Abstract
Background
Increasing rates of coccidioidomycosis among the general population are being described. Given the large number of military personnel stationed and training in endemic areas, data regarding infection trends among military members would be informative.
Methods
We performed a retrospective epidemiologic study concerning the incidence and severity of clinical cases of coccidioidomycosis at a Naval base located in an endemic area in California.
Results
Eighty-two military beneficiaries at the base were diagnosed with coccidioidomycosis from January 2002 to December 2006. Among active duty personnel, the rate of coccidioidomycosis rose 10-fold during the five-year study period: 29.88 to 313.71 cases per 100,000 person-years. The incidence of coccidioidal infections occurring in active duty members was higher than other military beneficiaries at the base. The median age of patients with a coccidioidal infection was 28 years, and 73% were male. Sixty-six had primary pulmonary disease, and 14 had disseminated disease; data were unavailable for two cases. The number of disseminated cases increased significantly over time; by 2006, 30% of the diagnosed cases were disseminated disease. Among cases of dissemination, 43% occurred among white/non-Hispanics. Disseminated disease was associated with high complement fixation titers and a more recent year of diagnosis. Although the sample size was small, we found no differences in rates of disseminated disease by race, likely due to the large number of cases among Caucasians.
Conclusions
Coccidioidomycosis incidence rates have significantly increased during the last five years among military beneficiaries. Active duty members were more likely to develop coccidioidomycosis than dependents or retirees, perhaps related to the number and intensity of exposures in this group.
Keywords: coccidioidomycosis, C. immitis, valley fever, military, epidemiology
INTRODUCTION
Coccidioidomycosis, known as “Valley Fever”, is a common fungal infection, which has gained notoriety over the past decade due to the increased incidence and severity - oftentimes, despite treatment [1-3]. It was first described in 1892, appearing in a soldier in Argentina, and became more apparent as a significant public health concern during the 1930s and 1940s, particularly in the San Joaquin Valley in southern California, with the “dust bowl” migrations [4-6]. Since then, coccidioidomycosis has become a well-described endemic mycotic infection in the Lower Sonoran Zone [7]. It is estimated that 150,000 infections occur annually, and epidemiologic studies show that approximately 40% develop symptomatic disease with protean manifestations ranging from mild pulmonary disease to more severe forms [6-8]. About 1−2% will develop disseminated disease, most frequently with involvement of the skin, bones, joints, and the meninges. The risk for dissemination appears to be increased in specific racial groups (especially African Americans and Filipinos), pregnant or postpartum women, elderly persons, and immunosuppressed individuals, such as those with AIDS or cancer, or who are transplant recipients [9-12].
Increasing numbers of infections have been reported in California and Arizona over the past 15 years [1, 5, 13, 14], and coccidioidomycosis is now one of the most frequent causes of community acquired pneumonia [15]. These findings may be partly due to environmental factors, construction activities, migration of increasing numbers of people into endemic areas, and the rising number of immunocompromised hosts in these areas. Trends of coccidioidomycosis among specific populations, such as the military, have not been reported.
Over 350,000 military personnel are stationed at bases within endemic regions of the U.S., including in California, Arizona, Nevada, New Mexico, Utah, and Texas; in addition, thousands more individuals conduct training exercises on temporary active duty order to these locations [16]. Military exercises often create dusty conditions which may aerosolize the infectious arthroconidia, which when inhaled lead to infection. Numerous outbreaks and sporadic cases among military members have been reported [6, 16-23]. Active surveillance and documentation of incidence rates of the disease in the military setting have not been recently evaluated. Whether military personnel are experiencing increasing rates of infections, similar to reports in the general population, is unknown, hence, we conducted an epidemiologic evaluation to determine the incidence and predictors of coccidioidomycosis among military members at a Naval base located in the San Joaquin Valley.
METHODS
A retrospective study was conducted for coccidioidomycosis cases at the Naval Air Station Lemoore (NASL), which is located in Kings County in the central valley of California (Figure 1). Cases were identified between January 1, 2002 and December 31, 2006 by recording patients presenting to the NASL internal medicine clinic with infection and by searching the laboratory's computerized database. A case was defined as a positive IgM and IgG ELISA test, a positive immunodiffusion test, or a positive complement fixation (CF) of ≥1:2 in the setting of a clinical illness consistent with coccidioidomycosis. An isolated positive IgM without IgG seroconversion, a CF of ≤1:2 or no histopathologic evidence of coccidioidomycosis were considered to have a false positive IgM test and was not included in our study.
Figure 1.
Location of the Lemoore Naval Air Station, Lemoore, California.
Previous laboratory data and medical record information were reviewed for each patient to ensure the case was a new diagnosis of coccidioidomycosis and not recurrent disease or a follow-up result from a previously diagnosed case; only new diagnoses of coccidioidomycosis were included in the study. Patient demographics and clinical information was obtained.
The patient population at NASL consists of approximately 17,046 persons, including 6,694 active duty members, 7,443 dependents, and 2,909 retirees; these numbers are estimated, as continuous turnover is seen among the military personnel at the base; however, we are not aware of any substantial changes in the number of personnel over the study time period. Demographic characteristics of the entire population served at the NASL hospital were: average age of 26 years (range one day-95 years); 47% were male and 53% were female. Of those with race recorded in the hospital database, 28.4% were white/non-Hispanic, 7.9% were white/Hispanic, 5.4% were African American, 5.1% were Asian/Pacific Islander, 2.0% were American Indian/Alaskan, and 0.7% were other. Of note, nearly one-half of enrollees did not report race/ethnicity on the medical registration form.
A sub-analysis was performed on the active duty population; this group (active duty personnel) represented 39% (6,694/17,046) of the total hospital population; the average age was 26 years, and 85.3% were male. The race distribution was 56.3% (n=3,769) white/non-Hispanic, 18.2% (n=1,219) white/Hispanic, 11% (n=740) African American, 5.1% (n=340) Filipino, 4.4% (n=297) American Indian/Alaskan Native, 3.8% (n=253) Asian/Pacific Islander, and 1.2% (n=76) other/unknown.
Statistical analyses included descriptive statistics on the cases of coccidioidomycosis. Comparisons between those with and without coccidioidomycosis utilized the Fishers exact test for categorical variables and the Wilcoxon rank test for continuous variables. Number of cases over time utilized the Chi-square test for trend. Variables significant in the univariate model (p<0.05) were included in a backward stepwise multivariate logistic regression model comparing those with pulmonary and disseminated disease (STATA software 9.0, College Station, Texas).
RESULTS
Incidence Rates
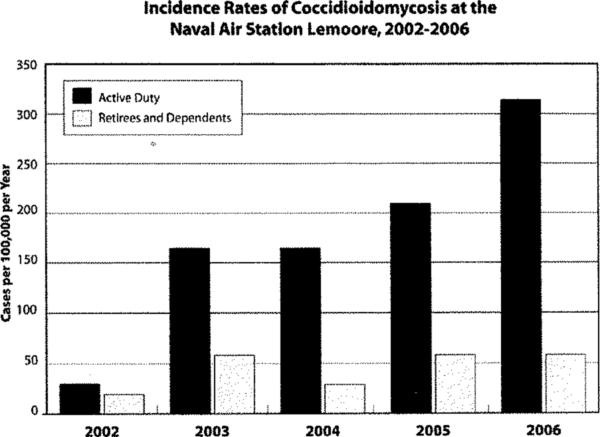
During the study period, 82 patients were newly diagnosed with coccidioidomycosis. The total number of cases increased 6.8-fold during the five year study period from four cases during 2002 to 27 cases by the year 2006 (X2=14.7, p<0.001) (Figure 2). The incidence rate for the overall population at the base was 96 cases per 100,000 person-years (PYs); the rate increased from 23 cases per 100,000 persons in 2002 to 158 cases/100,000 person-years in 2006. The incidence rate of coccidioidomycosis among active duty military members overall was 176 cases/100,000 PYs; the rate rose 10-fold during the five-year study period: 30 to 314 cases per 100,000 PYs. Notably, the incidence rate among non-active duty members treated at the medical facility did not significantly change over time (p=0.24). In 2006, the incidence rate of coccidioidal infections occurring in active duty members was 5.4-fold higher compared to military beneficiaries not serving on active duty at the base (Figure 2).
Figure 2.
Number of New Cases and Incidence Rates of Coccidioidomycosis at the Naval Air Station Lemoore, 2002−2006.
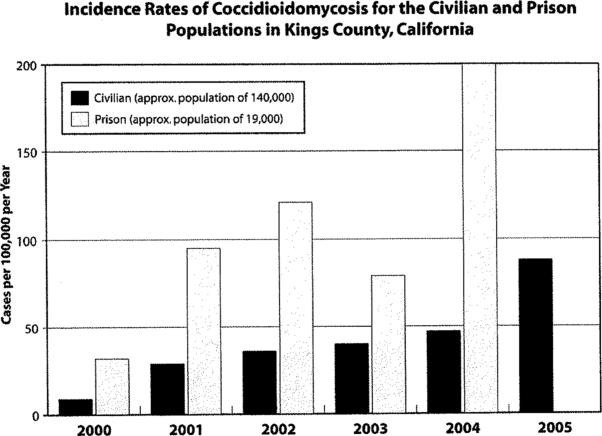
Similar trends have also been noted locally; the Kings County Public Health Department reported that coccidioidomycosis rates in both the local civilian and prison populations have increased over time (Figure 3). The number of cases among civilians in the county increased from 13 to 123 cases during the years 2000 to 2005 (incidence rate from nine to 88 cases/100,000 person-year). Similar, but higher rates occurred among the prison population as the incidence rates rose from 32 to 200 cases/100,000 person-year (Figure 3).
Figure 3.
Number of Cases and Incidence Rates of Coccidioidomycosis for the Civilian and Prison Populations in Kings County, California. Data from the Kings County Health Department.
Seasonal Trends
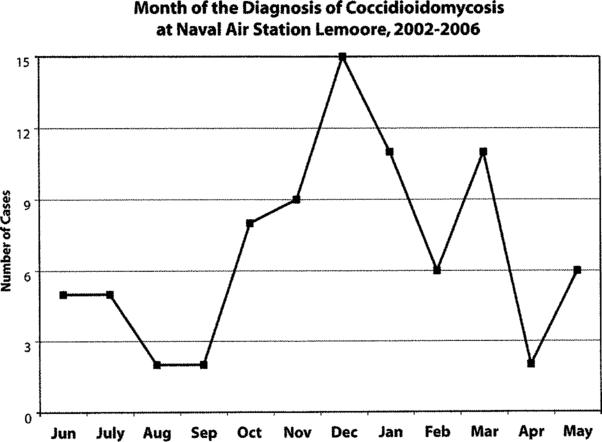
Coccidioidal cases were most commonly diagnosed during the late fall and winter months at Lemoore, with the highest number during the months of December and January. Seasonal trends are shown in Figure 4.
Figure 4.
Month of the Diagnosis of Coccidioidomycosis at the Naval Air Station Lemoore, 2002−2006.
Clinical Manifestations
Of the 82 cases of coccidioidomycosis, 80 (98%) had complete medical records; in two cases, the patient was referred to an outside medical facility, and information was not available. Of patients with complete information, sixty-six (82.5%) patients with coccidioidomycosis had pulmonary disease alone, and 14 (17.5%) had disseminated disease. At the time of presentation, 11 (13.4%) of the pulmonary cases had no evidence of dissemination and normal chest radiographs, but had a history of a respiratory infection. Of those with pulmonary disease with an infiltrate (n=55) on chest radiograph at presentation, 47/55 (85.5%) had single lobe involvement. Two-thirds of patients had right lung involvement. The lower segments were involved in 55% of cases, the upper lobes in 33%, and the middle lobe in 13%. Adenopathy was detected in seven chest radiographs; an effusion was found in two patients, both of whom were diagnosed with an empyema. Erythema nodosum accompanied two (3%) of the cases.
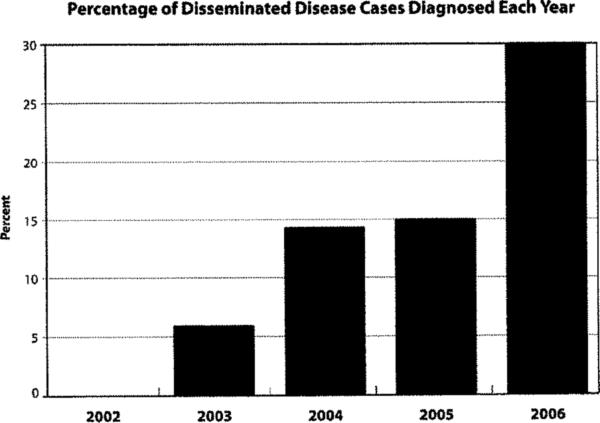
Of the 14 cases of disseminated disease, five (36%) had multiple areas of extrapulmonary involvement. Dissemination involved the skin (n=10), central nervous system (n=5), bone (n=5), and pericardium (n=1). Not only did the number of coccidioidomycosis cases increase over the study period, but the percentage of cases with dissemination (Chi-Square test 3.9, p=0.047) also increased. In 2002, no cases of disseminated disease were reported; in 2006, eight (30%) of the 27 were diagnosed with dissemination (Figure 5).
Figure 5.
Number and Percentage of Disseminated Disease Cases Diagnosed Each Year.
Demographic and Risk Factor Assessments
Of those with coccidioidomycosis, 59 (72.0%) were active duty members, 13 (15.8%) were dependents, and 10 (12.2%) were retirees (two of the retirees were guards at a local county prison). Among all military beneficiaries at the NASL, the median age of the patients with coccidioidomycosis was 28 years (range 9−69 years). Two patients were under the age of 18 years (dependent children) and seven were over the age of 50 years (retirees/dependents). Sixty (73.2%) of the patients were male; male gender was associated with a 3.1-fold higher rate of infection compared to females on the base (p<0.001). The race distribution of cases was white/non-Hispanic in 42.4%, white/Hispanic in 18.6%, Filipino in 18.6%, African American in 11.9%, and Asian in 8.5%; among those with race recorded in the medical profile; 22 of the patients did not have a race/ethnicity listed in their record.
Most (72%) of the coccidioidomycosis cases occurred among the active duty population. Among the 59 active duty members with coccidioidomycosis, the median age of active duty members was 26 years, and 49 (83.1%) were male. Among active duty members, males and females had similar risk for infection (OR 0.84, p=0.24). Race included: 18 (30.5%) Caucasians, 11 (18.6%) Hispanic, six (10.2%) African American, five (8.5%) Asian, five (8.5%) Filipino. In 14 (23.7%) cases, race was not recorded in the medical notes or registration information (Table 1).
Table 1.
Incidence Rates of Pulmonary and Disseminated Coccidioidomycosis Based on Race among Active Duty Personnel, Lemoore, Naval Air Station, Lemoore, California, 2002−2006.
| Race | AD Population at NAS No. | Pulmonary Disease No. cases | Rate of Pulmonary Disease per 100,000 PY | Disseminated Disease No. cases | Rate of Disseminated Disease per 100,000 PY | RR (p-value) of Pulmonary Disease | RR (p-value) of Disseminated Disease |
|---|---|---|---|---|---|---|---|
| All | 6,694 | 44 | 131.5 | 14 | 41.8 | ||
| White/non-Hispanic | 3,769 | 12 | 63.7 | 6 | 31.8 | Referent | Referent |
| African American | 740 | 3 | 81.1 | 2 | 54.1 | 1.27 (0.73) | 1.70 (0.62) |
| Hispanic | 1,219 | 10 | 164.1 | 1 | 16.4 | 2.59 (0.02)* | 0.51 (1.00) |
| Filipino | 340 | 2 | 117.6 | 2 | 117.6 | 1.85 (0.32) | 3.71 (0.14) |
| Asian | 253 | 3 | 237.1 | 2 | 158.1 | 3.76 (0.06) | 5.00 (0.09) |
PY, person-years; RR, relative risk.
Statistically significant with a p-value<0.05.
The incidence rates and relative risks of developing a pulmonary or disseminated coccidioidal infection by race are shown in Table 1. Among active duty members, the highest incidence rates for pulmonary disease occurred among Asians (237 cases per 100,000 PYs) and Hispanics (164 cases per 100,000 PYs). The relative risk for pulmonary disease was 2.6 for Hispanics (p=0.02) and 3.8 for Asians (p=0.06) compared to white/non-Hispanic military personnel. For each race, the number of uncomplicated pulmonary infections was more common than disseminated cases, except in Filipinos, in which the incidence was the same; these data are limited by the small sample size (Table 1).
Of those with disseminated disease (n=14), the median age was 24 years (mean 27.9, range 19−59); all cases occurred among active duty members. The age or sex of patients with disseminated versus pulmonary disease was not significantly different. The race among disseminated cases was white/non-Hispanic, six (42.9%); African American, two (14.3%); Filipino, two (14.3%); Asian, two (14.3%); Hispanic, one (7.1%); and unknown 1 (7.1%). Asians, Filipinos, and African Americans (in descending order) had the highest incidence rates of disseminated disease after accounting for the race of the personnel at the base; of note, Caucasians had a substantial rate of disseminated disease, with an incidence of 31.8 cases per 100,000 PYs. The risk of disseminated disease was not significantly predicted by race, perhaps due to the high rate among the comparator group of white/non-Hispanics.
Coccidioides spp. infections occurred in a relatively healthy population with only two patients with diabetes mellitus, one with cancer (multiple myeloma), and one with HIV infection with robust CD4 counts. Two patients were pregnant and/or postpartum during the time of diagnosis. Of this group of patients with comorbidities, only two developed disseminated disease (a diabetic male and a postpartum female). The other diabetic patient developed severe primary pulmonary disease requiring a prolonged ICU stay and partial lung resection due to an empyema. The others had uncomplicated pulmonary disease.
Laboratory Findings
Laboratory data showed complement fixation titers ranging from 0 to 1:256. Those with pulmonary disease had a median titer of 1:8 (range 0−1:64); 16% (n=7) had a titer of >1:16 but no clinical evidence of dissemination. Patients with proven disseminated disease had a median titer of 1:32 (range 1:4 to 1:256). Interestingly, 50% had a titer of ≤1:16; these cases usually involved the skin, however, one patient had pericardial involvement, and one had meningitis.
Overall, the median white blood count was 10,200 cells/mm3 (range 3,800−25,000); 46% had an elevated white blood count at the time of diagnosis. The erythrocyte sedimentation rate (ESR) was a median of 64/hour (range 1−122). The ESR was >20 in 81% of patients, >50 in 62%, and >100 in 16%. The eosinophil percentage ranged from 0 to 34% (median 4%), with 41% of patients having ≥6%. The absolute eosinophil count was as high at 6,630/mm3, and 40% had a count >500/mm3. Comparing patients with pulmonary and disseminated disease, no statistically significant differences were found in the white blood count, absolute eosinophil count, or ESR values. However, the CF titer was higher among disseminated than pulmonary cases (p=0.002).
Outcome Data
Sixteen (20.0%) of the 80 cases with complete data were hospitalized, including seven (50%) with disseminated disease and nine (14%) with pulmonary involvement. One active duty member underwent a pericardial window for coccidioidal pericarditis, but was able to remain on active duty service. Two patients required surgical partial resection of their lungs due to empyemas unresponsive to antifungal therapy alone. There were no deaths, but two persons had significant morbidity from the disease requiring medical retirement from the military.
Correlations and Multivariate Analyses
Variables which were correlated included disseminated disease with a more recent year of diagnosis, higher CF titers, and hospitalization. Other significant correlations included increasing age with ESR; male sex with active duty status; elevated ESR with both a high CF titer and hospitalization. In the multivariate analysis, a high CF titer remained predictive for dissemination (OR 1.04, p=0.003); a trend towards more recent year of coccidioidomycosis diagnosis and having disseminated disease (OR 2.39, p=0.06) was identified.
Discussion
This is the first surveillance study in several decades to describe clinical diagnoses of coccidioidomycosis among a defined military population residing at a Naval base. Our evaluation demonstrated that the incidence of coccidioidomycosis has significantly increased among military beneficiaries during the years 2002 to 2006. These trends are consistent with studies showing rising rates in the general population of King's County and other locations in California and Arizona [13, 14, 24, 25].
The overall incidence rate of clinical infections in our study was 96 cases per 100,000 person-years. This rate is higher than prior rates reported at Lemoore during the 1970s (approximately 10 cases/100,000 PYs) [17]. However, our rate is not considerably different from recent rates in the general U.S. population. Previously reported data using a similar diagnostic strategy demonstrated a rate of 86 cases per 100,000 PYs among the general population in Kern County; these data were from the late 1990s [12]. Higher incidence rates have been reported recently, including rates of 3,000 cases per 100,000 PYs among prison inmates in California during 2005 [25].
Among our study cohort, a greater proportion of active duty members was affected in comparison to the non-active duty (i.e., dependents and retirees). This may be due to greater soil exposures during work-related activities or training exercises. Previous studies have shown that exposure to dust is a risk factor for disease; professions at higher risk of infection include archeologists, as well as construction and agricultural workers [2]. Military personnel may experience dust exposure as part of military training exercises or other work-related activities; alternatively, the young military cohort may engage in recreational activities associated with dust exposure. This study did not quantify the risk of disease among specific military activities. Another reason that active duty members may have had more infections than retirees is that persons who recently moved into an endemic area are known to be at higher risk due to lack of protective immunity [10]; active duty personnel typically change duty stations every few years and may be at higher risk than retirees who have resided at the base for a longer duration of time.
As expected, most patients presented with respiratory symptoms and pulmonary involvement, as coccidioidomycosis is transmitted by inhalation of spores. However, the large proportion of cases that were disseminated disease is of interest. Traditionally, only 1% of cases diagnosed are disseminated, but in our cohort, 17.5% (14/80) had extrapulmonary involvement; in 2006, the figure was 30%. This is likely an indicator that only the “tip of the iceberg” of cases were clinically recognized and that the number of actual cases may have been several-fold higher than reported in this article. For instance, CE Smith estimated that 0.25% of all cases are disseminated among whites, and 2.5% are disseminated among blacks [6]; using these estimates, the rate of infections in this cohort would have been approximately 8,000 cases/100,000 person-years. Another possible explanation for the high proportion of disseminated cases is that the fungus, C. immitis, has increased in virulence and that more infections are resulting in disseminated forms of the disease. Although a higher percentage of dissemination can be seen among immunosuppressed hosts and the elderly [10, 12], our population was comprised of mainly young, healthy individuals who were immunocompetent. Finally, both clinically recognized cases and dissemination may be heightened among groups with intense, high–innoculum exposures [17, 19]. Further evaluation is warranted to better understand the epidemiology of coccidioidal infections in this population. One such strategy would be the use of a coccidioidal skin test preparation to more accurately identify infections which may not be clinically recognized. Such evaluations were performed in the 1970s at a different military base in California, Twenty-Nine Palms, and it was noted that among Marines permanently stationed at the base, 25.4% converted their skin test to positive over a six- to eight-month period [26]. This conversion rate was likely related to the fact that this group was in the tank battalion, which has a high likelihood of dust inhalation. More recent investigations, using ELISA testing [27], as well as unpublished data using skin tests, have shown much lower rates of infections among military personnel.
We observed a seasonal pattern, whereby most cases were identified during winter months. This is consistent with previous reports demonstrating that the highest number of exposures to the airborne arthroconidia occurs during the fall months, corresponding to dry, windy weather conditions [6, 25, 28]. A recent survey suggested that the peak exposure to fungal spores occurs in October-November [24]. Given the incubation period of one to three weeks and a possible delay in the clinical diagnosis of cases, the seasonal trends we noted appear to be consistent with these data. A second exposure peak, as recognized by Comrie, also occurs in the months of June-July (Figure 4) [24].
In our study, a higher percentage of male military beneficiaries were diagnosed with coccidioidomycosis. This trend remained after accounting for the number of males at the base. Other studies have also noted a higher rate of coccidioidomycosis among men, potentially due to occupational or recreational activity differences between the sexes. Of interest, the male-gender association with coccidioidal infections was mitigated when examining only those on active duty; this may have been due to the fact that the active duty females had similar work-related exposures than men, which placed them at equal risk. Further studies examining gender-specific work and recreational activities and their corresponding risk for infection would be useful.
Non-Caucasian ethnic groups, particularly Filipinos and African Americans, are thought to be more likely to develop disseminated disease [6, 22, 29]. White patients appear to be at the lowest risk of dissemination; in the current study, 43% of patients with dissemination were white. This is partially explained by the large number of Caucasians at the base. However, we were surprised at the high incident rates among this group. A similar experience was noted by Williams et al. in association with a dust storm at Lemoore NAS in the 1970s [17]. The increasing number of mixed racial backgrounds may make race associations difficult; for instance, although one patient reported being “white”, she had a significant Native American Indian ancestry. The phenotypic presentation of a patient does not fully represent the genetic factors that may predispose a patient for further complications. The ethnicity in this study most associated with dissemination was Asian; however, our small numbers limit any conclusions from these data.
Despite the fact that mostly young healthy persons were affected by this infection, 20% of the patients required hospitalization; this may incur significant medical costs estimated to be $23,000 to $34,000 per patient [11, 30]. Even among those not hospitalized, missed workdays and outpatient medical care results are important expenses. Serious disease requiring surgical procedures and/or military medical discharges occurred in a minority of our cases, perhaps due to the aggressive medical management provided in the cases at a nearby tertiary Naval hospital.
Prevention is paramount among patients at-risk for coccidioidomycosis. Methods to reduce dust formation are important considerations. Persons who cannot avoid dusty conditions should wear a facemask. Outdoor physical training sites should be modified for dust reduction, and aquatic exercise programs should be utilized when dust levels are high. Although prophylaxis using an azole is being studied to prevent clinical disease among high-risk patients (transplant recipients and AIDS patients) [9], the efficacy and cost-effectiveness of such a strategy is unclear, especially among large groups such as the military. A vaccine against Coccidioides spp. would be the best preventive strategy, but currently is not available.
Our study had some limitations. Cases included in our investigation were only those definitively diagnosed as coccidioidomycosis, which may have underestimated the true number of infections. Furthermore, some patients may have transferred to another base before the diagnosis of coccidioidomycosis was made. On the other hand, some military personnel are present on the base for short training exercises and are not accounted for in the total population of the base; however, to our knowledge, the cases in this report were among persons permanently stationed or residing at the base. Another limitation was the lack of complete ethnicity data; however, the authors are not aware of any specific biases regarding military personnel reporting ethnicity. Furthermore, in order to more accurately assess the racial impact on dissemination, a larger number of cases is needed. Finally, although we report seasonal trends in the diagnosis of coccidioidomycosis, many patients were treated with several courses of antibiotics prior to evaluation for coccidioidomycosis, which may have delayed the diagnosis. In one case, a patient was diagnosed with “pneumonia” while on a ship for six months; he was eventually found to have coccidioidomycosis after returning to the base.
In summary, there has been an increase in the number and severity of coccidioidomycosis cases among our military personnel. Further epidemiologic research studies on the driving forces of these trends are needed. In the meantime, clinicians should maintain a high index of suspicion for coccidioidomycosis, and workplaces in endemic areas should implement both educational programs and prevention strategies for this disease.
ACKNOWLEDGEMENT
The authors would like to thank Judy Christensen, Graphic Artist, for her work in the preparation of this manuscript.
Financial support: None.
Footnotes
The data in this article were presented in part at the Sixth International Symposium on Coccidioidomycosis August 23−27, 2006, Stanford University, Stanford, CA.
The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
Conflict of interest: None.
REFERENCES
- 1.Galgiani JN. Coccidioidomycosis: A regional disease of national importance: Rethinking approaches for control. Ann Intern Med. 1999;130:293–300. doi: 10.7326/0003-4819-130-4-199902160-00015. [DOI] [PubMed] [Google Scholar]
- 2.Kirkland TN, Fierer J. Coccidioidomycosis: a reemerging infectious disease. Emerging Infect Dis. 1996;2:192–9. doi: 10.3201/eid0203.960305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson RH, Einstein HE. Coccidioidal meningitis. Clin Infect Dis. 2006;42:103–7. doi: 10.1086/497596. [DOI] [PubMed] [Google Scholar]
- 4.Posada A. Uno Nuevo caso de micosis fungoidea con psorospermias. Ann Circulo Medico Argentino. 1892;15:585–96. [Google Scholar]
- 5.Rutherford GW, Barrett MF. Epidemiology and control of coccidioidomycosis in California.. West J Med. 1996;165:221–2. [PMC free article] [PubMed] [Google Scholar]
- 6.Smith CE. Coccidioidomycosis. In: Coates John B., Jr., editor. Preventive Medicine in World War II. IV. 1958. pp. 285–316. [Google Scholar]
- 7.Chiller TM, Galgiani JN, Stevens DA. Coccidioidomycosis. Infect Dis Clin North Am. 2003;17:41–57. doi: 10.1016/s0891-5520(02)00040-5. [DOI] [PubMed] [Google Scholar]
- 8.Galgiani JN, Ampel NM, Catanzaro A, Johnson RH, Stevens DA, Williams PL. Practice guidelines for the treatment of coccidioidomycosis. Clin Infect Dis. 2000;30:658–61. doi: 10.1086/313747. [DOI] [PubMed] [Google Scholar]
- 9.Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Johnson RH, Stevens DA, Williams PL. Infectious Diseases Society of America. Coccidioidomycosis. Clin Infect Dis. 2005;41:1217–23. doi: 10.1086/496991. [DOI] [PubMed] [Google Scholar]
- 10.Leake JAD, Mosley DG, England B, et al. Risk factors for acute symptomatic coccidioidomycosis among elderly persons in Arizona, 1996−1997. J Infect Dis. 2000;181:1435–40. doi: 10.1086/315400. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Coccidioidomycosis-Arizona, 1990−1995. MMWR Morb Mortal Wkly Rep. 1996;49:1069–73. [PubMed] [Google Scholar]
- 12.Rosenstein NE, Emery KW, Werner BS, Kao A, Johnson R, Rogers D, et al. Risk factors for severe pulmonary and disseminated coccidioidomycosis: Kern County, California, 1995−1996. Clin Infect Dis. 2001;32:708–15. doi: 10.1086/319203. [DOI] [PubMed] [Google Scholar]
- 13.Ampel NM, Mosely DM, England B, Vertz PD, Komatsu K, Hajjeh RA. Coccidioidomycosis in Arizona: increase in incidence from 1990 to 1995. Clin Infect Dis. 1998;27:1528–30. doi: 10.1086/515044. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention Increase in coccidioidomycosis-Arizona, 1998−2001. MMWR Morb Mortal Wkly Rep. 2003;52:109–12. [PubMed] [Google Scholar]
- 15.Valdivia L, Nix D, Wright M, et al. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg Infect Dis. 2006;12:958–62. doi: 10.3201/eid1206.060028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olivere JW, Meier PA, Fraser SL, Morrison WB, Parsons TW, Drehner DM. Coccidioidomycosis--the airborne assault continues: an unusual presentation with a review of the history, epidemiology, and military relevance. Aviat Space Environ Med. 1999;70:790–6. [PubMed] [Google Scholar]
- 17.Williams PL, Sable DL, Mendez P, Smyth LT. Symptomatic coccidioidomycosis following a severe natural dust storm at the Naval Air Station, Lemoore, Calif. Chest. 1979;76:566–70. doi: 10.1378/chest.76.5.566. [DOI] [PubMed] [Google Scholar]
- 18.Standaert SM, Schaffner W, Galgiani JN, Pinner RW, Kaufman L, Durry E, Hutcheson RH. Coccidioidomycosis among visitors to a Coccidioides immitis-endemic area: an outbreak in a military reserve unit. J Infect Dis. 1995;171:1672–5. doi: 10.1093/infdis/171.6.1672. [DOI] [PubMed] [Google Scholar]
- 19.Crum N, Lamb C, Utz G, Amundson D, Wallace M. Coccidioidomycosis outbreak among United States Navy SEALs training in a Coccidioides immitis-endemic area-Coalinga, California. J Infect Dis. 2002 Sep 15;186(6):865–8. doi: 10.1086/342409. Epub 2002 Aug 16. [DOI] [PubMed] [Google Scholar]
- 20.Rush WL, Dooley DP, Blatt SP, Drehner DM. Coccidioidomycosis: a persistent threat to deployed populations. Aviat Space Environ Med. 1993;64:653–7. [PubMed] [Google Scholar]
- 21.Olson PE, Bone WD, LaBarre RC, Martin CR, Utz GC, Miller LK, Gresham L. Coccidioidomycosis in California: regional outbreak, global diagnostic challenge. Mil Med. 1995;160:304–8. [PubMed] [Google Scholar]
- 22.Crum NF, Lederman ER, Stafford CM, Parrish JS, Wallace MR. Coccidioidomycosis: a descriptive survey of a reemerging disease. Clinical characteristics and current controversies. Medicine (Baltimore) 2004;83:149–75. doi: 10.1097/01.md.0000126762.91040.fd. [DOI] [PubMed] [Google Scholar]
- 23.Crum NF, Lederman ER, Hale BR, Lim ML, Wallace MR. A cluster of disseminated coccidioidomycosis cases at a US military hospital. Mil Med. Jun. 2003;168(6):460–4. [PubMed] [Google Scholar]
- 24.Comrie AC. Climate factors influencing coccidioidomycosis seasonality and outbreaks. Environ Health Perspect. 2005;113(6):688–92. doi: 10.1289/ehp.7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pappagianis D. Coccidioidomycosis in California State Correctional Institutions. Ann NY Acad Sci. 2007 doi: 10.1196/annals.1406.011. in press. Epub 2007 Mar 1. [DOI] [PubMed] [Google Scholar]
- 26.Hooper R, Poppell G, Curley R, Husted S, Schillaci R. Coccidioidomycosis among military personnel in Southern California. Mil Med. 1980;145:620–3. [PubMed] [Google Scholar]
- 27.Crum NF, Potter M, Pappagianis D. Seroincidence of Coccidioidomycosis during military desert training exercises. J Clin Microbiol. 2004;42:4552–5. doi: 10.1128/JCM.42.10.4552-4555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drutz DJ, Catanzaro A. Coccidioidomycosis. Part I. Am Rev Respir Dis. 1978;117:559–85. doi: 10.1164/arrd.1978.117.3.559. [DOI] [PubMed] [Google Scholar]
- 29.Pappagianis D, Lindsay S, Beall S, Williams P. Ethnic background and the clinical course of coccidioidomycosis. Am Rev Respir Dis. 1979;120:959–61. doi: 10.1164/arrd.1979.120.4.959. [DOI] [PubMed] [Google Scholar]
- 30.Park BJ, Sigel K, Vaz V, et al. An epidemic of coccidioidomycosis in Arizona associated with climatic changes 1998−2001. J Infect Dis. 2005;191:1981–7. doi: 10.1086/430092. [DOI] [PubMed] [Google Scholar]