Abstract
PURPOSE
In axial high myopes with “heavy eye” syndrome, orbital magnetic resonance imaging (MRI) demonstrates degeneration of the lateral rectus-superior rectus (LR-SR) band, so that the lateral rectus muscle slips inferiorly to produce esotropia and hypotropia. We asked if this degeneration might also cause strabismus in nonmyopic elderly patients.
METHODS
Three strabismic elderly patients, three strabismic high myopes, and 12 orthotropic elderly subjects underwent ophthalmic examinations and orbital MRI. Lateral rectus position was determined relative to globe center from quasicoronal images and correlated with LR-SR band structure. MRI was compared to histology of four cadaveric orbits ranging in age from 17 months to 93 years.
RESULTS
Two strabismic patients exhibited hypotropia and one exhibited esotropia. Their mean axial length was 24.1 ± 0.8 mm (mean ± SD), compared to 31.6 ± 1.4 mm for myopes. The lateral rectus muscle position of elderly strabismic subjects averaged 4.6 ± 1.7 mm inferior to globe center, which was significantly lower than that of orthotropic elderly subjects (2.1 ± 1.9 mm; P = 0.01) and similar to that of high myopes (5.1 ± 3.2 mm). By MRI, 100% of strabismic elderly orbits, 67% of strabismic myopic orbits, and 12.5% of control elderly orbits showed LR-SR band thinning, discontinuity, or displacement. LR-SR band degeneration was present histologically only in older cadavers.
CONCLUSION
Age-related LR-SR band degeneration permits the lateral rectus muscle to slip inferiorly in elderly non-myopes, a mechanism of strabismus similar to myopic “heavy eye” syndrome. Imaging may assist in diagnosing this mechanical cause of age-related strabismus.
Keywords: esotropia, pulley, hypotropia, heavy eye syndrome, high myopia, strabismus fixus, sagging eye syndrome
Introduction
Orbital connective tissues play an important role in eye movements, and abnormalities in orbital connective tissues can cause strabismus (1–6). Connective tissues couple the extraocular muscles to the orbital walls and to one another. Each rectus muscle is encircled by a pulley, a ring of primarily collagenous connective tissue located posterior to the globe equator and contiguous with Tenon’s fascia. Pulleys inflect rectus muscle paths, similar to the trochlea’s role in inflecting the superior oblique tendon path (7, 8). Connective tissue bands, which have also been called “intermuscular septa,” couple the pulleys to one another (7). One such band, the lateral rectus -superior rectus (LR-SR) band, joins the lateral and superior rectus muscles. The LR-SR band originates from the lateral border of the superior rectus pulley, and terminates in the superior border of the lateral rectus pulley (7).
Striking degeneration of orbital connective tissues occurs with aging (5, 7). For example, levator aponeurosis degeneration is a common cause of ptosis in the elderly (9–11). Degeneration of the LR-SR band may also occur in elderly people, allowing inferior displacement of the lateral rectus pulley and muscle. The lateral rectus pulley and muscle are under the tension of the inferior oblique muscle, whose orbital layer inserts on the lateral rectus pulley (12,13). Prior studies have confirmed inferior displacement of the horizontal rectus pulleys and muscles in elderly people, a possible explanation the common observation that older individuals have limitation in supraduction (14, 15).
Inferior displacement of the lateral rectus muscle is also a well-recognized cause of strabismus in high myopes (16). Known as “heavy eye” syndrome or myopic strabismus fixus, this syndrome is characterized by esotropia and hypotropia due to conversion of lateral rectus muscle action from abduction to infraduction (16–18). Patients with “heavy eye” syndrome have impaired abduction and supraduction. We hypothesized that inferior displacement of the lateral rectus muscle due to degeneration of the LR-SR band could cause strabismus in elderly nonmyopic subjects, a “sagging eye” syndrome. We expected these subjects to exhibit esotropias or hypotropias and abduction or supraduction deficits. We also expected to find evidence of inferior lateral rectus muscle displacement and LR-SR band degeneration on imaging and histological studies of elderly subjects.
Subjects and Methods
Clinical examination
Three strabismic elderly patients, three strabismic high myopes whose axial lengths exceeded 30 mm, and 12 orthotropic elderly control subjects underwent complete ophthalmic examinations. Written informed consent was prospectively obtained according to a protocol approved by the Institutional Review Board of the University of California Los Angeles and in conformity with the Health Information Portability and Accountability Act. Examinations included assessment of visual acuity, stereopsis, orthoptic examination, Hess screen testing, slit lamp and fundoscopic examination, cycloplegic and manifest refraction, and photography in diagnostic gaze positions (for strabismic subjects). Surgical records were reviewed.
Imaging
All subjects underwent high-resolution orbital magnetic resonance imaging (MRI) with a 1.5 T scanner (Signa; General Electric, Milwaukee, Wisconsin). The technique for obtaining high resolution orbital MRI with 2 to 3 mm sections has been previously described (19, 20). Images were digitally analyzed using the program Image J (21) on Macintosh computers (Apple Computer, Cupertino, California). We analyzed MRI during central gaze fixation by the eye under study. Muscle positions relative to globe centroids (equivalent to the centers of gravity) were determined from quasicoronal sections corrected for linear head orientation. For consistency in analysis, left orbits were digitally reflected into the configuration of “right” orbits. First, the globe – optic nerve junction was identified on each quasicoronal image. Next, the image most anterior to the globe – optic nerve junction where rectus muscles could still clearly be identified was selected (4 – 10 mm anterior to the globe–optic nerve junction, depending on gaze direction). This location corresponds well to rectus pulley locations (19). Positions of the centroids of the rectus muscles, the superior oblique muscle, and the globe were determined by outlining the structures visualized in cross-section. The difference between muscle and globe centroids gave muscle position relative to globe center. Positive differences reflected a superior or nasal position; negative differences reflected an inferior or temporal position. The LR-SR band was qualitatively assessed as continuous or discontinuous, thin or thick, and displaced or nondisplaced by examining adjacent quasicoronal image planes. Axial length was measured from axial and sagittal scans as the distance from corneal apex to the inner sclera perpendicular to the lens center. Axial length was taken to be the maximum measurement from three adjacent image planes. Means and standard deviations for rectus muscle positions and axial lengths were compared by Student’s t test for strabismic elderly eyes (n = 8) verses nonstrabismic elderly eyes (n = 24) and for strabismic elderly eyes versus strabismic highly myopic eyes (n = 6). Pearson’s Chi-square tests were used to compare LR-SR band characteristics between groups.
Histology
All cadaveric specimens were obtained in conformity with legal requirements and in compliance with the Declaration of Helsinki. Four human orbits ranging in age from 17 months to 93 years were studied. The three younger orbits were obtained from heads fresh-frozen shortly after death and stored by a tissue bank (IIAM, Scranton, PA), and the 93 year-old orbit was obtained via exenteration at autopsy within 24 hours of death. As previously described, the whole orbits were sectioned into 10 micron thick sections by a microtome (HM325, Carl-Zeiss, Thornwood, NY) after tissue processing, including formalin fixation, decalcification, dehydration, and embedding in paraffin (7). Masson trichrome stain was used to differentially color collagen (blue) and muscle (red). The LR-SR band was identified in coronal orbital sections as a collagenous band running between the lateral margin of the superior rectus/levator palpebrae superioris muscle complex and the superior margin of the lateral rectus muscle. Representative cross sections 12 – 18 mm posterior to the corneal apex were digitally photographed.
Case report
Elderly Strabismic Subject 1
An 85 year-old woman complained of vertical binocular diplopia since undergoing extracapsular cataract extraction and lens implantation under retrobulbar anesthesia in the right eye two years previously. She had also undergone Fasanella-Servat ptosis repair of the right upper eyelid, as well as three courses of radiation for a right-sided orbital lymphoma. She had medically-treated hypertension. On examination, visual acuity was 20/20 in each eye. The patient had −4 supraduction of the left eye (Fig. 1). The patient had 10Δ left hypotropia in central gaze. The left hypotropia increased to exceed 40Δ in sursumversion (Fig. 2). Upward saccadic velocity was clinically normal in the left eye. Forced duction testing showed no restriction to passive elevation of the left eye. The patient had a surgical scar of the right upper eyelid and mild ptosis with a high eyelid crease of the left upper eyelid. The remainder of the examination was notable only for a surgically irregular pupil and a posterior chamber lens in the right eye.
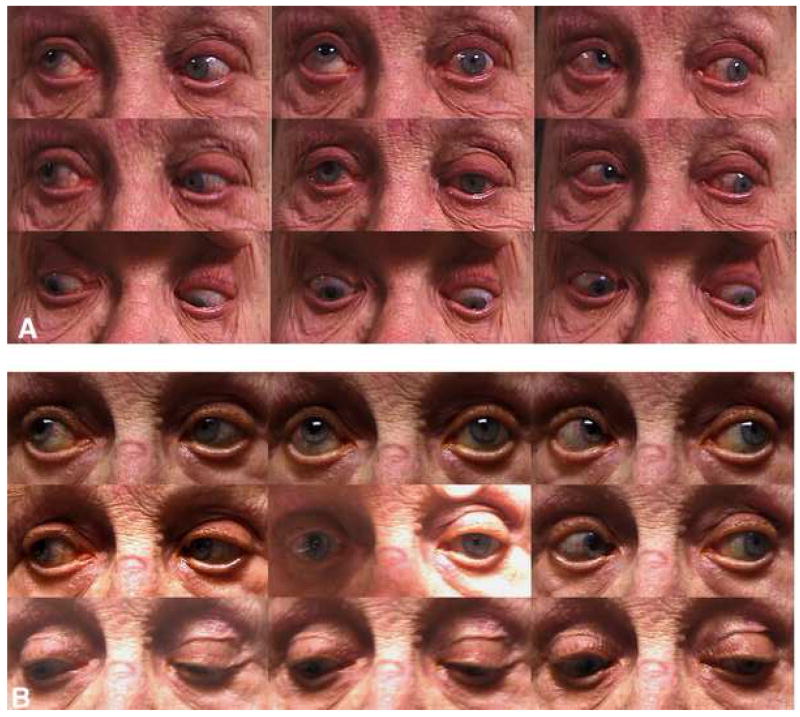
Fig. 1. Diagnostic gaze positions of elderly strabismic subject 1, who underwent surgical repair of the inferiorly displaced left lateral rectus muscle.
A - Preoperative photos show a –4 supraduction deficit of the left eye and a large left hypotropia in sursumversion. Note left upper eyelid ptosis and elevated eyelid crease. The right pupil was surgically mydriatic.
B – Postoperative photos show improved supraduction with smaller left hypotropia in sursumversion.
Fig. 2. Preoperative and postoperative Hess screen plots of elderly strabismic subject 1, showing left eye position with right eye fixating.
A – Preoperative: There is an incomitant left hypotropia increasing in sursumversion.
B – Postoperative: After surgical repair of the inferiorly displaced lateral rectus muscle, the left hypotropia improved.
Multipositional MRI showed normal contractile thickening of the left superior rectus muscle with attempted supraduction. The lateral rectus muscle was inferiorly displaced in both eyes, but the left lateral rectus muscle was displaced 7.6 mm and the right 6.0 mm. The left LR-SR band bowed superiorly and temporally, allowing anterior fat migration (Fig. 3B). Anterior fat migration was also visualized intraoperatively (Fig. 4). The patient underwent superior transposition of the left lateral rectus muscle by one tendon width along the spiral of Tillaux. Ligature of the left lateral rectus to the left superior rectus muscle belly was performed by passing a 5-0 non-absorbable suture through the superior third of the lateral rectus muscle and the temporal one third of the superior rectus muscle 10 mm posterior to their scleral insertions. The suture was tied, uniting the two rectus muscles and correcting the lateral rectus muscle path.
Fig. 3. Quasicoronal T1-weighted orbital MRIs of left orbits showing LR-SR bands and positions of the four rectus muscles relative to the globe.
A - Normal elderly control. In this 74 year-old male, the lateral rectus muscle was 2.2 mm inferior to globe center and was oriented nearly vertically. The LR-SR band was thick, continuous and nondisplaced. Axial length was 24.2 mm.
B - Elderly strabismic subject 1. In this 85 year-old female, the lateral rectus muscle was 7.6 mm inferior to globe center and was oriented obliquely rather than vertically. The LR-SR band was superotemporally displaced. Axial length was 23.3 mm.
C - Strabismic myopic subject 1. In this 63 year-old female, the lateral rectus muscle was 7.4 mm inferior to globe center and was obliquely oriented. The LR-SR band was thin and superotemporally displaced, but immediately adjacent to the enlarged globe. Axial length was 30.5 mm.
LPS/SR – levator palpebrae superioris/superior rectus, LR-SR – lateral rectus – superior rectus band, LR - lateral rectus, IR – inferior rectus, MR – medial rectus.
Fig. 4.
Intraoperative photo of left orbit of elderly strabismic subject 1 (left eye in adduction, surgeon’s view) showing absence of normal lateral rectus pulley connective tissues. The conjunctiva and Tenon’s are reflected temporally with a retractor and hook. Though no dissection of connective tissues was performed, there is prolapse of fat (arrow) into the surgical field. A small hook was used in an attempt to engage the connective tissues, which are absent. The thin line marks the lateral rectus muscle insertion.
Postoperatively, left eye supraduction improved markedly (Fig. 1). The patient was orthotropic in central gaze and had a smaller 14Δ left hypotropia in sursumversion that remained stable over 6 months of follow-up. Hess screen testing confirmed improvement in left hypotropia (Fig. 2).
Details of two additional cases are available online at jaapos.org.
Results
Clinical Characteristics
Two strabismic subjects exhibited hypotropia and one exhibited divergence insufficiency-type esotropia. All had decreased supraduction in one or both eyes, but no abduction deficits. All were female, ranging in age from 73 to 90 with a mean age of 83 ± 9 years. The 12 elderly control subjects, ranging in age from 56 to 74 (mean 64 ± 6 years), had normal versions and were orthotropic in central and secondary gazes. The difference in mean age in the elderly strabismic subjects compared to elderly control subjects did not reach statistical significance (P = 0.09). Seven control subjects were male and five were female. The three strabismic myopic subjects exhibited varying types of strabismus. One had a large esotropia and right hypotropia with absent abduction and supraduction in both eyes (strabismus fixus), one had an intermittent esotropia, and one had an exotropia with a right hypertropia and a mild infraduction deficit in the right eye. Ages were 50, 51 and 63 years. Two were female and one was male. A table of the clinical characteristics of the strabismic elderly and strabismic myopic subjects is available online at jaapos.org.
Rectus Muscle Displacement
While orthotropic elderly subjects had 2.1 ± 1.9 mm (mean ± SD) inferior lateral rectus muscle displacement relative to globe center, the lateral rectus muscle was significantly lower at 4.6 ± 1.7 mm in eyes of strabismic elderly subjects (P = 0.01, Fig. 6). Strabismic myopic eyes also had substantial inferior lateral rectus muscle displacement of 5.1 ± 3.2 mm, not significantly different from the eyes of strabismic elderly subjects (P = 0.8, Fig. 6).
Fig. 6.
Inferior displacement of lateral rectus muscle in strabismic elderly eyes compared to elderly control eyes and strabismic highly myopic eyes.
The medial rectus muscle was located 0.3 ± 2.1 mm inferior to globe center in strabismic elderly eyes, 0.9 ± 1.0 inferior to globe center in nonstrabismic elderly eyes, and 1.6 ± 1.4 inferior to globe center in strabismic myopic eyes. None of these differences was statistically significant. The superior rectus muscle was located 0.7 ± 1.9 mm temporally in strabismic elderly eyes, 0.2 ± 1.2 mm nasally in nonstrabismic elderly eyes, and 1.3 ± 1.7 mm nasally in strabismic myopic eyes. There was no significant difference between horizontal superior rectus muscle displacement between strabismic and nonstrabismic elderly eyes (P = 0.3). There was a trend toward a more nasal superior rectus muscle displacement in strabismic myopic eyes compared to strabismic elderly eyes (P = 0.09). In all groups, the inferior rectus muscle was located slightly nasal to globe center (strabismic elderly: 3.2 ± 1.1, nonstrabismic elderly: 2.6 ± 0.9, strabismic myopes 3.6 ± 1.8), but its location did not differ significantly among groups.
LR-SR Band
All (6 of 6) of strabismic elderly orbits, 67% (4 of 6) of strabismic myopic orbits, and 12.5% (3 of 24) of control elderly orbits had qualitatively abnormal LR-SR bands by MRI, characterized by thinning, discontinuity, displacement, or combinations of these findings (Fig. 5). A significantly higher proportion of orbits exhibited LR-SR band degeneration in the strabismic elderly group than the control elderly group (Fisher exact test, P = 0.0001).
Fig. 5. Examples of LR-SR band degeneration in elderly strabismic subjects visualized by T2-weighted orbital MRI.
A, B - Right and left orbits of elderly strabismic subject 3, showing thinned and superotemporally displaced LR-SR bands.
C, D - Right and left orbits of elderly strabismic subject 2, showing severely thinned and discontinuous LR-SR bands. Only fragments of the connective tissue are visible.
Axial Length
Mean axial length for elderly strabismic eyes was 24.1 ± 0.8 mm, for elderly nonstrabismic eyes 24.1 ± 1.0 mm, and for the strabismic myopic eyes 31.6 ± 1.4 mm. The mean axial length of elderly strabismic and nonstrabismic eyes was the same (P = 1.0). Mean axial length of elderly strabismic eyes was significantly shorter than mean axial length of strabismic myopic eyes (P < 0.0001).
Histology
The LR-SR band could be identified in coraonal histological sections of all four orbits as a collagenous band extending between the temporal border of the superior rectus/levator palpebrae superioris muscle complex and the superior border of the lateral rectus muscle (Fig. 7). It was contiguous with the collagenous sleeves encircling the superior and lateral rectus muscles, and could be identified along a broad anteroposterior extent in numerous serial sections of each orbit. The LR-SR band became progressive thinned and superotemporally displaced with age. In the 17 month-old and 4 year-old orbits, the LR-SR band closely followed the globe contour and was clearly distinct from the more peripheral lateral extension of the levator aponeurosis. In the 57 year-old orbit, the LR-SR band was superotemporally displaced and in close proximity to the lateral extension of the levator aponeurosis. In the 93 year-old orbit, the attenuated LR-SR band was indistinguishable from the lateral extension of the levator aponeurosis.
Fig. 7. Histological sections of whole orbits stained with Masson’s trichrome stain, showing progressive thinning and superotemporal displacement of the LR-SR band (black arrow) with age. 10 micron thick sections.
A - LR-SR band is thick and substantial in 17 month-old subject.
B - LR-SR band is thick and substantial in 4 year-old subject,
C - LR-SR band is thin and bulges superotemporally in 57 year-old subject.
D - LR-SR band is very thin, is virtually indistinguishable from the lateral levator aponeurosis, and bulges superotemporally in 93 year-old subject, The lateral rectus muscle has also shifted inferiorly as observed in the current patients using MRI.
LLA (green arrow) – lateral levator aponeurosis, LPS – levator palpebrae superioris, SR –superior rectus, LG – lacrimal gland, LR- lateral rectus, IO – inferior oblique.
Discussion
The three strabismic, elderly nonmyopic patients presented in this clinical and imaging study represent a previously unrecognized clinical entity, “sagging eye” syndrome. Similar to the “heavy eye” syndrome affecting some high axial myopes, these patients have hypotropia and esotropia, with MRI showing inferior lateral rectus muscle displacement. The evidence suggests that strabismus was caused by degeneration of a connective tissue ligament, the LR-SR band, permitting inferior slippage of the lateral rectus muscle and its pulley.
When LR-SR band degenerates unilaterally or asymmetrically, as in subject 1, hypotropia may result due to shift of some abducting force of the lateral rectus muscle to abnormal infraducting force. When inferior shift of the lateral rectus muscle occurs bilaterally and symmetrically, as in subject 2, the vertical effects may be balanced between the two eyes, but the loss of abducting force bilaterally additive, so that the predominant clinical feature is esotropia. While abduction deficits were not notable clinically, mildly impaired abducting force could contribute to divergence insufficiency in the setting of a pre-existing esophoria or deficient fusional divergence amplitudes. All elderly strabismic subjects exhibited supraduction deficits. The degree of supraduction deficit, which was assessed clinically prior to obtaining MRI, was generally concordant with the amount of inferior lateral rectus muscle displacement measured on MRI. However, the relationship was not linear: elderly strabismic subject 1, for example, had a profoundly asymmetric supraduction deficits yet only a 1.6 mm difference in lateral rectus muscle slippage, suggesting that slippage beyond a critical point may be important.
Because orbital connective tissue degeneration occurs in elderly people (7), coexisting ocular and systemic etiologies for strabismus are common. It is possible that inferior lateral rectus muscle displacement occurs gradually with time, and that the difference in displacement between the elderly strabismic and the elderly control groups is not the explanation for strabismus, but merely the consequence of additional aging. The strabismic elderly group was older (83 ± 9 years) than the elderly control group (64 ± 6 years). More exact age matching of strabismic elderly subjects and nonstrabismic elderly controls would have been optimal, but very elderly normal subjects are difficult to recruit for study. However, our understanding of the functional anatomy of orbital connective tissues, and the fact that these patients’ strabismus fit a pattern predicted by the imaging, make LR-SR band degeneration a plausible cause of their strabismus. Histological analyses of orbital connective tissues confirmed striking degeneration of the LR-SR band with age. Furthermore, we describe two patients who underwent surgical correction of the LR-SR band degeneration with one temporary and one long-term good outcome. Inferior lateral rectus muscle slippage is also a well-recognized cause of strabismus in axial high myopes (16, 22–24).
Though lateral rectus muscle position was similar in the strabismic elderly eyes and the highly myopic eyes, there are clinical differences in strabismus between these groups. “Heavy eye” syndrome is often characterized by profound esotropia with a severe abduction deficit in addition to hypotropia, whereas we found “sagging eye” syndrome to be characterized by hypotropia with supraduction deficits but less esotropia. In axial myopia, inferior lateral rectus muscle displacement is combined with an enlarged, irregularly-shaped globe. A staphylomatous globe may in some cases mechanically prevent abduction due to contact between the globe and the orbital wall (25). Staphylomatous globe shape may also explain the nasal superior rectus muscle displacement of high myopes (26), a trend reproduced in our study. The strabismic elderly subjects may have an exaggeration of normal age-related connective tissue wear-and-tear on orbital connective tissues, whereas highly myopic eyes may have a more widespread connective tissue degeneration involving both sclera and orbital connective tissues.
One would anticipate that orbital connective tissue degeneration due to aging would not be limited to the pulley connective tissues, but would involve adnexal connective tissues generally. Levator aponeurosis dehiscence causes mechanical blepharoptosis by a mechanism essentially identical to degeneration of the LR-SR band: the levator tendon thins and disinserts from the superior tarsus, elevating the upper lid crease as the lid margin descends. We noted that all elderly strabismic patients had previously undergone ptosis surgery or had high lid creases and blepharoptosis. Elevation of the upper lid crease and blepharoptosis may be useful clinical signs of co-existing dehiscence of the LR-SR band.
Acute onset of vertical or horizontal strabismus in older people is often interpreted as suggesting a stroke or mass lesion of the brain. Our study expands the differential diagnosis of strabismus in the elderly to include acute or chronic presentations of orbital connective tissue degeneration that have no neurological implications. The understanding of divergence insufficiency esotropia, a common entity in older patients, has evolved from the concept that it is due to a neurological lesion such as bilateral abducens paresis (27). In divergence insufficiency esotropia, abducting saccades are normal, and neuroimaging reveals no lesions (28). Whether divergence insufficiency esotropia may simply be due to bilateral and symmetrical degeneration of the LR-SR band, with balanced inferior shift of the lateral rectus muscles bilaterally, warrants further study.
One may consider obtaining orbital imaging in adult patients with hypotropias and esotropias of unclear etiology, especially if these patients exhibit clinical signs of levator aponeurosis dehiscence. High resolution orbital MRI has the advantage of directly demonstrating the LR-SR band in addition to showing inferior displacement of the lateral rectus muscle. Coronal orbital computed x-ray tomography (CT) does not show the LR-SR band, but can demonstrate large degrees of inferior lateral rectus muscle displacement. Most of the strabismic elderly subjects had displacement of the lateral rectus muscles 3.5 mm or more inferior to the globe center. This value might tentatively be regarded as a diagnostic threshold for this disorder.
Surgery to restore normal lateral rectus muscle path may be considered in cases of inferior lateral rectus muscle slippage due to LR-SR band degeneration. Two techniques performed in our subjects were (1) suturing the lateral and superior rectus muscle margins together approximately 10 mm posterior to their insertions, with superior transposition of the lateral rectus muscle insertion, and (2) suturing the margins of the lateral and superior rectus pulleys together using 5-0 non-absorbable suture. In subject 3, we suspect that vigorous eye rubbing may have disrupted the surgical union of the pulleys, which were already attenuated. It is unclear if the effect of pulley surgery will be durable, as connective tissues may continue to degenerate. Severe cases might require a loop of artificial material such as silicone between the superior and lateral rectus muscle bellies to suspend the lateral rectus muscle, a technique that has been described in “heavy eye” syndrome (23, 29). Additional clinical observations and surgical innovations are needed to treat strabismus resulting from connective tissue degeneration in the elderly.
Supplementary Material
Acknowledgments
USPHS NIH EY08313
Footnotes
Institution at which study was conducted: University of California Los Angeles
Meeting Presentation: 2008 AAPOS Annual Meeting, Washington, D.C.
Financial Interest: None
FDA Disclosure: MRI surface coils used in imaging are not FDA approved for this purpose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Demer JL. Pivotal role of orbital connective tissues in binocular alignment and strabismus: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2004;45:729–38. doi: 10.1167/iovs.03-0464. [DOI] [PubMed] [Google Scholar]
- 2.Demer JL. Current concepts of mechanical and neural factors in ocular motility. Curr Opin Neurol. 2006;19:4–13. doi: 10.1097/01.wco.0000198100.87670.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demer JL. Mechanics of the orbita. Dev Ophthalmol. 2007;40:132–57. doi: 10.1159/0000100353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hakim OM, El-Hag YG, Maher H. Persistence of eye movement following disinsertion of extraocular muscle. J AAPOS. 2008 doi: 10.1016/j.jaapos.2007.09.001. in press. [DOI] [PubMed] [Google Scholar]
- 5.Demer JL. More respect for connective tissues. J AAPOS. 2008 doi: 10.1016/j.jaapos.2007.12.003. in press. [DOI] [PubMed] [Google Scholar]
- 6.Hall LS, McCann J, Goldberg R, Santiago AP, Rosenbaum AL. Strabismus after orbital fractures and sinus surgery. In: Rosenbaum AL, Santiago AP, editors. Clinical Strabismus Management. Philadelphia: Saunders; 1999. pp. 309–22. [Google Scholar]
- 7.Kono R, Poukens V, Demer JL. Quantitative analysis of the structure of the human extraocular muscle pulley system. Invest Ophthalmol Vis Sci. 2002;43:2923–32. [PubMed] [Google Scholar]
- 8.Demer JL, Oh SY, Poukens V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 2000;41:1280–90. [PubMed] [Google Scholar]
- 9.Older JJ. Levator aponeurosis surgery for the correction of acquired ptosis. Analysis of 113 procedures. Ophthalmology. 1983;90:1056–9. doi: 10.1016/s0161-6420(83)80047-5. [DOI] [PubMed] [Google Scholar]
- 10.Frueh BR. The mechanistic classification of ptosis. Ophthalmology. 1980;87:1019–21. doi: 10.1016/s0161-6420(80)35135-x. [DOI] [PubMed] [Google Scholar]
- 11.Beard C. Ptosis. St. Louis: Mosby; 1976. [Google Scholar]
- 12.Demer JL. The anatomy of strabismus. In: Taylor D, Hoyt CS, editors. Pediatric Ophthalmology and Strabismus. Edinburgh: Elsevier Saunders; 2005. pp. 849–68. [Google Scholar]
- 13.Demer JL, Oh SY, Clark RA, Poukens V. Evidence for a pulley of the inferior oblique muscle. Invest Ophthalmol Vis Sci. 2003;44:3856–65. doi: 10.1167/iovs.03-0160. [DOI] [PubMed] [Google Scholar]
- 14.Clark RA, Isenberg SJ. The range of ocular movements decreases with aging. J AAPOS. 2001;5:26–30. doi: 10.1067/mpa.2001.111016. [DOI] [PubMed] [Google Scholar]
- 15.Clark RA, Demer JL. Effect of aging on human rectus extraocular muscle paths demonstrated by magnetic resonance imaging. Am J Ophthalmol. 2002;134:872–8. doi: 10.1016/s0002-9394(02)01695-1. [DOI] [PubMed] [Google Scholar]
- 16.Krzizok TH, Schroeder BU. Measurement of recti eye muscle paths by magnetic resonance imaging in highly myopic and normal subjects. Invest Ophthalmol Vis Sci. 1999;40:2554–60. [PubMed] [Google Scholar]
- 17.Kowal L, Troski M, Gilford E. MRI in the heavy eye phenomenon. Aust N Z J Ophthalmol. 1994;22:125–6. doi: 10.1111/j.1442-9071.1994.tb00779.x. [DOI] [PubMed] [Google Scholar]
- 18.Ward DM. The heavy eye phenomenon. Trans Ophthalmol Soc U K. 1967;87:717–26. [PubMed] [Google Scholar]
- 19.Clark RA, Miller JM, Demer JL. Location and stability of rectus muscle pulleys. Muscle paths as a function of gaze. Invest Ophthalmol Vis Sci. 1997;38:227–40. [PubMed] [Google Scholar]
- 20.Clark RA, Miller JM, Rosenbaum AL, Demer JL. Heterotopic muscle pulleys or oblique muscle dysfunction? J AAPOS. 1998;2:17–25. doi: 10.1016/s1091-8531(98)90105-7. [DOI] [PubMed] [Google Scholar]
- 21.Rasband WS. Image J. US National Institutes of Health; Bethesda, Maryland, USA: 1997–2008. [Accessed Nov. 3, 2007]. http://rsb.info.nih.gov/ij/ [Google Scholar]
- 22.Rowe FJ, Noonan CP. Surgical treatment for progressive esotropia in the setting of high-axial myopia. J AAPOS. 2006;10:596–7. doi: 10.1016/j.jaapos.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Wong I, Leo SW, Khoo BK. Loop myopexy for treatment of myopic strabismus fixus. J AAPOS. 2005;9:589–91. doi: 10.1016/j.jaapos.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Aoki Y, Nishida Y, Hayashi O, Nakamura J, Oda S, Yamade S, Kani K. Magnetic resonance imaging measurements of extraocular muscle path shift and posterior eyeball prolapse from the muscle cone in acquired esotropia with high myopia. Am J Ophthalmol. 2003;136:482–9. doi: 10.1016/s0002-9394(03)00276-9. [DOI] [PubMed] [Google Scholar]
- 25.Demer JL, Von Noorden GK. High myopia as an unusual cause of restrictive motility disturbance. Surv Ophthalmol. 1989;33:281–4. doi: 10.1016/0039-6257(82)90154-0. [DOI] [PubMed] [Google Scholar]
- 26.Krzizok T, Schroeder B. Quantification of recti eye muscle paths in high myopia. Strabismus. 2003;11:213–20. doi: 10.1076/stra.11.4.213.24306. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson DM. Divergence insufficiency revisited: natural history of idiopathic cases and neurologic associations. Arch Ophthalmol. 2000;118:1237–41. doi: 10.1001/archopht.118.9.1237. [DOI] [PubMed] [Google Scholar]
- 28.Lim L, Rosenbaum AL, Demer JL. Saccadic velocity analysis in patients with divergence paralysis. J Pediatr Ophthalmol Strabismus. 1995;32:76–81. doi: 10.3928/0191-3913-19950301-04. [DOI] [PubMed] [Google Scholar]
- 29.Krzizok TH, Kaufmann H, Traupe H. New approach in strabismus surgery in high myopia. Br J Ophthalmol. 1997;81:625–30. doi: 10.1136/bjo.81.8.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.