M, Xu Q. Association of Met439Thr substitution in heat shock protein 70 gene with postoperative atrial fibrillation and serum HSP70 protein levels. Cardiology. 2007;110:45–52. doi: 10.1159/000109406. [DOI] [PubMed] [Google Scholar]
- Ahn SG, Liu PC, Klyachko K, Morimoto RI, Thiele DJ. The loop domain of heat shock transcription factor 1 dictates DNA-binding specificity and responses to heat stress. Genes Dev. 2001;15:2134–2145. doi: 10.1101/gad.894801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammirante M, Rosati A, Gentilella A, et al. The activity of hsp90 alpha promoter is regulated by NF-kappa B transcription factors. Oncogene. 2008;27:1175–1178. doi: 10.1038/sj.onc.1210716. [DOI] [PubMed] [Google Scholar]
- Aosai F, Rodriguez Pena MS, Mun HS, et al. Toxoplasma gondii-derived heat shock protein 70 stimulates maturation of murine bone marrow-derived dendritic cells via toll-like receptor 4. Cell Stress Chaperones. 2006;11:13–22. doi: 10.1379/CSC-138R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archbold RA, Curzen NP. Off-pump coronary artery bypass graft surgery: the incidence of postoperative atrial fibrillation. Heart. 2003;89:1134–1137. doi: 10.1136/heart.89.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe N, Doh M, Simakova O, Kurganov B De Maio A. Hsc70 and Hsp70 interact with phosphatidylserine on the surface of PC12 cells resulting in a decrease of viability. FASEB J. 2004;18:1636–1645. doi: 10.1096/fj.04-2088com. [DOI] [PubMed] [Google Scholar]
- Asea A. Mechanisms of HSP72 release. J Biosci. 2007;32:579–584. doi: 10.1007/s12038-007-0057-5. [DOI] [PubMed] [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: Role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Bartling B, Hilgefort C, Friedrich I, Silber RE, Simm A. Cardio-protective determinants are conserved in aged human myocardium after ischemic preconditioning. FEBS Lett. 2003;555:539–544. doi: 10.1016/s0014-5793(03)01342-5. [DOI] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- Bausero MA, Gastpar R, Multhoff G, Asea A. Alternative mechanism by which IFN-gamma enhances tumor recognition: Active release of heat shock protein 72. J Immunol. 2005;175:2900–2912. doi: 10.4049/jimmunol.175.5.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausinger H, Lipsker D, Ziylan U, et al. Endotoxin-free heat-shock protein 70 fails to induce APC activation. Eur J Immunol. 2002;32:3708–3713. doi: 10.1002/1521-4141(200212)32:12<3708::AID-IMMU3708>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Becker T, Hartl FU, Wieland F. CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. J Cell Biol. 2002;158:1277–1285. doi: 10.1083/jcb.200208083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. doi: 10.1161/01.res.83.2.117. [DOI] [PubMed] [Google Scholar]
- Bethke K, Staib F, Distler M, Schmitt U, Jonuleit H, Enk AH, Galle PR, Heike M. Different efficiency of heat shock proteins (HSP) to activate human monocytes and dendritic cells: Superiority of HSP60. J Immunol. 2002;169:6141–6148. doi: 10.4049/jimmunol.169.11.6141. [DOI] [PubMed] [Google Scholar]
- Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, Udono H, Srivastava PK. Heat shock protein–peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315–1322. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgermann J, Flohe S, Scheubel RJ, Kuss O, Simm A, Schade FU, Friedrich I. Regulation of cytokine synthesis in cardiac surgery: role of extracorporeal circuit and humoral mediators in vivo and in vitro. Inflamm Res. 2007;56:126–132. doi: 10.1007/s00011-006-6152-5. [DOI] [PubMed] [Google Scholar]
- Boshoff T, Lombard F, Eiselen R, Bornman JJ, Bachelet M, Polla BS, Bornman L. Differential basal synthesis of Hsp70/Hsc70 contributes to interindividual variation in Hsp70/Hsc70 inducibility. Cell Mol Life Sci. 2000;57:1317–1325. doi: 10.1007/PL00000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbon A, Vionnet M, Leprince P, Vaissier E, Copeland J, McDonagh P, Debre P, Gandjbakhch I. The effect of methylprednisolone treatment on the cardiopulmonary bypass-induced systemic inflammatory response. Eur J Cardiothorac Surg. 2004;26:932–938. doi: 10.1016/j.ejcts.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Bowers DJ, Calvano JE, Alvarez SM, Coyle SM, Macor MA, Kumar A, Calvano SE, Lowry SF. Polymorphisms of heat shock protein-70 (HSPA1B and HSPA1L loci) do not influence infection or outcome risk in critically ill surgical patients. Shock. 2006;25:117–122. doi: 10.1097/01.shk.0000190826.36406.27. [DOI] [PubMed] [Google Scholar]
- Bulut Y, Michelsen KS, Hayrapetian L, Naiki Y, Spallek R, Singh M, Arditi M. Mycobacterium tuberculosis heat shock proteins use diverse toll-like receptor pathways to activate pro-inflammatory signals. J Biol Chem. 2005;280:20961–20967. doi: 10.1074/jbc.M411379200. [DOI] [PubMed] [Google Scholar]
- Campisi J, Leem TH, Fleshner M. Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell Stress Chaperones. 2003;8:272–286. doi: 10.1379/1466-1268(2003)008<0272:sehiaf>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlet J, Cohen J, Calandra T, Opal SM, Masur H. Sepsis: time to reconsider the concept. Crit Care Med. 2008;36:964–966. doi: 10.1097/CCM.0B013E318165B886. [DOI] [PubMed] [Google Scholar]
- Castellino F, Boucher PE, Eichelberg K, Mayhew M, Rothman JE, Houghton AN, Germain RN. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J Exp Med. 2000;191:1957–1964. doi: 10.1084/jem.191.11.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon JM, Adrie C, Fitting C, Adib-Conquy M. Reprogramming of circulatory cells in sepsis and SIRS. J Endotoxin Res. 2005;11:311–320. doi: 10.1179/096805105X58733. [DOI] [PubMed] [Google Scholar]
- Chan JY, Ou CC, Wang LL, Chan SH. Heat shock protein 70 confers cardiovascular protection during endotoxemia via inhibition of nuclear factor-kappaB activation and inducible nitric oxide synthase expression in the rostral ventrolateral medulla. Circulation. 2004;110:3560–3566. doi: 10.1161/01.CIR.0000143082.63063.33. [DOI] [PubMed] [Google Scholar]
- Chaney MA. Corticosteroids and cardiopulmonary bypass: a review of clinical investigations. Chest. 2002;121:921–931. doi: 10.1378/chest.121.3.921. [DOI] [PubMed] [Google Scholar]
- Chase MA, Wheeler DS, Lierl KM, Hughes VS, Wong HR, Page K. Hsp72 induces inflammation and regulates cytokine production in airway epithelium through a TLR4- and NF-kappaB-dependent mechanism. J Immunol. 2007;179:6318–6324. doi: 10.4049/jimmunol.179.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wu Y, Zhang Y, et al. Hsp70 inhibits lipopolysaccharide-induced NF-kappaB activation by interacting with TRAF6 and inhibiting its ubiquitination. FEBS Lett. 2006;580:3145–3152. doi: 10.1016/j.febslet.2006.04.066. [DOI] [PubMed] [Google Scholar]
- Cremer J, Martin M, Redl H, et al. Systemic inflammatory response syndrome after cardiac operations. Ann Thorac Surg. 1996;61:1714–1720. doi: 10.1016/0003-4975(96)00055-0. [DOI] [PubMed] [Google Scholar]
- Currie RW, Karmazyn M, Kloc M, Mailer K. Heat-shock response is associated with enhanced postischemic ventricular recovery. Circ Res. 1988;63:543–549. doi: 10.1161/01.res.63.3.543. [DOI] [PubMed] [Google Scholar]
- Delneste Y, Magistrelli G, Gauchat J, et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- Demidov ON, Tyrenko VV, Svistov AS, Komarova YY, Karpishenko AI, Margulis BA, Shevchenko YL. Heat shock proteins in cardiosurgery patients. Eur J Cardiothorac Surg. 1999;16:444–449. doi: 10.1016/s1010-7940(99)00291-2. [DOI] [PubMed] [Google Scholar]
- Dobbin CA, Smith NC, Johnson AM. Heat shock protein 70 is a potential virulence factor in murine toxoplasma infection via immunomodulation of host NF-kappa B and nitric oxide. J Immunol. 2002;169:958–965. doi: 10.4049/jimmunol.169.2.958. [DOI] [PubMed] [Google Scholar]
- Dybdahl B, Wahba A, Lien E, et al. Inflammatory response after open heart surgery: Release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation. 2002;105:685–690. doi: 10.1161/hc0602.103617. [DOI] [PubMed] [Google Scholar]
- Dybdahl B, Wahba A, Haaverstad R, Kirkeby-Garstad I, Kierulf P, Espevik T, Sundan A. On-pump versus off-pump coronary artery bypass grafting: more heat-shock protein 70 is released after on-pump surgery. Eur J Cardiothorac Surg. 2004;25:985–992. doi: 10.1016/j.ejcts.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Dybdahl B, Slordahl SA, Waage A, Kierulf P, Espevik T, Sundan A. Myocardial ischaemia and the inflammatory response: release of heat shock protein 70 after myocardial infarction. Heart. 2005;91:299–304. doi: 10.1136/hrt.2003.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azab SR, Rosseel PM, Lange JJ, Groeneveld AB, Strik R, Wijk EM, Scheffer GJ. Dexamethasone decreases the pro- to anti-inflammatory cytokine ratio during cardiac surgery. Br J Anaesth. 2002;88:496–501. doi: 10.1093/bja/88.4.496. [DOI] [PubMed] [Google Scholar]
- Mezayen R, Gazzar M, Seeds MC, McCall CE, Dreskin SC, Nicolls MR. Endogenous signals released from necrotic cells augment inflammatory responses to bacterial endotoxin. Immunol Lett. 2007;111:36–44. doi: 10.1016/j.imlet.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner L, Muppala V, Gehrmann M, et al. The heat shock protein HSP70 promotes mouse NK cell activity against tumors that express inducible NKG2D ligands. J Immunol. 2007;179:5523–5533. doi: 10.4049/jimmunol.179.8.5523. [DOI] [PubMed] [Google Scholar]
- Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2006;20:123–137. doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Feinstein DL, Galea E, Aquino DA, Li GC, Xu H, Reis DJ. Heat shock protein 70 suppresses astroglial-inducible nitric-oxide synthase expression by decreasing NFkappaB activation. J Biol Chem. 1996;271:17724–17732. doi: 10.1074/jbc.271.30.17724. [DOI] [PubMed] [Google Scholar]
- Fillinger MP, Rassias AJ, Guyre PM, et al. Glucocorticoid effects on the inflammatory and clinical responses to cardiac surgery. J Cardiothorac Vasc Anesth. 2002;16:163–169. doi: 10.1053/jcan.2002.31057. [DOI] [PubMed] [Google Scholar]
- Ganter MT, Ware LB, Howard M, Roux J, Gartland B, Matthay MA, Fleshner M, Pittet JF. Extracellular heat shock protein 72 is a marker of the stress protein response in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L354–L361. doi: 10.1152/ajplung.00405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Tsan MF. Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of tumor necrosis factor alpha release by murine macrophages. J Biol Chem. 2003;278:174–179. doi: 10.1074/jbc.M208742200. [DOI] [PubMed] [Google Scholar]
- Gastpar R, Gross C, Rossbacher L, Ellwart J, Riegger J, Multhoff G. The cell surface-localized heat shock protein 70 epitope TKD induces migration and cytolytic activity selectively in human NK cells. J Immunol. 2004;172:972–980. doi: 10.4049/jimmunol.172.2.972. [DOI] [PubMed] [Google Scholar]
- Giannessi D, Caselli C, Vitale RL, Crucean A, Murzi B, Ry SD, Vanini V, Biagini A. A possible cardioprotective effect of heat shock proteins during cardiac surgery in pediatric patients. Pharmacol Res. 2003;48:519–529. doi: 10.1016/s1043-6618(03)00193-2. [DOI] [PubMed] [Google Scholar]
- Gross C, Hansch D, Gastpar R, Multhoff G. Interaction of heat shock protein 70 peptide with NK cells involves the NK receptor CD94. Biol Chem. 2003;384:267–279. doi: 10.1515/BC.2003.030. [DOI] [PubMed] [Google Scholar]
- Gross C, Schmidt-Wolf IG, Nagaraj S, Gastpar R, Ellwart J, Kunz-Schughart LA, Multhoff G. Heat shock protein 70-reactivity is associated with increased cell surface density of CD94/CD56 on primary natural killer cells. Cell Stress Chaperones. 2003;8:348–360. doi: 10.1379/1466-1268(2003)008<0348:hspria>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Knowlton AA. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol. 2007;292:H3052–H3056. doi: 10.1152/ajpheart.01355.2006. [DOI] [PubMed] [Google Scholar]
- Hadley JS, Wang JE, Michaels LC, Dempsey CM, Foster SJ, Thiemermann C, Hinds CJ. Alterations in inflammatory capacity and TLR expression on monocytes and neutrophils after cardiopulmonary bypass. Shock. 2007;27:466–473. doi: 10.1097/01.shk.0000245033.69977.c5. [DOI] [PubMed] [Google Scholar]
- Halonen J, Halonen P, Jarvinen O, et al. Corticosteroids for the prevention of atrial fibrillation after cardiac surgery: a randomized controlled trial. JAMA. 2007;297:1562–1567. doi: 10.1001/jama.297.14.1562. [DOI] [PubMed] [Google Scholar]
- Hambsch J, Osmancik P, Bocsi J, Schneider P, Tarnok A. Neutrophil adhesion molecule expression and serum concentration of soluble adhesion molecules during and after pediatric cardiovascular surgery with or without cardiopulmonary bypass. Anesthesiology. 2002;96:1078–1085. doi: 10.1097/00000542-200205000-00009. [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Gupta S, Knowlton AA. Estrogen and regulation of heat shock protein expression in female cardiomyocytes: Cross-talk with NF kappa B signaling. J Mol Cell Cardiol. 2004;36:577–584. doi: 10.1016/j.yjmcc.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Hampton CR, Shimamoto A, Rothnie CL, Griscavage-Ennis J, Chong A, Dix DJ, Verrier ED, Pohlman TH. HSP70.1 and -70.3 are required for late-phase protection induced by ischemic preconditioning of mouse hearts. Am J Physiol Heart Circ Physiol. 2003;285:H866–H874. doi: 10.1152/ajpheart.00596.2002. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Sawa Y, Fukuyama N, Nakazawa H, Matsuda H. Preoperative glutamine administration induces heat-shock protein 70 expression and attenuates cardiopulmonary bypass-induced inflammatory response by regulating nitric oxide synthase activity. Circulation. 2002;106:2601–2607. doi: 10.1161/01.cir.0000035651.72240.07. [DOI] [PubMed] [Google Scholar]
- Hearse DJ. Myocardial protection during ischemia and reperfusion. Mol Cell Biochem. 1998;186:177–184. [PubMed] [Google Scholar]
- Hiesmayr MJ, Spittler A, Lassnigg A, et al. Alterations in the number of circulating leucocytes, phenotype of monocyte and cytokine production in patients undergoing cardiothoracic surgery. Clin Exp Immunol. 1999;115:315–323. doi: 10.1046/j.1365-2249.1999.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K. A comprehensive review of genetic association studies. Genet Med. 2002;4:45–61. doi: 10.1097/00125817-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Hutter MM, Sievers RE, Barbosa V, Wolfe CL. Heat-shock protein induction in rat hearts. A direct correlation between the amount of heat-shock protein induced and the degree of myocardial protection. Circulation. 1994;89:355–360. doi: 10.1161/01.cir.89.1.355. [DOI] [PubMed] [Google Scholar]
- Hutter JJ, Mestril R, Tam EK, Sievers RE, Dillmann WH, Wolfe CL. Overexpression of heat shock protein 72 in transgenic mice decreases infarct size in vivo. Circulation. 1996;94:1408–1411. doi: 10.1161/01.cir.94.6.1408. [DOI] [PubMed] [Google Scholar]
- Ito Y, Ando A, Ando H, Ando J, Saijoh Y, Inoko H, Fujimoto H. Genomic structure of the spermatid-specific hsp70 homolog gene located in the class III region of the major histocompatibility complex of mouse and man. J Biochem. 1998;124:347–353. doi: 10.1093/oxfordjournals.jbchem.a022118. [DOI] [PubMed] [Google Scholar]
- Jansen NJ, Oeveren W, Broek L, et al. Inhibition by dexamethasone of the reperfusion phenomena in cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1991;102:515–525. [PubMed] [Google Scholar]
- Jansen NJ, Oeveren W, Gu YJ, Vliet MH, Eijsman L, Wildevuur CR. Endotoxin release and tumor necrosis factor formation during cardiopulmonary bypass. Ann Thorac Surg. 1992;54:744–747. doi: 10.1016/0003-4975(92)91021-z. [DOI] [PubMed] [Google Scholar]
- Jayakumar J, Suzuki K, Khan M, et al. Gene therapy for myocardial protection: transfection of donor hearts with heat shock protein 70 gene protects cardiac function against ischemia–reperfusion injury. Circulation. 2000;102:III302–III306. doi: 10.1161/01.cir.102.suppl_3.iii-302. [DOI] [PubMed] [Google Scholar]
- Jayakumar J, Suzuki K, Sammut IA, et al. Heat shock protein 70 gene transfection protects mitochondrial and ventricular function against ischemia–reperfusion injury. Circulation. 2001;104:I303–I307. doi: 10.1161/hc37t1.094932. [DOI] [PubMed] [Google Scholar]
- Kalawski R, Bugajski P, Smielecki J, Wysocki H, Olszewski R, More R, Sheridan DJ, Siminiak T. Soluble adhesion molecules in reperfusion during coronary bypass grafting. Eur J Cardiothorac Surg. 1998;14:290–295. doi: 10.1016/s1010-7940(98)00197-3. [DOI] [PubMed] [Google Scholar]
- Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- Kimura F, Itoh H, Ambiru S, et al. Circulating heat-shock protein 70 is associated with postoperative infection and organ dysfunction after liver resection. Am J Surg. 2004;187:777–784. doi: 10.1016/j.amjsurg.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Kustanova GA, Murashev AN, Karpov VL, Margulis BA, Guzhova IV, Prokhorenko IR, Grachev SV, Evgen'ev MB. Exogenous heat shock protein 70 mediates sepsis manifestations and decreases the mortality rate in rats. Cell Stress Chaperones. 2006;11:276–286. doi: 10.1379/CSC-195R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 2005;280:23349–23355. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- Latchman DS. Heat shock proteins and cardiac protection. Cardiovasc Res. 2001;51:637–646. doi: 10.1016/s0008-6363(01)00354-6. [DOI] [PubMed] [Google Scholar]
- Lau SS, Griffin TM, Mestril R. Protection against endotoxemia by HSP70 in rodent cardiomyocytes. Am J Physiol Heart Circ Physiol. 2000;278:H1439–H1445. doi: 10.1152/ajpheart.2000.278.5.H1439. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- Lee KH, Lee CT, Kim YW, Han SK, Shim YS, Yoo CG. Heat shock protein 70 negatively regulates the heat-shock-induced suppression of the IkappaB/NF-kappaB cascade by facilitating IkappaB kinase renaturation and blocking its further denaturation. Exp Cell Res. 2005;307:276–284. doi: 10.1016/j.yexcr.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Lepore DA, Knight KR, Anderson RL, Morrison WA. Role of priming stresses and Hsp70 in protection from ischemia–reperfusion injury in cardiac and skeletal muscle. Cell Stress Chaperones. 2001;6:93–96. doi: 10.1379/1466-1268(2001)006<0093:ropsah>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Chen S, Lu E, Li Y. Ischemic preconditioning improves preservation with cold blood cardioplegia in valve replacement patients. Eur J Cardiothorac Surg. 1999;15:653–657. doi: 10.1016/s1010-7940(99)00070-6. [DOI] [PubMed] [Google Scholar]
- Liakopoulos OJ, Schmitto JD, Kazmaier S, Brauer A, Quintel M, Schoendube FA, Dorge H. Cardiopulmonary and systemic effects of methylprednisolone in patients undergoing cardiac surgery. Ann Thorac Surg. 2007;84:110–118. doi: 10.1016/j.athoracsur.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003;33:177–182. doi: 10.1038/ng1071. [DOI] [PubMed] [Google Scholar]
- Malagon I, Hogenbirk K, Pelt J, Hazekamp MG, Bovill JG. Effect of dexamethasone on postoperative cardiac troponin T production in pediatric cardiac surgery. Intensive Care Med. 2005;31:1420–1426. doi: 10.1007/s00134-005-2788-9. [DOI] [PubMed] [Google Scholar]
- Mandal K, Torsney E, Poloniecki J, Camm AJ, Xu Q, Jahangiri M. Association of high intracellular, but not serum, heat shock protein 70 with postoperative atrial fibrillation. Ann Thorac Surg. 2005;79:865–871. doi: 10.1016/j.athoracsur.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;95:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti-Carvajal A, Salanti G, Cardona AF (2008) Human recombinant activated protein C for severe sepsis. Cochrane Database Syst Rev (1):CD004388 [DOI] [PubMed]
- Massa C, Melani C, Colombo MP. Chaperon and adjuvant activity of hsp70: Different natural killer requirement for cross-priming of chaperoned and bystander antigens. Cancer Res. 2005;65:7942–7949. doi: 10.1158/0008-5472.CAN-05-0377. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- McBride WT, Armstrong MA, Gilliland H, McMurray TJ. The balance of pro and anti-inflammatory cytokines in plasma and bronchoalveolar lavage (BAL) at paediatric cardiac surgery. Cytokine. 1996;8:724–729. doi: 10.1006/cyto.1996.0096. [DOI] [PubMed] [Google Scholar]
- McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998;273:7523–7528. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- Millar DG, Garza KM, Odermatt B, Elford AR, Ono N, Li Z, Ohashi PS. Hsp70 promotes antigen-presenting cell function and converts T-cell tolerance to autoimmunity in vivo. Nat Med. 2003;9:1469–1476. doi: 10.1038/nm962. [DOI] [PubMed] [Google Scholar]
- Millo JL, Schultz MJ, Williams C, Weverling GJ, Ringrose T, Mackinlay CI, Poll T, Garrard CS. Compartmentalisation of cytokines and cytokine inhibitors in ventilator-associated pneumonia. Intensive Care Med. 2004;30:68–74. doi: 10.1007/s00134-003-2060-0. [DOI] [PubMed] [Google Scholar]
- Morariu AM, Loef BG, Aarts LP, Rietman GW, Rakhorst G, Oeveren W, Epema AH. Dexamethasone: benefit and prejudice for patients undergoing on-pump coronary artery bypass grafting: a study on myocardial, pulmonary, renal, intestinal, and hepatic injury. Chest. 2005;128:2677–2687. doi: 10.1378/chest.128.4.2677. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Jennen L, Schmidt J, Ellwart J, Issels R. Heat shock protein 72 on tumor cells: A recognition structure for natural killer cells. J Immunol. 1997;158:4341–4350. [PubMed] [Google Scholar]
- Multhoff G, Pfister K, Gehrmann M, Hantschel M, Gross C, Hafner M, Hiddemann W. A 14-mer Hsp70 peptide stimulates natural killer (NK) cell activity. Cell Stress Chaperones. 2001;6:337–344. doi: 10.1379/1466-1268(2001)006<0337:amhpsn>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mycko MP, Cwiklinska H, Szymanski J, et al. Inducible heat shock protein 70 promotes myelin autoantigen presentation by the HLA class II. J Immunol. 2004;172:202–213. doi: 10.4049/jimmunol.172.1.202. [DOI] [PubMed] [Google Scholar]
- Nathan N, Preux PM, Feiss P, Denizot Y. Plasma interleukin-4, interleukin-10, and interleukin-13 concentrations and complications after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2000;14:156–160. doi: 10.1016/s1053-0770(00)90010-7. [DOI] [PubMed] [Google Scholar]
- Oehler R, Pusch E, Zellner M, et al. Cell type-specific variations in the induction of hsp70 in human leukocytes by feverlike whole body hyperthermia. Cell Stress Chaperones. 2001;6:306–315. doi: 10.1379/1466-1268(2001)006<0306:ctsvit>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi Y, Taniguchi K, Matsumoto H, Ishihara A, Ohira Y, Roy RR. Muscle type-specific response of HSP60, HSP72, and HSC73 during recovery after elevation of muscle temperature. J Appl Physiol. 2002;92:1097–1103. doi: 10.1152/japplphysiol.00739.2001. [DOI] [PubMed] [Google Scholar]
- Okubo S, Wildner O, Shah MR, Chelliah JC, Hess ML, Kukreja RC. Gene transfer of heat-shock protein 70 reduces infarct size in vivo after ischemia/reperfusion in the rabbit heart. Circulation. 2001;103:877–881. doi: 10.1161/01.cir.103.6.877. [DOI] [PubMed] [Google Scholar]
- Palatianos GM, Balentine G, Papadakis EG, Triantafillou CD, Vassili MI, Lidoriki A, Dinopoulos A, Astras GM. Neutrophil depletion reduces myocardial reperfusion morbidity. Ann Thorac Surg. 2004;77:956–961. doi: 10.1016/j.athoracsur.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Park KJ, Gaynor RB, Kwak YT. Heat shock protein 27 association with the I kappa B kinase complex regulates tumor necrosis factor alpha-induced NF-kappa B activation. J Biol Chem. 2003;278:35272–35278. doi: 10.1074/jbc.M305095200. [DOI] [PubMed] [Google Scholar]
- Plumier JC, Ross BM, Currie RW, Angelidis CE, Kazlaris H, Kollias G, Pagoulatos GN. Transgenic mice expressing the human heat shock protein 70 have improved post-ischemic myocardial recovery. J Clin Invest. 1995;95:1854–1860. doi: 10.1172/JCI117865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG, Muthana M, Calderwood SK. The dual immunoregulatory roles of stress proteins. Trends Biochem Sci. 2008;33:71–79. doi: 10.1016/j.tibs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Prondzinsky R, Knupfer A, Loppnow H, et al. Surgical trauma affects the proinflammatory status after cardiac surgery to a higher degree than cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2005;129:760–766. doi: 10.1016/j.jtcvs.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Cohen IR. Heat shock proteins as endogenous adjuvants in sterile and septic inflammation. J Immunol. 2005;175:2777–2782. doi: 10.4049/jimmunol.175.5.2777. [DOI] [PubMed] [Google Scholar]
- Rafiee P, Shi Y, Pritchard KA, Jr, et al. Cellular redistribution of inducible Hsp70 protein in the human and rabbit heart in response to the stress of chronic hypoxia: Role of protein kinases. J Biol Chem. 2003;278:43636–43644. doi: 10.1074/jbc.M212993200. [DOI] [PubMed] [Google Scholar]
- Ran R, Lu A, Zhang L, et al. Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes Dev. 2004;18:1466–1481. doi: 10.1101/gad.1188204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico AI, Girones N, Fresno M, Alonso C, Requena JM. The heat shock proteins, Hsp70 and Hsp83, of leishmania infantum are mitogens for mouse B cells. Cell Stress Chaperones. 2002;7:339–346. doi: 10.1379/1466-1268(2002)007<0339:thspha>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Chavez FA, Wheeler H, Lindberg G, Munford RS, O'Keefe GE. Regional and systemic cytokine responses to acute inflammation of the vermiform appendix. Ann Surg. 2003;237:408–416. doi: 10.1097/01.SLA.0000055274.56407.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson-Malt S, Afrane B, El Barbary M (2007) Prophylactic steroids for pediatric open heart surgery. Cochrane Database Syst Rev (4): CD005550 [DOI] [PubMed]
- Rothenburger M, Tjan TD, Schneider M, et al. The impact of the pro- and anti-inflammatory immune response on ventilation time after cardiac surgery. Cytometry B Clin Cytom. 2003;53:70–74. doi: 10.1002/cyto.b.10027. [DOI] [PubMed] [Google Scholar]
- Rubens FD, Nathan H, Labow R, Williams KS, Wozny D, Karsh J, Ruel M, Mesana T. Effects of methylprednisolone and a biocompatible copolymer circuit on blood activation during cardiopulmonary bypass. Ann Thorac Surg. 2005;79:655–665. doi: 10.1016/j.athoracsur.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Sablotzki A, Friedrich I, Muhling J, Dehne MG, Spillner J, Silber RE, Czeslik E. The systemic inflammatory response syndrome following cardiac surgery: Different expression of proinflammatory cytokines and procalcitonin in patients with and without multiorgan dysfunctions. Perfusion. 2002;17:103–109. doi: 10.1177/026765910201700206. [DOI] [PubMed] [Google Scholar]
- Saito K, Dai Y, Ohtsuka K. Enhanced expression of heat shock proteins in gradually dying cells and their release from necrotically dead cells. Exp Cell Res. 2005;310:229–236. doi: 10.1016/j.yexcr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Satoh M, Shimoda Y, Akatsu T, Ishikawa Y, Minami Y, Nakamura M. Elevated circulating levels of heat shock protein 70 are related to systemic inflammatory reaction through monocyte toll signal in patients with heart failure after acute myocardial infarction. Eur J Heart Fail. 2006;8:810–815. doi: 10.1016/j.ejheart.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Schmitt JP, Schunkert H, Birnbaum DE, Aebert H. Kinetics of heat shock protein 70 synthesis in the human heart after cold cardioplegic arrest. Eur J Cardiothorac Surg. 2002;22:415–420. doi: 10.1016/s1010-7940(02)00327-5. [DOI] [PubMed] [Google Scholar]
- Schroder O, Schulte KM, Ostermann P, Roher HD, Ekkernkamp A, Laun RA. Heat shock protein 70 genotypes HSPA1B and HSPA1L influence cytokine concentrations and interfere with outcome after major injury. Crit Care Med. 2003;31:73–79. doi: 10.1097/00003246-200301000-00011. [DOI] [PubMed] [Google Scholar]
- Shaw AD, Stafford-Smith M, White WD, et al. The effect of aprotinin on outcome after coronary-artery bypass grafting. N Engl J Med. 2008;358:784–793. doi: 10.1056/NEJMoa0707768. [DOI] [PubMed] [Google Scholar]
- Shi Y, Tu Z, Tang D, Zhang H, Liu M, Wang K, Calderwood SK, Xiao X. The inhibition of LPS-induced production of inflammatory cytokines by HSP70 involves inactivation of the NF-kappaB pathway but not the MAPK pathways. Shock. 2006;26:277–284. doi: 10.1097/01.shk.0000223134.17877.ad. [DOI] [PubMed] [Google Scholar]
- Singh R, Kolvraa S, Bross P, Jensen UB, Gregersen N, Tan Q, Knudsen C, Rattan SI. Reduced heat shock response in human mononuclear cells during aging and its association with polymorphisms in HSP70 genes. Cell Stress Chaperones. 2006;11:208–215. doi: 10.1379/CSC-184R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton KD, Wischmeyer PE. Effects of HSP70.1/3 gene knockout on acute respiratory distress syndrome and the inflammatory response following sepsis. Am J Physiol Lung Cell Mol Physiol. 2006;290:L956–L961. doi: 10.1152/ajplung.00466.2005. [DOI] [PubMed] [Google Scholar]
- Singleton KD, Serkova N, Beckey VE, Wischmeyer PE. Glutamine attenuates lung injury and improves survival after sepsis: role of enhanced heat shock protein expression. Crit Care Med. 2005;33:1206–1213. doi: 10.1097/01.ccm.0000166357.10996.8a. [DOI] [PubMed] [Google Scholar]
- Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- St Rammos K, Koullias GJ, Hassan MO, Argyrakis NP, Voucharas CG, Scarupa SJ, Cowte TG. Low preoperative HSP70 atrial myocardial levels correlate significantly with high incidence of postoperative atrial fibrillation after cardiac surgery. Cardiovasc Surg. 2002;10:228–232. doi: 10.1016/s0967-2109(01)00138-7. [DOI] [PubMed] [Google Scholar]
- Storti S, Vittorini S, Iascone MR, et al. Analysis of the variation in the hsp70–1 and hsp90alpha mRNA expression in human myocardial tissue that has undergone surgical stress. Cell Stress Chaperones. 2003;8:18–25. doi: 10.1379/1466-1268(2003)8<18:aotvit>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Murtuza B, Sammut IA, et al. Heat shock protein 72 enhances manganese superoxide dismutase activity during myocardial ischemia–reperfusion injury, associated with mitochondrial protection and apoptosis reduction. Circulation. 2002;106:I270–I276. [PubMed] [Google Scholar]
- Szerafin T, Hoetzenecker K, Hacker S, et al. Heat shock proteins 27, 60, 70, 90alpha, and 20S proteasome in on-pump versus off-pump coronary artery bypass graft patients. Ann Thorac Surg. 2008;85:80–87. doi: 10.1016/j.athoracsur.2007.06.049. [DOI] [PubMed] [Google Scholar]
- Taggart DP, Bakkenist CJ, Biddolph SC, Graham AK, McGee JO. Induction of myocardial heat shock protein 70 during cardiac surgery. J Pathol. 1997;182:362–366. doi: 10.1002/(SICI)1096-9896(199707)182:3<362::AID-PATH879>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Tang D, Kang R, Xiao W, Wang H, Calderwood SK, Xiao X. The anti-inflammatory effects of heat shock protein 72 involve inhibition of high-mobility-group box 1 release and proinflammatory function in macrophages. J Immunol. 2007;179:1236–1244. doi: 10.4049/jimmunol.179.2.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnok A, Hambsch J, Emmrich F, Sack U, Son J, Bellinghausen W, Borte M, Schneider P. Complement activation, cytokines, and adhesion molecules in children undergoing cardiac surgery with or without cardiopulmonary bypass. Pediatr Cardiol. 1999;20:113–125. doi: 10.1007/s002469900417. [DOI] [PubMed] [Google Scholar]
- Temple SE, Cheong KY, Ardlie KG, Sayer D, Waterer GW. The septic shock associated HSPA1B1267 polymorphism influences production of HSPA1A and HSPA1B. Intensive Care Med. 2004;30:1761–1767. doi: 10.1007/s00134-004-2359-5. [DOI] [PubMed] [Google Scholar]
- Theriault JR, Mambula SS, Sawamura T, Stevenson MA, Calderwood SK. Extracellular HSP70 binding to surface receptors present on antigen presenting cells and endothelial/epithelial cells. FEBS Lett. 2005;579:1951–1960. doi: 10.1016/j.febslet.2005.02.046. [DOI] [PubMed] [Google Scholar]
- Tobian AA, Canaday DH, Harding CV. Bacterial heat shock proteins enhance class II MHC antigen processing and presentation of chaperoned peptides to CD4+ T cells. J Immunol. 2004;173:5130–5137. doi: 10.4049/jimmunol.173.8.5130. [DOI] [PubMed] [Google Scholar]
- Tomic V, Russwurm S, Moller E, et al. Transcriptomic and proteomic patterns of systemic inflammation in on-pump and off-pump coronary artery bypass grafting. Circulation. 2005;112:2912–2920. doi: 10.1161/CIRCULATIONAHA.104.531152. [DOI] [PubMed] [Google Scholar]
- Trost SU, Omens JH, Karlon WJ, Meyer M, Mestril R, Covell JW, Dillmann WH. Protection against myocardial dysfunction after a brief ischemic period in transgenic mice expressing inducible heat shock protein 70. J Clin Invest. 1998;101:855–862. doi: 10.1172/JCI265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan MF, Gao B. Endogenous ligands of toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- Molle W, Wielockx B, Mahieu T, Takada M, Taniguchi T, Sekikawa K, Libert C. HSP70 protects against TNF-induced lethal inflammatory shock. Immunity. 2002;16:685–695. doi: 10.1016/s1074-7613(02)00310-2. [DOI] [PubMed] [Google Scholar]
- Vega VL, Rodriguez-Silva M, Frey T, et al. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol. 2008;180:4299–4307. doi: 10.4049/jimmunol.180.6.4299. [DOI] [PubMed] [Google Scholar]
- Wang S, Diller KR, Aggarwal SJ. Kinetics study of endogenous heat shock protein 70 expression. J Biomech Eng. 2003;125:794–797. doi: 10.1115/1.1632522. [DOI] [PubMed] [Google Scholar]
- Wang R, Kovalchin JT, Muhlenkamp P, Chandawarkar RY. Exogenous heat shock protein 70 binds macrophage lipid raft microdomain and stimulates phagocytosis, processing, and MHC-II presentation of antigens. Blood. 2006;107:1636–1642. doi: 10.1182/blood-2005-06-2559. [DOI] [PubMed] [Google Scholar]
- Waterer GW, ElBahlawan L, Quasney MW, Zhang Q, Kessler LA, Wunderink RG. Heat shock protein 70–2+1267 AA homozygotes have an increased risk of septic shock in adults with community-acquired pneumonia. Crit Care Med. 2003;31:1367–1372. doi: 10.1097/01.CCM.0000063088.86079.03. [DOI] [PubMed] [Google Scholar]
- Weiss YG, Bromberg Z, Raj N, Raphael J, Goloubinoff P, Ben-Neriah Y, Deutschman CS. Enhanced heat shock protein 70 expression alters proteasomal degradation of IkappaB kinase in experimental acute respiratory distress syndrome. Crit Care Med. 2007;35:2128–2138. doi: 10.1097/01.ccm.0000278915.78030.74. [DOI] [PubMed] [Google Scholar]
- Wheeler DS, Fisher LE, Jr, Catravas JD, Jacobs BR, Carcillo JA, Wong HR. Extracellular hsp70 levels in children with septic shock. Pediatr Crit Care Med. 2005;6:308–311. doi: 10.1097/01.PCC.0000161075.97355.2E. [DOI] [PubMed] [Google Scholar]
- Whittall T, Wang Y, Younson J, Kelly C, Bergmeier L, Peters B, Singh M, Lehner T. Interaction between the CCR5 chemokine receptors and microbial HSP70. Eur J Immunol. 2006;36:2304–2314. doi: 10.1002/eji.200635953. [DOI] [PubMed] [Google Scholar]
- Williams RS, Benjamin IJ. Protective responses in the ischemic myocardium. J Clin Invest. 2000;106:813–818. doi: 10.1172/JCI11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, Benjamin IJ. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999;18:5943–5952. doi: 10.1093/emboj/18.21.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita N, Hoshida S, Nishida M, Igarashi J, Aoki K, Hori M, Kuzuya T, Tada M. Time course of tolerance to ischemia-reperfusion injury and induction of heat shock protein 72 by heat stress in the rat heart. J Mol Cell Cardiol. 1997;29:1815–1821. doi: 10.1006/jmcc.1997.0416. [DOI] [PubMed] [Google Scholar]
- Yared JP, Bakri MH, Erzurum SC, Moravec CS, Laskowski DM, Wagoner DR, Mascha E, Thornton J. Effect of dexamethasone on atrial fibrillation after cardiac surgery: Prospective, randomized, double-blind, placebo-controlled trial. J Cardiothorac Vasc Anesth. 2007;21:68–75. doi: 10.1053/j.jvca.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Ye Z, Gan YH. Flagellin contamination of recombinant heat shock protein 70 is responsible for its activity on T cells. J Biol Chem. 2007;282:4479–4484. doi: 10.1074/jbc.M606802200. [DOI] [PubMed] [Google Scholar]
- Yoo CG, Lee S, Lee CT, Kim YW, Han SK, Shim YS. Anti-inflammatory effect of heat shock protein induction is related to stabilization of I kappa B alpha through preventing I kappa B kinase activation in respiratory epithelial cells. J Immunol. 2000;164:5416–5423. doi: 10.4049/jimmunol.164.10.5416. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wang W, Qian L. Hsp70 may protect cardiomyocytes from stress-induced injury by inhibiting fas-mediated apoptosis. Cell Stress Chaperones. 2007;12:83–95. doi: 10.1379/CSC-231R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]