Abstract
It has been previously reported that serum levels of 70-kDa heat-shock protein (Hsp70) are elevated in preeclampsia. The aim of the present study was to examine whether increased serum Hsp70 levels are related to clinical characteristics and standard laboratory parameters of preeclamptic patients, as well as to markers of inflammation (C-reactive protein), endothelial activation (von Willebrand factor antigen) or endothelial injury (fibronectin), trophoblast debris (cell-free fetal DNA) and oxidative stress (malondialdehyde). Sixty-seven preeclamptic patients and 70 normotensive, healthy pregnant women were involved in this case-control study. Serum Hsp70 levels were measured with enzyme-linked immunosorbent assay (ELISA). Standard laboratory parameters (clinical chemistry) and C-reactive protein (CRP) levels were determined by an autoanalyzer using the manufacturer’s kits. Plasma von Willebrand factor antigen (VWF:Ag) levels were quantified by ELISA, and plasma fibronectin concentration by nephelometry. The amount of cell-free fetal DNA in maternal plasma was determined by quantitative real-time polymerase chain reaction analysis of the sex-determining region Y gene. Plasma malondialdehyde levels were measured by the thiobarbituric acid-based colorimetric assay. Serum Hsp70 levels were increased in preeclampsia. Furthermore, serum levels of blood urea nitrogen, creatinine, bilirubin and CRP, serum alanine aminotransferase and lactate dehydrogenase (LDH) activities, as well as plasma levels of VWF:Ag, fibronectin, cell-free fetal DNA and malondialdehyde were also significantly higher in preeclamptic patients than in normotensive, healthy pregnant women. In preeclamptic patients, serum Hsp70 levels showed significant correlations with serum CRP levels (Spearman R = 0.32, p = 0.010), serum aspartate aminotransferase (R = 0.32, p = 0.008) and LDH activities (R = 0.50, p < 0.001), as well as with plasma malondialdehyde levels (R = 0.25, p = 0.043). However, there was no other relationship between serum Hsp70 levels and clinical characteristics (age, parity, body mass index, blood pressure, gestational age, fetal birth weight) and laboratory parameters of preeclamptic patients, including markers of endothelial activation or injury and trophoblast debris. In conclusion, increased serum Hsp70 levels seem to reflect systemic inflammation, oxidative stress and hepatocellular injury in preeclampsia. Nevertheless, further studies are required to determine whether circulating Hsp70 plays a causative role in the pathogenesis of the disease.
Keywords: Biomarker, Heat-shock protein 70, Hepatocellular injury, Inflammation, Oxidative stress, Preeclampsia
Introduction
Preeclampsia is a severe complication of human pregnancy with a worldwide incidence of 2–10% (Duckitt and Harrington 2005). It is one of the leading causes of maternal and perinatal morbidity and mortality, even in developed countries. Despite intensive research efforts, the etiology and pathogenesis of preeclampsia are not completely understood. Preeclampsia is a two-stage disorder. Stage 1 of preeclampsia is poor placentation, which is followed by the development of the maternal syndrome (stage 2). According to our knowledge, maternal–fetal (paternal) immune maladaptation is the main cause of poor placentation. Subsequent uteroplacental insufficiency leads to placental ischemia and oxidative stress. The ischemic and oxidatively stressed placenta releases proinflammatory (Th1) cytokines, lipid peroxidation products and trophoblast debris (syncitiotrophoblast microfragments, cytokeratin, soluble DNA and RNA of fetal origin and even trophoblast cells) into the maternal circulation, which in turn trigger an excessive maternal systemic inflammatory response. The systemic inflammatory response with systemic oxidative stress and generalized endothelial dysfunction appears to be the cause of the maternal syndrome of preeclampsia (Redman et al. 1999; Redman and Sargent 2005). The development of preeclampsia is influenced by both genetic and environmental risk factors, suggesting its multifactorial inheritance (Roberts and Gammill 2005).
Heat-shock proteins (Hsps) are ubiquitous and phylogenetically conserved molecules, which indicate their functional importance. They are usually considered to be intracellular proteins with molecular chaperone and cytoprotective functions (Hightower 1991). However, 70-kDa heat-shock protein (Hsp70) is present in the peripheral circulation of healthy non-pregnant individuals (Pockley et al. 1998). Circulating Hsp70 levels were found to be elevated in peripheral and renal vascular disease (Wright et al. 2000), during and after exercise (Walsh et al. 2001; Fehrenbach et al. 2005), in sickle cell disease, particularly during vaso-occlusive crisis (Adewoye et al. 2005), in patients with acute infections (Njemini et al. 2003), with prostate cancer (Abe et al. 2004) or chronic heart failure (Genth-Zotz et al. 2004), in children with septic shock (Wheeler et al. 2005), after surgical procedures (Kimura et al. 2004), as well as following myocardial infarction (Dybdahl et al. 2005) and coronary artery bypass grafting (Dybdahl et al. 2002). We have recently reported that serum Hsp70 levels are significantly lower in healthy pregnant women than in healthy non-pregnant women (Molvarec et al. 2007b). In addition, we and other research groups observed increased circulating Hsp70 concentrations in preeclampsia (Jirecek et al. 2002; Fukushima et al. 2005; Molvarec et al. 2006). Furthermore, for the first time in the literature, we demonstrated that serum Hsp70 levels are significantly higher in patients with the syndrome of hemolysis, elevated liver enzymes and low platelet count (HELLP syndrome) than in severely preeclamptic patients without HELLP syndrome (Molvarec et al. 2007a). According to our findings, elevated serum Hsp70 level reflects tissue damage (hemolysis and hepatocellular injury) and disease severity in HELLP syndrome (Madach et al. in press). Nevertheless, the cause and clinical significance of increased circulating Hsp70 levels in preeclamptic patients without HELLP syndrome are currently unknown.
Therefore, the aim of the present study was to examine whether increased serum Hsp70 levels are related to clinical characteristics and standard laboratory parameters of preeclamptic patients, as well as to markers of inflammation (C-reactive protein), endothelial activation (von Willebrand factor antigen) or endothelial injury (fibronectin), trophoblast debris (cell-free fetal DNA) and oxidative stress (malondialdehyde).
Materials and methods
Study participants
Our study was designed employing a case-control approach. Sixty-seven preeclamptic patients and 70 normotensive (blood pressure <140 mmHg systolic and <90 mmHg diastolic), healthy pregnant women with uncomplicated pregnancies were involved in the study. The study participants were enrolled in the 1st Department of Obstetrics and Gynecology and in the Department of Obstetrics and Gynecology of Kútvölgyi Clinical Center, at the Semmelweis University, Budapest, Hungary. All women were Caucasian and resided in the same geographic area in Hungary. Exclusion criteria were multifetal gestation, chronic hypertension, diabetes mellitus, autoimmune disease, angiopathy, renal disorder, maternal or fetal infection and fetal congenital anomaly. The women were fasting, none were in active labor, and none had rupture of membranes. The subject group is a subgroup of one of our previously published studies (Molvarec et al. 2006). However, in that study, only serum Hsp70 levels were measured and reported.
Preeclampsia was defined by increased blood pressure (≥140 mmHg systolic or ≥90 mmHg diastolic on ≥2 occasions at least 6 h apart) that occurred after 20 weeks of gestation in a woman with previously normal blood pressure, accompanied by proteinuria (≥0.3 g/24 h). Blood pressure returned to normal by 12 weeks postpartum in each preeclamptic study patient. Pregnant women with eclampsia or HELLP syndrome were not enrolled in this study. Fetal growth restriction was diagnosed if the fetal birth weight was below the 10th percentile for gestational age and gender, based on Hungarian birth weight percentiles (Joubert 2000).
The study protocol was approved by the Regional, Institutional Committee of Medical Ethics at the Semmelweis University, and written informed consent was obtained from each patient. The study was conducted in accordance with the Declaration of Helsinki.
Biological samples
Maternal blood samples were obtained from an antecubital vein into native, as well as ethylenediaminetetraacetic acid or sodium citrate anticoagulated tubes and centrifuged at room temperature with a relative centrifugal force of 3,000 × g for 10 min. The aliquots of serum and plasma were stored at −80°C until the analyses were performed.
Laboratory methods
Serum Hsp70 levels were measured by using the enzyme-linked immunosorbent assay (ELISA) Kit of R&D Systems (DYC1663E, Minneapolis, MN, USA). Ninety-six-well microtiter plates were coated with mouse anti-human Hsp70 capture antibodies (100 μl; 2 μg/ml) in carbonate buffer (pH 9.5) overnight at 4°C. Plates were washed with phosphate-buffered saline (PBS) containing 0.1% Tween 20 three times and non-specific binding sites blocked by incubation with 200 μl of PBS containing 0.5% gelatin and Tween 20 for 1 h at room temperature. After washing, 100 μl of the reference preparation (recombinant human Hsp70, 0–10 ng/ml) or samples (1:1) was added and the plates were incubated for 2 h at room temperature. Plates were subsequently washed and Hsp70 binding was determined using biotinylated rabbit anti-human antibodies (100 μl; 0.5 μg/ml) in PBS gelatin. After 1.5 h at room temperature, plates were washed and incubated with streptavidin–horseradish-peroxidase (HRP, 1:200) in PBS gelatin for 20 min at room temperature. Plates were washed and 100 μl of o-phenylene-diamine (OPD) (Sigma, St. Louis, MO, USA) in citrate buffer was added. The optical density was measured at λ = 490 nm (reference at λ = 620 nm). The detection range of the assay was 0.05–10 ng/ml, the intra/inter-assay variability <10/<16%, respectively.
Standard laboratory parameters (clinical chemistry) and C-reactive protein (CRP) levels were determined by an autoanalyzer (Cobas Integra 800, Roche, Mannheim, Germany) using the manufacturer’s kits. Plasma von Willebrand factor antigen (VWF:Ag) levels were quantified by ELISA (Dakopatts, Glostrup, Denmark), while plasma fibronectin concentration by nephelometry (Dade Behring, Marburg, Germany), according to the manufacturer’s instructions. After extracting DNA with the silica adsorption method, the amount of cell-free fetal DNA in maternal plasma was determined in patients with male newborns by quantitative real-time PCR analysis of the sex-determining region Y (SRY) gene, as we described previously (Lazar et al. 2006). Plasma malondialdehyde levels were measured by the thiobarbituric acid-based colorimetric assay (Placer et al. 1966).
Statistical analysis
The normality of continuous variables was assessed using the Shapiro–Wilk’s W test. As the continuous variables were not normally distributed, nonparametric statistical methods were used. To compare continuous variables between two groups, the Mann–Whitney U test was applied. The Fisher exact and Pearson χ2 tests were performed to compare categorical variables between groups. As serum levels of Hsp70 and C-reactive protein, as well as plasma levels of VWF antigen, fibronectin, cell-free fetal DNA and malondialdehyde showed skewed distributions, we performed analyses of covariance (ANCOVA) with logarithmically transformed data. The Spearman rank order correlation was carried out to calculate correlation coefficients. The scatterplots were created, as a nonparametric method, with logarithmically transformed values of the dependent variable.
Statistical analyses were carried out using the following software: STATISTICA (version 7.1; StatSoft, Inc., Tulsa, OK, USA) and Statistical Package for the Social Sciences (version 15.0 for Windows; SPSS, Inc., Chicago, IL, USA). For all statistical analyses, p < 0.05 was considered statistically significant.
In the article, data are reported as median (range) for continuous variables and as number (percent) for categorical variables.
Results
Patient characteristics and standard laboratory parameters
The clinical characteristics of the study participants are shown in Table 1. There were no statistically significant differences in maternal age and the percentage of smokers and primiparas between the two study groups. The body mass index (BMI) and gestational age at blood draw were significantly higher in the preeclamptic group compared with the control group. The systolic and diastolic blood pressures were significantly higher, whereas the gestational age at delivery and the fetal birth weight were significantly lower in the preeclamptic group than in the control group. Fetal growth restriction was absent in control subjects, whereas the frequency of this condition was 16.4% in the preeclamptic group.
Table 1.
Clinical characteristics and laboratory parameters of normotensive, healthy pregnant women and preeclamptic patients
| Variable | Controls (n = 70) | Preeclampsia (n = 67) | Statistical significance (p value) |
|---|---|---|---|
| Age (years) | 30 (17–44) | 29 (19–42) | NS |
| BMI at blood draw (kg/m2) | 25.9 (19.0–42.0) | 30.0 (20.6–38.3) | <0.001 |
| Smokers | 0 (0%) | 3 (4.5%) | NS |
| Primiparas | 45 (64.3%) | 43 (64.2%) | NS |
| Systolic blood pressure (mmHg) | 110 (80–138) | 160 (135–220) | <0.001 |
| Diastolic blood pressure (mmHg) | 70 (55–86) | 100 (90–131) | <0.001 |
| Gestational age at blood draw (weeks) | 35 (20–40) | 38 (30–41) | <0.001 |
| Gestational age at delivery (weeks) | 39 (35–41) | 38 (33–41) | <0.05 |
| Fetal birth weight (grams) | 3500 (2650–4400) | 3200 (1400–4200) | <0.001 |
| Fetal growth restriction | 0 (0%) | 11 (16.4%) | <0.001 |
| Serum BUN level (mmol/l) | 2.7 (1.7–4.8) | 3.4 (0.8–6.5) | <0.001 |
| Serum creatinine level (µmol/l) | 47 (34–79) | 63 (36–95) | <0.001 |
| Serum bilirubin level (µmol/l) | 5.1 (1.8–15.2) | 7.4 (2.1–20.9) | <0.001 |
| Serum AST activity (U/l) | 19 (10–38) | 19 (10–148) | NS |
| Serum ALT activity (U/l) | 12 (7–32) | 15 (6–233) | <0.05 |
| Serum LDH activity (U/l) | 159 (93–211) | 192 (113–403) | <0.001 |
| Serum CRP level (mg/l) | 3.6 (0.5–28.0) | 6.7 (0.3–36.9) | <0.05 |
| Plasma VWF:Ag level (%) | 129.3 (47.8–297.1) | 187.1 (43.3–423.0) | <0.001 |
| Plasma fibronectin level (g/l) | 0.33 (0.14–0.84) | 0.58 (0.02–2.13) | <0.001 |
| Plasma fetal DNA level (pg/µl) | 0.001 (0.0–0.845) | 0.086 (0.002–3.200) | <0.001 |
| Plasma malondialdehyde level (nmol/ml) | 14.74 (6.19–36.52) | 18.62 (10.75–24.65) | <0.001 |
| Serum Hsp70 level (ng/ml) | 0.28 (0.03–0.59) | 0.58 (0.15–3.47) | <0.001 |
Data are presented as median (range) for continuous variables and as number (percent) for categorical variables
NS not significant, BMI body mass index, BUN blood urea nitrogen, AST aspartate aminotransferase, ALT alanine aminotransferase, LDH lactate dehydrogenase, CRP C-reactive protein, VWF:Ag von Willebrand factor antigen, DNA deoxyribonucleic acid, Hsp70 heat-shock protein 70
As depicted in Table 1, the serum blood urea nitrogen (BUN) and creatinine levels were significantly higher in the preeclamptic group compared to the control group, but these parameters were within the normal range in each patient in both groups. The preeclamptic patients had significantly higher serum bilirubin levels, as well as serum alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) activities than the control subjects. However, no significant differences were observed in serum aspartate aminotransferase (AST) activities between the two study groups.
Serum Hsp70 levels and markers of inflammation, endothelial activation or injury, trophoblast debris and oxidative stress
Serum levels of Hsp70 and C-reactive protein and plasma levels of VWF antigen, fibronectin, cell-free fetal DNA and malondialdehyde were significantly higher in preeclamptic patients than in normotensive, healthy pregnant women (Table 1). The differences in these variables between the two study groups remained significant even after adjustment for maternal age, BMI and gestational age at blood draw in analyses of covariance (ANCOVA).
Relationship of clinical characteristics, standard laboratory parameters and markers of inflammation, endothelial activation or injury, trophoblast debris and oxidative stress to serum Hsp70 levels in preeclampsia
We investigated whether clinical characteristics and laboratory parameters of preeclamptic patients are related to serum Hsp70 levels by calculating the Spearman rank order correlation coefficients (Table 2). In the preeclamptic group, serum Hsp70 levels showed significant correlations with serum CRP levels (Spearman R = 0.32, p = 0.010), serum AST (Spearman R = 0.32, p = 0.008) and LDH activities (Spearman R = 0.50, p < 0.001), as well as with plasma malondialdehyde levels (Spearman R = 0.25, p = 0.043). However, clinical characteristics of preeclamptic patients (maternal age, parity, BMI and gestational age at blood draw, systolic and diastolic blood pressure, gestational age at delivery and fetal birth weight) were not related to serum Hsp70 levels. Furthermore, serum levels of BUN, creatinine and bilirubin, serum ALT activity, as well as plasma levels of VWF antigen, fibronectin and cell-free fetal DNA did not show significant correlations with serum Hsp70 levels in the preeclamptic group.
Table 2.
Correlation coefficients with p values between clinical characteristics and laboratory parameters of preeclamptic patients and serum Hsp70 levels
| Variable | Correlation coefficient | Statistical significance (p value) |
|---|---|---|
| Age | 0.07 | 0.59 |
| BMI at blood draw | 0.07 | 0.64 |
| Systolic blood pressure | 0.12 | 0.35 |
| Diastolic blood pressure | 0.09 | 0.47 |
| Gestational age at blood draw | 0.14 | 0.25 |
| Gestational age at delivery | 0.03 | 0.79 |
| Fetal birth weight | 0.01 | 0.98 |
| Serum BUN level | 0.04 | 0.76 |
| Serum creatinine level | 0.10 | 0.46 |
| Serum bilirubin level | 0.20 | 0.12 |
| Serum AST activity | 0.32 | 0.008 |
| Serum ALT activity | 0.21 | 0.095 |
| Serum LDH activity | 0.50 | <0.001 |
| Serum CRP level | 0.32 | 0.010 |
| Plasma VWF:Ag level | −0.08 | 0.51 |
| Plasma fibronectin level | 0.11 | 0.37 |
| Plasma fetal DNA level | 0.04 | 0.81 |
| Plasma malondialdehyde level | 0.25 | 0.043 |
BMI body mass index, BUN blood urea nitrogen, AST aspartate aminotransferase, ALT alanine aminotransferase, LDH lactate dehydrogenase, CRP C-reactive protein, VWF:Ag von Willebrand factor antigen, DNA deoxyribonucleic acid, Hsp70 heat-shock protein 70
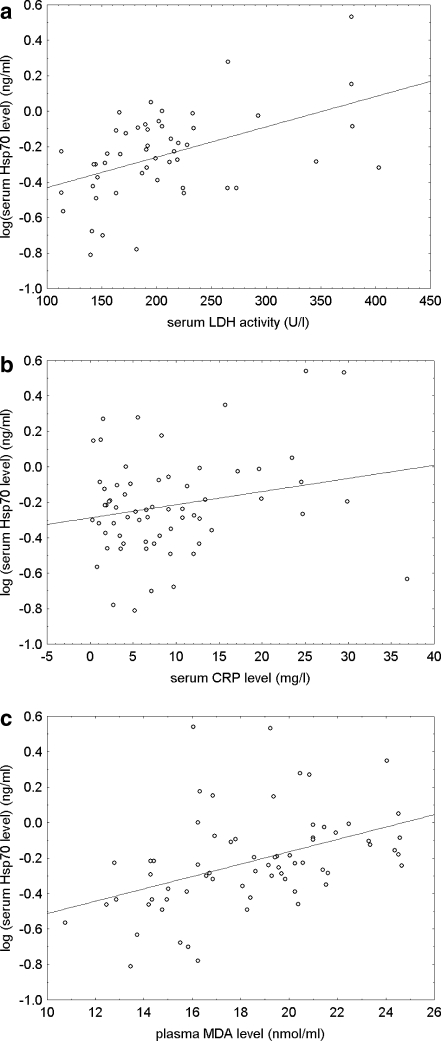
The relationship of serum Hsp70 levels to laboratory markers of hepatocellular injury, inflammation and oxidative stress in preeclamptic patients are visualized with scatterplots (Fig. 1). As can be seen in Fig. 1, the correlation between plasma malondialdehyde concentrations and serum Hsp70 levels was more pronounced with logarithmic transformation than calculating with the Spearman rank order statistics.
Fig. 1.
Scatterplots with linear fit and the regression line of logarithmically transformed values of serum heat-shock protein 70 (Hsp70) levels versus serum lactate dehydrogenase (LDH) activities (a), serum C-reactive protein (CRP) levels (b) and plasma malondialdehyde (MDA) concentrations (c) in preeclamptic patients
The sample size of the preeclamptic group allowed us to detect a correlation coefficient (rho) of 0.30 or higher, at a Type I error rate of 0.05, with a statistical power of at least 80%.
Discussion
In the present study, increased serum Hsp70 levels showed significant correlations with serum CRP levels, serum AST and LDH activities, as well as with plasma malondialdehyde levels in preeclampsia. However, there was no other relationship between serum Hsp70 levels and clinical characteristics (age, parity, BMI, blood pressure, gestational age, fetal birth weight) and laboratory parameters of preeclamptic patients, including markers of endothelial activation or injury and trophoblast debris.
The intracellular expression of Hsp70 can be induced by ischemia, reactive oxygen species and inflammatory cytokines, as well as by hemodynamic stress (acute hypertension) (Prohaszka and Fust 2004). Placental ischemia and oxidative stress, an excessive maternal systemic inflammatory response with systemic oxidative stress, as well as hemodynamic stress have been implicated in the pathogenesis of preeclampsia. Hsp70 may be released from viable cells exposed to stressful insults into the extracellular environment by non-classical (endoplasmic reticulum-Golgi-independent) protein transport mechanisms: within exosomes or lysosomes, as well as via intact lipid rafts (Broquet et al. 2003; Lancaster and Febbraio 2005; Mambula and Calderwood 2006). According to our findings, systemic inflammation and oxidative stress seem to be responsible – at least in part – for increased circulating Hsp70 levels in preeclampsia, as suggested by the significant positive correlations of serum Hsp70 levels with circulating levels of CRP (acute phase reactant) and malondialdehyde (lipid peroxidation product). Indeed, inflammatory cytokines have been reported to induce extracellular release of Hsp70 within exosomes (Bausero et al. 2005). Additionally, oxidative stress has been suggested to be involved in the exercise-induced circulating Hsp72 response and the supplementation with vitamin C and the vitamin E isoform γ-tocopherol completely blunted this response (Fischer et al. 2006). Furthermore, the antioxidant folic acid, which reduces oxidative stress in vivo, significantly decreased serum Hsp70 levels in patients with type 2 diabetes (Hunter-Lavin et al. 2004b).
Not only can Hsp70 be released from intact cells by active mechanisms, but it may also be discharged from damaged, necrotic cells in a passive manner (Basu et al. 2000). Circulating Hsp70 levels were found to be increased in several conditions where tissue damage is known to occur (Walsh et al. 2001; Dybdahl et al. 2002; Pittet et al. 2002; Kimura et al. 2004; Adewoye et al. 2005; Dybdahl et al. 2005; Fehrenbach et al. 2005; Suzuki et al. 2006). Trophoblast apoptosis and necrosis with increased shedding of syncytiotrophoblast microfragments and cell-free fetal DNA into the maternal circulation, as well as endothelial dysfunction/injury play a central role in the pathogenesis of preeclampsia (Redman and Sargent 2001). In severe cases, preeclampsia may be complicated by ischemic organ involvement, such as acute tubular or cortical necrosis leading to renal failure, hepatocellular necrosis or cerebral ischemia. However, increased serum Hsp70 levels were not related to the markers of trophoblast debris or endothelial dysfunction/injury in our preeclamptic group. Moreover, renal function did not affect circulating Hsp70 levels in our study, but we did not observe renal failure in our patients. Instead, liver enzymes – particularly serum LDH activities – showed the strongest correlations with serum Hsp70 levels in preeclampsia, which suggests that release of Hsp70 from necrotic hepatic cells might substantially contribute to the elevation in circulating Hsp70 levels found in preeclampsia. Interestingly, our research group has recently revealed that hepatocellular injury is associated with increased serum Hsp70 levels both in HELLP syndrome and chronic heart failure (Gombos et al. 2008; Madach et al. in press).
Although Hsp70 is present in the peripheral circulation of healthy subjects and circulating Hsp70 levels are altered in physiological and pathological circumstances, the origin of circulating Hsp70 in these conditions has not been fully elucidated. Our results denote that release of Hsp70 from trophoblast and endothelial cells might not account for increased serum Hsp70 levels in preeclampsia. Furthermore, hepatocellular necrosis occurs only in severe cases of preeclampsia and it explained only 25% of the variance in serum Hsp70 concentration in our preeclamptic group. Indeed, Hsp70 may also be released from intact hepatic cells, as was observed during semi-recumbent cycling (Febbraio et al. 2002). Interestingly, a number of acute phase proteins, such as CRP, are also synthesized and released by the liver. Thus, the significant correlation between serum Hsp70 and CRP levels raises the possibility that Hsp70 may originate from this organ in preeclampsia in the absence of hepatocellular necrosis. Moreover, innate immune cells (monocytes and granulocytes), which are exaggeratedly activated in preeclampsia and produce both proinflammatory cytokines and reactive oxygen species, are also capable of releasing Hsp70 into the extracellular space (Hunter-Lavin et al. 2004a) and might be additional sources of circulating Hsp70 in preeclampsia. Interestingly, circulating levels of both CRP (inflammatory marker) and malondialdehyde (marker of oxidative stress) showed significant correlations with serum Hsp70 levels in our preeclamptic group, which may support this hypothesis.
Elevated circulating Hsp70 level may not only be a marker of preeclampsia, but might also play a role in its pathogenesis. Extracellular Hsp70 derived from stressed and damaged, necrotic cells can act as an intercellular stress signalling molecule, representing an ancestral danger signal of a non-physiological condition, such as cellular stress or damage, to elicit innate and adaptive proinflammatory immune responses (Pockley 2003). Extracellular Hsp70 acts through binding to surface receptors (CD14, CD36, CD40, CD91, LOX-1, Toll-like receptor 2 and 4) on antigen-presenting cells, stimulating their proinflammatory cytokine (tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6) and chemokine production, as well as costimulatory molecule expression (Asea et al. 2000; Basu et al. 2001; Asea et al. 2002; Asea 2005). Furthermore, our research group has previously demonstrated that Hsp70 is a potent activator of the classical pathway of the human complement system (Prohaszka et al. 2002). The maternal systemic inflammatory response, which seems to be responsible for the signs and symptoms of preeclampsia, involves a rise in number and activation of leukocytes (monocytes and granulocytes) with production of proinflammatory cytokines leading to Th1 bias, as well as the activation of the complement system and the production of acute phase proteins (Redman et al. 1999). The relationship between increased serum Hsp70 and CRP levels found in our study suggests that circulating Hsp70 might be involved in the development of the maternal systemic inflammatory response in preeclampsia. Indeed, elevated circulating Hsp70 levels have already been observed to be associated with inflammatory responses in several pathological conditions, such as in acute infections, after liver resection and coronary artery bypass grafting, as well as following myocardial infarction (Dybdahl et al. 2002; Njemini et al. 2003; Kimura et al. 2004; Dybdahl et al. 2005). However, Hsp70 has also anti-inflammatory effects (Kingston et al. 1996; Tanaka et al. 1999; Wendling et al. 2000; House et al. 2001), and it might therefore also be involved in the resolution of inflammation. Although extracellular Hsp70 has been reported to bind to endothelial cells (Theriault et al. 2005) and Hsp70 has recently been found to be associated with endothelial activation in placental vascular diseases (Liu et al. in press), we could not establish any association between circulating Hsp70 levels and markers of endothelial activation or injury in preeclampsia.
Nevertheless, the lack of association between increased serum Hsp70 levels and several measured parameters in preeclampsia may also be due to genetic variations in Hsp70 production, release into the extracellular space or stability. Furthermore, Hsp70, with its intrinsic ability to act as a chaperone, can bind to other macromolecules, which might mask its detection by ELISA.
In conclusion, increased serum Hsp70 levels seem to reflect systemic inflammation, oxidative stress and hepatocellular injury in preeclampsia. However, further studies are required to determine whether circulating Hsp70 plays a causative role in the pathogenesis of preeclampsia or elevated serum Hsp70 levels are only consequences of the disease.
Acknowledgements
The skilful technical assistance of Szigeti Antalné and the support of Szilvia Walentin, Éva Imreh and Mónika Kleiber (Central Laboratory, Kútvölgyi Clinical Center, Semmelweis University, Budapest, Hungary) are acknowledged with many thanks. This work was supported by research grants from the Hungarian Scientific Research Fund (NF 72689) and the Faculty of Medicine of the Semmelweis University.
Abbreviations
- ALT
alanine aminotransferase
- ANCOVA
analysis of covariance
- AST
aspartate aminotransferase
- BMI
body mass index
- BUN
blood urea nitrogen
- CD
cluster of differentiation
- CRP
C-reactive protein
- DNA
deoxyribonucleic acid
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- HELLP
hemolysis, elevated liver enzymes, and low platelet count
- HRP
horseradish-peroxidase
- Hsp
heat-shock protein
- IL
interleukin
- LDH
lactate dehydrogenase
- LOX-1
lectin-like oxidised low-density lipoprotein receptor-1
- MDA
malondialdehyde
- OPD
o-phenylene-diamine
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- RNA
ribonucleic acid
- SRY
sex-determining region Y
- Th1
T helper 1
- TNF-α
tumor necrosis factor-α
- U
unit
- VWF:Ag
von Willebrand factor antigen
References
- Abe M, Manola JB, Oh WK, Parslow DL, George DJ, Austin CL, Kantoff PW. Plasma levels of heat shock protein 70 in patients with prostate cancer: a potential biomarker for prostate cancer. Clin Prostate Cancer. 2004;3:49–53. doi: 10.3816/cgc.2004.n.013. [DOI] [PubMed] [Google Scholar]
- Adewoye AH, Klings ES, Farber HW, et al. Sickle cell vaso-occlusive crisis induces the release of circulating serum heat shock protein-70. Am J Hematol. 2005;78:240–242. doi: 10.1002/ajh.20292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asea A. Stress proteins and initiation of immune response: chaperokine activity of hsp72. Exerc Immunol Rev. 2005;11:34–45. [PMC free article] [PubMed] [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/S1074-7613(01)00111-X. [DOI] [PubMed] [Google Scholar]
- Bausero MA, Gastpar R, Multhoff G, Asea A. Alternative mechanism by which IFN-gamma enhances tumor recognition: active release of heat shock protein 72. J Immunol. 2005;175:2900–2912. doi: 10.4049/jimmunol.175.5.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broquet AH, Thomas G, Masliah J, Trugnan G, Bachelet M. Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J Biol Chem. 2003;278:21601–21606. doi: 10.1074/jbc.M302326200. [DOI] [PubMed] [Google Scholar]
- Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybdahl B, Wahba A, Lien E, et al. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation. 2002;105:685–690. doi: 10.1161/hc0602.103617. [DOI] [PubMed] [Google Scholar]
- Dybdahl B, Slordahl SA, Waage A, Kierulf P, Espevik T, Sundan A. Myocardial ischaemia and the inflammatory response: release of heat shock protein 70 after myocardial infarction. Heart. 2005;91:299–304. doi: 10.1136/hrt.2003.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol. 2002;544:957–962. doi: 10.1113/jphysiol.2002.025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach E, Niess AM, Voelker K, Northoff H, Mooren FC. Exercise intensity and duration affect blood soluble HSP72. Int J Sports Med. 2005;26:552–557. doi: 10.1055/s-2004-830334. [DOI] [PubMed] [Google Scholar]
- Fischer CP, Hiscock NJ, Basu S, Vessby B, Kallner A, Sjoberg LB, Febbraio MA, Pedersen BK. Vitamin E isoform-specific inhibition of the exercise-induced heat shock protein 72 expression in humans. J Appl Physiol. 2006;100:1679–1687. doi: 10.1152/japplphysiol.00421.2005. [DOI] [PubMed] [Google Scholar]
- Fukushima A, Kawahara H, Isurugi C, Syoji T, Oyama R, Sugiyama T, Horiuchi S. Changes in serum levels of heat shock protein 70 in preterm delivery and pre-eclampsia. J Obstet Gynaecol Res. 2005;31:72–77. doi: 10.1111/j.1447-0756.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- Genth-Zotz S, Bolger AP, Kalra PR, Haehling S, Doehner W, Coats AJ, Volk HD, Anker SD. Heat shock protein 70 in patients with chronic heart failure: relation to disease severity and survival. Int J Cardiol. 2004;96:397–401. doi: 10.1016/j.ijcard.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Gombos T, Forhecz Z, Pozsonyi Z, Janoskuti L, Prohaszka Z. Interaction of serum 70-kDa heat shock protein levels and HspA1B (+1267) gene polymorphism with disease severity in patients with chronic heart failure. Cell Stress Chaperones. 2008;13:199–206. doi: 10.1007/s12192-007-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- House SD, Guidon PT, Jr., Perdrizet GA, et al. Effects of heat shock, stannous chloride, and gallium nitrate on the rat inflammatory response. Cell Stress Chaperones. 2001;6:164–171. doi: 10.1379/1466-1268(2001)006<0164:EOHSSC>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter-Lavin C, Davies EL, Bacelar MM, Marshall MJ, Andrew SM, Williams JH. Hsp70 release from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2004;324:511–517. doi: 10.1016/j.bbrc.2004.09.075. [DOI] [PubMed] [Google Scholar]
- Hunter-Lavin C, Hudson PR, Mukherjee S, Davies GK, Williams CP, Harvey JN, Child DF, Williams JH. Folate supplementation reduces serum hsp70 levels in patients with type 2 diabetes. Cell Stress Chaperones. 2004;9:344–349. doi: 10.1379/CSC-28R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirecek S, Hohlagschwandtner M, Tempfer C, Knofler M, Husslein P, Zeisler H. Serum levels of heat shock protein 70 in patients with preeclampsia: a pilot-study. Wien Klin Wochenschr. 2002;114:730–732. [PubMed] [Google Scholar]
- Joubert K. Standards of the body mass and body length of birth in Hungary on the basis of the 1990–1996 nation-wide liveborn data. Magy Noorv L. 2000;63:155–163. [Google Scholar]
- Kimura F, Itoh H, Ambiru S, et al. Circulating heat-shock protein 70 is associated with postoperative infection and organ dysfunction after liver resection. Am J Surg. 2004;187:777–784. doi: 10.1016/j.amjsurg.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Kingston AE, Hicks CA, Colston MJ, Billingham ME. A 71-kD heat shock protein (hsp) from Mycobacterium tuberculosis has modulatory effects on experimental rat arthritis. Clin Exp Immunol. 1996;103:77–82. doi: 10.1046/j.1365-2249.1996.929628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 2005;280:23349–23355. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- Lazar L, Nagy B, Ban Z, Nagy GR, Papp Z. Presence of cell-free fetal DNA in plasma of women with ectopic pregnancies. Clin Chem. 2006;52:1599–1601. doi: 10.1373/clinchem.2006.067587. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li N, You L, Liu X, Li H, Wang X (2008) HSP70 is Associated with Endothelial Activation in Placental Vascular Diseases. Mol Med (in press) doi:10.2119/2008.00009.Liu [DOI] [PubMed]
- Madach K, Molvarec A, Rigo J Jr, Nagy B, Penzes I, Karadi I, Prohaszka Z (2008) Elevated serum 70 kDa heat shock protein level reflects tissue damage and disease severity in the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Eur J Obstet Gynecol Reprod Biol (in press) doi:10.1016/j.ejogrb.2007.12.012 [DOI] [PubMed]
- Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol. 2006;177:7849–7857. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Prohaszka Z, Nagy B, Szalay J, Fust G, Karadi I, Rigo J., Jr Association of elevated serum heat-shock protein 70 concentration with transient hypertension of pregnancy, preeclampsia and superimposed preeclampsia: a case-control study. J Hum Hypertens. 2006;20:780–786. doi: 10.1038/sj.jhh.1002060. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Prohaszka Z, Nagy B, Kalabay L, Szalay J, Fust G, Karadi I, Rigo J., Jr Association of increased serum heat shock protein 70 and C-reactive protein concentrations and decreased serum alpha(2)-HS glycoprotein concentration with the syndrome of hemolysis, elevated liver enzymes, and low platelet count. J Reprod Immunol. 2007;73:172–179. doi: 10.1016/j.jri.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Rigo J, Jr., Nagy B, Walentin S, Szalay J, Fust G, Karadi I, Prohaszka Z. Serum heat shock protein 70 levels are decreased in normal human pregnancy. J Reprod Immunol. 2007;74:163–169. doi: 10.1016/j.jri.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Njemini R, Lambert M, Demanet C, Mets T. Elevated serum heat-shock protein 70 levels in patients with acute infection: use of an optimized enzyme-linked immunosorbent assay. Scand J Immunol. 2003;58:664–669. doi: 10.1111/j.1365-3083.2003.01341.x. [DOI] [PubMed] [Google Scholar]
- Pittet JF, Lee H, Morabito D, Howard MB, Welch WJ, Mackersie RC. Serum levels of Hsp 72 measured early after trauma correlate with survival. J Trauma. 2002;52:611–617. doi: 10.1097/00005373-200204000-00001. [DOI] [PubMed] [Google Scholar]
- Placer ZA, Cushman LL, Johnson BC. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem. 1966;16:359–364. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27:367–377. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- Prohaszka Z, Fust G. Immunological aspects of heat-shock proteins – the optimum stress of life. Mol Immunol. 2004;41:29–44. doi: 10.1016/j.molimm.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Prohaszka Z, Singh M, Nagy K, Kiss E, Lakos G, Duba J, Fust G. Heat shock protein 70 is a potent activator of the human complement system. Cell Stress Chaperones. 2002;7:17–22. doi: 10.1379/1466-1268(2002)007<0017:HSPIAP>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. The pathogenesis of pre-eclampsia. Gynecol Obstet Fertil. 2001;29:518–522. doi: 10.1016/S1297-9589(01)00180-1. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/S0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46:1243–1249. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Peake J, Nosaka K, et al. Changes in markers of muscle damage, inflammation and HSP70 after an Ironman Triathlon race. Eur J Appl Physiol. 2006;98:525–534. doi: 10.1007/s00421-006-0296-4. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kimura Y, Mitani A, et al. Activation of T cells recognizing an epitope of heat-shock protein 70 can protect against rat adjuvant arthritis. J Immunol. 1999;163:5560–5565. [PubMed] [Google Scholar]
- Theriault JR, Mambula SS, Sawamura T, Stevenson MA, Calderwood SK. Extracellular HSP70 binding to surface receptors present on antigen presenting cells and endothelial/epithelial cells. FEBS Lett. 2005;579:1951–1960. doi: 10.1016/j.febslet.2005.02.046. [DOI] [PubMed] [Google Scholar]
- Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6:386–393. doi: 10.1379/1466-1268(2001)006<0386:EISHIH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling U, Paul L, Zee R, Prakken B, Singh M, Eden W. A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10-producing T cells that cross-react with the mammalian self-hsp70 homologue. J Immunol. 2000;164:2711–2717. doi: 10.4049/jimmunol.164.5.2711. [DOI] [PubMed] [Google Scholar]
- Wheeler DS, Fisher LE, Jr., Catravas JD, Jacobs BR, Carcillo JA, Wong HR. Extracellular hsp70 levels in children with septic shock. Pediatr Crit Care Med. 2005;6:308–311. doi: 10.1097/01.PCC.0000161075.97355.2E. [DOI] [PubMed] [Google Scholar]
- Wright BH, Corton JM, El-Nahas AM, Wood RF, Pockley AG. Elevated levels of circulating heat shock protein 70 (Hsp70) in peripheral and renal vascular disease. Heart Vessels. 2000;15:18–22. doi: 10.1007/s003800070043. [DOI] [PubMed] [Google Scholar]