Abstract
Selection for higher production rate in cattle inhabiting challenging habitats may be considered disadvantageous because of possible deleterious effects on immunity and reproduction and, consequently, on calf crop percentage. In Israel, free-grazing high productive beef cows experience reduction in nutritional quality of forage during up to 8 months of the year. As milk production by dams dictates calf performance, dam’s nutritional needs and rebreeding rates, the aim of the present study was to test how lactating beef cows deal with combined caloric and protein stress both at the productive and self protective levels. For this purpose, we studied the effect of long-term caloric stress on milk characteristics and gene expression of stress and milk components producing proteins. Lactating dams responded to caloric stress by decreased body weight, milk, and milk protein production. To compensate for total energy loses in milk, they produced milk of higher fat concentration and shifted the proportions of its fatty acids towards long and unsaturated ones. This was reflected by increased mRNA transcription of the fatty acid binding protein. Prolonged low-energy diet promoted cell-specific heat shock protein (Hsp) response; whereas significant increase of Hsp90 but unchanged levels of Hsp70 proteins were observed in white blood cells, the expression of Hsp70 in milk somatic cells was markedly attenuated, in parallel with a marked increase of αs1-casein expression. At the mammary gland level, these results may indicate a decrease in turnover of proteins and a shift to an exclusive expression of milk components producing factors. Similar responses to caloric stress were revealed also in ketotic dairy cows. Ketosis promoted a shift towards long and unsaturated fatty acids and an increased expression of αs1-casein in milk somatic cells. These findings may reflect an evolutionary-preserved mechanism in lactating cows for coping with caloric restriction. Overall, our results provide an index to test suitability of beef cattle breeds to inadequate caloric demands.
Keywords: Caloric and protein stress, Ketosis, Hsp70, Hsp90, FABP3, αs1-casein, Milk somatic cells, Fatty acids profile, Beef cattle, Dairy cattle
Introduction
In the Mediterranean region, as well as in many countries all over the world, beef cattle herds are grown under extensive regime. Mediterranean ecosystems are distinguished by high seasonality in resource availability (Sternberg et al. 2000). This implies that free-grazing beef cattle may face a reduction in the nutritional quality of forage through its chemical ingredients, digestibility, and metabolized energy, especially during the hot and dry seasons (Aharoni et al. 2004; Brosh et al. 2004) which may last up to 8 months (Main 1986).
Poor quality feed may deleteriously affect reproduction (Randel 1990) and also interfere with calves’ weaning success through its adverse effect on milk production. Indeed, a continuous significant decrease in calf crop production of free-grazing beef cattle herds is recorded in Israel over the last two decades (Ungar et al. 2005). As decreased levels of energy intake shorten the duration and adversely affect the total milk yields during the lactation period (Jenkins and Ferrell 1992) and extend the period from calving to the first postpartum estrus (Randel 1990), it is highly probable that the continuous decrease in calf crop production may originate from the adverse effects of the reduced forage quality on milk characteristics of lactating beef cows.
Also dairy cattle may experience caloric stress (ketosis). Ketosis occurs in the first 2 months after calving and is caused by a severe negative energy balance that stems from high milk production, insufficient energy intake, and excessive body fat mobilization (de Roos et al. 2007). Over the last centuries, dairy cows have been selected for high milk yield regardless of the actual nutritional demands of the suckling calf. By comparing responses of beef and dairy cows to caloric stress and/or ketosis, it may be possible to follow whether changes in milk characteristics reflect evolutionary mechanisms by which lactating cows have coped with nutritional challenges in their habitat.
At the cellular level, induction of heat shock protein 70 (Hsp70) was associated with the development of tolerance to caloric stress (Kregel 2002). Hsp70 is a member of the Hsp super family that by rapid, specific, and massive synthesis assist organisms in coping with various stresses (Craig and Lindquist 1988; Welch 1990). However, members of this Hsp family are constitutively present in cells. The induced and the constitutively expressed Hsp families are well known as molecular chaperones which help in normal folding of various polypeptides, assist misfolded proteins to attain or regain their native states, regulate protein degradation, and help in translocation of proteins to different cellular compartments (Hartl and Hayer-Hartl 2002; Kovacs et al. 2005).
Milk production in beef cattle is considered the major determinant of maternal effects on growth rate of calves till weaning (Meyer et al. 1994). Milk greatly affects calf performance and the dam’s nutritional needs, thus indirectly affecting also rebreeding rates (Mallinckrodt et al. 1993).
Fat and, particularly, long-chain fatty acids (FAs) are the energy rich moiety of milk. Synthesis of milk fat by the mammary gland requires large quantities of fatty acids, of which 98% appear in the form of triglycerides (Whetstone et al. 1986; Palmquist et al. 1993). The supply of FAs for triglyceride synthesis in the ruminant mammary gland arises from two sources, the de novo synthesis of short-chain saturated fatty acids (C4–C16) and the uptake of long-chain fatty acids (C16, C18, C18:1, C18:2) from the blood (Dils 1983). Apart from its major components (fat, lactose, casein), mammalian milk is also comprised of many types of somatic cells, including neutrophils, macrophages, lymphocytes, eosinophils, and epithelial cells of the mammary gland (Kehrli and Shuster 1994). The epithelial cells are shed into the milk during the lactation process. They exhibit characteristics of viable and differentiated alveolar epithelial cells in various mammalian species, including bovines (Buehring 1990; Boutinaud et al. 2002). Primary cultures of epithelial cells from colostrum and milk, together with various sources of milk-derived cell lines, provide a good model for the study of lactogenesis, immunity transmission, cancer research, and viral infection. The RNA extracted from milk cells has been shown to represent gene expression in the mammary gland and thus provide a source of material for molecular studies of gene expression and environmental interactions (Boutinaud et al. 2002; German and Barash 2002).
In light of the above, developing a physiological and molecular index that would serve to test the response of free-grazing lactating beef cows to caloric stress both in the productive and self protective levels is inevitable. Such an index may be of great help when coming to test the suitability of beef cattle breeds to nutritional-challenging habitats. The present study focuses on the compensatory responses of lactating beef cows to a long-term intake of low energy and protein diet by studying milk production and characteristics (content, fatty acid composition) and milk somatic cells’ gene expression. Some of these responses (milk yield, fatty acid composition, and protein expression in milk somatic cells) are compared to ketotic dairy cows.
Materials and methods
Animals and treatments
Experiment 1 Ten gestating and lactating beef cows and their calves from the experimental Simmental dominated herd of Newe Ya’ar participated in this study. All cows were third parity or greater, with an average age of 6.5 years. Control cows (n = 4) were served ad lib diet of 11.5% crude protein (CP)–1.9 Mcal/kgDM metabolizable energy (ME) whereas the diet of experimental cows (n = 6) was comprised of 7% CP–1.45 Mcal/kgDM ME ad lib. The composition of the control diet in g/kg on dry matter (DM) basis was: barley grain—297, crushed maize grain—2.1, and wheat hay–poultry liter silage—503. The experimental diet in g/kg on DM basis was composed of: wheat hay—877 and poultry liter silage—386. The experimental low-energy protein (LEP) diet was designed to resemble natural forage during the hot and dry Mediterranean seasons. Both diets contained equal amounts of mineral and vitamin premix (16 g/kg of DM; Kofolk, Israel). While the control diet contained 82% degradable protein, the experimental diet contained 49% (calculations according to NRC 1996). The experiment lasted 3 months, and the average age of calves in each group at the initiation of the study was 4 months. The standard deviation of calves’ age at the initiation of the study was 10 and 14.5 days for the control and experimental groups, respectively. The initial body weigh (Mb) of the control dams was 599 ± 17 kg, while that of the experimental dams was 612 ± 79 kg. The initial Mb of the control calves was 251 ± 10 kg while that of the experimental calves was 270 ± 13 kg. Calves of both groups were supplemented with suckling ration (creep) ad lib.
Experiment 2 To study the effect of ketosis on milk fat characteristics of dairy cows, fresh milk was sampled from six control and ten ketotic Holstein–Friesian cows from a commercial dairy farm (Kibutz Yifat). Postcalving, the control and ketotic cows were fed with an identical diet, with ME—2.78 Mcal/kgDM and CP—18%, of which 51% was degradable. The ketotic status was determined by a veterinarian, using analysis of urinary keton bodies. Thereafter, milk samples were collected. All procedures involving animals were approved by the Israeli committee for animal care and experimentation.
Milk yields
To reveal the effect of continuous (3 months) LEP diet on beef cows’ potential to produce milk, we determined milk yields by the weigh-suckle-weigh technique. This technique is one of the most frequently cited methods for the measurement of milk production, and in spite of some limitations, similar result may be determined when using it in comparison with milking machine (Benson et al. 1999). Cows and calves were separated 16 h before sampling. The difference between calf weight before and after suckling, adjusted to a 24-h basis, provided an estimate of daily milk production of the cow. The suckling event continued for approximately 30 min after introduction of the calves to their dams.
Milk content
Hand-milked milk samples of control and 3-month caloric- and protein (CAP)-restricted beef cows were analyzed for fat, protein, urea, and lactose contents by the mid-infrared spectroscopic method (Milkoscan FT6000; Foss Food Technology Corp., DK; AOAC 1990). Somatic cell counts were determined by Fossomatic 5000 FC (Foss Food Technology Corp., DK).
Milk fatty acid composition
Milk samples were stored at −20°C until analysis of their fatty acid profile. The fat ring at the top of the tube was removed, and lipids were extracted in a hexane:isopropanol solvent mixture according to Hara and Radin (1978). An aliquot of 40 mg of the lipid fraction was transmethylated according to Christie (1982), with modifications as described by Chouinard et al. (1999). Gas chromatography of fatty acid methyl esters (FAME) was performed with a Hewlett Packard 6890 system, equipped with HP Chemstation software for peak integration. We used a Supelco SP-2560, 100-m fused silica capillary column of 0.25 mm i.d., with ultrahigh-purity helium carrier gas at a flow rate of 20 ml/min. Injector and flame-ionization detector (FID) temperatures were 250°C and 260°C, respectively. The splitting ratio to the detector was 1:50. The oven temperature schedule was 140°C for 5 min, T increase to 175°C at 4°C /min, constant 175°C for 25 min, T increase to 220°C at 4°C/min, and constant 220°C for 20 min. The total run time was 70 min. Standard FAME preparations (Sigma-Aldrich) were injected separately to relate the peaks to fatty acids. The FAME preparations used were methyl esters of: C14:0, C14:1, C16:0, C16:1, C18:0, C18:1t9, C18:1t10, C18:1t12, C18:1c9, C18:1c11, C18:1c12, C18:2c9c12, C18:3c6c9c12 (γ-linolenic), C18:3c9c12c15 (α-linolenic), conjugated linoleic acid (CLA), and C20:4 (arachidonic). The CLA preparation (Sigma O5632) contained a racemic mixture of four isomers: C18:2t9c11, C18:2c9t11, C18:2t10c12, and C18:2c10t12, which were detected by the chromatography: the first two as overlapping peaks and the last two as separated peaks. Then, a standard mixture of equal concentrations of these 18 FAME was injected to facilitate the calculation of correction factors for each acid. These correction factors accounted for both the differing sensitivity of the FID to the different FAME and for the ratio of FAME to FA molecular weights, in the calculation of FA concentrations from the areas of the corresponding FAME peaks.
Processing of milk somatic cells
Somatic cells from fresh milk of beef and dairy cows (150–200 ml) were pelleted by centrifugation at 1,000 × g for 10 min at 4°C in the presence of 0.5 mM ethylenediaminetetraacetic acid (EDTA; final concentration) to reduce the levels of casein–fat emulsion. The cell pellet was washed three (in the case of RNA extraction) to five (in the case of cellular protein extraction) times with cold phosphate buffered saline (PBS)-EDTA (0.5 mM) to eliminate casein and fat globules. Protein and RNA were extracted from the somatic cells within 30 min from sampling. Until then, samples were kept in a chilled box.
Blood sampling
Blood was sampled from the caudal vein of dams, using evacuated tubes (Greiner bio-one GmbH, Austria) containing EDTA as anticoagulant. The blood was centrifuged at 1,000 × g, 4°C, to separate the cells from the plasma. The buffy coat was transferred to a new chilled Eppendorf tube. Remaining red blood cells were removed by RBC lysis buffer (Roche, cat # 1-814-389). Leukocytes were then washed with cold PBS and immediately used for protein extraction.
SDS-PAGE and Western blot
Whole-cell lysates were boiled in sample application buffer containing 2-mercaptoethanol. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel (10%) and transferred onto nitrocellulose membranes (Schleicher & Schuell Gmbh, Dassel, Germany). The membranes were probed with monoclonal anti-actin (Sigma, cat # A1978), anti-Hsp70 (recognizing the constitutive and the inducible forms of the protein; Sigma H5147), and anti-Hsp90 (Stressgen, cat # SPA-830), followed by appropriate secondary antibodies. Proteins were visualized by enhanced chemiluminescence.
αs1-casein identification—mass spectrometry analysis
Protein extracts of somatic cells were run on a 10% acrylamide gel and stained with Coomassie blue. The stained protein bands, at a molecular mass of ∼29 kDa, were cut from the gel with a clean razor blade and the proteins were reduced with 10 mmol l−1 dithiothreitol and modified with 100 mmol l−1 iodoacetamide in 10 mmoll·l−1 ammonium bicarbonate. The gel pieces were treated with 50% acetonitrile in 10 mmol l−1 ammonium bicarbonate to remove the stain, followed by drying the gel pieces. The dried gel pieces were rehydrated with 10% acetonitrile in 10 mmol·l–1 ammonium bicarbonate containing 0.005 μg·μl−1 trypsin and then incubated overnight at 37°C. The resulting peptides were recovered with 60% acetonitrile with 0.1% trifluoroacetate. The tryptic peptides were resolved by reverse-phase high-performance liquid chromatography on 0.1 × 300-mm fused silica capillaries (J&W, Folsom, CA, USA; 100 μm i.d.) home-filled with porous R2 (Persepective, Framingham, MA, USA).
The peptides were eluted using an 80-min linear gradient of 5–95% acetonitrile with 0.1% acetic acid in water at a flow rate of ∼1 μl·min−1. The liquid from the column was electrosprayed into an ion-trap mass spectrometer (LCQ; Finnigan, San Jose, CA, USA). Mass spectrometry (MS) was performed in the positive ion mode using repetitively full MS scan followed by collision induced dissociation (CID) of the most dominant ion selected from the first MS scan. The mass spectrometry data were compared to simulated proteolysis and CID of the proteins in the NR-National Center for Biotechnology Information database using the Sequest software (J. Eng and J. Yates, University of Washington and Finnigan, San Jose, CA, USA). The amino terminal of the protein was sequenced on a Peptide Sequencer 494A [Perkin Elmer, (Applied Biosystems), Foster City, CA, USA] according to the manufacturer’s instructions. The migration of αs1-casein in the gel was verified by running a pure αs1-casein as standard (Sigma).
RNA isolation and RT-PCR
Total RNA was extracted from milk somatic cells by using the TRI REAGENT LS (MRC, Cincinnati, OH, USA Cat. # TS-120), according to the manufacturer’s recommendation. To remove genomic DNA contamination, samples were treated with DNase (Epicenter, cat # DB0711k) according to the manufacturer’s recommendation. The concentration of RNA was measured by Nano-Drop (ND-1000) as well as its quality. Quality of total RNA was additionally estimated by nondenaturating agarose gel.
The RNA was stored in −80°C or immediately utilized for reverse transcriptase polymerase chain reaction (RT-PCR) reactions using the Verso cDNA Kit (Thermo Fisher Scientific Inc. Cat # AB1453/A). The T-Personal PCR machine (Biometra) was programmed as follows: 42°C for 60 min for the RT step followed by 95°C for 2 min and the amplification steps of 94°C for 2 min, 60°C for 40 s, and 72°C for 1:30 min. A master mix was prepared and aliquoted to test tubes, each of which was amplified for 30 cycles.
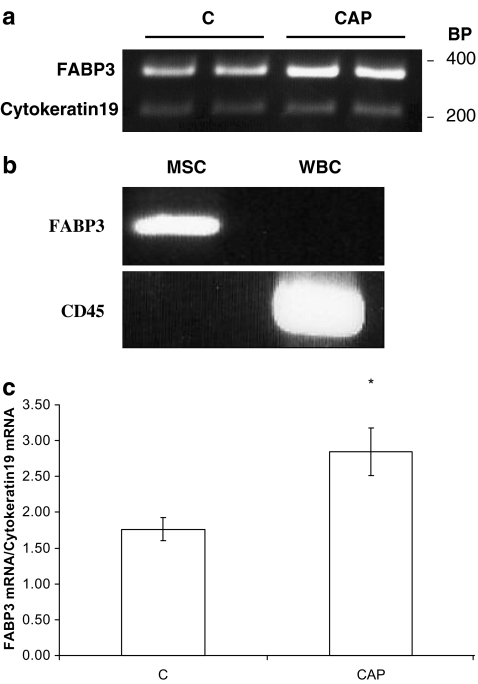
The comparative RT-PCR reactions in Fig. 3 were performed using two pairs of primers in a same reaction mix: For FABP3, the forward primer TTCGTGGGTACCTGGAAG and the reverse primer CGAGTGCAAACTGCAGTG amplified a 367-bp fragment, while for Cytokeratin19, the forward primer AGATGACTTCCGCACCAAGT and the reverse primer GCCCTTCAGCACACTCATTT amplified a 196-bp fragment. For CD45, the forward primer was ATGTATCTGTGGCTTAAAC while the reverse primer was CATTACACT TGAATTGTCC. Except for the annealing temperature which was 49°C, the PCR program for the amplification of CD45 was identical to the above mentioned.
Fig. 3.
Representative RT-PCR analysis of fatty acid binding protein 3 (FABP3) expression in milk somatic cells (MSC) in response to caloric and protein stress (CAP). Cytokeratin19 was used as a loading control (a). Differential expression of FABP3 in MSC but not in leukocytes (WBC). CD45 was used as a specific marker of WBC (b). c Densitometry ratios of FABP3/Cytokeratin19 mRNA. Results are presented as means ± SD. Asterisk indicates significant increase of the ratio in CAP treatment (p < 0.001). BP Base pairs as size markers to indicate the length of the amplified fragment
Statistical analysis
One- and two-tailed two-sample t-tests were performed to test for differences in calves weight gain, body weight loss of dams, fatty acid composition of milk, protein, and mRNA expression between control and experimental groups. We used Pearson correlation coefficient (r) to test the correlation between calves’ weight gains and weight losses of their dams, milk production and fat concentration, milk fatty acids and calves’ weight gain, milk yield and calves’ weight gain, and milk production and fat concentration. Proportions were arcsin square-root-transformed so that they conformed more closely to a normal distribution. Statistical analyses were performed using SPSS 14.0 software (SPSS Inc. 2005).
Results
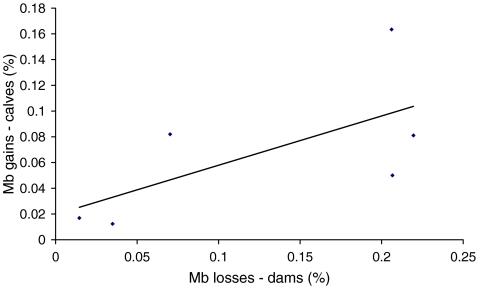
At the end of the entire experimental period, weight gains of the control beef calves (49 ± 2.7 kg) were higher than those of the experimental beef calves (34.7 ± 10.6 kg); however, the difference was not significant (p = 0.241), probably due to a large variability among gains of the experimental beef calves. Weight loss of the experimental beef dams also varied widely (7–100 kg; standard deviation (SD) = 35) in comparison to a slight increase (3 ± 2 kg) in the weight of the control beef dams group. Within the experimental beef group, claves’ body weight (Mb) gain was significantly correlated (r = 0.75, p = 0.04, n = 6) with Mb loss of their dams (Fig. 1).
Fig. 1.
Correlation between body weight loss of lactating beef dams and body weight gain of their calves, in response to caloric and protein stress (r = 0.75, p = 0.04, n = 6)
In addition to the negative effect on Mb, caloric stress revealed also a significant reduction (p = 0.027, n = 10) in milk production (1.9 ± 0.43 kg and 0.5 ± 0.09 kg, for control and experimental beef cows, respectively). Also, ketosis has significant effect on milk yield. In our study, 1 week after calving (on the day of ketosis determination by the veterinarian), milk yield of ketotic dairy cows was 37 ± 12 l, while the yield of nonketotic dairy cows was 45 ± 8 l (p = 0.08).
The effect of CAP stress on milk content is shown in Table 1. Interestingly, dams of the LEP diet group produced significantly higher concentration of milk fat and significantly lower concentration of lactose. The levels of urea tended to decrease, whereas protein concentration was unaffected.
Table 1.
The effect of caloric stress on milk content (% fresh weight) of lactating beef cows
| Milk component | Control diet | Low-energy diet | [t]a (df = 7) | p value |
|---|---|---|---|---|
| Fat | 4 ± 0.22 | 5.2 ± 0.18 | −3.96 | 0.003 |
| Protein | 3.69 ± 0.28 | 4.1 ± 0.34 | −0.789 | 0.228 |
| Lactose | 5.06 ± 0.08 | 4.04 ± 0.31 | 2.216 | 0.031 |
| Urea | 0.38 ± 0.002 | 0.308 ± 0.002 | 1.883 | 0.051 |
Results are presented as means ± standard error of the mean.
aOne-tailed t-test
A negative correlation between milk production and fat concentration was revealed for the control (r = −0.998, p = 0.042, n = 4) and the combined (r = −0.883, p = 0.004, n = 10) groups. No correlation between milk production and fat concentration was revealed in the LEP group (r = −0.122, p = 0.845, n = 6). In the same manner, no significant correlation was obtained between milk yield and calves’ weight gain in this group (r = −0.53, p = 0.3, n = 6).
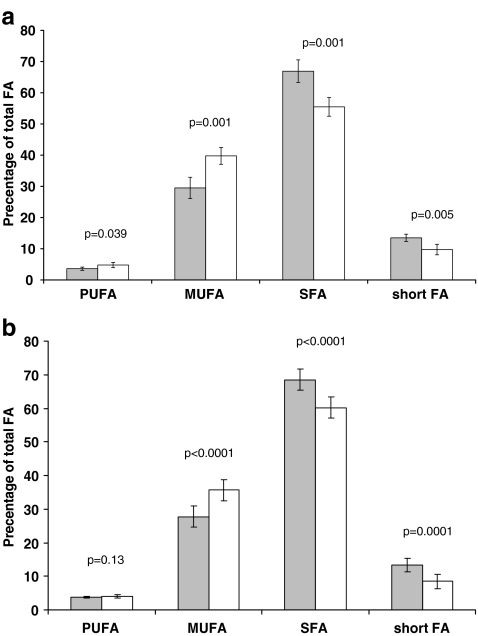
The effect of LEP diet on FAs composition of milk is presented in Fig. 2a. For the statistical analysis, we have grouped the FAs into four groups: (a) C4:0–C12:0 (short fatty acids; shortFA); (b) C4:0–C14:0, C16:0, C18:0 (saturated fatty acids, SFA); (c) C14:1, C16:1, and isomers of C18:1 (monounsaturated fatty acids, MUFA); and (d) C18:2, C18:3, CLA isomers, and C20:4 (polyunsaturated fatty acids, PUFA). In general, the FAs profile had a reciprocal tendency: The proportions of short FAs and SFA were significantly higher in the control milk, whereas MUFA and PUFA proportions were significantly higher in the experiment milk. A significant negative correlation was revealed between the experimental beef calves’ weight gain and SFA (r = −0.975, p = 0.001, n = 6), while the MUFA was significantly positively correlated with the experimental beef calves’ weight gain (r = 0.915, p = 0.011, n = 6). A similar significant change in the proportions of milk FAs was also observed in ketotic dairy cows. Ketosis promoted a significant increase in MUFA, accompanied by significant decrease in short FA and SFA (Fig. 2b).
Fig. 2.
Fatty acids profile of milk fat of calorie–protein-restricted lactating beef dams (a) and ketotic dairy cows (b). Gray bars—control group, white bars—CAP stress and ketosis for panel a and b, respectively. PUFA Polyunsaturated fatty acids, MUFA monounsaturated fatty acids, SFA saturated fatty acids, short FA short-chain fatty acids. Lines above bars indicate t-test scores and significances. Results are presented as means ± SD
Among other reasons, the shift in milk FAs profile could arise from changes in the mobilization of long-chain FAs from the blood to the mammary gland. To test this possibility, we followed the expression of fatty acid binding protein 3 (FABP3). As shown in Fig. 3a,c, CAP stress significantly increased FABP3 mRNA level in milk somatic epithelial cells (p < 0.001). This increase in FABP3 mRNA is exclusively contributed by the epithelial fraction within the milk somatic cells, since leukocytes do not seem to express this gene (Fig. 3b).
Prolonged LEP diet promoted cell-specific Hsp response in lactating beef cows; whereas no significant induction of Hsp70 (p = 0.62) and mild but significant induction of Hsp90 (p = 0.03) was observed in white blood cells (Fig. 4a, d), long-term caloric stress markedly attenuated the expression of Hsp70 in milk somatic cells (Fig. 4b). In parallel with the attenuation of Hsp70, a marked increase in the levels of αs1-casein was observed in the same cell extracts (Fig. 4b). Similarly, a pronounced induction of αs1-casein was seen in milk somatic cells of ketotic dairy cows (Fig. 4c).
Fig. 4.
Representative Western blot for a Hsp70, Hsp90, and actin (as loading control) in leukocytes and b Hsp70 in milk somatic cells of lactating beef dams in response to caloric stress. In b, c αs1-casein was detected by mass spectrometry analysis in milk somatic cells of beef and dairy cows, respectively. d Densitometry ratios of Hsp70/actin and Hsp90/actin at the protein level. Results are presented as means ± SD. C Control, CAP caloric and protein stress, Ket ketosis, Commer purified αs1-casein (Sigma)
Discussion
Mother milk production (quantity and quality) is likely to affect weaning success of offspring. Its responses to challenging nutritional demands in the natural habitat may play a key role in the continuous decrease in calf crop percentage of free-grazing beef cattle herds in Israel and other countries with extensive grazing regime. To explore this possibility, we investigated the effect of CAP stress on the performance of gestating lactating beef cows by means of calves’ development, milk characteristics, and protein expression in milk somatic cells. To compare the effect of CAP stress on milk characteristics of other cattle breed, we followed the impact of ketosis on milk fatty acid composition and αs1-casein expression in dairy cows’ milk.
Verification of negative energy balance in lactating cows
The calculated ME of the experimental diet was 75% of the control. To confirm that beef cows attained negative energy balance in response to the LEP diet, we followed the changes in their Mb and milk production. Experimental lactating beef dams lost considerable weight due to the decreased ME in their diet. However, their Mb varied widely. The variability in beef dams’ Mb loses was further reflected in weight gains of their calves. These results could imply on a differential strategy for coping with energy stress among individual lactating beef cows. Although calves’ creep intake was not recorded individually in this study, it is clear from the results that it did not fully compensate for energy and protein restriction in dam’s diet. The nearly fourfold reduction in milk production was the second confirmation that in response to 75% ME of the control diet, the lactating beef cows attained negative energy balance.
Effects of caloric stress on milk content and fat composition
The effect of feed on milk composition has been extensively studied in the last decades. Most of the attention was directed to fat concentration, mostly because of all three major milk solids, fat is the most sensitive to dietary influences (Sutton 1988). Generally, reducing the forage to concentrate ratio in diets, which favors the formation of glucogenic volatile fatty acids (diets with increased energy input), negatively affects milk fat concentration (Sutton 1988; Crocker et al. 1997; Hurtaud et al. 1998). To the best of our knowledge, no studies have focused on the effect of caloric and protein restriction on milk fatty acid profile. We checked whether CAP stress may have triggered compensatory responses, in terms of milk content or fat composition. Indeed, the significantly higher concentration of milk fat and the lower concentration of lactose may indicate of such responses.
Being the agent in milk that accounts for the osmotic pressure (Morrissey 1985; Sutton 1988), the decreased lactose concentration can explain, at least partially, the drop in milk volume. Unlike the effect on milk fat observed when higher concentrate feed is served low-protein intake causes only minor reduction in milk fat and protein concentrations (Sutton 1988). However, in dairy cows, a decrease in the rumen availability of protein increases milk production when crude protein intake is less than 14% (Nocek and Russell 1988). Similarly, fat concentration is inversely relates to milk yield (Sutton 1988).
In the current study, the drop in milk volume together with the unaffected levels of milk protein reflects an overall reduction in milk protein production by the beef cows. As milk urea highly correlates with plasma urea and as plasma urea levels reflect changes in protein metabolism (Roseler et al. 1993), the decreased levels of milk urea in response to CAP stress, in the present study, may indicate a general decrease in protein synthesis.
We examined the effect of diet on the correlation between milk production (obtained from the weigh-suckle-weigh technique) and milk fat concentration. The obtained outcome suggests that unlike in control, the increased fat concentration in the experimental beef dam’s milk is not a result of a decrease in milk production but rather reflects a compensatory response to LEP diet. A further support in the hypothesis that lactating cows adapt to CAP stress by changing fat characteristics arises from its FAs profile. Here, a significant shift towards the energy-rich, long-chain unsaturated FAs was favorable in the case of energy- and protein-deprived diet. Moreover, SFA and MUFA which constitute a conspicuous portion of FAs in milk fat correlated with calves’ weight gain, but in opposite directions. Taken together, the overall changes in milk fat may reflect physiological responses to assure energy supply to the suckling calf when the ME and CP of the diet is reduced.
Changes in expression of FABP3, Hsp70, and αs1-casein in milk somatic cells
Milk FAs originate from two sources, de novo synthesis by mammary cells (short-chain FAs; C < 16) and uptake of longer-chain FAs from the circulation; the origin of which are the diet and body reserves (Baumgard et al. 2002). Previous studies have shown that manipulations causing milk fat depression resulted in decreased secretion of both FAs classes but with pronounced effect on the short-chain FAs (Baumgard et al. 2000, 2002; Chouinard et al. 1999; Loor and Herbein 1998). It was well demonstrated, though, that the reduction was caused by modulating the expression and activity of key enzymes of milk fat synthesis (Baumgard et al. 2002). Based on these findings and taking together the data from Figs. 1 and 2 and Table 1, it is tempting to hypothesize that during lactation, cows respond to caloric and protein restriction by altering gene expression of at least part of these key enzymes. This, in turn, might favor the mobilization of unsaturated FA into the mammary gland to compensate for the decreased milk production and thus to support the energy needs of the developing calf at the expense of own body reserves. To check this possibility, we followed the mRNA expression of FABP3 in milk somatic cells. FABP3 is a low molecular weight, cytosolic protein, which binds FAs with high affinity and participates in intracellular FAs transport (Simpson et al. 1999). Indeed, it appeared evident that the shift of milk fat towards long-chain energy-rich FAs may be explained, at least partially, by the increased levels of FABP3 in the mammary gland. As the experimental beef dams lost weight, the increased levels of FABP3 may also indicate that the higher proportions of long-chain energy-rich FAs in their milk fat originated from lipid mobilization of body reserves.
In many experimental systems, induction of Hsp70 has been implicated in mediating the beneficial effects noted with caloric restriction (Duffy et al. 1997; Yu and Chung 2001; Patel and Finch 2002). We followed Hsp expression, at the protein level, in blood and milk somatic cells, in response to 3 months of CAP stress, and revealed a cell-specific response; whereas Hsp70 and Hsp90 were slightly induced in white blood cells, the expression of Hsp70 was markedly attenuated in milk somatic cells. Since the expression of Hsp70 in blood leukocytes was not reduced, we assume that the Hsp70 attenuation in milk somatic cells was contributed by the epithelial cells. This assumption is strengthened by the fact that in the same cell extracts, αs1-casein is presenting a mirror image to that of Hsp70.
Furthermore, since the LEP diet contained only 7% crude protein (instead of 11.5% in control) and as Hsp70 assists newly synthesized proteins to attain their native states, regulates protein degradation, and is an important component of cellular networks (Arya et al. 2007), it cannot be excluded that the attenuation of Hsp70, seen in milk somatic cells, reflects a decrease in the turnover of self proteins in favor of milk components producing factors, such as FABP3 and αs1-casein. αs1-casein is one of four acidic phosphoproteins that comprise 75% of milk protein in ruminants. The concentration of calcium and phosphate in milk is highly correlated with that of casein. As the other members of the family, αs1-casein is present in ruminant milk in a micellar structure responsible for the calcium and phosphate transport to the neonates (Sørensen et al. 2003). In light of the above, it is tempting to speculate that under CAP stress the mammary gland may function as a buffered productive autonomous entity, where the genetic program is shifted towards support of the calf’s demands.
Hints for preserved evolutionary mechanism
Milk production in dairy cattle is not devoted to the developing calf but rather to human benefits. It was therefore interesting to study whether their selection, over the last centuries, for high milk production, would trigger similar responses to caloric stress as those observed in lactating beef cows. Indeed, similar responses, at the level of fatty acid composition and αs1-casein expression were revealed in beef and dairy cows. It is thus suggested that at least in the case of high productive cattle breeds, such as Simental and Holstein, altered milk fat characteristics reflect physiological responses to caloric stress. In a broader sense, this common response may reflect a preserved evolutionary mechanism by which lactating cows have coped with nutritional challenges in their habitat. This hypothesis, however, should be further investigated with more cattle breeds and candidate genes.
Summary
Milk production by beef cows is the major determinant that motivates the herd’s performance. However, when taking into account the adaptation of domestic animals to their habitat, selection for higher production rate is considered disadvantageous in harsh environments. In this regard, the introduction of highly productive breeds at the cost of local breeds less productive but better adapted to CAP restriction is questionable. As differential responses for lactation traits exist among cattle breeds, the parameters tested in the present study (changes in Mb, milk production, milk components, milk fat FA profile, FABP3, αs1-casein, and Hsp70 expression) may serve as an index to compare the suitability of beef cattle breeds to nutritional challenges in their habitat.
Acknowledgements
We thank Professor Zeev Arad for critically reviewing this manuscript. This research was supported by funds of the Israeli Milk Marketing Board. Contribution No. 525/08 from the ARO, The Volcani Center, Bet Dagan, Israel.
References
- Aharoni Y, Brosh A, Orlov A, Shargal E, Gutman M. Energy balance of grazing beef cows in Mediterranean pasture, the effects of stocking rate and season: 1. Digesta kinetics, faecal output and digestible dry mater intake. Livest Prod Sci. 2004;90:89–100. doi: 10.1016/j.livprodsci.2004.03.007. [DOI] [Google Scholar]
- Arya R, Mallik M, Lakhotia SC. Heat shock genes—integrating cell survival and death. J Biosci. 2007;32:595–610. doi: 10.1007/s12038-007-0059-3. [DOI] [PubMed] [Google Scholar]
- Official methods of analysis. 15. Arlington, VA: AOAC; 1990. [Google Scholar]
- Baumgard LH, Corl BA, Dwyer DA, Saebo A, Bauman DE. Identification of the conjugated linoleic isomer that inhibits milk fat synthesis. Am J Physiol. 2000;278:R179–R184. doi: 10.1152/ajpregu.2000.278.1.R179. [DOI] [PubMed] [Google Scholar]
- Baumgard LH, Matitashvili E, Corl BA, Dwyer DA, Bauman DE. trans-10, cis-12 conjugated linoleic acid decreases lipogenic rates and expression of genes involved in milk lipid synthesis in dairy cows. J Dairy Sci. 2002;85:2155–2163. doi: 10.3168/jds.S0022-0302(02)74294-X. [DOI] [PubMed] [Google Scholar]
- Benson ME, Henry MJ, Cardellino RA. Comparison of weigh-suckle-weigh and machine milking for measuring ewe milk production. J Anim Sci. 1999;77:2330–2335. doi: 10.2527/1999.7792330x. [DOI] [PubMed] [Google Scholar]
- Boutinaud M, Rulquin H, Keisler DH, Djiane J, Jammes H. Use of somatic cells from goat milk for dynamic studies of gene expression in the mammary gland. J Anim Sci. 2002;80:1258–1269. doi: 10.2527/2002.8051258x. [DOI] [PubMed] [Google Scholar]
- Brosh A, Aharoni Y, Shargal E, Choshniak I, Sharir B, Gutman M. Energy balance of grazing beef cows in Mediterranean pasture, the effects of stocking rate and season: 2. Energy expenditure estimated from heart rate and oxygen consumption, and energy balance. Livest Prod Sci. 2004;90:101–115. doi: 10.1016/j.livprodsci.2004.03.008. [DOI] [Google Scholar]
- Buehring GC. Culture of mammary epithelial cells from bovine milk. J Dairy Sci. 1990;73:956–963. doi: 10.3168/jds.S0022-0302(90)78752-8. [DOI] [PubMed] [Google Scholar]
- Chouinard PY, Corneau L, Barbano DM, Metzger LE, Bauman DE. Conjugated linoleic acids alter milk fatty acid composition and inhibit milk fat secretion in dairy cows. J Nutr. 1999;129:1579–1584. doi: 10.1093/jn/129.8.1579. [DOI] [PubMed] [Google Scholar]
- Christie WW. A simple procedure of rapid trans-methylation of glycerolipids and cholesteryl esters. J Lipid Res. 1982;23:1072–1075. [PubMed] [Google Scholar]
- Craig EA, Lindquist S. The heat shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.000453. [DOI] [PubMed] [Google Scholar]
- Crocker LM, DePeters EJ, Fadel JG, Perez-Monti H, Taylor SJ, Wyckoff JA, Zinn RA. Influence of processed corn grain in diets of dairy cows on digestion of nutrients and milk composition. J Dairy Sci. 1997;81:2394–2407. doi: 10.3168/jds.S0022-0302(98)70131-6. [DOI] [PubMed] [Google Scholar]
- Roos APW, Bijgaart HJCM, Hørlyk J, Jong G. Screening for subclinical ketosis in dairy cattle by Fourier transform infrared spectrometry. J Dairy Sci. 2007;90:1761–1766. doi: 10.3168/jds.2006-203. [DOI] [PubMed] [Google Scholar]
- Dils RR. Milk fat synthesis. In: Mepham TB, editor. Biochemistry of lactation. Amsterdam: Elsevier; 1983. pp. 141–157. [Google Scholar]
- Duffy PH, Leakey JEA, Pipkin JL, Turturro A, Hart RW. The physiologic, neurologic, and behavioral effects of caloric restriction related to aging, disease, and environmental factors. Environ Res. 1997;73:242–248. doi: 10.1006/enrs.1997.3714. [DOI] [PubMed] [Google Scholar]
- German T, Barash I. Characterization of an epithelial cell line from bovine mammary gland. In Vitro Cell Dev Biol. 2002;38:282–292. doi: 10.1290/1071-2690(2002)038<0282:COAECL>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hara A, Radin NS. Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem. 1978;90:420–426. doi: 10.1016/0003-2697(78)90046-5. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Hurtaud C, Rulquin H, Vérité R. Effects of level and type of energy source (volatile fatty acids or glucose) on milk yield, composition and coagulating properties in dairy cows. Reprod Nutr Dev. 1998;38:315–330. doi: 10.1051/rnd:19980312. [DOI] [PubMed] [Google Scholar]
- Jenkins TG, Ferrell CL. Lactation characteristics of nine breeds of cattle fed various quantities of dietary energy. J Anim Sci. 1992;70:1652–1660. doi: 10.2527/1992.7061652x. [DOI] [PubMed] [Google Scholar]
- Kehrli ME, Shuster DE. Factors affecting milk somatic cells and their role in health of the bovine mammary gland. J Dairy Sci. 1994;77:619–627. doi: 10.3168/jds.S0022-0302(94)76992-7. [DOI] [PubMed] [Google Scholar]
- Kovacs IA, Szalay MS, Csermely P. Water and molecular chaperones act as weak links of protein folding networks: energy landscape and punctuated equilibrium changes point towards a game theory of proteins. FEBS Lett. 2005;579:2254–2260. doi: 10.1016/j.febslet.2005.03.056. [DOI] [PubMed] [Google Scholar]
- Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- Loor JJ, Herbein JH. Exogenous conjugated linoleic acid isomers reduce bovine milk fat concentration and yield by inhibiting de novo synthesis. J Nutr. 1998;128:2411–2419. doi: 10.1093/jn/128.12.2411. [DOI] [PubMed] [Google Scholar]
- Main AR. Resilience on the level of individual animal. In: Dell B, Hopkins AJM, Lamont BB, editors. Resilience in mediterranean type ecosystems. Dordrecht, Netherlands: Junk; 1986. pp. 83–94. [Google Scholar]
- Mallinckrodt CH, Bourdon RM, Golden BL, Schalles RR, Odde KG. Relationship of maternal milk expected progeny differences to actual milk yield and calf weaning weight. J Anim Sci. 1993;71:355–362. doi: 10.2527/1993.712355x. [DOI] [PubMed] [Google Scholar]
- Meyer K, Carrick MJ, Donnelly BJP. Genetic parameters for milk production of Australian beef cows and weaning weight of their calves. J Anim Sci. 1994;72:1155–1165. doi: 10.2527/1994.7251155x. [DOI] [PubMed] [Google Scholar]
- Morrissey PA. In: Developments in dairy chemistry, vol 3. Fox PF, editor. New York: Elsevier; 1985. pp. 1–34. [Google Scholar]
- Nocek JE, Russell JB. Protein and energy as an integrated system. Relationship of ruminal protein and carbohydrate availability to microbial synthesis and milk production. J Dairy Sci. 1988;71:2070–2107. [Google Scholar]
- Nutrient requirements of beef cattle. 7. Washington, DC, USA: National Academies; 1996. [Google Scholar]
- Palmquist DL, Beaulieu AD, Barbano DM. Feed and animal factors influencing milk fat composition. J Dairy Sci. 1993;76:1753–1771. doi: 10.3168/jds.s0022-0302(93)77508-6. [DOI] [PubMed] [Google Scholar]
- Patel NV, Finch CE. The glucocorticoid paradox of caloric restriction in slowing brain aging. Neurobiol Aging. 2002;23:707–717. doi: 10.1016/S0197-4580(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Randel RD. Nutrition and postpartum rebreeding in cattle. J Anim Sci. 1990;68:853–862. doi: 10.2527/1990.683853x. [DOI] [PubMed] [Google Scholar]
- Roseler DK, Ferguson JD, Sniffen CJ, Herrema J. Dietary protein degradability effects on plasma and milk urea nitrogen and milk nonprotein nitrogen in Holstein cows. J Dairy Sci. 1993;76:525–534. [Google Scholar]
- Simpson MA, LiCata VJ, Ribarik Coe N, Bernlohr DA. Biochemical and biophysical analysis of the intracellular lipid binding proteins of adipocytes. Mol Cell Biochem. 1999;192:33–40. doi: 10.1023/A:1006819715146. [DOI] [PubMed] [Google Scholar]
- Sørensen ES, Møller L, Vinther M, Petersen TE, Rasmussen LK. The phosphorylation pattern of human as1-casein is markedly different from the ruminant species. Eur J Biochem. 2003;270:3651–3655. doi: 10.1046/j.1432-1033.2003.03755.x. [DOI] [PubMed] [Google Scholar]
- SPSS 14.0 for Windows. Chicago, IL: SPSS; 2005. [Google Scholar]
- Sternberg M, Gutman M, Perevolotsky A, Ungar ED, Kigel J. Vegetation response to grazing management in a Mediterranean herbaceous community: a functional group approach. J Appl Ecol. 2000;37:224–237. doi: 10.1046/j.1365-2664.2000.00491.x. [DOI] [Google Scholar]
- Sutton JD. Altering milk composition by feeding. J Dairy Sci. 1988;72:2801–2814. [Google Scholar]
- Ungar ED, Karlibach Y, Yehuda Y, Baram H, Gutman M. Multi-year analysis of production in free grazing beef cattle herd in the Golan height. Yediot Labokrim. 2005;113:13–19. [Google Scholar]
- Welch WJ. The mammalian stress response: cell physiology and biochemistry of stress proteins. In: Morimoto RI, Tissieres A, Georgopolous C, editors. Stress proteins in biology and medicine. New York: Cold Spring Harbor Laboratory; 1990. pp. 223–278. [Google Scholar]
- Whetstone HD, Hurley WL, Davis CL. Identification and Characterization of a fatty acid binding protein in bovine mammary gland. Comp Biochem Physiol B. 1986;85:687–692. doi: 10.1016/0305-0491(86)90068-4. [DOI] [PubMed] [Google Scholar]
- Yu BP, Chung HY. Stress resistance by caloric restriction for longevity. Ann N Y Acad Sci. 2001;928(1):39–47. doi: 10.1111/j.1749-6632.2001.tb05633.x. [DOI] [PubMed] [Google Scholar]