Abstract
Background
The stress-related neuropeptide corticotropin-releasing factor (CRF) is involved in determining behavioral strategies for responding to stressors, in part through its regulation of the dorsal raphe (DR)-serotonin (5-HT) system. CRF1 and CRF2 receptor subtypes have opposing effects on this system that are associated with active vs. passive coping strategies, respectively.
Methods
Immunoelectron microscopy and in vivo single unit recordings were utilized to assess CRF receptor distribution and neuronal responses, respectively, in the DR of stressed and unstressed rats
Results
Here we show that in unstressed rats CRF1 and CRF2 are differentially distributed within DR cells, with CRF1 being prominent on the plasma membrane, and CRF2 being cytoplasmic. Stress experience reverses this distribution, such that CRF2 is recruited to the plasma membrane and CRF1 tends to internalize. As a consequence of this stress-induced cellular redistribution of CRF receptors, neuronal responses to CRF change from inhibition to a CRF2-mediated excitation.
Conclusions
Given evidence that CRF1 and CRF2 activation are associated with distinct behavioral responses to stress, the stress-triggered reversal in receptor localization provides a cellular mechanism for switching behavioral strategies for coping with stressors.
Keywords: receptor trafficking, serotonin, antalarmin, corticotropin-releasing hormone, immunoelectron microscopy, behavioral coping strategies
Introduction
Stress is associated with diverse psychiatric diseases (eg., affective disorders, substance abuse) (1, 2). Dysfunctions of corticotropin-releasing factor (CRF), a critical neuromediator of the stress response, have been implicated in the link between stress and psychiatric disorders (3). One mechanism by which CRF links stress and depression is through its regulation of major biogenic amine brain systems that are implicated in these disorders, including the norepinephrine-containing nucleus, locus coeruleus, and the serotonin (5-HT)-containing dorsal raphe nucleus (DR) (4, 5).
CRF regulates the DR-5-HT system through CRF1 and CRF2 receptor subtypes that have opposing effects on the activity of this system (6, 7). Low doses of CRF that are more selective for CRF1 decrease DR neuronal activity and extracellular 5-HT in certain DR forebrain targets (6, 8, 9). CRF1-mediated inhibition of the DR-5-HT system is engaged during an initial exposure to swim stress (10). Alternatively, higher doses of CRF or engaging CRF2 receptors, activates DR-5-HT neurons (6, 7, 9). The opposing actions of CRF1 and CRF2 receptors in the DR are hypothesized to facilitate active and passive behavioral coping styles, respectively (4). For example CRF2-mediated activation of the DR promotes the passive behavior that characterizes learned helplessness (i.e., deficits in escape responses) (11). In contrast, low doses of CRF, that are more selective for CRF1 receptors, have an opposing effect to promote the active escape response (12). Similarly, active escape responses during an initial exposure to swim stress are associated with CRF-mediated inhibition of the DR (10).
Previous exposure to stress can change the magnitude or quality of neuronal responses to subsequent stress or CRF (10, 13, 14). In the case of the DR, previous exposure to swim stress changes the response to both a subsequent swim stress or CRF, such that inhibition is no longer apparent and there is evidence of neuronal activation (10). Given that these effects have been suggested to facilitate different coping styles (i.e., active vs. passive, respectively), the mechanisms underlying this shift may explain how prior exposure to stress promotes the passive behavior that characterizes depression. One mechanism through which this can occur is through stress-induced CRF receptor cellular trafficking, which was recently associated with changes in locus coeruleus neuronal sensitivity to CRF (15).
The present study used electron microscopic visualization of immunogold labeled receptors to examine the cellular distribution of CRF1 and CRF2 in DR neurons of unstressed rats and show how this distribution is altered by prior stress. Electrophysiological studies demonstrated the functional consequences of the stress-induced changes in CRF receptor localization. The results demonstrate a novel cellular mechanism whereby stress can qualitatively change neuronal responses to the same agonist and thereby promote different behaviors.
Methods and Materials
Subjects
Adult male Sprague-Dawley rats weighing 250–300g were housed 2–3 per cage on a 12-hour light schedule in a temperature-controlled (20°C) colony room with free access to standard chow and water. Rats used in fluorescent microscopy studies were obtained from Harlan Laboratories (Indianapolis, IN) and rats used in swim stress studies were from Taconic Laboratories (Germantown, NY). All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Thomas Jefferson University and The Children’s Hospital of Philadelphia and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Immunohistochemical Localization of CRF receptors in the DR
Rats used for immunofluorescence studies were transcardially perfused with ice cold 4% paraformaldehyde and coronal sections (40 µm) through the DR were made using a vibratome. Sections were processed to visualize 5-HT and CRF1 or CRF2 (see Supplemental Methods).
For electron microscopic studies, immunohistochemical detection of CRF receptors was performed using immunogold detection, identical to methods described previously (15, 16). For immunodetection of CRF2 with 5-HT at the ultrastructural level, CRF2 and 5-HT were detected using immunogold and immunoperoxidase, respectively (See Supplemental Methods).
Swim Stress
For the electron microscopy studies of CRF receptor trafficking, rats were administered vehicle (aCSF) or the CRF1 antagonist, antalarmin (20 mg/kg, i.p.), 30 min prior to swim stress. The procedures used for swim stress were identical to those described previously (15). Rats were subsequently returned to their home cage where they remained until perfusion (24 h later). Non-swim control animals were handled briefly and returned to their home cage until perfusion, which occurred 24 hours later.
DR neuronal recording
For electrophysiological recordings, rats were exposed to swim stress or handled as described above. These rats were not pretreated with vehicle or antalarmin. Twenty-four hours after swim stress or handling, rats were anesthetized with isofluorane (1–2% in air) and surgically prepared for recording extracellular single unit activity from DR neurons as described (6) (See also, Supplemental Methods). Spontaneous discharge rate was recorded for 3–5 min prior to CRF (30 ng in 30 nl) microinfusion into the DR and then for a period of 5–15 min after CRF administration. In some rats, the CRF2 antagonist, antisauvagine-30 (3 µg in 3 µl) was administered through an intracerebroventricular cannula (i.c.v.) 10 min prior to CRF.
Data analysis
Sampling for CRF receptors, alone and in conjunction with 5-HT, was done in the mid-caudal dorso- and ventromedial subregions of the DR extending from approximately −7.80mm to −8.3mm bregma in regions where mRNA signal has been reported for CRF2 (17). All immunofluorescence and electron microscopy imaging and data analysis were performed in this region (See Supplemental Methods, Fig S1,A). Immunohistochemical analysis of 5-HT and CRF1 or CRF2 was carried out in sections through the DR of 3 rats. Dual immunolabel electron microscopy studies for CRF2/5-HT in the DR were sampled from 4 rats. Ultrastructural studies examining the distribution of CRF receptors in unstressed and stressed rats were carried out in DR sections obtained from nine rats (3 unstressed, 3 vehicle/stressed, 3 antalarmin/stress).
Semi-quantitative analysis of data from CRF1, CRF2, or CRF2/5-HT labeled sections was performed in areas of the neuropil with labeling detected at the ultrastructural level. Profiles were considered immunolabeled when containing two or more immunogold particles. Tissue was collected such that multiple pools representing a coronal series through the DR could be processed in parallel for the immunodetection of CRF1 and CRF2. Each pool consisted of several 40 µm sections through the DR, of which a minimum of two sections per immunolabel (CRF1, CRF2, or CRF2/5-HT) per animal were used in the analysis. From each of these thicker sections, approximately 10 grids each containing 4–8 ultrathin (70–80nm) sections were collected and analyzed at the ultrastructural level. Assessment of CRF receptor distribution was carried out on the most superficial portions of the tissue (18) and cellular elements were defined based on characterizations described by Peters et al. (19). Sampling throughout the neuropil was done at random and only dendrites containing at least 2–3 immunogold particles were included in the analysis.
Analysis of CRF receptor trafficking in handled and swim stressed rats was quantified in immunolabeled dendritic processes by calculating the ratio of cytoplasmic to total gold particles for each dendrite in individual rats. A mean ratio of cytoplasmic to total gold particles was determined for each rat (See Table S1) and the average ratio per group was calculated. Group means were compared by either a two-tailed unpaired student’s t-test (CRF1) or one-way ANOVA with Tukey’s multiple comparison post hoc test (CRF2) and p<0.05 was determined to be statistically significant.
Mean DR discharge rate determined over the 200 s prior to CRF administration was taken as the mean basal discharge rate with subsequent rates at individual timepoints after CRF expressed as a percentage of this mean. The mean basal rates were compared between groups by one-way ANOVA. Timecourses of the effect of CRF on DR activity in different experimental groups were compared using a two-way repeated measures ANOVA with Bonferroni’s post hoc test.
Drugs
Ovine CRF (Dr. Jean Rivier, The Salk Institute, La Jolla, CA) and antisauvagine-30 (Sigma Chemical Co., St. Louis, MO) were diluted in water (1 mg/ml) and 10 µl aliquots were concentrated, stored at −70°C and reconstituted in artificial cerebrospinal fluid (aCSF) on the day of the experiment. Antalarmin (Kenner C. Rice, NIH/NIDDK, Bethesda, MD) was suspended in a solution containing 5% cremaphor and 5% ethanol and injected i.p. (20 mg/kg) in a volume of 1 ml/kg.
Results
CRF receptor localization in DR neurons of unstressed rats: Light microscopy
CRF1 immunoreactivity was robust in the perikarya of DR neurons where it was associated with both 5-HT- and non-5-HT-containing cell bodies (Fig 1A,C,E). Within these profiles, CRF1 immunoreactivity was most prominent near the periphery, consistent with a localization on the plasma membrane (Fig. 1A,C,E).
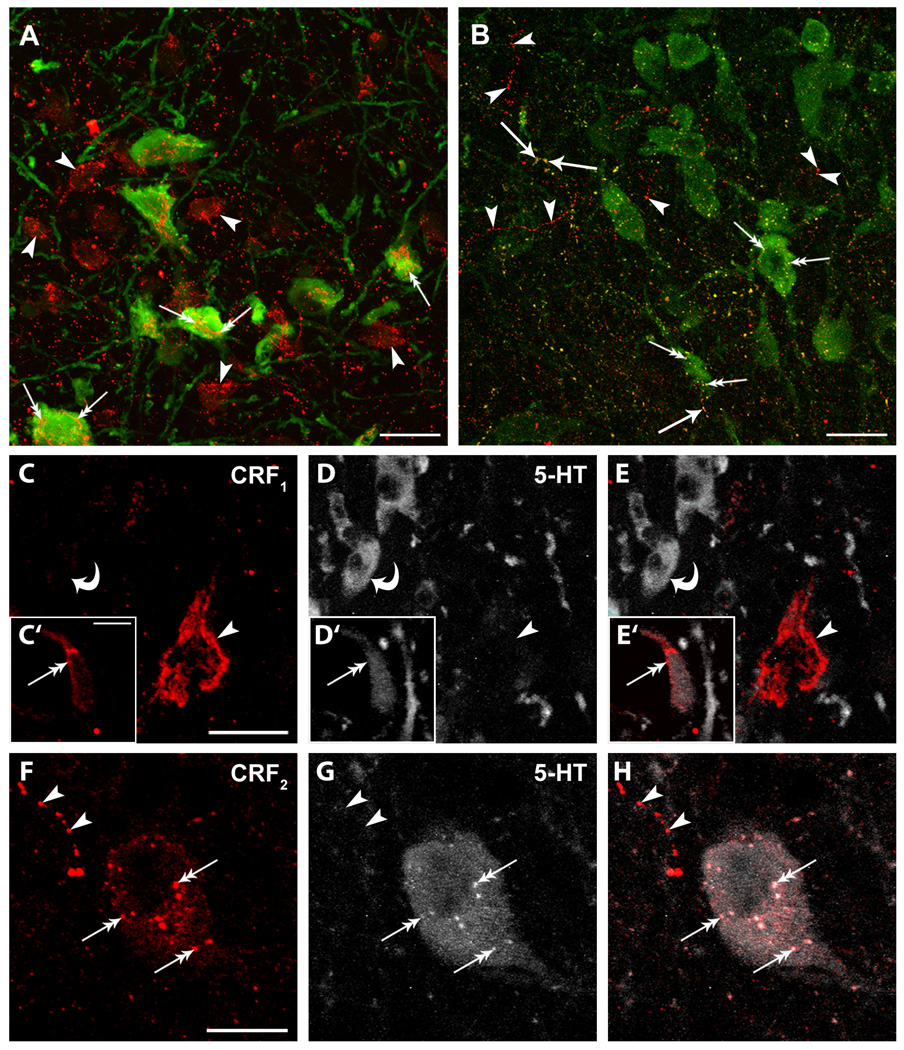
Figure 1. Immunofluorescent labeling of CRF1 and CRF2 in rat DR.
A: Immunofluorescent labeling of CRF1 (red) and 5-HT (green) in the DR. CRF1 was associated with 5-HT (double arrows) and non-5-HT profiles (arrowheads). B: Immunofluorescent labeling of CRF2 (red) and 5-HT (green) in the DR. CRF2 had a punctuate distribution and was associated with 5-HT immunoreactive cell bodies (double arrows), 5-HT containing fibers (single arrows) and non-5-HT containing fibers (arrowheads). C–H: CRF receptor localization and distribution within DR cellular profiles. As in A–B, sections were dual-labeled for 5-HT and either CRF1 (C–E) or CRF2 (F–H). C–E: CRF1 (arrowheads) is prominent near the periphery of profiles lacking detectable 5-HT immunoreactivity (D,E) where 5-HT containing cells are present in the same field (curved arrows). CRF1 is also enriched near the periphery of 5-HT positive cells (C’–E’; double arrow). F–H: CRF2 (double arrows) is distributed within the cytoplasm of this representative 5-HT-containing neuron. This pattern was remarkably different from CRF1 which was concentrated at the membrane. Punctate fibers immunoreactive for CRF2 (arrowheads) but lacking detectable 5-HT immunoreactivity are present within the same field. Scale bars = 20 µm (A–B); 10 µm (C–H)
The appearance of CRF2 immunolabeling in the DR differed from that of CRF1 in being more punctate (Fig. 1B,F,H). Like CRF1, CRF2 was associated with 5-HT- and non-5-HT-containing cell bodies in the DR (Figs. 1B,H). However, CRF2 was also apparent in varicose fibers either alone or in combination with 5-HT (Fig. 1B,H). Unlike CRF1 immunolabeling (Fig. 1 C,E), CRF2 immunolabeling appeared cytoplasmic as opposed to being localized to the periphery of neuronal profiles (Figs. 1 F,H).
CRF receptor localization in DR neurons of unstressed rats: Electron microscopy
Because CRF2 was apparent in both perikarya and fibers using fluorescent microscopy, the presence of CRF2 immunoreactive pre- and postsynaptic profiles was analyzed using electron microscopy. Many CRF2-containing profiles were axon terminals (n=85 of 294 total profiles, 29%), an example of which is shown in Figure 2A. However, the majority of CRF2-containing profiles were somatodendritic (n=209 of 294 total, 71%). In sections processed for dual immunohistochemical detection of both CRF2 and 5-HT, over half of the 5-HT-labeled dendrites expressed CRF2 immunoreactivity (n=393 of 665 total, 59%) and likewise the majority of CRF2 labeled dendrites were 5-HT-containing (n=393 of 582 total CRF2 dendrites, 69%). A dual labeled CRF2/5-HT dendrite is depicted in Figure 2B.
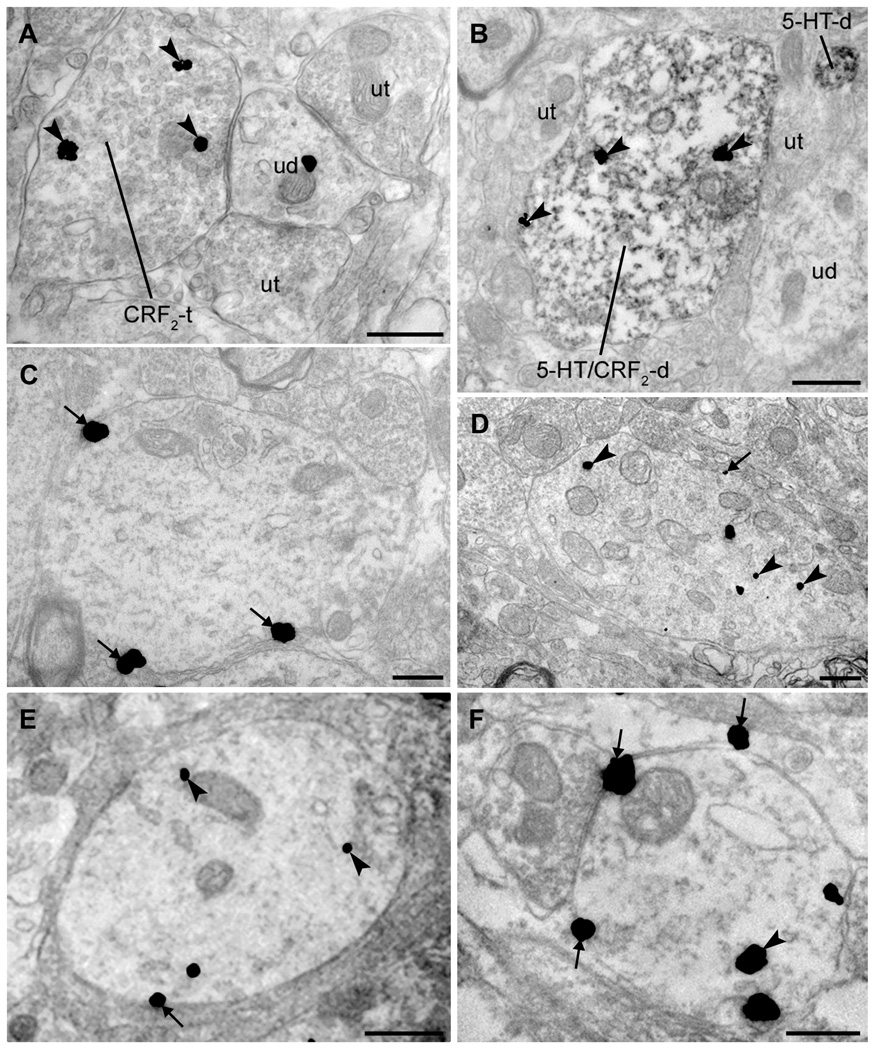
Figure 2. Ultrastructural examination of CRF receptors in the rat DR: distribution and effects of swim stress.
A: Some DR axon terminals contained evidence of CRF2 immunoreactivity (immunogold particles; arrowheads). CRF2-containing axon terminals (CRF2-t) were often found in synaptic contact with unlabeled dendrites (ud). Here, the ud targeted by the CRF2-t is also contacted by axon terminals lacking CRF2 immunoreactivity (ut). B: CRF2 is present in 5-HT-containing dendrites in the DR. Dual immunolabeling for CRF2 (immunogold; arrowheads) and 5-HT (immunoperoxidase) indicated that some 5-HT-containing dendrites colocalized CRF2 (5-HT/CRF2-d) in the DR. In this field, numerous unlabeled axon terminals (ut), a dendrite lacking detectable immunoreactivity (ud) and a dendrite containing 5-HT (5-HTd) but not CRF2 are also present. C–D: CRF1 and CRF2 have markedly different associations with the plasma membrane in unstressed rats. C: CRF1 was often found in association with the plasma membrane (arrows). D: In contrast to CRF1, CRF2 immunoreactivity was located predominantly in the cytoplasm of dendrites (arrowheads) although occasionally observed at the plasma membrane (arrows). E–F: Distribution of immunogold labeling for CRF1 and CRF2 in DR dendrites 24 h after swim stress. E: CRF1 is largely contained within the cytoplasm (arrowheads) with some receptor still present at the plasma membrane (arrows) following stress. F: CRF2 was redistributed to the plasma membrane (arrows) following swim stress, though some CRF2 immunoreactivity remained within the cytoplasm (arrowheads). Scale bars = 500 nm (A–F).
In unstressed rats, CRF1 immunoreactivity was localized to the plasma membrane of dendrites (Fig. 2C) and also distributed within the cytoplasm (not shown). The mean ratio of cytoplasmic-to-total CRF1 immunogold particles indicated a relatively equal distribution between plasma membrane and cytoplasmic compartments in unstressed rats (Table 1). This is comparable to the cellular distribution of CRF1 within locus coeruleus neurons in unstressed rats (15, 16). In contrast to CRF1, CRF2 immunogold labeling in DR dendrites of unstressed rats was mostly cytoplasmic (Fig. 2D), consistent with what was observed using fluorescence microscopy (Fig. 1B, H). Quantification of the ratio of cytoplasmic to total gold particles verified the predominant localization of CRF2 in the cytoplasm of DR neurons of unstressed rats (Table 1).
Table 1.
Ratio of cytoplasmic to total immunogold particles representing the cellular distribution of CRF1 and CRF2 in the DR.
| Ratio of cytoplasmic to total immunogold label | |||
|---|---|---|---|
| Unstressed | Stress | Stress/Antagonist | |
| CRF1 | 0.54 ± 0.04 | 0.66 ± 0.03 | Not determined |
| CRF2 | 0.85 ± 0.01* | 0.56 ± 0.03 | 0.77 ± 0.04* |
Total number of dendrites from 3 rats ± S.E.M.
One way ANOVA; F(2,8)=20.9, p<0.002
p<0.01 vs. stress
Swim stress shifts CRF receptor localization in DR neurons
In locus coeruleus neurons, swim stress results in significant CRF1 internalization that is apparent 1 and 24 h later (15). In DR neurons, there was a tendency for increased CRF1 in the cytoplasmic compartment twenty-four hours following swim stress (Fig. 2E). However, the mean ratio of cytoplasmic to total CRF1-immunogold particles in stressed rats was not statistically different from that determined in handled controls (p=0.07) (Table 1). This ratio was also somewhat less than that determined in LC neurons (i.e., 0.8) at a similar time after swim stress (15).
In contrast to the tendency for CRF1 to internalize after swim stress, CRF2 was recruited from its predominant localization within the cytoplasm to the plasma membrane 24 hours following swim stress (Fig. 2F). The mean ratio of cytoplasmic-to-total gold particles in CRF2-immunolabeled dendrites was significantly decreased after swim stress and matched that of CRF1 in unstressed rats (Table 1). The schematic in Figure 3C depicts how the net shift in receptor distribution changes the primary receptor subtype on the plasma membrane from CRF1 to CRF2, setting up a condition in which CRF2 actions can predominate.
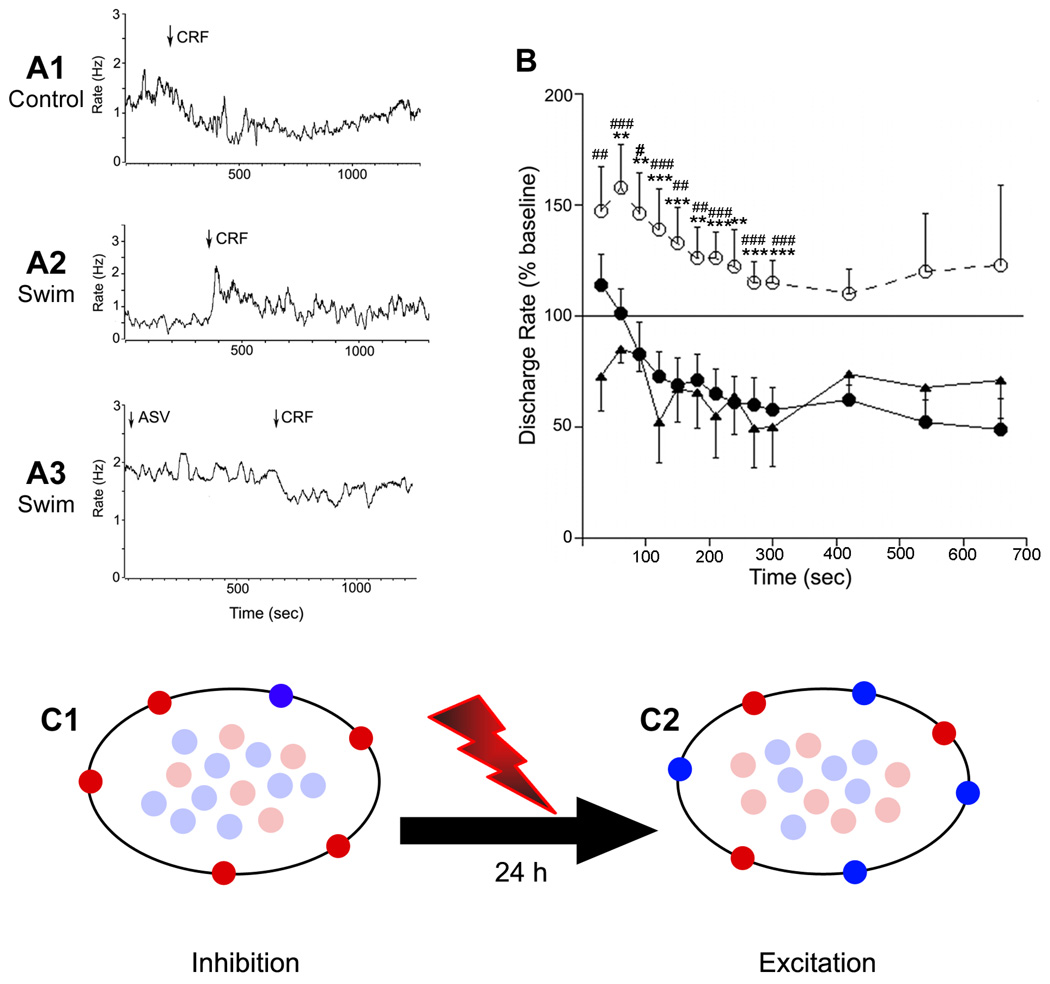
Figure 3. Swim stress results in a qualitative change in CRF effects on DR neuronal activity that favors CRF2 regulation.
A1–A3: Traces indicating the mean LC frequency of individual DR units recorded in an unstressed rat (A1), a rat exposed to swim stress 24 h prior to recording (A2) and a swim stress rat pretreated with antisauvagine-30 (A3). Arrows indicate the administration of CRF or antisauvagine (ASV); note the opposing effects produced by CRF in control vs. the swim stress rat. B: Time course of the effect of CRF on DR neuronal activity. The abscissa indicates the time after injection and the ordinate indicates the mean discharge rate expressed as a percentage of the mean rate determined over 200 seconds prior to injection. Shown are the mean effect of CRF in control rats (solid circles, n=11), swim stressed rats (open circles, dashed line, n=9) and swim stressed rats pretreated with antisauvagine-30 (solid triangles, n=6). Repeated measures ANOVA indicated a statistically significant effect of group (F(2,23)=8.4, p<0.002) and time (F(6,138)=7.0, p<0.0001 but no interaction (F(12,138)=1.0). Asterisks (*) indicate differences between control vs swim determined by Bonferroni post hoc test: **p<0.01; ***p<0.005; Number signs (#) indicate differences between swim vs ASV determined by Bonferroni post hoc test: #p<0.05; ##p<0.01; ### p<0.005. The mean basal discharge rates of cells in the three groups were 1.04±0.21 Hz, 1.94±1.02 and 1.18±0.05 for control, swim stress rats and swim stress rats that were administered ASV before CRF, respectively. These values were not different from one another (F(2,25)=1.2, p=0.3). C: Working model depicting how stress-induced redistribution of CRF receptors can result in a qualitatively different response to CRF. Schematic depicts cytoplasmic vs. plasma membrane localization of CRF1 (red) and CRF2 (blue) in unstressed rats (C1) and rats 24 h after swim stress (C2). In the unstressed condition CRF1 predominates over CRF2 on the plasma membrane and the neuronal response to CRF is inhibition. Twenty-four hours after swim stress the receptors are redistributed such that CRF2 predominates on the plasma membrane and the response to CRF switches to excitation.
Stress-induced CRF1 trafficking in LC neurons required CRF1 activation during the stress (15). Similarly, in the DR the stress-induced recruitment of CRF2 to the plasma membrane of DR neurons required CRF1 receptor activation as it was prevented by pretreatment with the selective CRF1 antagonist, antalarmin, administered prior to swim stress. In rats pretreated with antalarmin before swim stress, the ratio of CRF2 immunolabel in cytoplasm-to-total label was similar to that of unstressed rats and significantly increased compared to stressed rats administered vehicle (Table 1). As swim stress did not produce a statistically significant change in CRF1 cellular localization, this was not examined in antalarmin-treated rats.
Electrophysiological consequence of stress-induced shifts in CRF receptor localization
The shift in CRF receptor localization seen 24h after swim stress is of interest given previous reports that at this time the response to typically inhibitory doses of CRF changes to excitation (10). To determine whether the physiological changes were a functional consequence of receptor trafficking, electrophysiological recordings were performed with the selective CRF2 antagonist, antisauvagine-30. Spontaneous discharge rates of DR neurons tended to be higher in rats with a history of swim stress (1.64 ± 0.4, n=15) compared to control rats 1.04 ± 0.25, n=11) although this was not statistically significant (p=0.23, Student’s t-test for unpaired samples). CRF (30 ng) had opposing effects on DR neuronal activity depending on the history of stress (Fig. 3A,B). In previously unstressed subjects, CRF inhibited DR neuronal activity by approximately fifty percent (Fig. 3A1,B closed circles). In contrast, this same dose of CRF had predominantly excitatory effects in rats with a history of swim stress (Fig. 3A2,B open circles), confirming previous findings (10). Pretreatment of stressed rats with the selective CRF2 antagonist, antisauvagine-30 (30 min before CRF) reinstated the CRF-induced inhibition of DR neurons, such that the response to CRF resembled that observed in unstressed rats (Fig. 3A3, B closed triangles). This demonstrates that the qualitative change in the neuronal response to CRF (from inhibition to excitation) produced by stress was a consequence of CRF2 recruitment to the plasma membrane. The finding that antisauvagine-30 unmasked CRF-induced inhibition in stressed rats is consistent with continued presence of sufficient CRF1 receptor on plasma membrane to mediate an inhibitory response (Fig. 3B).
Discussion
The present findings are the first to describe the cellular localization of CRF receptor subtypes, CRF1 and CRF2, in the DR at an ultrastructural level and demonstrate that these are differentially distributed in cellular compartments, with CRF1 having an increased presence on the plasma membrane compared to CRF2 in unstressed animals. Swim stress results in trafficking of the CRF receptor subtypes in opposing directions such that CRF1 tends to move to the cytoplasm and CRF2 is recruited to the plasma membrane. The cellular redistribution of CRF receptor subtypes corresponds to a shift in neuronal response to CRF from inhibition to excitation. It is significant that these cellular and physiological changes occur at a time after swim stress when the behavioral response to a subsequent challenge changes to become less active and more passive. Thus, stress-induced redistribution of CRF receptor subtypes in the DR may serve as a cellular switch that facilitates alternate coping strategies.
Convergent findings have implicated brain DR-5-HT dysfunctions in affective disorders. These include alterations in biomarkers of 5-HT function in depression, decreased responses to pharmacological challenge with 5-HT receptor agonists and antidepressant or antianxiety efficacy of drugs that modify 5-HT neurotransmission (20–24). Additionally, polymorphisms in genes that encode for crucial components of 5-HT neurotransmission have been associated with affective disorders (25). Hypersecretion and/or other dysfunctions of CRF are thought to link stress and affective disorders and one route by which this can occur is through CRF regulation of the DR-5-HT system (1, 26).
CRF-containing axon terminals densely innervate the DR, synapsing with both GABA and 5-HT neurons here (27–29). CRF2 mRNA is highly expressed in the DR, whereas CRF1 mRNA is relatively low (17, 30). Nonetheless, pharmacologically engaging either CRF1 or CRF2 affects DR neuronal activity, 5-HT release in DR targets, and behavior, and evidence supports a neurotransmitter role for endogenous CRF through actions at both receptor subtypes (4). The effects of CRF on the DR-5-HT system are complex because of the regional and neurochemical heterogeneity of the nucleus and the presence of both CRF receptor subtypes. In spite of this heterogeneity, a story has emerged based on findings from several different laboratories that posits that low levels of CRF engage CRF1 receptors in the DR and tone down activity of the system, whereas higher levels that engage CRF2 activate the system, and these two actions facilitate contrasting behaviors. Relatively low doses of ovine CRF that are more selective for the CRF1 subtype inhibit DR neuronal activity and decrease 5-HT extracellular levels in many forebrain targets (6, 9, 31). DR inhibition is attenuated by the selective CRF1-antagonists further supporting a role for CRF1 in the presence of low levels of CRF (6,31). CRF1-mediated inhibition is engaged during an initial exposure to swim stress when rats exhibit active escape behaviors (10). As doses of CRF increase to a level that is less selective for CRF1, inhibitory effects are lost and there is evidence of neuronal activation that is likely CRF2 mediated (6, 9, 31). Consistent with this, activating CRF2 receptors in the DR with the selective agonist, urocortin 2, increases DR neuronal activity, 5-HT efflux in forebrain targets, and c-fos expression in DR neurons (7, 31–33). CRF2 activation in the DR is engaged during the uncontrollable stress that is used in the learned helplessness model and is necessary for the behavioral consequence of learned helplessness that is characterized by a passive behavior, i.e., a deficit in escape from an adverse event (11). Interestingly, doses of CRF that inhibit DR neurons interfere with the development of learned helplessness (12). Together, these sets of findings support a scheme whereby CRF1 and CRF2 regulate the DR-5-HT system in opposing manners (inhibition and excitation, respectively) with contrasting behavioral consequences, i.e., facilitating active vs. passive coping strategies, respectively (4).
Whether the DR-5-HT system is activated or inhibited when endogenous CRF is released during stress and the behavioral consequence of this is determined in part by the receptor subtype that predominates on the plasma membrane. Although CRF1 is relatively evenly distributed in cytoplasmic and membrane compartments, as reported in LC neurons of unstressed rats (15, 16), the present study reveals that it is more abundant on the plasma membrane of DR neurons compared to CRF2. Interestingly, the CRF2 differs from CRF1 in containing a pseudo signal peptide that affects cellular trafficking (34) and this may contribute to its differential distribution in DR neurons. With the prominence of CRF1 receptors on the plasma membrane of unstressed rats, relatively low levels of CRF that might correspond to acute stress would be predicted to inhibit the system. This is well illustrated in neuronal and behavioral responses to an initial exposure to swim stress, in which the DR-5-HT system is inhibited by endogenous CRF and this is associated with active escape behavior (10, 35). A more severe or prolonged stress may release sufficient CRF to engage the few CRF2 receptors on the cell surface and activate the system, as occurs during the uncontrollable stress that produces learned helplessness (11). Upon subsequent exposure to swim stress, CRF- and stress-induced inhibition of the DR-5-HT system are lost and this correlates to a shift from active escape behavior to a passive response (i.e., floating) (10, 35, 36). The present study revealed a cellular mechanism for this functional shift. Thus, swim stress initiates the cellular redistribution of CRF receptor subtypes with the result that CRF2 predominates on the plasma membrane. This cellular effect qualitatively changes the neuronal response to CRF and can account for the shift from active to passive behavior. This novel cellular mechanism that allows cells to have distinct responses to CRF depending on the history of stress may serve to promote alternate coping strategies if the original response is not appropriate or sufficient in dealing with a persistent or repeated stress.
Both CRF1 and CRF2 are coupled to the GTP-binding protein, Gs, and transduce activation of adenylate cyclase in many systems. Although this intracellular signaling pathway has been the most well characterized for these receptor subtypes, there is substantial evidence for coupling of these receptors to diverse signaling pathways (37). This could account for opposing responses engaged by the two receptors in the same neurons. It is also possible that the receptors are in different neuronal populations, e.g., GABA vs. 5-HT that could explain differential effects on 5-HT activity. Even in this scenario, increasing the influence of CRF2 by recruitment to the plasma membrane would still change the general neuronal response to CRF. Future studies using immunogold particles of varying sizes to double label DR neurons for both receptors and immunoperoxidase labeling for 5-HT or GABA will address this question.
CRF1 receptor activation is a requirement for CRF receptor redistribution because pretreatment with a selective CRF1 antagonist prior to swim stress prevented the effect. This indicates that cellular signaling engaged by CRF1 activation is a critical step in plasma membrane recruitment of CRF2. Administration of a CRF receptor antagonist (subtype non-selective) after the first swim stress also reinstates the ability of a subsequent swim stress to inhibit the DR-5-HT system (10). Together these findings underscore the link between receptor trafficking and the physiological response.
In rats with a history of prior swim stress, antidepressants reinstate both swim stress-induced inhibition of the DR-5-HT system and the active escape behavior, indicating that the response of the DR-5-HT system is directly related to the behavioral response to swim stress and that this is a target of antidepressant efficacy (10, 35, 36). That antidepressant efficacy may involve interference with stress-induced CRF receptor trafficking is a potential mechanism that deserves further exploration.
In summary, we identified the redistribution of CRF receptor subtypes as a unique cellular mechanism through which the response of a system to CRF can qualitatively change. This can trigger a shift in coping mechanisms that may be adaptive in responding to repeated stressors. Alternatively, the persistence of a passive behavioral strategy induced by this mechanism may be involved in the pathogenesis of depression.
Supplementary Material
Acknowledgements
This work was supported by NIH grants MH58250 to RJV and DA15395 to EJV. MW was supported by both the Foerderer and Mary Smith Fellowships from TJU. The authors would like to thank Beverly S. Reyes, DVM, Ph.D. for helpful discussions regarding the electron microscopy analyses. We also thank Mulan Li and Ni Zhai for their technical assistance with electron microscopy processing and confocal imaging, respectively. The authors have no biomedical financial interests or potential conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Maria Waselus, Department of Neurosurgery, Thomas Jefferson University, Farber Institute for Neurosciences, Philadelphia, PA 19107.
Cristiano Nazzaro, Department of Anesthesiology, The Children’s Hospital of Philadelphia, Abramson Pediatric Research Center, Philadelphia, PA 19104.
Rita J. Valentino, Department of Anesthesiology, The Children’s Hospital of Philadelphia, Abramson Pediatric Research Center, Philadelphia, PA 19104
Elisabeth J. Van Bockstaele, Department of Neurosurgery, Thomas Jefferson University, Farber Institute for Neurosciences, Philadelphia, PA 19107
References
- 1.Gold PW, Chrousos GP, Kellner CH. Overview: Psychiatric implications of basic and clinical studies with corticotropin-releasing factor. Am J Psych. 1984;141:619–627. doi: 10.1176/ajp.141.5.619. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 3.Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, et al. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci. 1986;6:2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valentino RJ, Commons KG. Peptides that fine-tune the serotonin system. Neuropeptides. 2005;39:1–8. doi: 10.1016/j.npep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Valentino RJ, Van Bockstaele EJ. Convergent regulation of the locus coeruleusnorepinephrine system as an adaptive response to stress. Eur J Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirby LG, Rice K, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- 7.Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identifed neurons in the rat dorsal raphe nucleus reveals dual actions. J Neurosci. 2004;24:1305–1311. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- 9.Price ML, Lucki I. Regulation of serotonin release in the lateral septum and striatum by corticotropin-releasing factor. J Neurosci. 2001;21:2833–2841. doi: 10.1523/JNEUROSCI.21-08-02833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price ML, Kirby LG, Valentino RJ, Lucki I. Evidence for corticotropin-releasing factor regulation of serotonin in the lateral septum during acute swim stress: adaptation produced by repeated swim. Psychopharmacology. 2002;162:406–414. doi: 10.1007/s00213-002-1114-2. [DOI] [PubMed] [Google Scholar]
- 11.Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, et al. Corticotropin-releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammack SE, Pepin JL, DesMarteau JS, Watkins LR, Maier SF. Low doses of corticotropin-releasing hormone injected into the dorsal raphe nucleus block the behavioral consequences of uncontrollable stress. Behav Brain Res. 2003;147:55–64. doi: 10.1016/s0166-4328(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 13.Curtis AL, Pavcovich LA, Valentino RJ. Previous stress alters corticotropin-releasing factor neurotransmission in the locus coeruleus. Neuroscience. 1995;65:541–550. doi: 10.1016/0306-4522(94)00496-r. [DOI] [PubMed] [Google Scholar]
- 14.Curtis AL, Pavcovich LA, Valentino RJ. Long term regulation of locus coeruleus sensitivity to corticotropin-releasing factor by swim stress. J Pharmacol Exp Ther. 1999;289:1211–1219. [PubMed] [Google Scholar]
- 15.Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reyes BA, Fox K, Valentino RJ, Van Bockstaele EJ. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci. 2006;23:2991–2998. doi: 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- 17.Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, et al. Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leranth C, Pickel VM. Electron Microscopic Preembedding Double-Immunostaining Methods. In: Heimer L, Zaborszky L, editors. Neuroanatomical tract-tracing methods, 2: recent progress. 2 ed. New York: Plenum; 1989. pp. 129–172. [Google Scholar]
- 19.Peters A, Palay SL, Webster HD. The Fine Structure of the Nervous System: Neurons and Their Supporting Cells. 3 ed. Oxford University Press; 1991. [Google Scholar]
- 20.van Praag HM. Depression, suicide, and serotonin metabolism in the brain. In: Post RM, Ballenger JC, editors. Neurobiology of Mood Disorders. Baltimore, MD: Williams and Wilkins; 1984. pp. 601–618. [Google Scholar]
- 21.Nordstrom P, Asperg M. Suicide risk and serotonin. Int Clin Psychopharmacol. 1992;6 Suppl 6:6–21. doi: 10.1097/00004850-199206006-00003. [DOI] [PubMed] [Google Scholar]
- 22.Cowen PJ. Serotonin receptor subtypes in depression: evidence from studies in neuroendocrine regulation. Clin Neuropharmacol. 1993;16 Suppl. 3:S6–S18. [PubMed] [Google Scholar]
- 23.Lesch KP. 5-HT1A receptor responsivity in anxiety disorders and depression. Prog Neuropsychopharmacol Biol Psychiat. 1991;15:723–733. doi: 10.1016/0278-5846(91)90001-h. [DOI] [PubMed] [Google Scholar]
- 24.Mann JJ, Malone KM, Diegl DJ, PErel J, Cooper TB, Mintun MA. Demonstration in vivo of reduced serotonin responsivity in the brain of untreated depressed patients. Am J Psych. 1996;153:174–182. doi: 10.1176/ajp.153.2.174. [DOI] [PubMed] [Google Scholar]
- 25.Heils A, Mossner R, Lesch KP. The human serotonin transporter gene polymorphism-basic research and clinical implications. J Neural Transm. 1997;104:1005–1014. doi: 10.1007/BF01273314. [DOI] [PubMed] [Google Scholar]
- 26.Nemeroff CB. The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry. 1996;1:336–342. [PubMed] [Google Scholar]
- 27.Swanson LW, Sawchenko PE, Rivier J, Vale W. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 28.Valentino RJ, Liouterman L, Van Bockstaele EJ. Evidence for regional heterogeneity in corticotropin-releasing factor interactions in the dorsal raphe nucleus. J Comp Neurol. 2001;435:450–463. doi: 10.1002/cne.1043. [DOI] [PubMed] [Google Scholar]
- 29.Waselus M, Valentino RJ, Van Bockstaele EJ. Ultrastructural evidence for a role of gamma-aminobutyric acid in mediating the effects of corticotropin-releasing factor on the rat dorsal raphe serotonin system. J Comp Neurol. 2005;482:155–165. doi: 10.1002/cne.20360. [DOI] [PubMed] [Google Scholar]
- 30.Van Pett K, Viau V, bittencourt JC, Chan RK, Li HY, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 31.Lukkes JL, Forster GL, Renner KJ, Summers CH. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphe differentially affect serotonin release in the nucleus accumbens. Eur J Pharmacol. 2008;578:185–193. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staub DR, Spiga F, Lowry CA. Urocortin 2 increases c-Fos expression in topographically organized subpopulations of serotonergic neurons in the rat dorsal raphe nucleus. Brain Res. 2005;1044:176–189. doi: 10.1016/j.brainres.2005.02.080. [DOI] [PubMed] [Google Scholar]
- 33.Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, et al. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 34.Rutz C, Renner A, Alken M, Schulz K, Beyermann M, Wiesner B, et al. The corticotropin-releasing factor receptor type 2a contains an N-terminal pseudo signal peptide. J Biol Chem. 2006;281:24910–24921. doi: 10.1074/jbc.M601554200. [DOI] [PubMed] [Google Scholar]
- 35.Kirby LG, Lucki I. Interaction between the forced swimming test and fluoxetine treatment on extracellular 5-HT and 5-HIAA in the rat. J Pharmacol Exp Ther. 1997;282:967–976. [PubMed] [Google Scholar]
- 36.Kirby LG, Lucki I. The effect of repeated exposure to forced swimming on extracellular levels of 5-hydroxytryptamine in the rat. Stress. 1998;2:251–263. doi: 10.3109/10253899809167289. [DOI] [PubMed] [Google Scholar]
- 37.Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.