Abstract
Both protein kinase C (PKC) activation and Hsp70 expression have been shown to be key components for exercise-mediated myocardial protection during ischemia–reperfusion injury. Given that Hsp70 has been shown to undergo inducible phosphorylation in striated muscle and liver, we hypothesized that PKC may regulate myocardial Hsp70 function and subsequent exercise-conferred cardioprotection through this phosphorylation. Hence, acute exercise of male Sprague–Dawley rats (30 m/min for 60 min at 2% grade) was employed to assess the role of PKC and its selected isoforms in phosphorylation of Hsp70 and protection of the myocardium during ischemia–reperfusion injury. It was observed that administration of the PKC inhibitor chelerythrine chloride (5 mg/kg) suppressed the activation of three exercise-induced PKC isoforms (PKCα, PKCδ, and PKCɛ) and attenuated the exercise-mediated reduction of myocardial infarct size during ischemia–reperfusion injury. While this study also demonstrated that exercise led to an alteration in the phosphorylation status of Hsp70, this posttranslational modification appeared to be dissociated from PKC activation, as exercise-induced phosphorylation of Hsp70 was unchanged following inhibition of PKC. Taken together, these results indicate that selected isoforms of PKC play an important role in exercise-mediated protection of the myocardium during ischemia–reperfusion injury. However, exercise-induced phosphorylation of Hsp70 does not appear to be a mechanism by which PKC induces this cardioprotective effect.
Keywords: Signal transduction, Rat, Heart, Treadmill running, Heat shock proteins
Introduction
Acute physical exercise has been shown to improve functional recovery, enhance contractility, and preserve metabolic function of the myocardium during ischemia–reperfusion injury (I/R-injury) (Brown et al. 2005b; Locke et al. 1995; Paroo et al. 2002; Yamashita et al. 1999). While the intracellular mechanism(s) involved in this exercise-conferred cardioprotection remain unclear, several studies have indicated an important role for the Ca2+-dependent intracellular protein kinase, protein kinase C (PKC) (Yamashita et al. 2001). It has been demonstrated that prior administration of the PKC inhibitor chelerythrine chloride (CHEL), attenuates the exercise-mediated reduction of infarct size within the myocardium during I/R-injury (Carson and Korzick 2003; Yamashita et al. 2001).
In myocardial cells, PKC is a ubiquitously expressed serine-threonine kinase that is divided into a family of isozymes consisting of three major subgroups: the conventional, calcium-dependent (α, βI, βII, and γ); the novel, calcium-independent (δ, ɛ, η, θ, and possibly μ); and the atypical PKCs (ζ and τ/λ) (for review, see Newton 1995). Selected PKC isoforms have been suggested to play important roles in both the early (2–3 h) and late (24–48 h) phases of cardioprotection following sublethal cardiac perturbations, including preischemia preconditioning (Carroll and Yellon 1999; Pagliaro et al. 2001). Carson and Korzick (2003) demonstrated that acute exercise mediates the activation of several of these cardioprotective PKC isoforms immediately and 24 h postexercise in a biphasic manner reminiscent of these windows of protection. PKC has been implicated in the posttranslational phosphorylation and regulation of several key cardioprotective cytosolic proteins (Ping et al. 1999) and one potential target is the inducible member of the 70-kDa heat-shock family, Hsp70, which has been demonstrated to be at least partially responsible for exercise-induced myocardial protection during I/R-injury (Paroo et al. 2002). It has been reported that activation of PKC is not responsible for the exercise-induced, transcriptional expression of Hsp70 (Melling et al. 2004); however, it may play a role in the posttranslational modification of cardiac Hsp70 and its subsequent function (Joyeux et al. 1997). In other tissues, including the liver (Gonzalez and Manso 2004), and extensor digitorum longus and soleus muscles (Hernando and Manso 1997), it has been shown that Hsp70 undergoes posttranslational modification through phosphorylation following exercise. Further, the time course of Hsp70 phosphorylation (Gonzalez and Manso 2004) is similar to that of the transient activation of PKC following exercise (Carson and Korzick 2003). Hence, this may represent a mechanism by which PKC could regulate the functional capacity of myocardial Hsp70 following exercise. Indeed, posttranslational modifications have been reported to regulate other heat-shock proteins. For instance, phosphorylation of myocardial Hsp27 is an important regulatory step for its function in the protection of actin fragmentation during I/R-injury (Loktionova and Kabakov 1998).
In the present investigation, we examined the possible role of PKC in the phosphorylation of Hsp70 and the significance of this potential posttranslational modification on the exercise-mediated reduction of myocardial infarct size during I/R-injury. To do so, we examined the activation of specific PKC isoforms at both early (immediately postexercise) and late (24 h postexercise) time points of PKC activation to assess their influence on the posttranslational modification of Hsp70 and their role in the delayed cardioprotective effects of exercise. We hypothesized that PKC-mediated phosphorylation of Hsp70 would represent a mechanism by which PKC would confer cardioprotection following exercise.
Methods and materials
Animal characteristics This study was approved by the University of Western Ontario Council on Animals Care and was performed in accordance with the Guidelines of the Canadian Council on Animal Care. Adult (12-week-old) male Sprague–Dawley (400–450 g) rats obtained from Charles River (St. Constant, Quebec) were housed in standard rat cages maintained at constant temperature and humidity with a 12:12 h light–dark cycle. Rats were fed and watered ad libitum.
Experimental protocol Forty animals were randomly assigned to one of three experimental groups (groups 1, 2, and 3). Group 1 (n = 15) and group 2 (n = 15) underwent 60 min of continuous running (30 m/min; 2% grade) on a motor-driven treadmill (Melling et al. 2004), while group 3 (n = 10) consisted of control (CON) animals, which were handled similarly to the exercised groups (Ex) but did not undergo the exercise protocol. All animals underwent a light exercise familiarization on the treadmill 5 and 3 days prior to the exercise protocol. Group 1 and group 2 animals were either treated with a PKC inhibitor (PKC−) CHEL [5 mg/kg body weight in 5% dimethyl sulphoxide (DMSO)] or a vehicle treatment (SHAM; 5% DMSO), respectively. Drug treatments were administered 10 min prior to the initiation of the exercise protocol as per previously reported data (Melling et al. 2004; Nadruz et al. 2004). Animals in groups 1 and 2 were killed either immediately (5–10 min postexercise; n = 5/group) or 24 h (n = 5/group) following the completion of the exercise protocol to examine the activation of specific PKC isoforms and Hsp70 content and phosphorylation. The remaining five animals in groups 1 and 2 were killed 24 h postexercise and underwent the I/R-injury protocol (see below) to assess the effect of exercise on infarct size. At the time of death, animals were anesthetized via an intraperitoneal injection of sodium pentobarbital (Somnotol; 65 mg/kg) and the hearts were extirpated. Colonic temperatures were taken prior to and immediately following the exercise protocol.
Ischemia–reperfusion protocol Animals killed 24 h following the exercise were euthanized and hearts were isolated, placed in cold Krebs–Henseleit buffer, and mounted on a cannula by the aorta for retrograde perfusion with the use of a modified Langendorff procedure (Paroo et al. 2002). Perfusion was maintained at 10 mL/min with 95% O2/5% CO2-gassed Krebs–Henseleit buffer at 37°C. Hearts were paced at 300 bpm throughout the experiment and were equilibrated for 30 min prior to global ischemia, which was induced by terminating flow for 30 min. Hearts were subsequently reperfused for 30 min.
Determination of infarct size Following the ischemia–reperfusion protocol, hearts were immediately frozen and stored overnight at −70°C. To determine infarct size, hearts were serially sliced into ∼2-mm sections and incubated in 1% triphenyltetrazolium chloride for 15 min. Tissue slices were then fixed in 10% formalin solution. Tissue staining brick red (noninfarcted tissue) and white (infarcted tissue) were scanned and then quantified using Scion Image Software. Infarct area ratio was defined as [infarct area]/[total left ventricular area] (Nakajima et al. 2001).
Cellular fractionation and protein quantification Membrane/cytosolic and whole cell extracts were prepared according to Carson and Korzick (2003) and Locke et al. (1995), respectively. Total protein concentrations were determined using the Bradford protein method (Bradford 1976).
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Western blotting Fractionated cellular samples were mixed with equal volumes of sample buffer [0.5 M Tris Base, 13% glycerol, 0.5% sodium dodecyl sulfate (SDS), 13% β-mercaptoethanol, Bromophenol Blue] and separated according to their molecular weight on gels consisting of 12% acrylamide separating gel overlaid by 4% acrylamide stacking gel. A molecular weight standard (BIO-RAD precision marker standard) and a recombinant control of each protein being detected were run concurrently on each gel for accurate quantification of the protein, as well as proper determination of its molecular weight. After electrophoresis, the proteins were transferred to nitrocellulose membranes and blocked in 3% milk blotto in Tris-buffered saline (TBS) for 2 h and then washed twice in Tris-Tween buffered saline (TTBS) (0.01% Tween-20 in TBS) for 5 min each wash. Membranes were then incubated in primary antibody specific to either Hsp70 [Anti-Hsp70 (1:5,000) polyclonal antibody, Stressgen SPA-901], PKCɛ, PKCα, PKCβ, PKCγ, PKCδ, PKCη, PKCθ, PKCι, PKCλ [Anti-PKCɛ (1:1,000), -PKCα (1:1,000), -PKCβ (1:250), -PKCγ (1:1,000), -PKCδ (1:500), -PKCη (1:250), -PKCθ (1:250), -PKCι (1:250), -PKCλ (1:250) polyclonal antibodies, Tranduction 611–421], overnight in TTBS (2% blotto). Following incubation, membranes were washed in TTBS and treated with secondary antibody according to the manufacturers’ instructions. For all isoforms of PKC, antibody detection was performed using the enhanced chemiluminescence method (ECL, Amersham), while Hsp70 antibody detection was performed colorimetrically (BIORAD). To ensure equal protein loading, the Ponceau staining method was performed as described by Ping et al. (1997). Briefly, membranes were incubated in Ponceau S solution (Sigma) for 5 min immediately following antibody detection. The largest nonspecific band was subjected to densitometric analysis and the average density was determined across the lanes. Antibody detection was then normalized to the ratio of Ponceau stain density in that lane and the average density of the Ponceau stain and represented as a percentage of the recombinant standard of that protein.
Two-dimensional electrophoresis and detection of Hsp70 phosphorylation Two-dimensional (2D) electrophoresis and immunoblotting procedures were performed according to Gonzalez and Manso (2004). Briefly, approximately 80 μg of protein, from heart homogenates prepared in lysis buffer (10 mM KCl, 10 mM Tris/HCl, pH 7.6, 180 mM 2-mercaptoethanol, 50% glycerol and 1 mM phenylmethylsulfonyflouride), were separated electrophoretically through isoelectric focusing (IEF) gels using a pH range of 4–6.5. IEF gels were run with a maximum current of 0.33 mA/gel for the duration of 18 h at 800 V, followed by 30 min at 900 V. At the completion of the run, each gel was equilibrated for 10 min in 60 mM Tris/HCl pH 6.8, 2% SDS, 100 mM dithiothreitol, and 10% glycerol prior to being loaded onto a SDS-polyacrylamide gel electrophoresis (PAGE) for the second dimension electrophoresis. SDS-PAGE was performed using 12% acrylamide gels polymerized in the presence of 15% glycerol. After electrophoresis, the proteins were transferred to nitrocellulose membranes and Hsp70 detection was performed as described above.Phosphorylated Hsp70 was assessed by examining the transition of Hsp70 variants towards less acidic isoelectric points (Hernando and Manso 1997). Briefly, heart tissue homogenates were incubated at 35°C for 2 h with or without alkaline phosphatase (AP) from calf intestine (Sigma-Aldrich) at a concentration of 50 mU/μg protein. Following incubation, heart homogenates were separated through IEF and then subjected to SDS-PAGE and transferred to nitrocellulose membranes for Hsp70 detection, as described above.
Statistical analyses All blots were quantified using Scion Image Analysis software. Results are reported as mean ± SE and values were compared using a one-way analysis of variance. Upon confirmation of a significant main effect, individual differences were determined with the use of least squares difference post hoc test. A value of p < 0.05 was considered significant.
Results
Animal characteristics All Ex animals completed the 60-min moderate-intensity exercise protocol and demonstrated a significant elevation in colonic temperature immediately postexercise (Table 1; p < 0.05). This increase in colonic temperature was not different between the Ex groups, indicating that administration of CHEL prior to exercise did not influence their ability to perform the exercise protocol.
Table 1.
Animal weight and colonic temperatures postexercise
| Group | Body mass (g) | Temperature (°C) | |
|---|---|---|---|
| Pre-exercise | Postexercise | ||
| CON | 408.6 ± 4.52 | 37.8 ± .1 | – |
| Ex SHAM | 406.1 ± 4.51 | 37.6 ± 0.1 | 40.0 ± 0.9a |
| Ex PKC(−) | 397.9 ± 4.46 | 37.2 ± 0.1 | 40.5 ± 1.1a |
Values are means ± SE.
CON control, Ex SHAM vehicle-treated animals exercised for 60 min at 30 m/min at 2% grade, EX PKC(−) exercised in the presence of the PKC inhibitor CHEL; see text for details
aSignificantly different from pre-exercise temperatures (p < 0.05)
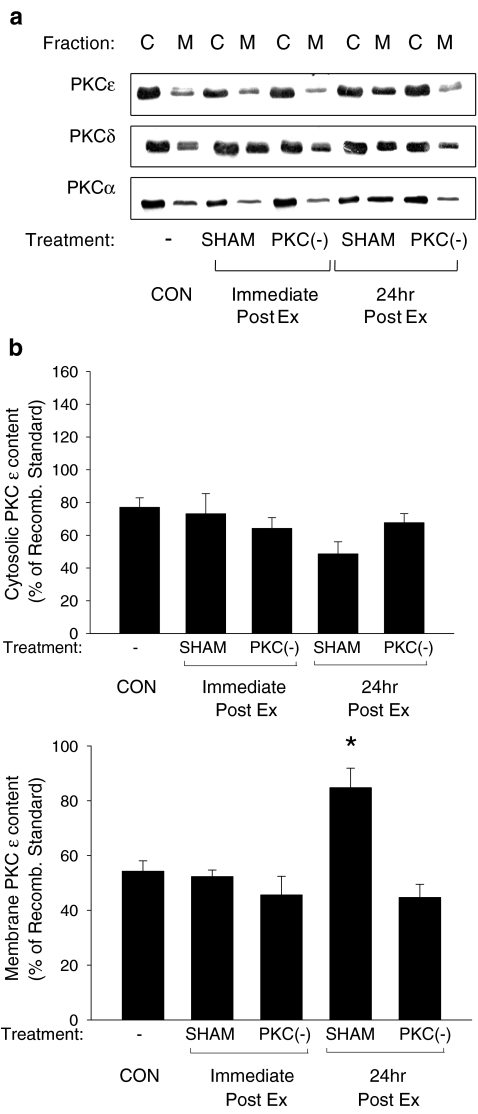
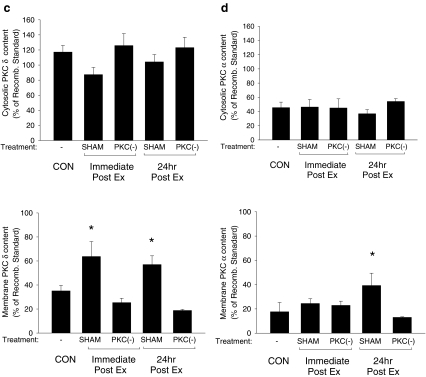
Translocation of PKC isoforms following exercise Immunoblots probed with antibodies specific to different PKC isoforms were used to examine their subcellular localization following exercise. Following the exercise protocol, PKCɛ (Fig. 1B) and PKCα (Fig. 1D) demonstrated a significant increase in the membrane fraction at 24 h postexercise only, while membrane levels of PKCδ were significantly elevated both immediately and 24 h postexercise (Fig. 1C). Total amounts of individual PKC isoforms (cytosolic plus membrane fractions) did not change following acute exercise, nor did the subcellular localization of PKCγ, PKCη, PKCθ, PKCι, and PKCλ (data not shown). Administration of the PKC inhibitor CHEL suppressed the exercise-induced translocations of PKCδ, PKCɛ, and PKCα (Fig. 1), indicating the efficacy of the PKC inhibitor.
Fig. 1.
Translocation of myocardial PKCɛ, PKCδ, and PKCα following acute exercise. A Representative blots of PKC isoforms of cytosol (C) and membrane (M) fractions in control (CON) and exercised (Ex) animals are shown. PKCɛ (B), PKCδ (C), and PKCα (D) translocation in vehicle-treated (SHAM) and PKC-inhibited (PKC(−)) animals was examined immediately and 24 h following the completion of the exercise protocol (Ex; 30 m/min, 60 min at 2% grade) using commercially available antibodies on cytosol (C) and membrane (M) fractions. Quantitative analysis of PKCɛ, PKCδ, and PKCα are represented as a percentage of a recombinant PKCɛ, PKCδ, and PKCα standards, respectively. Data are means ± SE of five animals per group. Asterisks, significantly different from control (CON) membrane fraction, p < 0.05
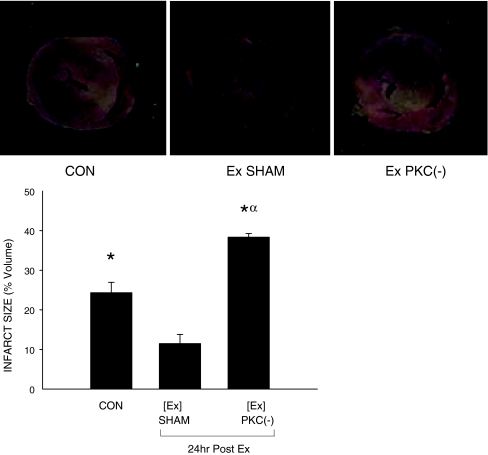
Infarct size Infarct size (percent of total ventricular area) was significantly reduced from 50% in the CON group to 20% in the Ex group (Fig. 2). Prior administration of the PKC inhibitor CHEL increased infarct size in the exercise-treated animals to 75%, a significant elevation compared to CON and SHAM-treated animals.
Fig. 2.
Effect of PKC inhibition on myocardial infarct size following acute exercise. Determination of infarct ratio in vehicle-treated (SHAM) and PKC-inhibited (PKC(−)) exercised (Ex) animals (30 m/min for 60 min; 2% grade) was performed using triphenyltetrazolium chloride staining following I/R-injury. Tissue-stained brick red (noninfarcted tissue) and white (infarcted tissue) were scanned and were quantified using Scion Image Software. Infarct area ratio was defined as [infarct area]/[total left ventricular area]. Data are means ± SE of five animals per group. α Significantly different from control (CON), p < 0.05; asterisks, significantly different from Ex alone, p < 0.05
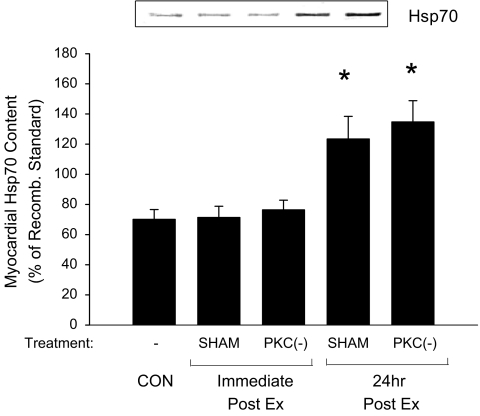
Hsp70 content and phosphorylation Immunoblots probed with an antibody specific to Hsp70 were used to examine the myocardial content and phosphorylation status of Hsp70 following 1D- (Fig. 3) and 2D-electrophoresis (Fig. 4), respectively. Following exercise, total myocardial Hsp70 content was significantly elevated at 24 h, but not immediately postexercise. Prior administration of CHEL did not alter the postexercise elevation in Hsp70 content within the myocardium (Fig. 3), suggesting that PKC is not involved in the exercise-mediated expression of Hsp70 content.
Fig. 3.
Effect of PKC on exercise-induced myocardial Hsp70 protein content. Hsp70 content in vehicle-treated (SHAM) and PKC inhibited (PKC(−)) animals was examined immediately and 24 h following the completion of the exercise protocol (Ex; 30 m/min, 60 min at 2% grade). Determination of Hsp70 protein content was performed using 100 μg of myocardial tissue run on a SDS-PAGE and transferred onto a nitrocellulose membrane where it was detected using an antibody specific to Hsp70. Quantitative analysis of Hsp70 in myocardial tissue was represented as a percentage of a recombinant Hsp70 standard. A representative blot is shown above. Data are means ± SE of five animals per group. Asterisks, significantly different from control (CON), p < 0.05
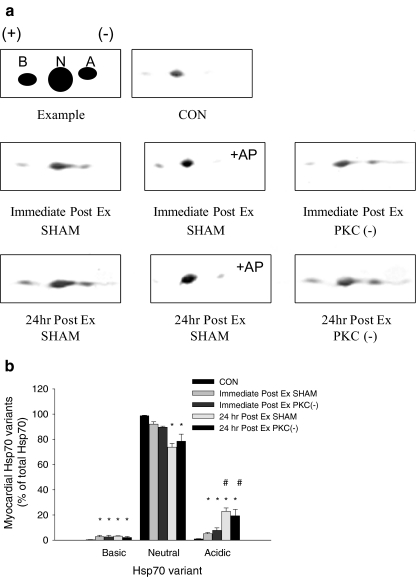
Fig. 4.
Relative amount of myocardial Hsp70 variants immediately and 24 h following acute exercise. Analysis of basic (B), neutral (N), and acidic (A) Hsp70 variants of vehicle-treated (SHAM) and PKC inhibited (PKC(−)) and exercised animals (Ex: 30 m/min for 60 min; 2% grade) was performed following 2D electrophoresis using an antibody specific to Hsp70. To identify phosphorylated variants of Hsp70, samples were incubated with alkaline phosphatase (+AP) prior to 2D-electrophoresis (see “Methods and materials” section). A An illustration of the 2D pattern of Hsp70 variants with representative blots of control (CON) and Ex animals. B Relative amounts (percent of total Hsp70) of each Hsp70 variant immediately and 24 h postexercise. Data are means ± SE of five animals per group. Asterisks, significantly different from control, p < 0.05. Sharp signs, significantly different from 30 min Post Ex, p < 0.05
Following 2D-electrophoresis, three different isoelectric point variants of the Hsp70 protein were detected within the myocardium (Fig. 4A). In CON animals, Hsp70 expression was low and consisted for the most part of the dominant neutral variant. However, following exercise, in addition to the neutral variant, more acidic and basic variants were detected. The increase in the acidic variant was significantly greater at 24 h compared to immediately postexercise, while no significant differences in the basic variant were observed between these time points. This increase in the acidic variant at 24 h corresponded with a significantly lower expression of neutral variant compared to immediately postexercise (Fig. 4).
To determine if these variants were phosphorylated forms of Hsp70, samples were incubated with AP prior to 2D-electrophoresis. Immediately and 24 h postexercise, AP treatment led to an increase in the expression of the dominant neutral variant and a reduction in the expression of the acidic Hsp70 variant. Inhibition of PKC prior to exercise did not alter the phosphorylation status of Hsp70, suggesting that PKC did not mediate the phosphorylation of Hsp70 following exercise.
Discussion
Consistent with previous literature, the current investigation supports a role for acute exercise in the enhanced protection of the myocardium following cardiovascular stress (Brown et al. 2005b; Locke et al. 1995; Paroo et al. 2002). In this instance, the protection is demonstrated by a significant reduction in myocardial infarct size during I/R-injury compared to sedentary subjects at 24 h postexercise. Interestingly, protection against global ischemia 24 h following an acute bout of exercise was comparable to that observed previously following several weeks of exercise training (Brown et al. 2003; Chaves et al. 2006), suggesting that, in males at least, this may be an acute rather than accumulating effect of repeated bouts of exercise. As noted by others (Carson and Korzick 2003; Yamashita et al. 2001), this exercise-induced cardioprotective effect appears to be directly associated with the activation of PKC, as exercise-conferred cardioprotection was attenuated following the prior administration of the PKC inhibitor, CHEL.
Myocardial Hsp70 phosphorylation postexercise
PKC plays an essential role in the phosphorylation and modification of several important cellular cytosolic proteins (Ping et al. 1999). Posttranslational mechanisms have been shown to be important processes in the expression and function of Hsp70 (Theodorakis and Morimoto 1987). In the liver, EDL, and soleus, Hsp70 undergoes inducible phosphorylation following acute exercise (Gonzalez and Manso 2004; Hernando and Manso 1997), which may impact the functional characteristics of Hsp70 within the cell (Lakshmikuttyamma et al. 2004). However, it is not presently clear as to the significance of this modification of Hsp70 in the myocardium. As demonstrated in Figs. 3 and 4, immediately postexercise, there was a significant increase in the amount of acidic and basic Hsp70 variants (represented as a percentage of total Hsp70), while total expression of Hsp70 was not altered (Figs. 3 and 4). The rapid increase in these variants without a concomitant increase in total Hsp70 suggests posttranslational modification rather than an elevation in transcriptional expression. Further, it is likely that the increase in acidic and basic Hsp70 variants was the result of posttranslational modifications of the neutral Hsp70 variant, as a significant decrease in the amount of the dominant neutral variant was observed. In contrast, the continued increase in the acidic variant, coupled with the elevation in total Hsp70 expression and an absence of changes in the amount of both neutral and basic Hsp70 variants at 24 h postexercise, suggests a selective increase in expression of the acidic Hsp70 variant at this time point. Discrepancies do exist, however, in the expression of individual Hsp70 variants at the two time periods postexercise, which may suggest that the role of Hsp70 undergoes subtle changes over time. Such a possibility remains to be investigated.
Contrary to our original hypothesis, our results do not demonstrate a role for PKC in the posttranslational modification of Hsp70 following exercise. While phosphorylation of the Hsp70 molecule does correspond to the windows reported for PKC activation (Carson and Korzick 2003), CHEL did not alter the exercise-mediated increase in the expression of either of the phosphorylated variants (Fig. 4), nor did it alter the expression of Hsp70 postexercise (Fig. 3) despite PKC inactivation (Fig. 1). Given prior observations on the protection of left ventricular function by Hsp70 (Marber et al. 1995; Paroo et al. 2002), these results indicate that both Hsp70 and PKC mediate the protection of the myocardium during I/R-injury though independent mechanisms.
Myocardial PKC activation following exercise
Several PKC isoforms have been implicated in key cellular functions that are believed to be beneficial to the myocardium during cardiovascular stress. For example, PKC isoforms δ, ɛ, α, and β have been implicated in the regulation of such processes as Ca2+-handing (Venema and Kuo 1993), contraction (Meldrum et al. 1996), and intracellular signaling (Ping et al. 1999). In the current study, our results demonstrate that three of these isoforms, PKCδ, PKCɛ, and PKCα, undergo activation immediately and 24 h postexercise, as indicated by an increase in their membrane translocation (Fig. 1). The first, PKCɛ, has received the most attention as a potential cardioprotective agent (Ping et al. 1997) due to its downstream function in the NO-mediated signaling pathway (Ping et al. 1999). Following ischemic preconditioning, there is a late phase of enhanced tolerance of the heart to I/R-injury whereby NO exerts a number of beneficial actions, including inhibition of calcium influx through L-type channels, decreased myocardial contractility, antagonistic effects on the β-adrenergic receptor, opening of ATP-sensitive potassium (KATP) channels, and antioxidant properties (Hoffman et al. 2003). Interestingly, NO has also been shown to play a role in the late phase of exercise-mediated cardioprotection (Bolli 2001). Moreover, selective inhibition of exercise-mediated production of NO through the suppression of NOS attenuates exercise-induced protection of the myocardium during I/R-injury. Consistent with these data, our results demonstrate a delayed activation of PKCɛ postexercise (Fig. 1B).
PKCδ has also been reported to be an important factor in the protection of the myocardium during I/R-injury through the regulation of mitochondrial ATP-sensitive potassium (mitoKATP) channels (Wang and Ashraf 1999). Activation of PKCδ is believed to be directly involved in the opening of mitoKATP channels leading to the phosphorylation of several key mitochondrial proteins (Wang et al. 1999). It has been reported that the activation of mitoKATP improves mitochondria energy production after ischemia (Kowaltowski et al. 2001), blunts mitochondria Ca2+ accumulation during ischemia (Holmuhamedov et al. 1999), and decreases the production of harmful reactive oxygen species during reperfusion injury (Ozcan et al. 2002). Indeed, ischemia–reperfusion studies have demonstrated that cardioprotection has been lost when mitoKATP are blocked using either a direct KATP channel inhibitor; 5-hydroxydecanoate; or, indirectly, the PKC inhibitor CHEL (Loubani et al. 2004). In the current study, we see an elevation in the membrane localization of PKCδ both immediately and 24 h following exercise (Fig. 1C), which supports previous reports indicating a role for enhanced mitoKATP channel function during both early and late phases of cardioprotection (Ockaili et al. 1999). Interestingly, exercise-induced alterations in KATP channels have been reported in cardiomyocytes from trained rats (Jew and Moore 2002), but sarcolemmal rather than mitochondrial KATP channels appear to be most important in this case (Brown et al. 2005a).
While much less information is available regarding the role of PKCα in protection of the myocardium, PKCα plays an essential role in control of cardiac contractility through the regulation of Ca2+-handling (Venema and Kuo 1993). It has been shown that a reduction in intracellular Ca2+ overload via a PKCα-mediated improvement in Ca2+-handling is likely to enhance cardioprotection (Zucchi et al. 1995). Indeed, treatment with CHEL abolishes the beneficial effects of ischemic preconditioning on sarcoplasmic reticulum Ca2+-pump function and the subsequently improved Ca2+ homeostasis (Kawabata et al. 2000). However, controversy exists as to the beneficial role of long-term activation of PKCα in myocardial function. It has been reported that the upregulation of PKCα activation leads to a diminished baseline ejection fraction, resulting in a subsequent terminal cardiomyopathy (D’Angelo et al. 1997). Further, gene ablation studies have demonstrated that chronic inhibition of PKCα not only improved myocardial contractility but inhibited Gq-mediated cardiac hypertrophy (Hahn et al. 2003). Hence, the increase in PKCα observed in the present investigation (Fig. 1D) may mark a beneficial adaptation to a single bout of exercise through increased Ca2+ handling ability, while many bouts of exercise may result in decreased activation of this isoform (Carson and Korzick 2003), thereby avoiding inappropriate functional or hypertrophic stimuli.
In summary, the current results demonstrate that, following acute exercise, posttranslational modification of Hsp70 occurs through phosphorylation, suggesting several layers of regulation of myocardial Hsp70 function and its potential involvement in myocardial protection. In addition, we demonstrate that select PKC isoforms (PKCɛ, PKCδ, and PKCα) are also active postexercise but are not involved in this exercise-mediated phosphorylation of Hsp70. While our results support an important role for PKC in exercise-conferred cardioprotection (Yamashita et al. 2001), Hsp70 phosphorylation is not the mechanism by which PKC mediates this protection. Further work is needed to elucidate the mechanisms associated with the phosphorylation of Hsp70 and its functional role in the protection of the myocardium during I/R-injury.
Acknowledgements
This work was supported by grant 8170-05 RGPIN from the National Science and Engineering Research Council of Canada to E.G. Noble and by Ontario Graduate Scholarships for Science and Technology to C.W.J. Melling.
References
- Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown DA, Jew KN, Sparagna GC, Musch TI, Moore RL. Exercise training preserves coronary flow and reduces infarct size after ischemia–reperfusion in rat heart. J Appl Physiol. 2003;95:2510–2518. doi: 10.1152/japplphysiol.00487.2003. [DOI] [PubMed] [Google Scholar]
- Brown DA, Chicco AJ, Jew KN, Johnson MS, Lynch JM, Watson PA, Moore RL. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J Physiol. 2005;569:913–924. doi: 10.1113/jphysiol.2005.095729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Lynch JM, Armstrong CJ, Caruso NM, Ehlers LB, Johnson MS, Moore RL. Susceptibility of the heart to ischaemia–reperfusion injury and exercise-induced cardioprotection are sex-dependent in the rat. J Physiol. 2005;564:619–630. doi: 10.1113/jphysiol.2004.081323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R, Yellon DM. Myocardial adaptation to ischaemia—the preconditioning phenomenon. Int J Cardiol. 1999;68(Suppl 1):S93–101. doi: 10.1016/S0167-5273(98)00297-6. [DOI] [PubMed] [Google Scholar]
- Carson LD, Korzick DH. Dose-dependent effects of acute exercise on PKC levels in rat heart: is PKC the heart’s prophylactic? Acta Physiol Scand. 2003;178:97–106. doi: 10.1046/j.1365-201X.2003.01131.x. [DOI] [PubMed] [Google Scholar]
- Chaves EA, Pereira-Junior PP, Fortunato RS, Masuda MO, Carvalho AC, Carvalho DP, Oliveira MF, Nascimento JH. Nandrolone decanoate impairs exercise-induced cardioprotection: role of antioxidant enzymes. J Steroid Biochem Mol Biol. 2006;99:223–230. doi: 10.1016/j.jsbmb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- D’Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, Dorn GW. Transgenic Galphaq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci U S A. 1997;94:8121–8126. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez B, Manso R. Induction, modification and accumulation of HSP70s in the rat liver after acute exercise: early and late responses. J Physiol. 2004;556:369–385. doi: 10.1113/jphysiol.2003.058420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn HS, Marreez Y, Odley A, Sterbling A, Yussman MG, Hilty KC, Bodi I, Liggett SB, Schwartz A, Dorn GW. Protein kinase Calpha negatively regulates systolic and diastolic function in pathological hypertrophy. Circ Res. 2003;93:1111–1119. doi: 10.1161/01.RES.0000105087.79373.17. [DOI] [PubMed] [Google Scholar]
- Hernando R, Manso R. Muscle fibre stress in response to exercise: synthesis, accumulation and isoform transitions of 70-kDa heat-shock proteins. Eur J Biochem. 1997;243:460–467. doi: 10.1111/j.1432-1033.1997.0460a.x. [DOI] [PubMed] [Google Scholar]
- Hoffman A, Goldstein S, Samuni A, Borman JB, Schwalb H. Effect of nitric oxide and nitroxide SOD-mimic on the recovery of isolated rat heart following ischemia and reperfusion. Biochem Pharmacol. 2003;66:1279–1286. doi: 10.1016/S0006-2952(03)00441-6. [DOI] [PubMed] [Google Scholar]
- Holmuhamedov EL, Wang L, Terzic A. ATP-sensitive K+channel openers prevent Ca2+ overload in rat cardiac mitochondria. J Physiol. 1999;519(Pt 2):347–360. doi: 10.1111/j.1469-7793.1999.0347m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jew KN, Moore RL. Exercise training alters an anoxia-induced, glibenclamide-sensitive current in rat ventricular cardiocytes. J Appl Physiol. 2002;92:1473–1479. doi: 10.1063/1.1485110. [DOI] [PubMed] [Google Scholar]
- Joyeux M, Baxter GF, Thomas DL, Ribuot C, Yellon DM. Protein kinase C is involved in resistance to myocardial infarction induced by heat stress. J Mol Cell Cardiol. 1997;29:3311–3319. doi: 10.1006/jmcc.1997.0556. [DOI] [PubMed] [Google Scholar]
- Kawabata KI, Netticadan T, Osada M, Tamura K, Dhalla NS. Mechanisms of ischemic preconditioning effects on Ca(2+) paradox- induced changes in heart. Am J Physiol Heart Circ Physiol. 2000;278:H1008–H1015. doi: 10.1152/ajpheart.2000.278.3.H1008. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD. Bioenergetic consequences of opening the ATP-sensitive K(+) channel of heart mitochondria. Am J Physiol Heart Circ Physiol. 2001;280:H649–H657. doi: 10.1152/ajpheart.2001.280.2.H649. [DOI] [PubMed] [Google Scholar]
- Lakshmikuttyamma A, Selvakumar P, Anderson DH, Datla RS, Sharma RK. Molecular cloning of bovine cardiac muscle heat-shock protein 70 kDa and its phosphorylation by cAMP-dependent protein kinase in vitro. Biochemistry. 2004;43:13340–13347. doi: 10.1021/bi049036k. [DOI] [PubMed] [Google Scholar]
- Locke M, Tanguay RM, Klabunde RE, Ianuzzo CD. Enhanced postischemic myocardial recovery following exercise induction of HSP 72. Am J Physiol. 1995;269:H320–H325. doi: 10.1152/ajpheart.1995.269.1.H320. [DOI] [PubMed] [Google Scholar]
- Loktionova SA, Kabakov AE. Protein phosphatase inhibitors and heat preconditioning prevent Hsp27 dephosphorylation, F-actin disruption and deterioration of morphology in ATP-depleted endothelial cells. FEBS Lett. 1998;433:294–300. doi: 10.1016/S0014-5793(98)00920-X. [DOI] [PubMed] [Google Scholar]
- Loubani M, Hassouna A, Galinanes M. Delayed preconditioning of the human myocardium: signal transduction and clinical implications. Cardiovasc Res. 2004;61:600–609. doi: 10.1016/j.cardiores.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;95:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum DR, Cleveland JC, Jr, Sheridan BC, Rowland RT, Banerjee A, Harken AH. Cardiac surgical implications of calcium dyshomeostasis in the heart. Ann Thorac Surg. 1996;61:1273–1280. doi: 10.1016/0003-4975(95)00952-3. [DOI] [PubMed] [Google Scholar]
- Melling CW, Thorp DB, Noble EG. Regulation of myocardial heat shock protein 70 gene expression following exercise. J Mol Cell Cardiol. 2004;37:847–855. doi: 10.1016/j.yjmcc.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Nadruz W, Jr, Kobarg CB, Kobarg J, Franchini KG. c-Jun is regulated by combination of enhanced expression and phosphorylation in acute-overloaded rat heart. Am J Physiol Heart Circ Physiol. 2004;286:H760–H767. doi: 10.1152/ajpheart.00430.2003. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Hangaishi M, Ishizaka N, Taguchi J, Igarashi R, Mizushima Y, Nagai R, Ohno M. Lecithinized copper, zinc-superoxide dismutase ameliorates ischemia-induced myocardial damage. Life Sci. 2001;69:935–944. doi: 10.1016/S0024-3205(01)01188-2. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.43.25526. [DOI] [PubMed] [Google Scholar]
- Ockaili R, Emani VR, Okubo S, Brown M, Krottapalli K, Kukreja RC. Opening of mitochondrial KATP channel induces early and delayed cardioprotective effect: role of nitric oxide. Am J Physiol. 1999;277:H2425–H2434. doi: 10.1152/ajpheart.1999.277.6.H2425. [DOI] [PubMed] [Google Scholar]
- Ozcan C, Bienengraeber M, Dzeja PP, Terzic A. Potassium channel openers protect cardiac mitochondria by attenuating oxidant stress at reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282:H531–H539. doi: 10.1152/ajpheart.00552.2001. [DOI] [PubMed] [Google Scholar]
- Pagliaro P, Gattullo D, Rastaldo R, Losano G. Ischemic preconditioning: from the first to the second window of protection. Life Sci. 2001;69:1–15. doi: 10.1016/S0024-3205(01)01113-4. [DOI] [PubMed] [Google Scholar]
- Paroo Z, Haist JV, Karmazyn M, Noble EG. Exercise improves postischemic cardiac function in males but not females: consequences of a novel sex-specific heat shock protein 70 response. Circ Res. 2002;90:911–917. doi: 10.1161/01.RES.0000016963.43856.B1. [DOI] [PubMed] [Google Scholar]
- Ping P, Zhang J, Cao X, Li RC, Kong D, Tang XL, Qiu Y, Manchikalapudi S, Auchampach JA, Black RG, Bolli R. PKC-dependent activation of p44/p42 MAPKs during myocardial ischemia-reperfusion in conscious rabbits. Am J Physiol. 1999;276:H1468–H1481. doi: 10.1152/ajpheart.1999.276.5.H1468. [DOI] [PubMed] [Google Scholar]
- Ping P, Zhang J, Qiu Y, Tang XL, Manchikalapudi S, Cao X, Bolli R. Ischemic preconditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res. 1997;81:404–414. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- Theodorakis NG, Morimoto RI. Posttranscriptional regulation of hsp70 expression in human cells: effects of heat shock, inhibition of protein synthesis, and adenovirus infection on translation and mRNA stability. Mol Cell Biol. 1987;7:4357–4368. doi: 10.1128/mcb.7.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema RC, Kuo JF. Protein kinase C-mediated phosphorylation of troponin I and C-protein in isolated myocardial cells is associated with inhibition of myofibrillar actomyosin MgATPase. J Biol Chem. 1993;268:2705–2711. [PubMed] [Google Scholar]
- Wang Y, Ashraf M. Role of protein kinase C in mitochondrial KATP channel-mediated protection against Ca2+ overload injury in rat myocardium. Circ Res. 1999;84:1156–1165. doi: 10.1161/01.res.84.10.1156. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hirai K, Ashraf M. Activation of mitochondrial ATP-sensitive K(+) channel for cardiac protection against ischemic injury is dependent on protein kinase C activity. Circ Res. 1999;85:731–741. doi: 10.1161/01.res.85.8.731. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Hoshida S, Otsu K, Asahi M, Kuzuya T, Hori M. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med. 1999;189:1699–1706. doi: 10.1084/jem.189.11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita N, Baxter GF, Yellon DM. Exercise directly enhances myocardial tolerance to ischaemia–reperfusion injury in the rat through a protein kinase C mediated mechanism. Heart. 2001;85:331–336. doi: 10.1136/heart.85.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi R, Ronca-Testoni S, Yu G, Galbani P, Ronca G, Mariani M. Postischemic changes in cardiac sarcoplasmic reticulum Ca2+ channels. A possible mechanism of ischemic preconditioning. Circ Res. 1995;76:1049–1056. doi: 10.1161/01.res.76.6.1049. [DOI] [PubMed] [Google Scholar]