Abstract
Large conductance K+ channels (BKCa) are expressed in uterine artery (UA) smooth muscle from nonpregnant and pregnant sheep and contribute to the regulation of basal vascular tone and responses to estrogen and vasoconstrictors. To determine if BKCa are expressed in women and contribute to UA function, we collected UA from nonpregnant women (n=31) at elective hysterectomy and analyzed for subunit protein, localization with immunohistochemistry and function using endothelium-denuded rings. UA expresses BKCa α-, β1- and β2-subunit protein. KCl and phenylephrine (PE, an α1-agonist) caused dose-dependent vasoconstriction (P<0.001), and UA precontracted with PE dose-dependently relaxed with sodium nitroprusside (SNP; P<0.001). Tetraethylammonium chloride (TEA, 0.2–1.0 mM), a BKCa inhibitor, dose-dependently increased resting tone (P=0.004; 28±5.3% with 1.0 mM), enhanced PE-induced (10−6 M) vasoconstriction (P<0.04), and attenuated SNP-induced relaxation at 1.0 mM (P=0.02). BKCa are expressed in human UA and modulate vascular function by attenuating vasoconstrictor responses and contributing to nitric oxide-induced vasorelaxation.
Keywords: Nitric oxide, α-agonists, basal tone, myometrium, β1/β2-regulatory subunits
INTRODUCTION
Understanding the mechanisms that modulate uterine blood flow (UBF) are of great interest as this may provide insight into the control of prepregnancy UBF and uteroplacental (UPBF) and myometrial blood flow during pregnancy, the latter enhancing our understanding of the mechanisms that ensure fetal growth and well-being. Studies of UBF in women are generally descriptive (1), and studies of vascular function are represented by in vitro studies of uterine arteries (UA). Thus most of our understanding of the in vivo regulation of UBF has been derived from studies using various animal models, in particular, nonpregnant and pregnant sheep (2,3). Nonetheless, pregnancy is associated with marked uterine vasodilation and a >30-fold rise in UPBF at term (1,2,4,5), reflecting substantial decreases in basal vascular tone. It also is notable that although angiotensin II and α-agonists have different effects on the uterine circulation, the latter being the most potent vasoconstrictor (2,6,7), uterine vascular responses to both vasoconstrictors are attenuated in pregnant women and sheep compared to nonpregnant (7–9). The mechanisms responsible for these observations remain unclear.
We (10–13) recently identified the large conductance, Ca+2-activated K+ channels (BKCa) in the vascular smooth muscle (VSM) of ovine UA (UVSM). The channel is a product of the slo gene and member of the voltage-gated K+ channel superfamily (14–16). It consists of 4 α-subunits that comprise the pore, and as many as 4 regulatory β-subunits that modify channel function and provide phenotypic and functional diversity (17–19). In general, the β1 subunit is the major regulatory subunit in VSM, but β2 may also be present. The β1 regulatory subunit modifies Ca+2 and voltage sensitivity and confers estrogen responsiveness (20–22). Channel activity increases acutely after estrogen exposure, resulting in membrane hyperpolarization, vasorelaxation and in sheep, increases in UBF in sheep (10,11,23,24). Channel activity also increases in the presence of increased intracellular Ca+2 and submembrane sparks, resulting in dampening or inhibition of voltage-gated Ca+2 channels and decreases in intracellular Ca+2 due to movement of Ca+2 into the sarcoplasmic reticulum (17,25). These events modify vasoconstrictor responses and contribute to the maintenance of basal arterial tone (16,17,25). Thus may contribute to uterine vascular regulation.
In nonpregnant sheep, BKCa inhibition with tetraethylammonium (TEA), which has high specificity for the channel at doses ≤1 mM (26), minimally alters basal UBF and dose-dependently inhibits estrogen-induced uterine vasodilation, which is in part due to increases in vascular nitric oxide synthase expression (NOS), nitric oxide (NO) and cGMP synthesis (27,28). In the last third of ovine pregnancy, uterine arterial infusions of TEA block estrogen-induced vasodilation at low doses (11) and dose-dependently decrease basal UPBF (11,12). More recently, we (29) have observed that BKCa also contribute to the attenuated responses by the uteroplacental vascular bed to α-agonists. Thus, BKCa appear to be an important modulator of uterine vascular function, reactivity and basal blood flow in ovine pregnancy. Its role in the human UA, however, has not been examined. The purpose of the present study was to determine if BKCa are present in large and small UA from nonpregnant women and to assess their role in the maintenance of basal tone, responses to α-stimulation and NO-mediated relaxation. We hypothesized that BKCa are in UVSM and contribute to uterine vascular function.
METHODS
Tissue collection
UA were collected from 32 nonpregnant women undergoing elective hysterectomy for benign gynecologic disorders (leiomyomas, 16; pelvic organ prolapse, 5; menorrhagia/adenomyosis, 2; endometriosis, 1; chronic pelvic pain, 1; and dysfunctional uterine bleeding, 7). Menstrual status was subsequently determined by endometrial histology and date of last menstrual period if known. Of the 27 premenopausal women, 13 were in the follicular and 10 in the secretory phase, whereas 4 had atrophic endometrium. Five samples were obtained from menopausal women (pelvic organ prolapse, 2; leiomyomas, 3). None were on hormone replacement therapy at the time of tissue collection. UA were dissected from the parametrium and paracervical connective tissues and adjacent myometrium, placed in chilled physiologic based saline (118.3 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 14.9 mM NaHCO3, 11.1 mM Glucose and 2.5 mM CaCl2) and transported to the laboratory within 30min, where they were placed in iced PBS and the adventitia removed by blunt and sharp dissection. Sections of UA, 3–4 cm long were placed in PBS and stored overnight at 4°C with continuous 95%O2:5%CO2 gas bubbling. If additional tissue remained, arteries were opened longitudinally, the endothelium removed with a soft-tipped cotton swab, frozen in liquid nitrogen and stored at −80°C (13). Other samples were prepared for immunohistochemistry as described below.
Study protocol
Samples from 26 women were available for studies of UA function. These oxygenated UA were removed from the refrigerator the following morning, cut into 4–5 mm rings and the endothelium removed by turning the tip of a small forceps in the lumen. Endothelium removal was verified histologically, and the basal lamina and underlying VSM were intact. Each ring was placed on a stirrup attached to a transducer (Grass FT-03, Quincy, MA and iWorx FT-302, Dover, NH) to measure force generation in a 25-ml volume chamber as previously reported (30). The PBS (120.5mM NaCl, 4.8mM KCl, 1.2mM MgSO4, 1.2 mM NaH2PO4, 20.4 NaHCO3, 1.6mM CaCl2, 10.0mM Dextrose, and 1.0 Pyruvate) in the chamber was maintained at 37°C throughout the experiment by a circulating water pump. After a 30min equilibration period, rings were progressively stretched to obtain optimal length (Lo), as determined with progressive increases in KCl-induced contractile responses with 65 mM KCl. Not all UA segments were included in each protocol because of a limitation in available baths and the need to perform studies with each ring in duplicate. Dose-response curves were constructed at Lo with KCl (n=4; 10–120 mM) and cumulative doses of PE (n=5; 10−8 to 10−5 M), an α1-agonist, to examine nonreceptor and adrenergic receptor-mediated responses, respectively. At the end of the PE dose-response and while rings remained contracted, a relaxation dose-response curve was generated in selected rings with cumulative doses of the NO donor sodium nitroprusside (SNP; n=5; 10−8 to 10−5 M) to measure NO-mediated vasorelaxation; responses are expressed as the per cent relaxation after the PE contraction. To determine the effect of channel inhibition on PE responses, selected rings were washed (n=9), allowed to return to baseline and contracted with a single dose of 10−6 M PE followed by the addition of TEA at 0.2, 0.5 or 1.0 mM, adding only one dose per pair. Other rings received only PE and served as a control for the second exposure, demonstrating the absence of tachyphylaxis (P>0.1). After stabilization of the PE+TEA response, which demonstrates the effects of BKCa inhibition on the PE vasoconstrictor response, 10−7 M SNP was added to the bath to determine if BKCa inhibition also modified NO-mediated relaxation. The effects of channel inhibition on basal tone were examined in selected rings washed with PBS and allowed to return to baseline tone for 20–30min. The three doses of TEA were then added to the bath of duplicate rings to examine the effects of BKCa inhibition on basal tone (n=8). Data were recorded on an electronic data-acquisition system (Summit ACQuire and Summit DaStar, Gould Systems, Valley View, OH) in grams of force generated at Lo. When appropriate, responses were normalized to the response to 65mM KCl for each ring. At the completion of studies, 3 women were identified as being postmenopausal, and their UA studies were removed from all functional analyses. These studies were approved by the Institutional Review Board for Human Research at the UT Southwestern Medical Center at Dallas.
Western Analysis
Samples of endothelium denuded UA (n=6; 30 mg) that had been frozen in liquid nitrogen and stored at −80°C were weighed and homogenized in 40x volumes of sodium dodecyl sulfate (SDS) buffer as previously described (13). Homogenates were centrifuged at 10,000 × g for 2min. The supernatant was removed, and an aliquot used to determine cellular or soluble protein by BCA reagent (Pierce, Rockford, IL). Bromophenol blue and 2-mercaptoethanol were added to aliquots, and equal amounts of soluble protein were loaded (20 µg) on 7.5–10% polyacrylamide minigels and subjected to SDS-polyacrylamide gel electrophoresis as previously described (13). Proteins were electrophoretically transferred to nitrocellulose paper at 100 volts for 1h. Blots were incubated overnight with antisera to BKCa α-subunit (1:300; α1098–1196, Chemicon International, Temecula, CA), the β1- (1:200; Alomone Labs Ltd, Jerusalem, Israel) or the β2-subunit (1:200; Alomone Labs Ltd, Jerusalem, Israel). After 1h incubation with anti-rabbit immunoglobulin G conjugated with horseradish peroxidase (1:2000), immunoreactive protein was visualized by chemiluminescence.
Immunohistochemistry
In order to determine the localization of BKCa within the UA wall and the extent of BKCa expression in UA ranging from 2–4 mm diameter to small intramyometrial branches, additional intact segments of UA with paired myometrium were washed in PBS, fixed in 4% paraformaldehyde for 6h at room temperature and embedded in paraffin as previously described (13). Sections were mounted on slides, deparaffinized, hydrated, incubated with avidin-biotin blocking agent for 30min and incubated overnight at room temperature with polyclonal antibodies to the BKCa α-, β1- or β2-subunits (1:25). After endogenous peroxidases were quenched with 3% H2O2 in H2O for 30min, immunostaining was detected with standard strepavidin-biotin-horseradish peroxidase and hematoxylin counter staining. At least 3 UA and sections of myometrium were examined.
Statistical Analysis
Dose-responses were analyzed using analysis of variance (ANOVA) for multiple groups followed by an appropriate pairwise multiple comparison procedure to compare doses. EC50 was determined using GraphPad Prism 5 (Graphpad Software, Inc., San Diego, CA). Paired t test was used to compare responses in the absence and presence of TEA. Data are presented as the mean ± SEM.
RESULTS
Immunoblot Analysis
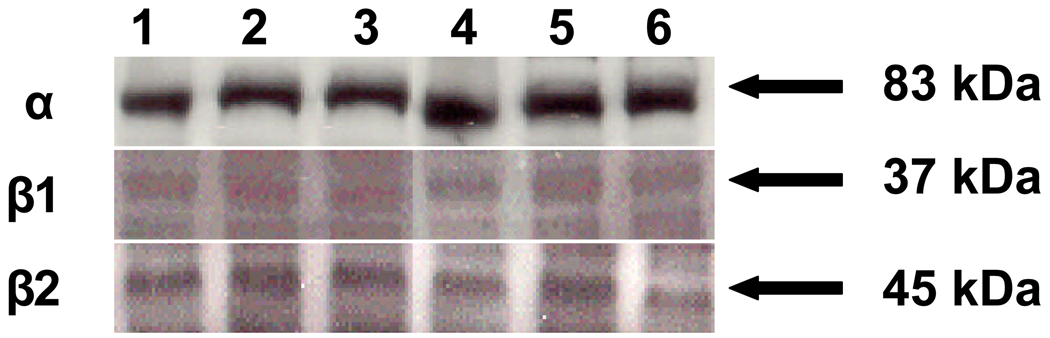
To determine if BKCa are expressed in human UVSM, we performed immunoblotting for the α-, β1- and β2-subunits in samples available from 6 women with an average age of 43 7/12 yrs. The α-subunit was identified in all samples as a single protein species at 83 kDa (Figure 1). The β1- and β2-subunits were also present in all samples at 37 and 45 kDa, respectively. Of note, the density of the β2-subunit appears to equal that of the β1-subunit; however, a direct comparison is not possible since samples are run on separate gels. When samples were available from proliferative, secretory and inactive phases and run on the same gel, there was no difference in the density of the 3 subunits.
Figure 1.
Immunoblot analysis demonstrating BKCa α-, β1- and β2-subunits in uterine artery smooth muscle from 6 randomly selected nonpregnant women. Proliferative endometrium is in lanes 1, 2 and 4, inactive in lanes 3 and 5, and secretory in lane 6.
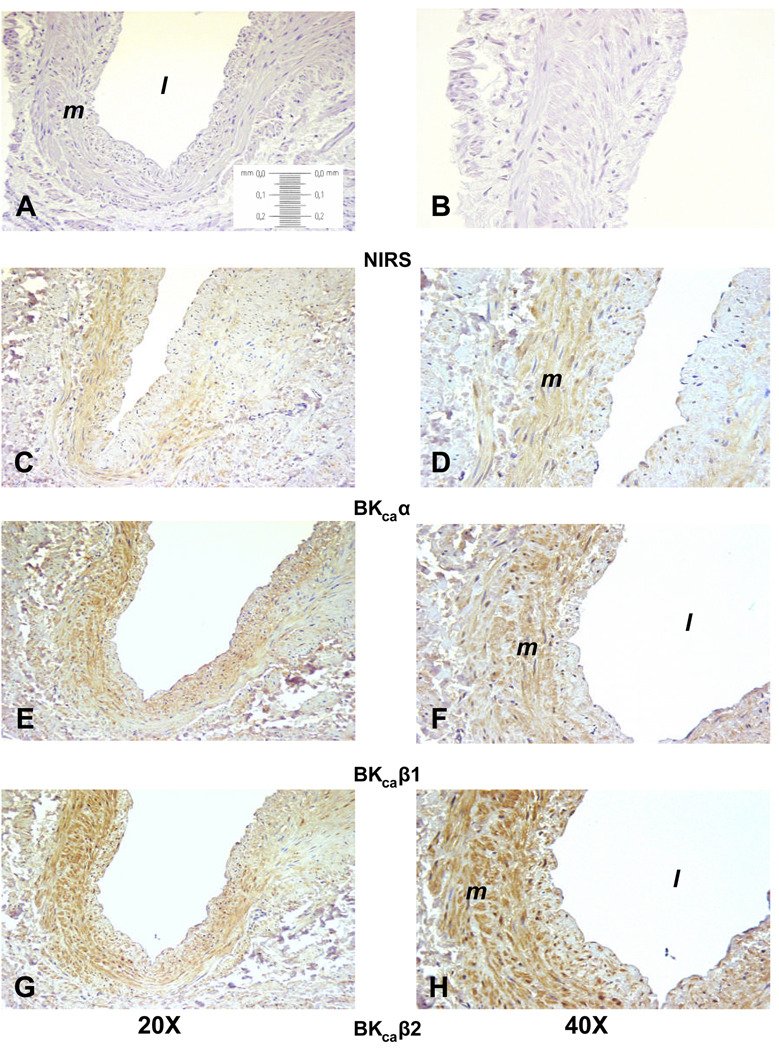
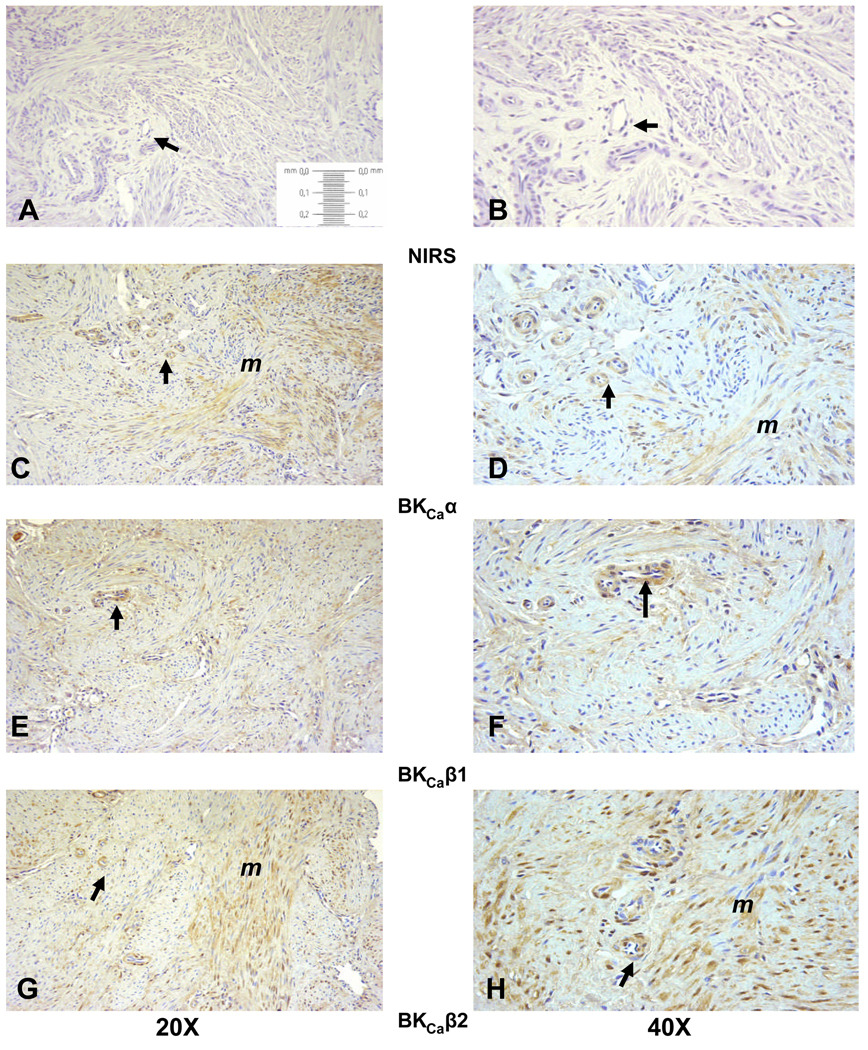
To determine BKCa localization within the UA wall, immunohistochemistry was performed in UA from 4 women and blinded for endometrial status. There was variable immunostaining in the 4 samples, with decreased staining in women whose endometrium was considered to be in the secretory phase, i.e., high progesterone. There was excellent immunostaining throughout the UA media in one sample (Figure 2) that allowed for the localization of α- (panels C and D), β1- (panels E and F) and β2-subunits (panels G and H). Immunostaining was specific to the VSM and was not in the endothelium or lamina propria. Notably, β2-subunit immunostaining appears to equal or exceed that for β1. In order to assess the extent of subunit expression in the small intramyometrial branches of the UA, we examined the paired myometrial samples available from these women. The variability in myometrium paralleled that in the VSM. The myometrium paired with the UA in Figure 2 demonstrated immunostaining for all 3 subunits in small arterioles throughout the myometrium (Figure 3). Notably, immunostaining for the 3 subunits was observed in myometrial smooth muscle, and as in UVSM, β2-subunit immunostaining is at least equal to or greater than the β1-subunit.
Figure 2.
Representative immunohistochemistry localizing BKCa subunits in the uterine artery from a nonpregnant woman. Panels A, C, E and G are at 20X magnification; panels B, D, F and H are at 40X. Panels A and B are controls with nonimmune serum, C and D are the pore α-subunit, E and F the β1-subunit, and G and H the β2-subunit. The vessel lumen is noted by the l and the media by m.
Figure 3.
Representative immunohistochemistry localizing BKCa subunits in intramyometrial arteries and myometrial smooth muscle from a nonpregnant woman. Tissue is paired with the uterine artery in Figure 2. Panels A, C, E and G are at 20X magnification; panels B, D, F and H are at 40X. Panels A and B are controls with nonimmune serum, C and D are the pore α-subunit, E and F the β1-subunit, and G and H the β2-subunit. Intramyometrial arteries are denoted by the arrows and myometrial smooth muscle by m.
Contraction-Relaxation Responses
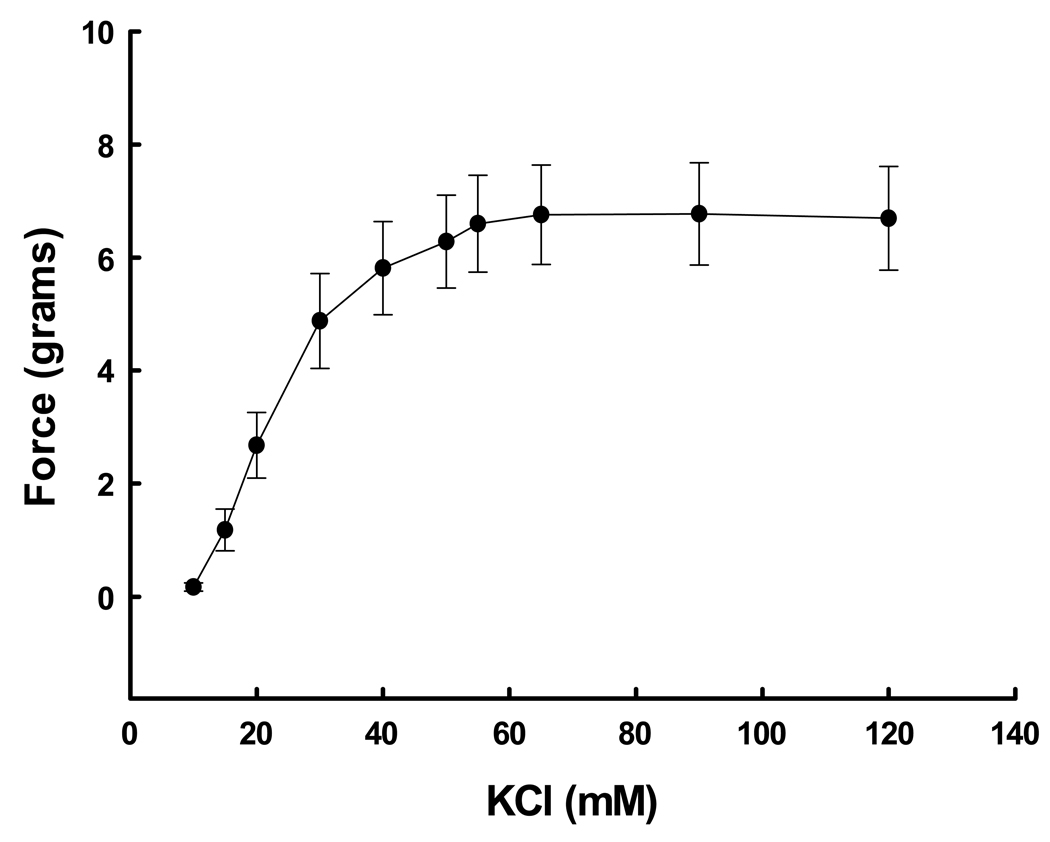
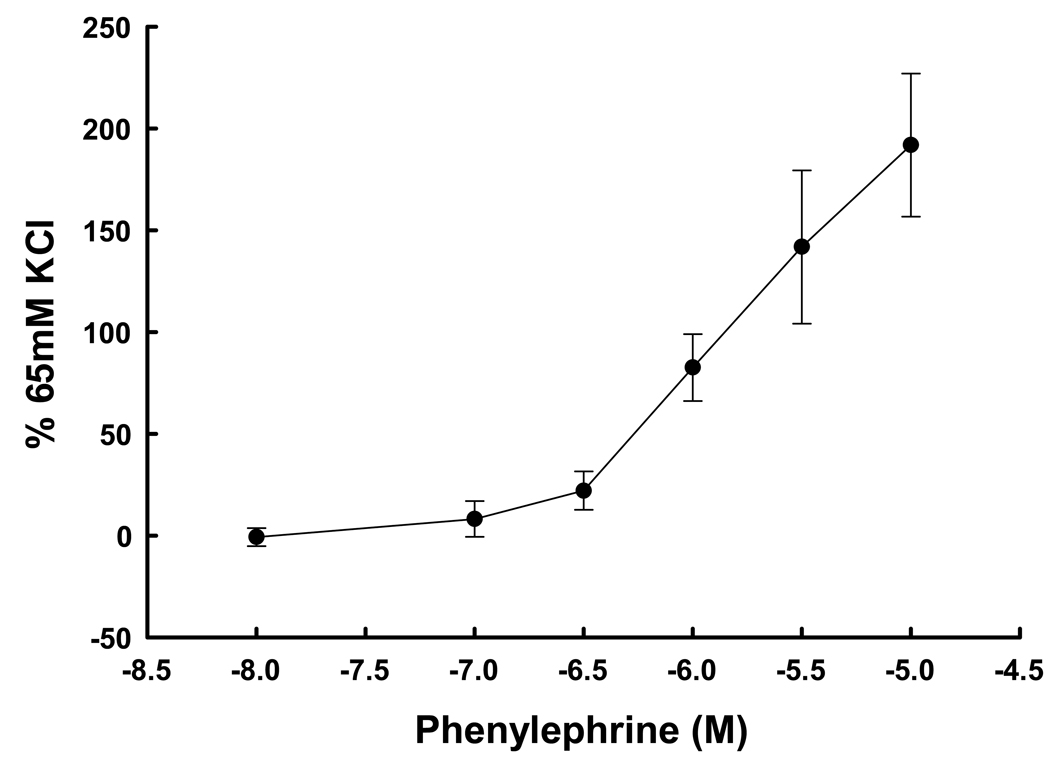
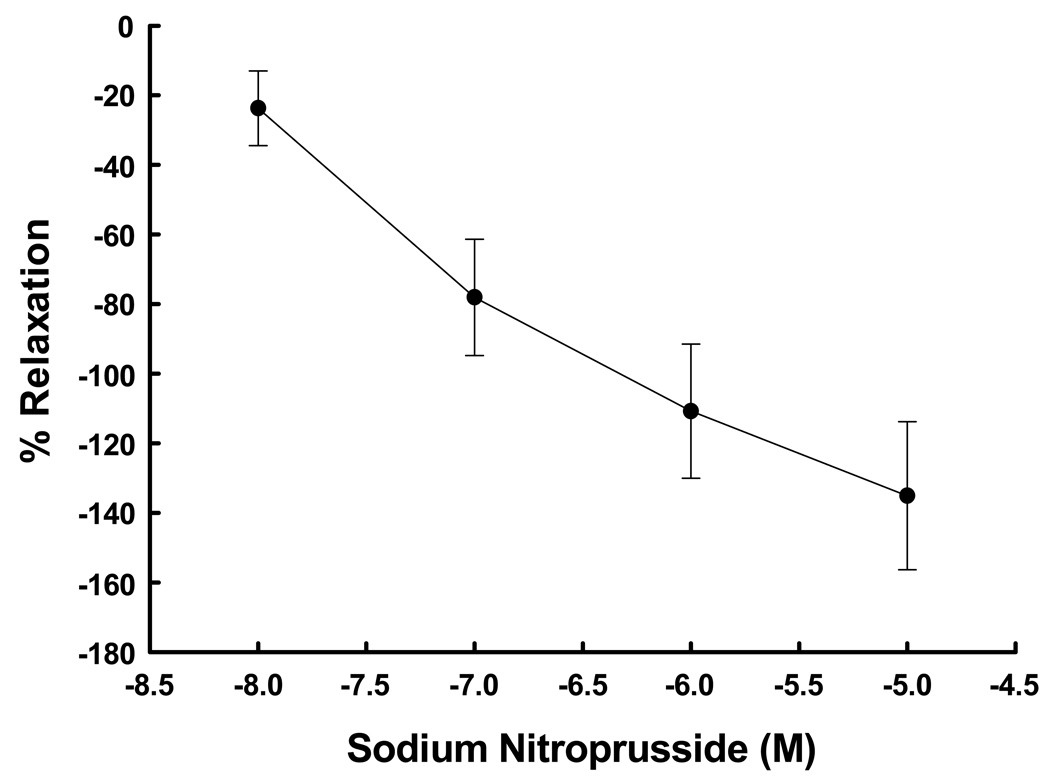
Denuded UA rings demonstrated a dose-response (P<0.001, ANOVA) to both KCl at 10–120 mM (Figure 4) and cumulative doses of PE at 10−8 to 10−5 M (Figure 5). The EC50 for each agent was 22.2 mM and 1.35 × 10−6 M, respectively. Maximum responses occurred at 40 mM and 10−5.5 M, respectively. The addition of SNP after precontraction with PE resulted in dose-dependent relaxation (Figure 6; P=0.004, ANOVA). Thus the contraction-relaxation apparatus in the samples of UVSM was intact and functional. We determined if there was a relationship between responses in UA from women subsequently shown by endometrial histology to be in the secretory versus proliferative phase. There were no differences in the responses to 65 mM KCl or 10−6 M PE (P≥0.2, t-test)
Figure 4.
Dose-response curve to KCl in denuded uterine artery rings from 4 nonpregnant women. The differences between doses were significant (P<0.001, ANOVA).
Figure 5.
Dose-response curve to the α-agonist phenylephrine in denuded uterine artery rings from 5 nonpregnant women normalized to the response to 65 mM KCl. The differences between doses were significant (P<0.001, ANOVA).
Figure 6.
Dose-response curve to the NO donor sodium nitroprusside in denuded uterine arteries precontracted with phenylephrine from 4 nonpregnant women. Responses are the per cent relaxation after exposure to each dose of sodium nitroprusside. The differences between doses were significant (P=0.004, ANOVA).
Effects of BKCa Blockade
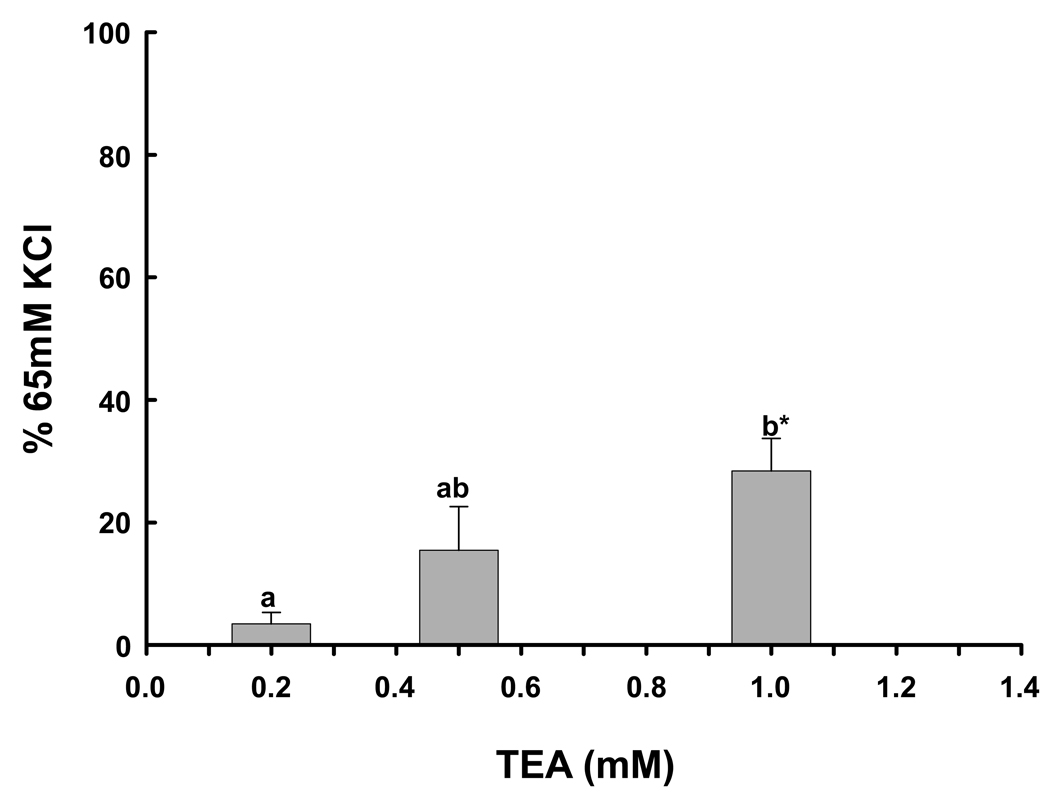
To ascertain if BKCa blockade with TEA resulted in dose-related effects, we used 3 doses, 0.2, 0.5 and 1.0 mM, all of which have high specificity for BKCa (26). To determine the role of BKCa in the regulation of basal vascular tone, duplicate rings were exposed to only one of the 3 doses of TEA. There was a significant increase in basal tone compared to vehicle following TEA exposure at 1.0 mM (Figure 7; P=0.002) after normalizing for the response to 65 mM KCl. Furthermore, there was a significant dose affect (P=0.004, ANOVA).
Figure 7.
The effect of BKCa blockade with 0.2 (n=7), 0.5 (n=7) and 1.0 (n=8) mM TEA on basal tone in denuded human uterine artery rings from 8 women. Different letters denote significant differences between doses of TEA (P=0.004, ANOVA). *denotes a significant difference from basal force, P=0.002.
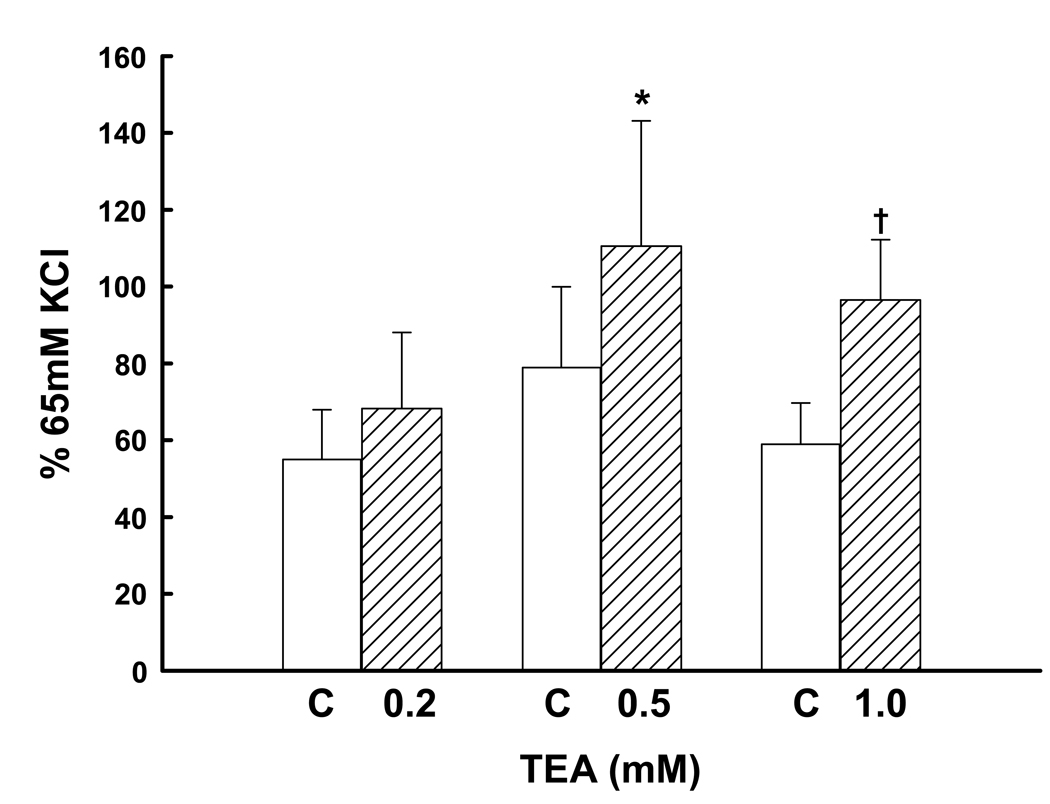
BKCa modulate constrictor responses to several agonists, thereby contributing to the maintenance of tissue or organ blood flow (16,17,25). Since the uterine vasculature is very sensitive to α-agonists in vivo (7), we examined the effect of channel blockade with TEA on the constriction responses to the α1-agonist PE. In the absence of TEA, PE increased UA force normalized as a per cent of the 65 mM KCl response (Figure 8), and there were no differences in the responses by control rings to PE alone, P=0.5. The addition of TEA enhanced PE-mediated vasoconstriction, the significance paralleling the increasing dose from P=0.2, to 0.04 and 0.003 (paired t test); but a dose effect could not be demonstrated for the 3 doses studied (P=0.5, ANOVA).
Figure 8.
The effect of BKCa blockade with 0.2 (n=6), 0.5 (n=7) and 1.0 (n=7) mM TEA on phenylephrine-induced vasoconstriction in denuded human uterine artery rings from 9 women. Open bars represent control responses that do not differ from each other (P=0.5, ANOVA). Cross-hatched bars are responses in the presence of one dose of TEA; † P=0.04 and *P=0.003 compared to control, paired t test.
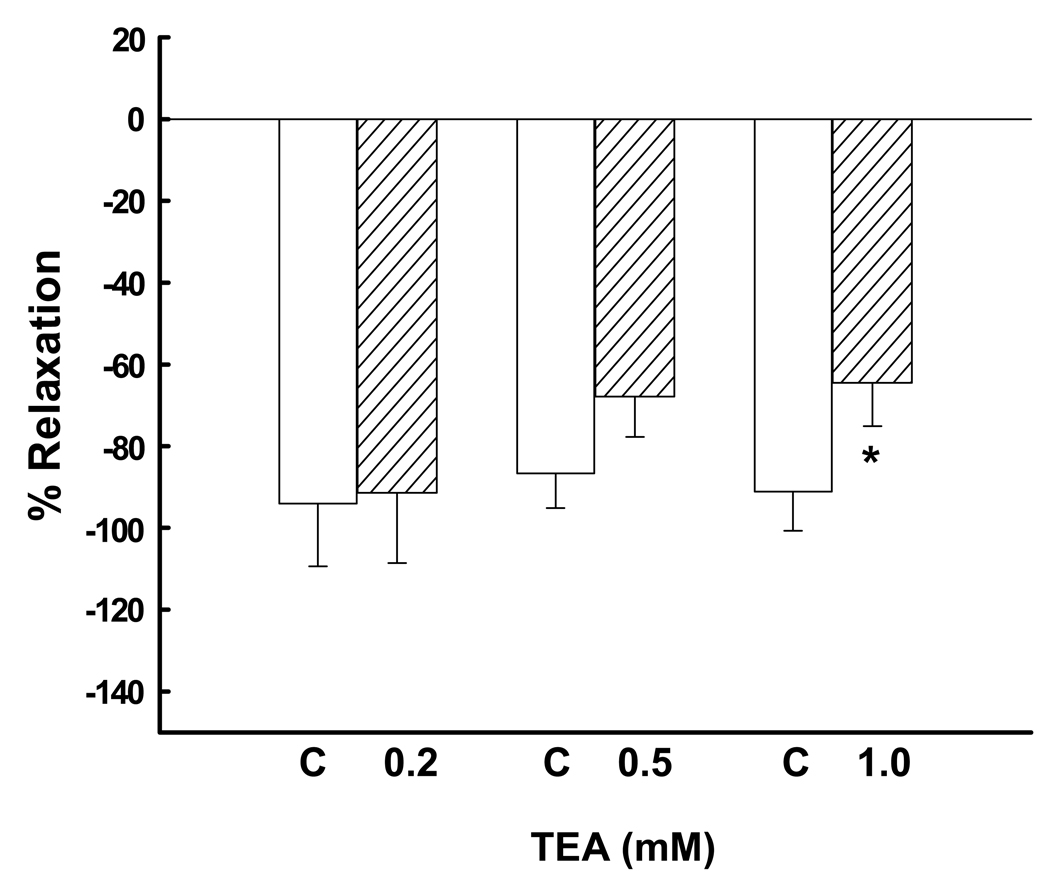
BKCa also modulate relaxation responses to NO (10,24). Thus, we examined the effect of channel blockade on NO-induce relaxation, comparing the responses to the NO donor SNP in the presence of PE-induced vasoconstriction with 10−5 M without and with TEA, using the 3 doses noted above. In the absence of TEA, 10−7 M SNP resulted in significant relaxation responses that did not differ between the 3 control groups, 88–98% decreases in tone (Figure 9; P=0.2, ANOVA). Although 0.2 and 0.5 mM TEA did not alter the relaxation responses (P>0.1, paired t test), 1.0 mM TEA caused a 28% reduction in the relaxation response, P=0.019.
Figure 9.
The effect of BKCa blockade with 0.2 (n=6), 0.5 (n=7) and 1.0 (n=7) mM TEA on relaxation responses to sodium nitroprusside after preconstriction with a single dose of 10−5 M phenylephrine in denuded human uterine artery rings from 9 women. Open bars represent control responses in the absence of blockade and do not differ (P=0.5, ANOVA). Responses with 0.2 and 0.5 mM TEA do not differ from control. *P=0.018 (paired t test) compared to control.
DISCUSSION
The mechanisms that contribute to the regulation of UBF in nonpregnant and pregnant women remain unclear. Studies in animal models, in particular sheep, suggest several mechanisms are involved, e.g., NO, cyclooxygenase products endothelial derived hyperpolarizing factor and differences in receptor subtype expression (31–34). However, these represent proximal events in the signaling pathways and do not address the downstream effectors involved. We (10,11) reported the presence of BKCa in UVSM from nonpregnant and pregnant ewes. Furthermore, we (10,12,29) demonstrated that BKCa play an essential role in the uterine vascular responses to estrogen and vasoconstrictors and in the regulation of basal UPBF in the last third of ovine pregnancy. To date, no one has examined the presence or role of BKCa in human UA. In the present report, we identified the essential subunits that comprise the BKCa in human UVSM, i.e., the pore forming α-subunit and the β1 and β2 regulatory subunits. Moreover, we observed that channel blockade increases basal tone, enhances responses to α-stimulation, and decreases NO-mediated relaxation. Thus, we present the first evidence of BKCa expression in human UA and their role in the regulation of UA function, suggesting BKCa may contribute to the regulation of UBF in nonpregnant and pregnant women, thereby opening a new vista for studies of UBF regulation in women.
BKCa consist of 4 α-subunits that form the channel pore and up to 4 regulatory β-subunits that modify phenotypic and functional diversity (14,35). The α-subunit is derived from a single gene, but may exist as several protein species varying from 83 to 105 kDa, demonstrating the occurrence of post-translational modification (13,36). Although we previously identified 4 protein species in UVSM from nonpregnant ewes, only a single species at 83 kDa was observed in human UVSM. This may be related to species differences or the intriguing idea that this relates to the advanced age of the women included in the present study (16,37); the significance of this finding, however, is unclear. There are 4 regulatory subunits derived from separate genes, and their tissue distribution differs. β3 and β4 are found in the nervous system, whereas β1 and β2 are in smooth muscle, the former predominantly in VSM (15,16). β1 is abundant in UVSM of young nonpregnant reproductively active ewes, whereas β2 is minimally expressed (13). Further, β1 is up-regulated by estrogen exposure (13,38,39) and required for estrogen responsiveness (20,21). Although it is difficult to quantify, β1 expression appears to be decreased in the population of nonpregnant women studied compared to that previously seen in reproductively active ewes (13). Further, β2 appears up-regulated, whereas it is quite low in the nonpregnant ewe. Aging has been associated with decreases in channel density and the β1-subunit (16,37); this, however, has not been studied in women or in the uterine vasculature. Although both β-subunits modify voltage and calcium sensitivity, the β2 increases inactivation of BK currents and recovery from inactivation (19,40,41). It is possible the differences in regulatory subunit stoichiometry between young ewes and the women studied is associated with alter UA channel function, but this is unknown. It is tempting to speculate that estrogen treatment in these women would increase β1:β2 stoichiometry and thus channel function. Notably, BKCa were in both large UA and small intramyometrial branches of nonpregnant women, the latter consistent with studies in pregnant women (38,34). Thus BKCa extend throughout the uterine vascular bed.
Although the effects of BKCa inhibition with TEA on basal UBF in nonpregnant sheep are minimal (10), this results in substantial increases in basal uterine vascular resistance and decreases UPBF in intact pregnant sheep (29). In the present study, BKCa inhibition with TEA increased basal UA tone dose-dependently, values increasing ~30% with 1.0 mM. This is modest compared to the ~80% fall in UPBF with 0.3 mM TEA in pregnant ewes (29) and the >60% fall in UBF in nonpregnant ewes during estrogen-mediated uterine vasodilation (10). However, it is consistent with the modest effect seen in nonpregnant ewes in the absence of estrogen, which is also associated with low β1 expression (10). Thus, the role of the BKCa as a modulator of vascular tone increases in the presence of NO-mediated vasodilation (10,11) and during pregnancy, when basal UPBF is increased substantially, suggests that BKCa contribute to UBF (11,12). Studies are presently underway to determine their role during the estrous cycle.
BKCa modulate vascular responses to vasoconstrictors through Ca+2 sensitive mechanisms (16,17,25,40,41). The c-terminal end of α-subunit is able to sense changes in intracellular Ca+2 and the occurrence of localized submembrane Ca+2 sparks (16). Channel activity increases when intracellular Ca+2 rises, increasing membrane hyperpolarization, dampening or inhibiting of voltage-activated Ca+2 channels, which decreases in cytosolic Ca+2 and attenuates constrictor responses. We (29) recently observed enhanced vasoconstrictor responses to infused PE during local BKCa uterine inhibition in pregnant sheep. In the present study, BKCa blockade with TEA also enhanced PE-induced contractions by human UA. Thus, BKCa contribute to UA vasoconstrictors responses in women and sheep, suggesting BKCa contribute to the attenuated uterine vascular responses to α-agonists in pregnancy (7,9). If so, alterations in channel function could enhance uterine vascular responses to vasoconstrictors in hypertensive pregnancies and increase the risk for fetal growth restriction.
The rise in UBF in cycling ewes and near-term pregnant sheep are due to vasodilation (5,42). In both instances, UA derived NO is believed to play an important role, reflecting increases in UA NOS expression due to ovarian and placental estrogen synthesis during the estrous cycle and pregnancy, respectively (27,28,32,42). SNP, a NO donor, increases VSM cGMP, which should activate BKCa and cause UA vasorelaxation, thereby mimicking the endogenous NO-mediated vasodilation discussed (16). SNP dose-dependently relaxed precontracted denuded human UA, and BKCa inhibition attenuated these relaxation responses. This is similar to that seen in nonpregnant ewes after estrogen exposure (10). Thus, BKCa also contribute to NO-mediated relaxation in human UA. It is now possible to design studies to explore the signaling pathways in human UA after estrogen and NO exposure.
In this report, we have shown that the essential components of BKCa are expressed in UVSM from nonpregnant women. Unlike young reproductively active ewes (13), channel expression appears to be decreased and β2 exceeds β1 expression, but the physiologic consequences are unclear. It also is unclear if estrogen and progesterone modulate subunit expression in women, necessitating further studies to delineate this. Importantly, BKCa contribute to the maintenance of basal UA tone and the UA responses to α-agonists and NO. Thus BKCa likely contribute to the regulation of UBF during the estrous cycle and pregnancy. If so, channel dysfunction may contribute to abnormalities in implantation, placentation, and increases in UPBF and fetal growth in pregnancy.
Acknowledgments
Support: This study was supported by NIH grants HD-08783 (CRR) and HD11149 (RAW).
REFERENCES
- 1.Lunell NO, Nylund LE, Lewander R, Sarby B. Uteroplacental blood flow in pre-eclampsia measurements with indium-113m and a computer-linked gamma camera. Clin Hypertens B. 1982;1:105–117. doi: 10.3109/10641958209037184. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld CR. The Uterine Circulation. Vol. 1989. Ithaca, NY: Perinatology Press; 1989. pp. 113–134. 135–156, 208–238, 239–271. [Google Scholar]
- 3.Nathanielsz PW. Animal Models in Fetal Medicine (IV) Ithaca, NY: Perinatology Press; 1984. [Google Scholar]
- 4.Rosenfeld CR. Distribution of cardiac output in ovine pregnancy. Am J Physiol. 1977;232:H231–H235. doi: 10.1152/ajpheart.1977.232.3.H231. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld CR, Morris FH, Jr, Makowski EL, Meschia G, Battaglia FC. Circulatory changes in the reproductive tissues of ewes during pregnancy. Gynecol Invest. 1974;5:252–268. doi: 10.1159/000301658. [DOI] [PubMed] [Google Scholar]
- 6.Naden RP, Rosenfeld CR. Effect of angiotensin II on uterine and systemic vasculature in pregnant sheep. J Clin Invest. 1981;68:468–474. doi: 10.1172/JCI110277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magness RR, Rosenfeld CR. Systemic and uterine responses to alpha-adrenergic stimulation in pregnant and nonpregnant ewes. Am J Obstet Gynecol. 1986;155:897–904. doi: 10.1016/s0002-9378(86)80047-3. [DOI] [PubMed] [Google Scholar]
- 8.Erkkola RU, Pirhonen JP. Uterine and umbilical flow velocity waveforms in normotensive and hypertensive subjects during the angiotensin II sensitivity test. Am J Obstet Gynecol. 1992;166:910–916. doi: 10.1016/0002-9378(92)91361-d. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld CR, Gant NF., Jr The chronically instrumented ewe, a model for studying vascular reactivity to angiotensin II in pregnancy. J Clin Invest. 1981;67:486–492. doi: 10.1172/JCI110057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenfeld CR, White RE, Roy T, Cox BE. Calcium-activated potassium channels and nitric oxide coregulate estrogen-induced vasodilation. Am J Physiol. 2000;279:H319–H328. doi: 10.1152/ajpheart.2000.279.1.H319. [DOI] [PubMed] [Google Scholar]
- 11.Rosenfeld CR, Cornfield DN, Roy T. Ca2+-activated K+ channels modulate basal and E2β-induced rises in uterine blood flow in ovine pregnancy. Am J Physiol. 2001;281:H422–H431. doi: 10.1152/ajpheart.2001.281.1.H422. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld CR, Roy T, DeSpain K, Cox BE. Large-conductance Ca2+-dependent K+ channels regulate basal uteroplacental blood flow in ovine pregnancy. J Soc Gynecol Investig. 2005;12:402–408. doi: 10.1016/j.jsgi.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Nagar D, Liu X-t, Rosenfeld CR. Estrogen regulates β1-subunit expression in Ca2+-activated K+ channels in arteries from reproductive tissues. Am J Physiol Heart Circ Physiol. 2005;289:H1417–H1427. doi: 10.1152/ajpheart.01174.2004. [DOI] [PubMed] [Google Scholar]
- 14.Toro L, Wallner M, Meera P, Tanaka Y. Maxi-KCa, a unique member of the voltage-gated K+ channel superfamily. News Physiol Sci. 1998;13:112–117. doi: 10.1152/physiologyonline.1998.13.3.112. [DOI] [PubMed] [Google Scholar]
- 15.Kaczorowski GJ, Knaus HG, Leonard RJ, McManus OB, Garcia ML. High-conductance calcium-activated potassium channels; structure, pharmacology, and function. J Bionerg Biomembr. 1996;28:255–267. doi: 10.1007/BF02110699. [DOI] [PubMed] [Google Scholar]
- 16.Ledoux J, Werner ME, Brayden, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology. 2006;21:69–79. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 17.Brenner R, Perez GJ, Bonev AD, et al. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 18.Wang YW, Ding JP, Xia XM, Lingle CJ. Consequences of the stoichiometry of Slo1 alpha and auxiliary beta subunits on functional properties of large-conductance Ca2+-activated K+ channels. J Neurosci. 2002;22:1550–1561. doi: 10.1523/JNEUROSCI.22-05-01550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orio P, Latorre R. Differential effects of β1 and β2 subunits on BK channel activity. J Gen Physiol. 2005;125:395–411. doi: 10.1085/jgp.200409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dick GM, Rossow CF, Smirnov S, Horowitz B, Sanders KM. Tamoxifen activates smooth muscle BK channels through the regulatory β1 subunit. J Biol Chem. 2001;26:34594–34599. doi: 10.1074/jbc.M104689200. [DOI] [PubMed] [Google Scholar]
- 21.Valverde MA, Rojas P, Amigo J, et al. Acute activation of maxi-K channels (hSlo) by estradiol binding to the β subunit. Science. 1999;285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- 22.Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel β-subunits. News Physiol Sci. 2002;17:156–161. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- 23.White RE, Darkow DJ, Lang JL. Estrogen relaxes coronary arteries by opening BKCa channels through a cGMP-dependent mechanism. Circ Res. 1995;77:936–942. doi: 10.1161/01.res.77.5.936. [DOI] [PubMed] [Google Scholar]
- 24.Darkow DJ, Lu L, White RE. Estrogen relaxation of coronary artery smooth muscle is mediated by nitric oxide and cGMP. Am J Physiol Heart Circ Physiol. 1997;272:H2765–H2773. doi: 10.1152/ajpheart.1997.272.6.H2765. [DOI] [PubMed] [Google Scholar]
- 25.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 26.Nelson MT, Quayle JM. Physiologic roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 27.Rosenfeld CR, Cox BE, Roy T, Magness RR. Nitric oxide contributes to estrogen-induced vasodilation of the ovine uterine circulation. J Clin Invest. 1996;98:2158–2166. doi: 10.1172/JCI119022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Buren GA, Yang DS, Clark KE. Estrogen-induced uterine vasodilation is antagonized by L-nitroarginine methyl ester, an inhibitor of nitric oxide synthesis. Am J Obstet Gynecol. 1992;167:828–833. doi: 10.1016/s0002-9378(11)91597-x. [DOI] [PubMed] [Google Scholar]
- 29.Rosenfeld CR, Roy TA. Large conductance Ca2+-dependent K+ channels contribute to attenuated uterine vascular responses to α-stimulation in pregnant sheep. Reprod Sciences. 2007;14:110A. [Google Scholar]
- 30.Hutuna C, Cox BE, Liu X-t, DeSpain K, Rosenfeld CR. Vascular development beginning in early ovine gestation: carotid smooth muscle function, phenotype and biochemical markers. Am J Physiol Regulatory Integrative Comp Physiol. 2007;293:323–333. doi: 10.1152/ajpregu.00851.2006. [DOI] [PubMed] [Google Scholar]
- 31.Magness RR, Osei-Boaten K, Mitchell MD, Rosenfeld CR. In vitro prostacyclin production by ovine uterine and systemic arteries: Effects of angiotensin. J Clin Invest. 1985;76:2206–2212. doi: 10.1172/JCI112229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol Regulatory Integrsative Comp Physiol. 1997;272:R441–R463. doi: 10.1152/ajpregu.1997.272.2.R441. [DOI] [PubMed] [Google Scholar]
- 33.Cox BE, Roy TA, Rosenfeld CR. Angiotensin II mediates uterine vasoconstriction through α-stimulation. Am J Physiol Heart Circ Physiol. 2004;287:H126–H134. doi: 10.1152/ajpheart.00046.2003. [DOI] [PubMed] [Google Scholar]
- 34.Gillham JC, Myers JE, Baker PN, Taggart MJ. Regulation of endothelial-dependent relaxation in human systemic arteries by SKCa and IKCa channels. Reprod Sci. 2007;14:43–50. doi: 10.1177/1933719106298197. [DOI] [PubMed] [Google Scholar]
- 35.Knaus HG, Garcia-Calvo M, Kaczorowski GJ, Garcia ML. Subunit composition of the high conductance calcium-activated potassium channel from smooth muscle, a representative of mSlo and slowpoke family of potassium channels. J Biol Chem. 1994;269:3921–3924. [PubMed] [Google Scholar]
- 36.Benkusky NA, Fergus DJ, Zucchero TM, England SK. Regulation of the Ca2+-sensitive domains of the maxi-K channel in the mouse myometrium during gestation. J Biol Chem. 2000;275:27712–27719. doi: 10.1074/jbc.M000974200. [DOI] [PubMed] [Google Scholar]
- 37.Nishimaru K, Eghbali M, Lu R, Marijic J, Stefani E, Toro L. Functional and molecular evidence of MaxiK channel β1 subunit decrease with coronary artery ageing in the rat. J Physiol. 2004;559:849–862. doi: 10.1113/jphysiol.2004.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benkusky NA, Korovkina VP, Brainard AM, England SK. Myometrial maxi-K channel β1 subunit modulation during pregnancy and after 17β-estradiol stimulation. FEBS Lett. 2002;524:97–102. doi: 10.1016/s0014-5793(02)03011-9. [DOI] [PubMed] [Google Scholar]
- 39.Holdiman AJ, Fergus DJ, England SK. 17β-estradiol upregulates distinct maxi-K channel transcripts in mouse uterus. Mol Cell Endocrinol. 2002;192:1–6. doi: 10.1016/s0303-7207(02)00136-3. [DOI] [PubMed] [Google Scholar]
- 40.Nelson MT, Bone AD. The β1 subunit of the Ca2+-sensitive K+ channel protects against hypertension. J Clin Invest. 2004;113:955–957. doi: 10.1172/JCI21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagen BM, Bayguinov O, Sanders KM. β1-subunits are required for regulation of coupling between Ca2+ transients and Ca2+-activated K+ (BK) channels by protein kinase C. Am J Physiol Cell Physiol. 2003;285:C1270–C1280. doi: 10.1152/ajpcell.00153.2003. [DOI] [PubMed] [Google Scholar]
- 42.Gibson TC, Phernetton TM, Wiltbank MC, Magness RR. Development and use of an ovarian synchronization model to study the effects of endogenous estrogen and nitric oxide on uterine blood flow during ovarian cycles in Sheep. Bio Reprod. 2004;70:1886–1894. doi: 10.1095/biolreprod.103.019901. [DOI] [PubMed] [Google Scholar]