Abstract
Increased expression of heparanase stimulates the progression of various human cancers including breast cancer. Therefore a deeper understanding of the mechanisms involved in regulating heparanase is critical in developing effective treatments for heparanase overexpressing cancers. In this study, we investigated the potential use of extracellular superoxide dismutase (EcSOD) to enhance the inhibitory effects of heparin/LMWH in breast cancer cells. EcSOD binds to cell surfaces and the ECM through its Heparin Binding Domain (HBD). Deleting this HBD rendered the protein a more potent inhibitor of breast cancer growth, survival, and invasion. Amongst the treatment combinations examined, EcSODΔHBD plus LMWH provided the best tumor suppressive effects in inhibiting breast cancer growth and invasion in vitro. We have further shown that overexpression of EcSOD decreased accumulation of VEGF in the culture medium and increased the level of intact cell surface-associated heparan sulfate (HS), thus implicating inhibition of heparanase expression as a potential mechanism. Overexpression of EcSOD inhibited steady state heparanase mRNA levels by more than 50% as determined by quantitative RT-PCR. Moreover, heparanase promoter activation was suppressed by EcSOD as indicated by a luciferase reporter assay. These findings provide a molecular pathway showing that regulation of heparanase transcription can be mediated by oxidative stress which was previously unrecognized. Our study implies that overexpression of EcSOD is a promising strategy to enhance the efficacy of heparin/LMWH by inhibiting heparanase as a novel treatment for breast cancer.
Keywords: invasion, heparanase, extracellular superoxide dismutase, heparan sulfate, reactive oxygen species
Introduction
Heparanase has been widely implicated as an important regulator of proliferation, invasion, and metastasis as well as a significant promoter of malignancy-associated angiogenesis in breast cancer (1, 2). Heparanase is an endoglucuronidase that is involved in the degradation of heparan sulfate (HS), a linear sulfated glycosaminoglycan (3). As a key component of the cell surface proteoglycans and the extracellular matrix (ECM), HS participates in the self-assembly, insolubility, and barrier properties of the basement membrane (4). Heparan sulfate also serves as a storage depot for various members of the heparin-binding family of growth factors, sequestering them in inactive forms (5). Cleavage of HS by heparanase releases these signaling molecules, which can then be activated through binding to their corresponding receptors, thereby promoting cancer growth, angiogenesis, and invasion (6). Furthermore, by degrading the constituents of the basement membranes and the ECM, heparanase participates in invasion of cancer cells into the underlying stroma and metastasis to distal sites via vascular and lymphatic routes (7). In addition, heparanase has been shown to simulate angiogenesis by inducing vascular endothelial growth factor (VEGF).
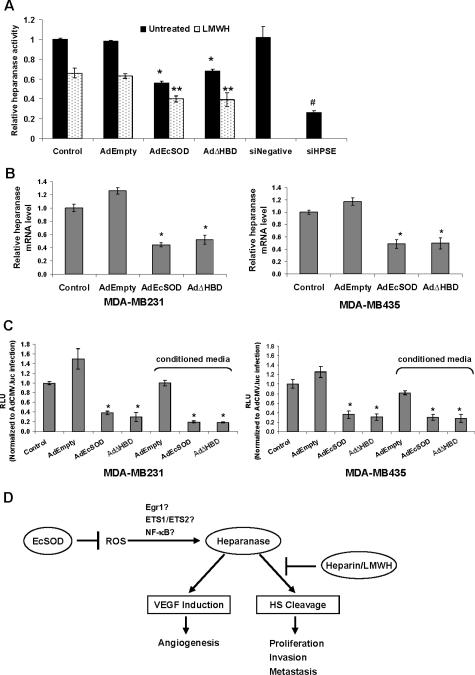
The findings that heparanase is elevated in a wide variety of human cancer cells, including breast cancer (8) has led to an explosion of therapeutic strategies to inhibit its enzymatic activity. These inhibitors include neutralizing antibodies, siRNAs, HS mimetics such as polyanionic molecules, as well as the anti-coagulants heparin and low molecular weight heparin (LMWH) (7, 9). Due to its highly sulfated structure, heparin is the most negatively charged biological molecule, rendering it a potent inhibitor of heparanase as a HS mimic. LMWHs, in general, are considered more effective anti-angiogenic and antimetastatic agents than the unfractionated heparin due to their smaller variation in fragment sizes, longer half lives, and greater bio-availabilities (10). In addition to heparanase, HS degradation has also been shown to be mediated by reactive oxygen species (ROS) (11). Superoxide dismutases (SODs) are cellular antioxidant enzymes that provide the primary defense against these ROS, by converting superoxide radicals (O2.-) into hydrogen peroxide (H2O2), which is subsequently removed by cellular catalase and peroxidases (12). This suggests that scavenging ROS with SOD may help in protecting the integrity of HS to inhibit heparanase-mediated cancer progression as illustrated in our proposed model in Figure 6D.
Figure 6. Inhibition of heparanase expression and total activity levels by EcSOD in breast cancer cells.
(A) Total heparanase activity was evaluated by a homogeneous time resolved fluorescence assay in control or adenovirus infected MDA-MB231 cells. (B) Quantitative real time RT-PCR analysis of heparanase mRNA expression in MDA-MB231 (left) and MDA-MB435 (right) after 72 h of infection. Each value is shown as a relative value compared to the amount in the control and normalized to 18S transcription level. Two-tailed Student's t-test, * P < 0.05 vs. AdEmpty. (C) Heparanase promoter activity was evaluated using a luciferase reporter assay system. Luciferase activity resulting from heparanase promoter activity is expressed as relative light units (RLU) as a percentage of the CMV promoter-driven activity. Error bars = SD of the means. For A and C, a one way ANOVA followed by a post-hoc Tukey's test was used; * P <0.05 vs. AdEmpty, ** P <0.05 vs. AdEmpty plus LMWH, # P <0.001 vs. siNegative. (D) A schematic representation of proposed model illustrating the potential synergistic therapeutic application of heparin/LMWH plus EcSOD by targeting heparanase in breast cancer.
As the name implies, extracellular superoxide dismutase (EcSOD) is the predominant superoxide scavenger in the extracellular space. An important and unique feature of this enzyme is that it contains a heparin binding domain (HBD) (13). Once produced and secreted into the extracellular space, the full length EcSOD binds to cell surfaces and the ECM via this HBD. Removal of this domain occurs naturally by post-translational proteolysis, producing a truncated form of the protein (EcSODΔHBD) that remains in the circulation but has lost the ability to associate with HS (14-16). Since the full length EcSOD has a stronger affinity for heparin/LMWH than HS, addition of heparin/LMWH will result in redistribution of this EcSOD from cell surfaces and ECM into the circulation system (17). These circulating EcSODs will modulate the oxidative stress in the tumor microenvironment. In addition to increasing the circulating level of EcSOD, heparin has also been shown to induce the expression of this antioxidant enzyme (18). It is therefore logical to speculate that the anti-tumor effect of heparin and LMWH may extend to their effects in increasing the circulating level of EcSOD.
We investigated whether overexpression of EcSOD could enhance the inhibitory effect of heparin/LMWH in breast cancer cells. Our data showed that overexpression of EcSOD, particularly the EcSODΔHBD inhibited the in vitro growth and invasion of two aggressive breast cancer cell lines. These inhibitory effects of either forms of EcSOD were greatly enhanced with the addition of heparin/LMWH. We have further shown that EcSOD down-regulated heparanase transcription hence protecting the cleavage of cell surface HS proteoglycan and decreasing VEGF accumulation in the medium.
Materials and Methods
Cell culture
Immortalized, non-malignant breast epithelial MCF-10A cells, human mammary adenocarcinoma cell lines, MCF-7 cells, MDA-MB231 cells, and MDA-MB435 cells were purchased from American Type Culture Collection (Rockville, Maryland). These cells were cultured as described previously (19).
Adenovirus transduction
Overexpression of EcSODs was achieved by adenovirus infection as previously described with MOI of 50 for 72 h (20). The adenovirus constructs used were generated to overexpress a human full-length wild-type EcSOD gene, the EcSOD gene with a deletion in the heparin binding domain, EcSODΔHBD, or the mutated EcSOD gene with a R213G mutation in the heparin binding domain, EcSOD/R213G, as described (20-23).
Heparin and LMWH treatment
After 24 h of adenovirus infection in serum free media, cells were incubated in complete media supplemented with heparin (0.5 mg/mL, Sigma Aldrich) and LMWH (50 IU/mL, Pharmion, CO) for 48 h.
RNA interference
Pre-designed double stranded siRNA against heparanase (siHPSE) was purchased from Ambion Inc. (Austin, Texas) with the sequence of 5' GCAAUUGCUACUCCGAGAAtt 3'. As a negative control, a siNegative siRNA was obtained from Ambion. Transfection of siRNAs was performed as previously described (20).
Real Time RT-PCR analysis
Quantitative real time RT-PCR assay for heparanase (HPSE) mRNA expression was performed as previously described with gene-specific HPSE primer/probe mix (Assays-on-Demand, Applied Biosystems Inc.)
Western Analysis
Protein expression of EcSOD was determined by Western blot analysis as previously described (20)
Antioxidant enzyme activity gels
Conditioned media was harvested from cells transduced with adenovirus vectors for 72 h. Activity of EcSOD was evaluated by native SOD activity gel assay by the method of Beauchamp and Fridovich (24) as previously described (20).
Cell growth
After 72 h of adenoviral infection or siRNA transfection, cells were seeded at a density of 5 × 103 cells in 24-well plates in complete media or media supplemented with heparin/LMWH. For the growth analysis, cells were counted daily for 10 days using a Coulter counter. Cell population doubling times in hours (DT) were determined as previously described (25).
Clonogenic assay
After 72 h of adenoviral vector infection or siRNA transfection, cells were plated in triplicate into 60-mm dishes in complete media or media supplemented with heparin/LMWH. The dishes were maintained in the incubator for 12-14 days. The colonies containing greater than 50 cells were scored.
Invasion assay
The in vitro invasive properties of the aggressive breast cancer cells were performed using the BD Bio-Coat Matrigel invasion assay system (BD Biosciences). Briefly, 2 × 105 of cells were suspended in serum free medium and seeded into the Matrigel chambers consisting with 8-μm pores. The transwell chambers were then placed into 24-well plates, into which we added complete medium or medium containing heparin/LMWH. After incubating cells for 18 h, the upper surface of the transwell chambers was removed with a cotton swab and the invading cells were fixed and stained with Giemsa stain. Photographs were taken under a light microscope and the number of invaded cells was counted in 5 random microscopic fields.
Determination of extracellular superoxide level
Conditioned media were incubated at room temperature with 500 μM hypoxanthine (Sigma) and 40 Units/mL xanthine oxidase (Sigma, St Louis, MO) in the presence of 50 mM DMPO (Dojindo Labs, Kumamoto, Japan). The mixed samples were then placed into a High Sensitivity cavity using an AquaX (4-bore) sample cell (Bruker instruments, Billerica, MA). ESR spectra were obtained with a Bruker EMX spectrometer. Signal heights were measured using the 2nd down field peak of the 4-line DMPO spin adduct and are reported in arbitrary units.
VEGF assay
The effects of EcSOD on VEGF levels in the media were determined by a Quantikine Human VEGF Immunoassay kit (R&D Systems, Minneapolis). After 72 h of adenovirus infection or siRNA transfection, cells were harvested and seeded at 1 × 104 cells in 24-well in complete media or media supplemented with heparin/LMWH. After 24 h of incubation, cell culture supernatant was collected for the VEGF analysis as described by the manufacturer.
Flow cytometry analysis of intact HS
Cells were trypsinized, washed with PBS containing 0.1% BSA, and incubated with anti-heparan sulfate antibody (10E4, Seikagaku, Japan) for 30 min. They were then washed and labeled with FITC-conjugated-goat-antimouse-Ig (Beckton Dickinson). A suspension of stained cells was subjected to FACSCalibur flow cytometry (BD Biosciences, CA) to evaluate the fluorescence intensity.
Heparanase activity
The ability of endogenous heparanase to digest exogeneously added HS was determined with Heparanase Assay kit utilizing a homogeneous time resolve fluorescence (HTRF) technology (Cisbio US, MA). In brief, cell lysates were added to 384-well assay plate followed by an addition of biotinylated HS labeled with europium cryptate. After incubation at 37°C for 60 min fluorescent labeled streptavidin was then added. The HTRF value in each well was measured using a SpectraMax M5e (Molecular Devices) plate reader.
Heparanase promoter activity assay
EcSODs were overexpressed by adenovirus infection as described above with or without co-infection with an adenovirus vector constructed to express the luciferase gene driven by a heparanase promoter (AdHPSE.luc) or a CMV promoter (AdCMV.luc) (26) at an MOI of 50 of each virus for 48 h. Luciferase activity of the infected cell lysate (20 ug total protein) was evaluated with Luciferase Assay System with Reporter Lysis Buffer (Promega, Madison, WI) using the Tecan SpectraFluor plate reader.
Statistical analysis
Statistical analyses were performed using SYSTAT. For some experiments, a single factor ANOVA followed by post-hoc Tukey test was used to determine statistical differences between means. Statistical analyses were assessed using a 2-tailed Student's t test. Results shown are representative of at least three separate experiments each performed in triplicate.
Results
Heparanase expression is up-regulated in breast cancer cell lines
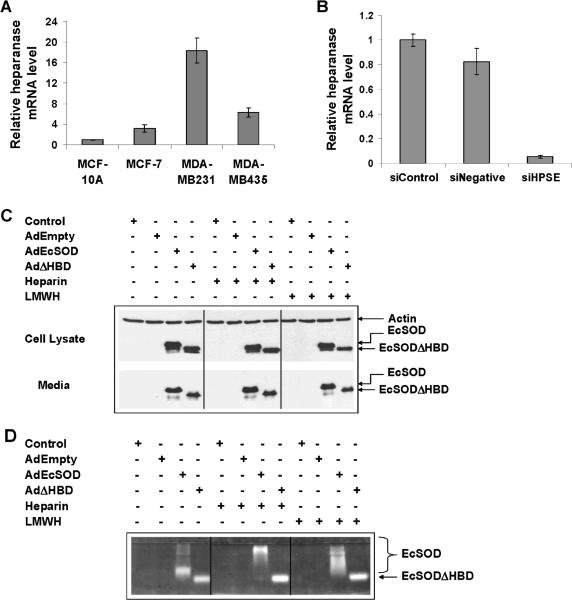
In comparison to the non malignant mammary epithelial cell, MCF-10A, breast cancer cell lines showed elevated heparanase mRNA expression as determined by a quantitative real time RT-PCR (Figure 1A). The highest expression was detected in MDA-MB231 cells followed by MDA-MB435 cells which are both aggressive and invasive breast cancer cells. In an attempt to show that heparanase is the main contributing factor in stimulating breast cancer growth and invasion, we have identified a siRNA against human heparanase, siHPSE, which efficiently inhibited the heparanase expression in MDA-MB231 cells as shown in Figure 1B.
Figure 1. Determination of heparanase mRNA levels and confirmation of EcSOD expression in breast cancer cells.
(A) The levels of heparanase mRNA were measured by quantitative real time RT-PCR. Each value is shown as a relative value compared to the amount in the MCF-10A and normalized to 18S transcription level. Results shown are from three independent experiments. Error bars are the SEMs for inter-experimental error. (B) Short interfering RNA specific to heparanase (siHPSE) was used to down-regulate heparanase expression in MDA-MB231 cells. SiNegative is a non-targeting siRNA control. (C) Western blot analysis showing overexpression of full length EcSOD (33 kD) and truncated EcSODΔHBD (30 kD) in MDA-MB231 cells after 72 h of adenovirus infection in both the cell lysate (top) and the conditioned media (bottom). (D) Native gel assay showing the activity of EcSOD from the conditioned media harvested from adenovirus infected cells.
Overexpression of EcSODs in MDA-MB231 cells
Western blot analysis in Figure 1C shows that MDA-MB231 cells do not express EcSOD but high levels of both forms of EcSOD were detected from the cell lysates after 72 h of AdEcSOD and AdΔHBD infection. Note that although this cell line does not express this antioxidant enzyme by nature, it is able to process the ~31 kD full length protein into a ~27 kD truncated form, which is presumably the EcSODΔHBD. The lower panel of the Western blot analysis indicates that this breast cancer cell line is able to secrete both forms of EcSOD into the conditioned media.
The overexpressed EcSODs are catalytically active
Figure 1D clearly indicates that both the full length and the truncated form of EcSODs are catalytically active in the conditioned media harvested from the AdEcSOD and AdΔHBD infected MDA-MB231 cells. Addition of heparin and LMWH shifted the mobility of the full length EcSOD in the gel, indicating that the HBD of this protein is intact and functional in associating with these negatively charged compounds. It is noteworthy that the heparin has an average molecular weight of 12-15 kDa while the LMWH used in this study ranges from 5.5-7.5 kD in size. The mobility of the truncated EcSOD, on the other hand, was not affected by heparin/LMWH, consistent with it lacking the HBD.
EcSOD plus heparin/LMWH inhibited the growth of breast cancer cells
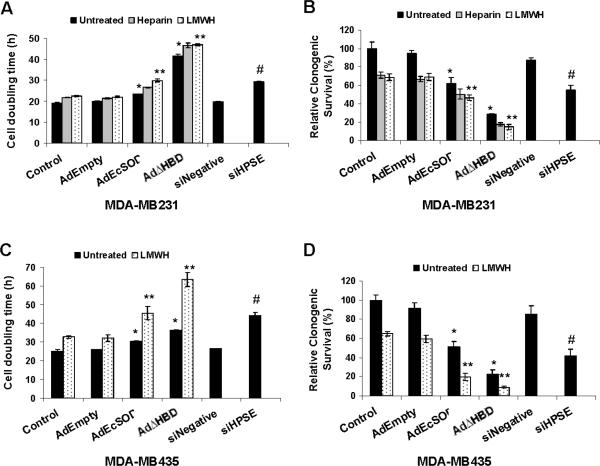
To investigate the effect of EcSODs with or without heparin/LMWH on breast cancer cell growth, we determined the cell doubling time from growth rate analysis. Overexpression of the full length EcSOD moderately prolonged the cell doubling time of MDA-MB231 cells (Figure 2A) and MDA-MB435 cells (Figure 2C). Interestingly, overexpression of the truncated EcSOD dramatically slowed the growth of both aggressive breast cancer cell lines by more than two fold in comparison to control or AdEmpty infected cells. Heparin and LMWH slightly increased the cell doubling time in both cell lines and they provided additive effects in cells overexpressing either forms of the EcSODs. To show that the reduction in cell growth is due to inhibition of heparanase, we down-regulated the expression of heparanase with RNA interference and showed that siHPSE transfection resulted in an increase in cell doubling time of both cell lines.
Figure 2. Overexpression of EcSOD increased the inhibitory effects of heparin/LMWH on breast cancer cell growth and clonogenic capacity.
(A and C) Cells were infected with adenovirus vectors in complete media or media supplemented with heparin/LMWH and cell doubling times were calculated from the growth curves as described in Materials and Methods. (B and D) The colony forming ability of cells was evaluated by counting colonies 14 days post seeding. Inhibition of heparanase expression was achieved via siHPSE transfection. Non-targeting siNegative was used as a siRNA transfection control. Error bars = SD of the mean of three growth curves. Using one way ANOVA followed by post-hoc Tukey's test, *P <0.05 vs. AdEmpty, ** P <0.05 vs. LMWH plus AdEmpty, # P<0.01 vs. siNegative transfection.
EcSODs plus heparin/LMWH additively reduced clonogenic survival
Consistent with the growth inhibition, Figure 2 shows that EcSOD overexpression greatly reduced the clonogenic survival of both MDA-MB231 cells (Figure 2B) and MDA-MB435 cells (Figure 2D), with a more dramatic effect seen with EcSODΔHBD overexpression. Again, EcSODΔHBD plus LMWH was the best combination treatment in inhibiting the clonogenic survival of breast cancer cells.
EcSODs enhanced the inhibitory effect of heparin/LMWH on the invasion of breast cancer cells
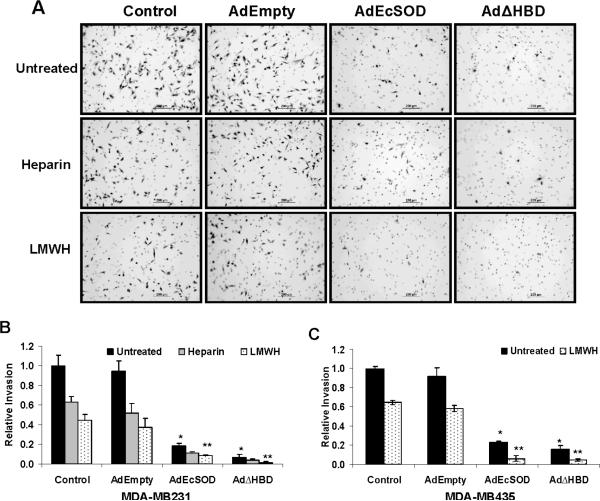
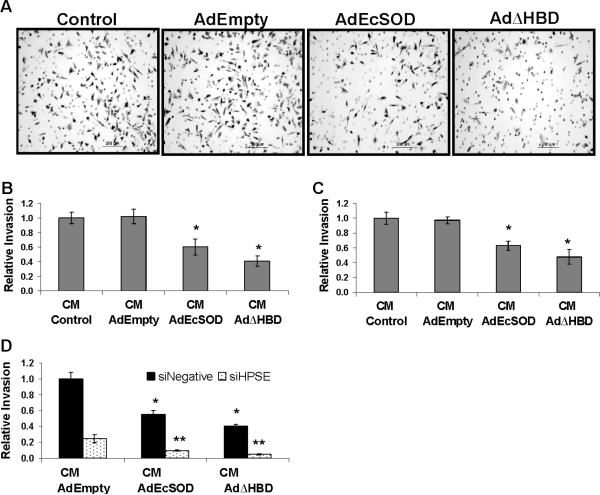
The photomicrographs in Figure 3A show representative fields of adenovirus vector transduced MDA-MB231 cells which have invaded through the Matrigel invasion chamber pores (round 8 μM circles). This invasion assay convincingly illustrates the drastic effect of EcSODs plus heparin/LMWH in suppressing the invasion of MDA-MB231 cells. The results of this invasion assay were quantified as shown in Figure 3B. Cells overexpressing the full length and the truncated EcSODs showed more than 5.2 and 13.5 fold reduction (vs. AdEmpty infected cells) in invasion potential, respectively. Heparin and LMWH treatment alone also reduced the invasion but most importantly, dramatic inhibitory effects were observed when cells simultaneously overexpress either the full length EcSOD or EcSODΔHBD. Similar abrogation of invasion was also observed in MDA-MB435 cells (Figure 3C).
Figure 3. Overexpression of EcSOD suppressed the invasive properties of breast cancer cells in vitro.
After 72 h of control or adenovirus infection, the invasive potential of MDA-MB231 cells was determined as described in the Materials and Methods. (A) Representative photographs of cells invaded through the Matrigel membranes. (B) The numbers of invaded cells from five random fields were counted and presented relative to control cells. (C) Similar results were obtained from MDA-MB435 cells. Error bars = SD of the mean of three invasion assays. Using one way ANOVA followed by post-hoc Tukey's test, * P <0.05 vs. AdEmpty, ** P <0.05 vs. AdEmpty plus LMWH.
Secreted EcSODs (in conditioned media) suppressed breast cancer invasion
Since EcSOD is an extracellular protein, we investigated whether the secreted EcSOD in the conditioned media will affect the invasion of breast cancer cells. Indeed, our data showed that breast cancer cells are less invasive in conditioned media containing the full length and the truncated EcSODs in comparison to the conditioned media obtained from control and AdEmpty infected cells (Figure 4A). Quantitative analyses of the invasion assay are shown in Figure 4B for MDA-MB231 cells and Figure 4C for MDA-MB435 cells. In the presence of soluble full length and truncated EcSODs in the media, suppressing heparanase expression with siHPSE provided a further inhibition of the invasion to 10 ± 1.2 % and 5 ± 0.8 %, respectively, relative to the siNegative transfected cells in the AdEmpty conditioned media (Figure 4D).
Figure 4. Exogenous EcSOD inhibited invasion of breast cancer cells.
(A) The ability of wild type MDA-MB231 cells to invade through Matrigel in conditioned media harvested from adenovirus EcSOD infected cells was evaluated. (B) Cells from five random microscopic fields were counted and plotted in relative to control cells. (C) Similar experiments were repeated with MDA-MB435 cells. (D) Invasion of siRNA transfected MDA-MB231 cells through the Matrigel membrane in conditioned media harvested from adenovirus vector infection. Error bars= SD of the mean of three assays. Using one way ANOVA followed by post-hoc Tukey's test,* P <0.05 vs. conditioned media from AdEmpty infection. ** P <0.05 vs. siNegative tranfected cells in conditioned media of AdEmpty.
Inhibition of heparanase modulated cellular oxidative stress
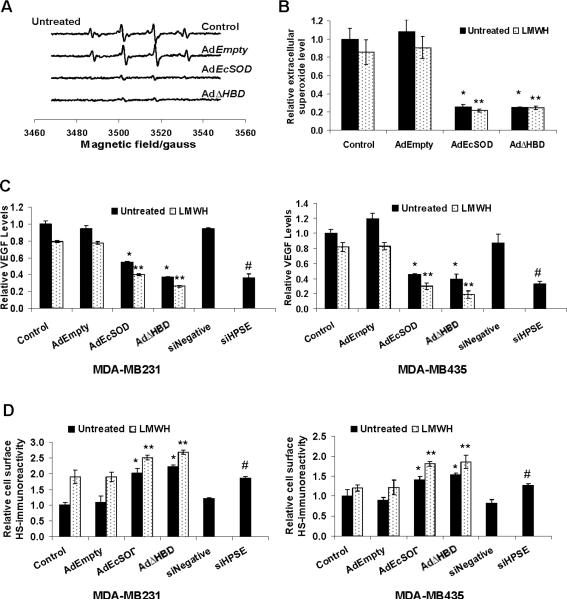
We then performed a DMPO-adduct spin trapping EPR assay to further confirm and quantify the specificity of the superoxide scavenging ability of the EcSODs in the conditioned media. The relative rates of superoxide formation correspond to the intensities of the DMPO-OH spin adduct as shown in Figure 5A. EPR spectra from control and AdEmpty treated conditioned media showed typical peak patterns specific to superoxide generation in MDA-MB231 cells. The intensities of the peak heights were significantly diminished in conditioned media containing either the full length EcSOD or the EcSODΔHBD. The relative extracellular superoxide levels were quantified from the peak heights and are shown in Figure 5B.
Figure 5. Scavenging extracellular superoxide by EcSOD decreased the levels of VEGF in the media and prevented HS cleavage in MDA-MB231 cells.
(A) Conditioned media of MDA-MB231 cells harvested from either control or adenovirus infection were analyzed for superoxide radicals with EPR assay. Representative EPR spectra showing the DMPO-OH spin adduct of the media. (B) The EPR peak height calculated from the conditioned media as a relative measurement of extracellular superoxide levels. (C) VEGF levels in the media were determined by an ELISA assay. (D) EcSOD overexpression increased the immunoreactivity of intact cell surface HS in breast cancer cells. Cells were infected with adenovirus for 72 h and the content of intact cell surface HS was evaluated by immunostaining and flow cytometry analysis. Errror bars = SD of the mean of at least three experiments. Using one way ANOVA followed by post-hoc Tukey's test, *P <0.05 vs. AdEmpty, ** P <0.05 vs. AdEmpty plus LMWH, # P<0.005 vs. siNegative.
EcSOD and heparanase inhibitors decreased levels of VEGF in the media
Since VEGF is one of the effecter molecules affected by heparanase, we determined whether EcSOD will inhibit the bioavailability of this angiogenic factor. As shown in Figure 5C, cells that overexpressed either the long form of EcSOD or the EcSODΔHBD significantly decreased the VEGF protein levels in the media. Addition of LMWH to these EcSOD overexpressing cells resulted in a further reduction in the VEGF levels.
EcSOD and LMWH increased intact HS on the breast cancer cell surface
To investigate whether scavenging the extracellular superoxide radicals with EcSOD overexpression will prevent the heparanase-mediated HS chain degradation, we assessed the immunoreactivity of breast cancer cell surface HS by flow cytometry with monoclonal antibody 10E4. This antibody reacts with epitopes containing N-sulfated glucosamine residues on intact HS side chains of proteoglycans but not with the heparanase-digested HS proteoglycan (27, 28). Figure 5D shows that cells overexpressing either form of the EcSOD have higher content of intact cell surface HS than the control and AdEmpty infected cells. This protective effect against HS degradation was enhanced with the addition of LMWH.
Overexpression of EcSOD inhibited heparanase activity
We then examined whether EcSOD prevents HS degradation by inhibiting the activity of endogenous heparanase. Figure 6A shows that EcSOD overexpression inhibited heparanase activity by more than 50%. This inhibitory effect is enhanced in the presence of LMWH. The ability of heparin and LMWH to block the activity of heparanase is well documented (3) but the effect of EcSOD on heparanase activity has not been reported.
EcSOD down-regulated heparanase transcription in breast cancer cells
We then determined whether EcSOD inhibited the heparanase activity by affecting its gene expression. Results from quantitative real time RT-PCR analysis in Figure 6B (left) shows that full-length EcSOD overexpressing cells had less than half the steady state levels of heparanase mRNA compared to AdEmpty infected MDA-MB231 cells. AdΔHBD infection also resulted in a reduction of heparanase mRNA expression. We have also observed similar down-regulation of heparanase gene expression by these EcSODs in MDA-MB435 cells (Figure 6B, right) indicating that this inhibitory effect is not cell line specific. These results imply that the inhibition of heparanase activity seen in EcSOD/EcSODΔHBD overexpressing cells (Figure 6A) is likely due to the decreased expression level of heparanase; that is, overexpression of these antioxidant enzymes is unlikely to have changed the specific activity of heparanase.
EcSOD inhibited heparanase promoter activity
To address the mechanism underlying decreased heparanase gene expression by EcSODs, we used a human heparanase promoter-luciferase reporter construct. When the heparanase promoter activity was determined, we found that endogenous overexpression of EcSODs via adenovirus vectors as well as exogenous EcSODs in conditioned media decreased the expression of luciferase by more than 50% compared to both the control and the AdEmpty infection in both breast cancer cell lines (Figure 6C). These data clearly demonstrate that EcSOD may provide an antitumor effect in breast cancer cells by impairing the expression level of heparanase and thus limiting the activity of this HS degrading enzyme.
Discussion
A critical event in the process of cancer invasion and metastasis is the degradation of various components of ECM, including HS proteoglycan. Heparanase is the only known endoglycosidase involved in HS cleavage and it has been shown to play an important role in sustaining the pathology of malignant tumors (8). Recent experimental and clinical evidence shows that inhibiting heparanase with heparin/LMWH significantly reduced the growth and metastasis of various human cancers including breast cancer (2, 29). In this report, we have demonstrated that overexpression of EcSOD significantly inhibited heparanase expression and total activity and enhanced the inhibitory effects of heparin/LMWH in two aggressive and invasive breast cancer cell lines.
Inhibiting heparanase expression with either the pharmaceutical drugs (heparin and LMWH) or by genetic manipulation (siHPSE) leads to a slower growth, reduced clonogenic capacity, and invasive potential of aggressive breast cancer cell lines (Figure 2-4), which is in agreement with numerous reports. However, this is the first investigation showing that overexpression of the antioxidant enzyme EcSOD has a profound anti-tumor effect in breast cancer cells. Consistent with our previous findings in pancreatic cancer cells (20), AdEcSOD transduction resulted in a slower growth and a decreased clonogenic capacity in breast carcinoma cells (Figure 2). We have further shown that overexpression of full length EcSOD interfered with the invasion potential of these cells (Figure 3 and 4). Furthermore, exogeneous EcSOD in the conditioned media had the ability to inhibit invasion of breast cancer cells. This exciting observation suggests that systemic application of EcSOD may have promise in the therapeutic intervention for breast cancer treatment. In support of this, Wheeler et al (30) have shown that intramuscular injection of an adenoviral vector with EcSOD construct prior to tumor implantation blunted melanoma growth in mice, suggesting that secreted EcSOD has tumor suppressive properties.
It is intriguing yet puzzling that removal of the HBD from EcSOD rendered the truncated antioxidant enzyme a better inhibitor of growth and invasion in breast cancer cells. These findings do not parallel the evidence provided by other reports showing that deleting HBD from EcSOD resulted in a loss of its protective effects in reducing arterial pressure during hypertension and preventing vascular dysfunction (21, 23, 31), pointing to the importance of physical interaction between EcSOD and cell surfaces. Having said that, considering the fact that cells naturally produce this truncated EcSOD devoid of the HBD, EcSODΔHBD must serve some biological roles that have yet to be elucidated. In this report, we have demonstrated that liberating EcSOD from HS interaction resulted in a greater inhibition of growth, clonogenic survival, and invasion of breast cancer cells (Figures 2-4). The mechanisms involved in these suppressive effects of EcSODΔHBD are unclear but we speculate that deleting this domain imposes less physical restriction compared to the full length EcSOD, which are mainly sequestered on cell surfaces. In other words, the free, non-HS bound EcSODΔHBD may possess greater suppressive effects than its full length counterpart due to a greater bioavailability. It has also been suggested that the HBD of this antioxidant enzyme participates in internalization of the protein by endocytosis and subsequent degradation of EcSOD by lysosomal proteases (32, 33). Therefore, removal of this domain may increase the half life of the truncated protein. However, considering the variety of the binding partners for EcSOD's HBD which include but are not limited to heparin (34), HS (35), hyaluronan (36), collagen type I (37), and fibulin 5 (38), further investigations are needed to understand the biological significance of this domain of EcSOD. Moreover, identification of the protease involved in the processing of this domain will accelerate our understanding of the role played by this antioxidant enzyme.
Interestingly, a natural mutation, Arg213 to Gly (R213G), located in the HBD has been described for human EcSOD. This polymorphism, which has been found in 4% of Swedish, 3% of Australian, and 6% of Japanese people, resulted in an increase in the concentration of plasma EcSOD by 10-30 fold due to a reduced heparin affinity of this mutant (39, 40). Although the consequences of this polymorphism are unclear, it has been shown to be associated with the poor outcome in diabetic patients who require hemodialysis and an increased risk factor associated with cardiovascular disease (41, 42). However, this R213G variant has also been shown to confer resistance to the development of chronic obstructive pulmonary disease in some smokers, which was suggested to be linked to a greater availability of the circulating EcSOD-R213G, thereby providing a better antioxidant and inflammatory response (43). These rather conflicting results imply that the R213G variant or an increased circulating EcSOD level may contribute to different outcomes under varying pathophysiological conditions, and our study indeed provides evidence that increased circulating EcSOD may be therapeutically beneficial in breast cancer treatment. In an attempt to further characterize the role of the HBD or heparin affinity of EcSOD, we have examined the effect of EcSOD-R213G in breast cancer tumorigenesis via adenovirus mediated gene transduction (AdR213G). Overexpression of this variant EcSOD resulted in an intermediate tumor phenotype in inhibiting growth, clonogenic survival, and invasion compared to AdEcSOD and AdΔHBD infected MDA-MB231 cells (Supplemental Figure 1). This study provides further evidence that association with cell surface HS is not essential in the growth and invasion suppressive effects of EcSOD.
Furthermore, we have illustrated that in the presence of heparin/LMWH, the inhibitory effects of EcSOD overexpression on growth, clonogenic capacity, and invasion is greatly enhanced in breast cancer cells. However, this additive effect can not be attributed to the interaction between heparin/LMWH and EcSOD since deleting the HBD rendered the truncated form of this antioxidant enzyme a more potent inhibitor of malignant behavior in breast carcinoma cells in the presence of heparin/LMWH (Figure 2-4). These data strongly suggest that increased circulating EcSOD, in a truncated form or in combination with heparin/LMWH, maybe therapeutically beneficial for breast cancer.
In addition to scavenging extracellular superoxide radicals (Figure 6), we have provided evidence that EcSOD enhanced the anti-tumor effects of heparin/LMWH in part by inhibiting heparanase mRNA expression in breast cancer cells (Figure 6). This down-regulation of heparanase transcription likely led to the observed decrease in the bioavailability of the angiogenic VEGF as well as higher content of intact cell surface HS (Figure 5). Previous reports have demonstrated that EcSOD can prevent oxidative fragmentation of HS (44) and the negatively charged extracellular matrix component, hyaluronan (36) in the lung. However, this is the first report illustrating the ability of EcSOD to regulate the expression of heparanase, thus providing a potential mechanism for the observed anti-tumor effects of EcSOD. Although further work is needed to show how this occurs, we suggest that EcSOD may exert its effect on heparanase expression by blocking activation of redox-sensitive transcription factors that participate in regulating heparanase transcription such as Egr1, NFκB and Ets1/2 (45). These transcription factors are known to be regulated by oxidative stress. It has been reported that EcSOD inhibited the expression of VEGF in an in vivo melanoma study by preventing oxidative activation of NFκB (30) and activation of this transcription factor has been shown to correlate with heparanase expression in various carcinomas (46). In addition, ROS and the reninangiotensin system (RAS) have been shown to up-regulate glomerular heparanase expression in rats with adriamycin nephropathy further supporting that modulation of oxidative stress with an antioxidant enzyme such as EcSOD can inhibit the expression of heparanase. We have also demonstrated that EcSOD inhibited the expression of heparanase independent of its HBD since deleting this domain provided similar inhibitory effect on heparanase transcription and promoter activity (Figure 6). While this study showed the promising use of EcSOD expression to amplify the therapeutic efficacy of heparin/LMWH in breast cancer cells, these combination treatments did not completely abolish the clonogenic survival of the cells suggesting that there are limitations to these strategies and that multiple strategies will be needed.
In conclusion, this study indicates that scavenging ROS with EcSOD down-regulated heparanase expression and activity and prevented loss of cell surface HS in breast cancer cells. This property of EcSOD enhanced the inhibitory effects of heparin/LMWH in breast cancer cells, indicating that EcSOD plus heparin/LMWH combination could be used as promising therapeutic agents in breast cancer treatment by targeting heparanase-mediated pathways as represented in Figure 5D. Most significantly, this report provides evidence showing that the HBD of EcSOD is not essential in its tumor-suppressive effects, implying a biological role for the truncated EcSODΔHBD or the circulating EcSOD released by heparin/LMWH.
Supplementary Material
Acknowledgements
The authors thank Dr. James Crapo (National Jewish Medical and Research Center, Denver, CO) for providing antibodies to EcSOD. We thank Brett Wagner (University of Iowa, Iowa City, IA) for EPR studies supported by grant P30CA086862. This study was supported by NIH grants CA73612 and CA115438, and Komen for the Cure grant KG080437. We dedicate this work to the late Dr. Larry Oberley whose pioneering studies on SOD in cancer we honor.
Financial support: M. Teoh is supported by Komen for The Cure Postdoctoral Fellowship Award KG080437. This study was supported by NIH grants CA115438 and CA073612 to FED.
References
- 1.Cohen I, Pappo O, Elkin M, et al. Heparanase promotes growth, angiogenesis and survival of primary breast tumors. Int J Cancer. 2006;118:1609–17. doi: 10.1002/ijc.21552. [DOI] [PubMed] [Google Scholar]
- 2.Gotte M, Yip GW. Heparanase, hyaluronan, and CD44 in cancers: a breast carcinoma perspective. Cancer Res. 2006;66:10233–7. doi: 10.1158/0008-5472.CAN-06-1464. [DOI] [PubMed] [Google Scholar]
- 3.Vlodavsky I, Ilan N, Naggi A, Casu B. Heparanase: structure, biological functions, and inhibition by heparin-derived mimetics of heparan sulfate. Curr Pharm Des. 2007;13:2057–73. doi: 10.2174/138161207781039742. [DOI] [PubMed] [Google Scholar]
- 4.Iozzo RV. Heparan sulfate proteoglycans: intricate molecules with intriguing functions. J Clin Invest. 2001;108:165–7. doi: 10.1172/JCI13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernfield M, Gotte M, Park PW, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–77. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 6.Vlodavsky I, Abboud-Jarrous G, Elkin M, et al. The impact of heparanese and heparin on cancer metastasis and angiogenesis. Pathophysiol Haemost Thromb. 2006;35:116–27. doi: 10.1159/000093553. [DOI] [PubMed] [Google Scholar]
- 7.Boyd DD, Nakajima M. Involvement of heparanase in tumor metastases: a new target in cancer therapy? J Natl Cancer Inst. 2004;96:1194–5. doi: 10.1093/jnci/djh256. [DOI] [PubMed] [Google Scholar]
- 8.Vlodavsky I, Elkin M, Abboud-Jarrous G, et al. Heparanase: one molecule with multiple functions in cancer progression. Connect Tissue Res. 2008;49:207–10. doi: 10.1080/03008200802143281. [DOI] [PubMed] [Google Scholar]
- 9.McKenzie EA. Heparanase: a target for drug discovery in cancer and inflammation. Br J Pharmacol. 2007;151:1–14. doi: 10.1038/sj.bjp.0707182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norrby K. Low-molecular-weight heparins and angiogenesis. Apmis. 2006;114:79–102. doi: 10.1111/j.1600-0463.2006.apm_235.x. [DOI] [PubMed] [Google Scholar]
- 11.Raats CJ, Bakker MA, van den Born J, Berden JH. Hydroxyl radicals depolymerize glomerular heparan sulfate in vitro and in experimental nephrotic syndrome. J Biol Chem. 1997;272:26734–41. doi: 10.1074/jbc.272.42.26734. [DOI] [PubMed] [Google Scholar]
- 12.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–49. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 13.Sandstrom J, Carlsson L, Marklund SL, Edlund T. The heparin-binding domain of extracellular superoxide dismutase C and formation of variants with reduced heparin affinity. J Biol Chem. 1992;267:18205–9. [PubMed] [Google Scholar]
- 14.Lookene A, Stenlund P, Tibell LA. Characterization of heparin binding of human extracellular superoxide dismutase. Biochemistry. 2000;39:230–6. doi: 10.1021/bi991512x. [DOI] [PubMed] [Google Scholar]
- 15.Enghild JJ, Thogersen IB, Oury TD, Valnickova Z, Hojrup P, Crapo JD. The heparin-binding domain of extracellular superoxide dismutase is proteolytically processed intracellularly during biosynthesis. J Biol Chem. 1999;274:14818–22. doi: 10.1074/jbc.274.21.14818. [DOI] [PubMed] [Google Scholar]
- 16.Sandstrom J, Karlsson K, Edlund T, Marklund SL. Heparin-affinity patterns and composition of extracellular superoxide dismutase in human plasma and tissues. Biochem J. 1993;294(Pt 3):853–7. doi: 10.1042/bj2940853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlsson K, Marklund SL. Heparin-induced release of extracellular superoxide dismutase to human blood plasma. Biochem J. 1987;242:55–9. doi: 10.1042/bj2420055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adachi T, Hara H, Yamada H, et al. Heparin-stimulated expression of extracellular-superoxide dismutase in human fibroblasts. Atherosclerosis. 2001;159:307–12. doi: 10.1016/s0021-9150(01)00512-3. [DOI] [PubMed] [Google Scholar]
- 19.Weydert CJ, Y Z, Sun W, et al. Increased oxidative stress creased by MnSOD or CuZnSOD plus BCNU inhibited breast cancer cell growth. Free Radic Biol Med. 2007 doi: 10.1016/j.freeradbiomed.2007.11.009. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teoh MLT, Sun W, Smith B, Oberley LW, Cullen JJ. Modulation of ROS in pancreatic cancer: Insight into tumor suppression by superoxide dismutases. Clinical Cancer Research. 2007 (in press) [Google Scholar]
- 21.Chu Y, Iida S, Lund DD, et al. Gene transfer of extracellular superoxide dismutase reduces arterial pressure in spontaneously hypertensive rats: role of heparin-binding domain. Circ Res. 2003;92:461–8. doi: 10.1161/01.RES.0000057755.02845.F9. [DOI] [PubMed] [Google Scholar]
- 22.Nakane H, Chu Y, Faraci FM, Oberley LW, Heistad DD. Gene transfer of extracellular superoxide dismutase increases superoxide dismutase activity in cerebrospinal fluid. Stroke. 2001;32:184–9. doi: 10.1161/01.str.32.1.184. [DOI] [PubMed] [Google Scholar]
- 23.Chu Y, Alwahdani A, Iida S, Lund DD, Faraci FM, Heistad DD. Vascular effects of the human extracellular superoxide dismutase R213G variant. Circulation. 2005;112:1047–53. doi: 10.1161/CIRCULATIONAHA.104.531251. [DOI] [PubMed] [Google Scholar]
- 24.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–87. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 25.Lam EW, Zwacka R, Engelhardt JF, et al. Adenovirus-mediated manganese superoxide dismutase gene transfer to hamster cheek pouch carcinoma cells. Cancer Res. 1997;57:5550–6. [PubMed] [Google Scholar]
- 26.Breidenbach M, Rein DT, Schondorf T, et al. A new targeting approach for breast cancer gene therapy using the heparanase promoter. Cancer Lett. 2006;240:114–22. doi: 10.1016/j.canlet.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 27.David G, Bai XM, Van der Schueren B, Cassiman JJ, Van den Berghe H. Developmental changes in heparan sulfate expression: in situ detection with mAbs. J Cell Biol. 1992;119:961–75. doi: 10.1083/jcb.119.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solic N, Wilson J, Wilson SJ, Shute JK. Endothelial activation and increased heparan sulfate expression in cystic fibrosis. Am J Respir Crit Care Med. 2005;172:892–8. doi: 10.1164/rccm.200409-1207OC. [DOI] [PubMed] [Google Scholar]
- 29.Mellor P, Harvey JR, Murphy KJ, et al. Modulatory effects of heparin and short-length oligosaccharides of heparin on the metastasis and growth of LMD MDA-MB 231 breast cancer cells in vivo. Br J Cancer. 2007;97:761–8. doi: 10.1038/sj.bjc.6603928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wheeler MD, Smutney OM, Samulski RJ. Secretion of extracellular superoxide dismutase from muscle transduced with recombinant adenovirus inhibits the growth of B16 melanomas in mice. Mol Cancer Res. 2003;1:871–81. [PubMed] [Google Scholar]
- 31.Brown KA, Chu Y, Lund DD, Heistad DD, Faraci FM. Gene transfer of extracellular superoxide dismutase protects against vascular dysfunction with aging. Am J Physiol Heart Circ Physiol. 2006;290:H2600–5. doi: 10.1152/ajpheart.00676.2005. [DOI] [PubMed] [Google Scholar]
- 32.Chu Y, Piper R, Richardson S, Watanabe Y, Patel P, Heistad DD. Endocytosis of extracellular superoxide dismutase into endothelial cells: role of the heparin-binding domain. Arterioscler Thromb Vasc Biol. 2006;26:1985–90. doi: 10.1161/01.ATV.0000234921.88489.5c. [DOI] [PubMed] [Google Scholar]
- 33.Ohta H, Adachi T, Hirano K. Internalization of human extracellular-superoxide dismutase by bovine aortic endothelial cells. Free Radic Biol Med. 1994;16:501–7. doi: 10.1016/0891-5849(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 34.Adachi T, Kodera T, Ohta H, Hayashi K, Hirano K. The heparin binding site of human extracellular-superoxide dismutase. Arch Biochem Biophys. 1992;297:155–61. doi: 10.1016/0003-9861(92)90654-f. [DOI] [PubMed] [Google Scholar]
- 35.Karlsson K, Lindahl U, Marklund SL. Binding of human extracellular superoxide dismutase C to sulphated glycosaminoglycans. Biochem J. 1988;256:29–33. doi: 10.1042/bj2560029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao F, Koenitzer JR, Tobolewski JM, et al. Extracellular superoxide dismutase inhibits inflammation by preventing oxidative fragmentation of hyaluronan. J Biol Chem. 2008;283:6058–66. doi: 10.1074/jbc.M709273200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen SV, Oury TD, Ostergaard L, et al. Extracellular superoxide dismutase (EC-SOD) binds to type i collagen and protects against oxidative fragmentation. J Biol Chem. 2004;279:13705–10. doi: 10.1074/jbc.M310217200. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen AD, Itoh S, Jeney V, et al. Fibulin-5 is a novel binding protein for extracellular superoxide dismutase. Circ Res. 2004;95:1067–74. doi: 10.1161/01.RES.0000149568.85071.FB. [DOI] [PubMed] [Google Scholar]
- 39.Juul K, Tybjaerg-Hansen A, Marklund S, et al. Genetically reduced antioxidative protection and increased ischemic heart disease risk: The Copenhagen City Heart Study. Circulation. 2004;109:59–65. doi: 10.1161/01.CIR.0000105720.28086.6C. [DOI] [PubMed] [Google Scholar]
- 40.Sandstrom J, Nilsson P, Karlsson K, Marklund SL. 10-fold increase in human plasma extracellular superoxide dismutase content caused by a mutation in heparin-binding domain. J Biol Chem. 1994;269:19163–6. [PubMed] [Google Scholar]
- 41.Yamada H, Yamada Y, Adachi T, et al. Protective role of extracellular superoxide dismutase in hemodialysis patients. Nephron. 2000;84:218–23. doi: 10.1159/000045580. [DOI] [PubMed] [Google Scholar]
- 42.Marklund SL, Nilsson P, Israelsson K, Schampi I, Peltonen M, Asplund K. Two variants of extracellular-superoxide dismutase: relationship to cardiovascular risk factors in an unselected middle-aged population. J Intern Med. 1997;242:5–14. doi: 10.1046/j.1365-2796.1997.00160.x. [DOI] [PubMed] [Google Scholar]
- 43.Young RP, Hopkins R, Black PN, et al. Functional variants of antioxidant genes in smokers with COPD and in those with normal lung function. Thorax. 2006;61:394–9. doi: 10.1136/thx.2005.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kliment CR, Tobolewski JM, Manni ML, Tan RJ, Enghild J, Oury TD. Extracellular superoxide dismutase protects against matrix degradation of heparan sulfate in the lung. Antioxid Redox Signal. 2008;10:261–8. doi: 10.1089/ars.2007.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vreys V, David G. Mammalian heparanase: what is the message? J Cell Mol Med. 2007;11:427–52. doi: 10.1111/j.1582-4934.2007.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao HJ, Fang Y, Zhang X, et al. Tumor metastasis and the reciprocal regulation of heparanase gene expression by nuclear factor kappa B in human gastric carcinoma tissue. World J Gastroenterol. 2005;11:903–7. doi: 10.3748/wjg.v11.i6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.