Abstract
Context: One underappreciated consequence of modern clinical and public health practices is that the experience of being at risk for disease has been converging with the experience of disease itself. This is especially true for certain chronic diseases, in which early diagnosis and aggressive treatment have led to symptom-less and sign-less disease and in which treatments have largely been aimed at altering the disease's future course.
Methods: This article reviews the historical scholarship and medical literature pertinent to transformations in the chronic disease and risk experiences.
Findings: The experience of chronic disease increasingly resembles or has become indistinguishable from risk because of (1) new clinical interventions that have directly changed the natural history of disease; (2) increased biological, clinical, and epidemiological knowledge about the risk of chronic disease; (3) the recruitment of larger numbers into chronic disease diagnoses via new screening and diagnostic technology and disease definitions; (4) new ways of conceptualizing efficacy; and (5) intense diagnostic testing and medical interventions.
Conclusions: The converged experience of risk and disease has led to some unsettling and generally underappreciated consequences that might be subjected to more clinical and policy reflection and response: (1) some puzzling trends in medical decision making, such as the steep and uniform increase in the numbers of women across a broad spectrum of risk/disease in breast cancer who have opted for prophylactic mastectomies; (2) a larger and highly mobilized disease/risk population, resulting in an expanded market for interventions and greater clout for disease advocates; (3) shifts in the perceived severity of the disease, with ripple effects on how people experience and understand their illness and risk of disease; and (4) interventions that promise both to reduce the risk of disease and to treat its symptoms.
Keywords: Risk, chronic disease, history of medicine, efficacy, medical decision making, medicalization, prevention, screening
As a second-year medical student twenty-five years ago, I watched an attending physician approach one senior house officer after another asking for the names of hospitalized patients on whom students could practice taking medical histories and performing physical examinations. A suitable patient would have been admitted to the hospital with an as yet undiagnosed collection of symptoms and signs (in addition to being conscious and able to speak English). Each house officer was in charge of thirty to fifty patients but could find only one or two who were suitable.
My medical teachers had evoked in their lectures and at the bedside the sick patient who experienced illness uncorrupted by medical knowledge and prior medical intervention. That few such patients existed in the hospital did little damage to this ideal type, whose existence was sustained by older assumptions about clinical practice and medical education.
In the intervening years, the gap between our idealized view of the sick patient and the actual one who attends our hospitals and clinics has only grown. Hospital and outpatient care is less often about new, undiagnosed, symptomatic problems than it is about existing chronic disease, especially the expectant management of problems predicted or found by other interventions and earlier medical surveillance, for example, the placement of pacemakers in patients previously found to have slow or irregular heartbeats or colonoscopies done after an abnormal fecal occult blood test.
Central to patients' actual presenting complaints has been a new “risky” way of experiencing health and disease. While many observers have noted medicine's increasing focus on disease prevention and risk among the erstwhile healthy, few have recognized the profound parallel developments among the already sick or diagnosed.
Some observers have highlighted the important role of the pharmaceutical industry and medical research in jointly producing both new preventive medicines and the new risk factors that these medicines target (Greene 2007). Others have stressed a new style of medical surveillance in which normal populations have been surveyed and subjected to a growing number of demands to comply with medical directives (Armstrong 1995). Although these accounts focus on the radical expansion of preventive medicine into the otherwise healthy population, much less attention has been paid to parallel developments among the chronically ill. There has been a profound, if largely unnoticed, shift in who is understood to be suffering from chronic disease and the disease experience itself. In many instances, chronic disease has become a kind of risk state in which diagnosis, treatment, and “disease management” are directed at reducing the chances of anticipated, feared developments.
This shift has resulted in a converged experience of risk and chronic disease. On one side of this convergence, the number of otherwise healthy individuals who are considered to be “at risk for,” or have risk factors for, a particular disease has grown immensely; their bodies have been subjected to increased surveillance; and the risk state itself has become more embodied and, in other ways, more disease-like. In large measure, these changes have resulted from the expanding medicalization processes noted by sociologists and others over the past few decades. From the other side, the experience of chronic disease increasingly resembles the experience of people at risk for disease. Because this latter change has received much less attention than have the different ways in which previously healthy people have been recruited into and reshaped by new risk categories, I will focus on it here.
Distinguishing the disease experience from the risk experience is difficult because so many developments are obliterating this difference. In everyday usage, disease is understood to be a pathological process producing ill health, including symptoms. The risk of disease is some statistical probability that ill health might happen. As an imminent state, there can be no illness experience that emanates from risk in any direct, physiological sense (of course, emotional distress and other psychological consequences often follow from knowledge of and beliefs about risk; see Barsky 1988). If readers are imagining exceptions to these definitions, they are probably thinking about the processes I am describing. Screening and early detection of HIV, for example, can, in a very short time, transform someone from feeling healthy to being “positive for HIV,” leading to preventive drug treatments that cause symptoms.
In this article, I first describe the convergence of the experience of risk and chronic disease in modern American medicine and society. Then I analyze some of the reasons why the chronic disease experience has become so risky, followed by a discussion of the consequences for individuals and populations, along with some clinical and policy implications. Most of my observations are from a small but very prevalent set of risks and diseases: breast cancer, asthma, diabetes, and hypertension.
My focus is on medical developments and their effects on the patient's experience of disease. These developments have not occurred in a vacuum, but in order to highlight those influences that are subject to some clinical and policy response, I will not discuss the larger social, economic, and political changes that constitute sociologists' and others' observations that Western societies are increasingly becoming risk societies. Such societies are engaging in the politics of risk distribution as much as resource allocation, are facing dangers of borderless and often invisible global threats (bird flu, terrorism, extensively resistant tuberculosis, global warming), and thus are turning inward—reflexively—to make sense of these dangers and responses (Beck 1992; Giddens 1990).
Some, but by no means all (see, e.g., Conrad 2007), sociologists feel that the medicalization concept—so closely tied to the transformed risk experience—has lost its utility.1 They note that medicalization has strayed far from its original meaning and context: the labeling of deviance as disease in order to expand medical authority (Davis 2006). In contrast, the term medicalization has come to denote actions by many nonphysician actors (patients, disease advocates, bureaucrats, drug companies, etc.) that, through myriad processes, result in the expansion of medical entities and the numbers of individuals recruited into them. While I understand the importance of recovering some conceptual clarity and political bite, social science scholarship and health policy would be poorly served if we tried to set back the clock and narrow the term's scope. It is not simply that the sociologists' concept has expanded in the intervening years. Rather, there has been a transformation in how ill health has been produced, labeled, managed, and ultimately experienced.
What Is the Converged Experience of Chronic Disease and Risk?
Imagine two women, one who is suffering from breast cancer and the second, “merely” at risk for the disease. The first woman is fifty-eight years old. Two years earlier, she detected a lump in her left breast. After an aspiration biopsy revealed cancerous cells, she had a lumpectomy and removal of lymph nodes in her armpit (none of which contained cancer), followed by a course of local radiation and then six months of chemotherapy. After this acute treatment, she was put on a five-year course of the “anti-estrogen” Tamoxifen. She now closely follows developments in breast cancer. At the moment, she is concerned about whether to start another kind of hormonal therapy after her course of Tamoxifen ends and whether she should begin getting screening breast MRIs and/or more frequent mammography. For these and other questions, she frequently searches the web and attends meetings of breast cancer survivor and advocacy groups.
The second woman also is fifty-eight years old. She took birth control pills during her twenties, had her first child at age thirty-four, and, at the urging of her gynecologist, took supplementary estrogen pills starting at age fifty because of menopausal symptoms and to prevent heart disease and osteoporosis. A few years later her doctor told her to stop taking these pills because new medical evidence had conclusively shown that their risks—especially an increased risk of developing breast cancer—outweighed their putative benefits. Since age forty, she has been getting annual mammograms. Four years ago, she had an abnormal mammogram, which led to an aspiration biopsy that did not show cancer. Fearful of developing breast cancer, she is attentive to media reports and periodically browses the Internet for new information on cancer prevention. She has seen direct-to-consumer advertisements for Tamoxifen as a preventive measure for women at high risk of breast cancer. She understands that she has multiple risk factors for breast cancer, such as being middle-aged, being postmenopausal, having had her first child after thirty, having earlier used hormone replacement therapy, and having a history of a benign breast biopsy. She has sought advice from friends, doctors, and breast cancer advocacy groups about whether to take Tamoxifen and/or to find other means of reducing her risk of breast cancer.
At present, the first woman does not experience any symptoms of cancer but nonetheless undergoes intensive surveillance, has concerns about the long-term effects of previous treatments, and faces the future with caution. The experience of the second women is not very different. She may well decide to take Tamoxifen to prevent breast cancer. Like the first woman, she undergoes frequent surveillance and faces the future with caution. Both women face an array of similar choices and seek guidance in similar places. They share fears for the future, feelings of randomness and uncertainty, and pressures for self-surveillance. Both seek ways to regain a sense of control and face difficult decisions about preventive treatment and consumption. They are part of a larger breast cancer continuum, both in how scientists understand breast cancer and as a mobilized group for advocacy, fund-raising, and awareness (Klawiter 2002).
What Has Made Chronic Disease More Risky?
The transition from acute to chronic disease in the twentieth century is both a historical and an epidemiological truism. Yet not as obvious has been the way in which the management of risk has become a dominant feature of the disease experience. How has the chronic disease experience become so risky?
I suggest five reasons: (1) new clinical interventions that have directly changed the natural history of disease; (2) greater biological, clinical, and epidemiological knowledge of chronic disease risk; (3) recruitment of larger numbers into chronic disease diagnoses through new screening and diagnostic technology and disease definitions; (4) new ways of conceptualizing efficacy; and (5) intense diagnostic testing and medical intervention.
Clinical Interventions That Have Directly Changed the Natural History of Disease
The most self-evident way that chronic disease has become more risky has been through the direct effects of new clinical interventions on the natural history of disease. Beginning in the early twentieth century, some diseases were transformed by interventions that removed or alleviated signs and symptoms of acute pathological processes but did not entirely eradicate the underlying disease. Perhaps the most dramatic and earliest example was the transformative role of insulin on the diabetes experience (Feudtner 1996).2 Insulin treatment gave doctors and patients a way to control hyperglycemia. For many children with type 1 diabetes, life spans changed from a few months or years after diagnosis to many decades. At the same time, however, the insulin treatment itself did not simply substitute for the normal operations of the diseased pancreas. It produced its own life-threatening problems, required constant monitoring and decision making, and often became an arena of conflict over control and responsibility among children, parents, and their doctors. Moreover, by allowing diabetics to avoid diabetic coma and live with their disease for decades, insulin therapy uncovered a host of more difficult-to-manage and largely hidden metabolic and other abnormalities that diabetes produces, including damage to the kidney, heart, nerves, and eyes.
The disease experience was similarly transformed for breast cancer at the beginning of the twentieth century (Aronowitz 2007). Surgical innovators like William Halsted promoted radical cancer surgery, and life after radical surgery was often very different from the breast cancer experience of earlier eras. Many women no longer suffered from growing and recurring tumors in their chest. In the absence of detectable “external” disease and after punishing treatment with often severe side effects, they often believed that their suffering bought them a greater chance of survival. But the radical surgery did not appreciably change patients' ultimate prognosis. Halsted understood this incomplete and frustrating reality, reluctantly acknowledging that radical surgery had little effect on the late, metastatic stage of cancer's natural history, which was responsible for its deadliness. Indeed, Halsted generally avoided discussing with his patients the possibility of future metastatic disease until he was forced to when the cancer recurred. Even though he encouraged his breast cancer patients to get on with their lives after radical surgery and keep a stiff upper lip, they did not generally follow this advice. Postmastectomy patients were understandably fearful that their cancer would return and sought reassurance and examinations by physicians, surveyed their bodies, and wondered about steps they could take to prevent future disease. Many women believed their cancer surgery had been effective because their attentiveness to changes in their bodies had led them to seek medical attention in the nick of time. After surgery, many understandably surveyed their own bodies systematically and wanted frequent checkups.
During this same period, new medical interventions such as radiation and radium therapy were developed to deal with recurring cancer. Although these treatments were unlikely to cure the cancer, their high scientific status and powerful effects led many patients to believe in their efficacy and strengthened their vigilance to catch cancer recurrences in time for these treatments to succeed. In sum, many of these early twentieth-century patients after surgery lived a “life at risk,” one filled with fear, close surveillance of their bodies, and increased demand for medical examinations. They hungered for some means to reassert control over their fears that their cancer would return. At the end of their life, patients with recurrences also experienced a highly medicalized last stage of illness.
The breast cancer experience, like the experience of many diabetes patients, had been transformed. Unlike diabetes but perhaps more like the majority of other twentieth-century diseases subject to new medical treatments, this transformation was due to many indirect and collateral effects of new treatments as well as to the direct effects on the natural history of disease.
Increased Biological, Clinical, and Epidemiological Knowledge of Chronic Disease Risk
A key driver of the risky chronic disease experience has been the explosion in knowledge, such as new details and models of the natural history of disease, associations among laboratory, radiological, and other test findings and disease etiology and prognosis, new frameworks for understanding disease, and molecular and other insights into disease mechanisms. As a result of clinical and epidemiological study, for example, we have learned that patients with inflammatory bowel disease have an increased risk of colon cancer. Knowledge of this increased risk has led to efforts at the secondary prevention of cancer (secondary prevention means the early detection of disease or other efforts to ward off the harmful effects of disease progression, and primary prevention implies efforts to avoid the disease in the first place). Almost all inflammatory bowel disease patients are urged to get annual colonoscopies and sometimes to get prophylactic surgery.
Clinical and epidemiological study and intense diagnostic testing (discussed later) have combined to create a web of knowledge in which the variation in any number of laboratory tests and physiological parameters is associated with a higher risk of some untoward outcome. Increasingly, having one disease puts you at risk for another. For example, routine diagnostic blood testing may uncover a high serum protein level, leading to serum electrophoresis, and ultimately a diagnosis of a monoclonal gammopathy, which is an abnormal pattern of antibody production that does not itself produce symptoms and occurs in 2 to 4 percent of adults over fifty. Through epidemiological, clinical, and laboratory study, we have come to understand this condition as part of a continuum of abnormalities that include the highly malignant multiple myeloma. An individual found to have a monoclonal gammopathy understandably enters into a world of concern about the future and close surveillance so that interventions might be deployed early. Each year, entirely new conditions are created based on observations of potential clinical outcomes following abnormal tests performed in the course of routine medical care and screening, for example, the antiphospholipid antibody syndrome.
A greater knowledge of the risks associated with existing or new diagnoses understandably leads to difficult choices and uncertainties. After being tested for abnormal incidental laboratory tests or donating blood, millions of Americans have learned that they have silent hepatitis C infection (National Digestive Diseases Information Clearing House 2006). Clinical and epidemiological studies have revealed that hepatitis C infection is likely to remain asymptomatic but that some of those infected will later develop serious and possibly fatal chronic liver disease. There have been many attempts to reach a consensus on how to deal with this uncertainty. Should the general population be screened? Among those with serological evidence of infection, who should have more advanced tests and procedures (including liver biopsy)? Who should be treated with costly and dangerous treatments such as interferon and ribavirin?
We are only at the beginning of the road to new and redefined diseases leading from the exponential rise in correlations between variations in the human genome and various states of health and disease and the likely profound impact on the risk experience (Novas and Rose 2000). The result will likely be more people who are aware for longer periods of time about possible future ill health and who will be advised to modify their lifestyle and undergo different types of surveillance and medical prevention.
The Recruitment of Larger Numbers into Chronic Disease Diagnoses via New Screening and Diagnostic Technology and Disease Definitions
Larger numbers of people are experiencing risky chronic disease because of the creation and diffusion of increasingly sensitive screening technologies, “earlier” definitions of pathological states, and lowered thresholds for clinical diagnosis. In some diseases, such as breast cancer, “early” diagnosis, often picked up by more sensitive screening tests (such as the recent use of MRIs to screen for breast cancer), has grown almost independently of any change in the numbers of women with recurrent and often fatal disease. In such cases, I would argue that the emergence of the risky chronic disease experience is, in aggregate, an addition to rather than an exchange of one characteristic disease experience for another, such as occurred in type 1 diabetes mellitus in the early twentieth century.
More people experience risky chronic disease when they are recruited into new disease categories that are constructed as “earlier” and less prototypical presentations of existing diseases. Peter Kramer (1993), for example, has argued for such formes frustes psychiatric diagnoses, like low self-esteem, chronic minor depression, social inhibition, and anhedonia. Much of this diagnosis creep is driven by pharmaceutical companies that want to expand markets for their products in league with physicians and other moral entrepreneurs who champion the expanded disease categories.
Similarly, many examples of new predisease states constitute boundary processes on the border of medicalizing the previously healthy and extending the diagnosis of preexisting disease to “earlier” points in a disease's natural history. Defined by pathologists but sustained by screening campaigns, different cervical and breast precancers have been discovered and widely diagnosed throughout the latter half of the twentieth century. Breast cancer has had an exponential growth of precancers, largely driven by the widespread use of screening mammography. The incidence of lobular and ductal carcinoma in situ, for example, jumped from a rate of 11.3 per 100,000 women per year in 1975 to an astounding 91.2 per 100,000 women in 2002 (Ries et al. 2005).
Each year, more than 3 million American women are found to have a Pap smear abnormality, typically ASCUS, “atypical squamous cells of uncertain significance.” ASCUS is part of a continuum of precancerous abnormalities that have constantly been redefined and reclassified over the past decades. As a result, women with this very common abnormality enter a continuum of risk, which often requires more frequent surveillance and more invasive diagnostic procedures.
Prehypertension and prediabetes are, in some ways, simpler phenomena. They are defined by lower thresholds along the same continuous axis—blood pressure and serum glucose, respectively—that have been used to define the fully formed disease states. Hypertension, I should note, is itself an asymptomatic risk factor for heart disease and stroke. But its long history, objective definition, and medical treatment have given it a borderline disease status in common medical usage and practice.
The experience of people diagnosed with the different precancers, prehypertension, and prediabetes can be very similar to that of people with cancer, hypertension, and diabetes. In hypertension and diabetes mellitus, the medical treatment for both the disease and the predisease diagnosis and treatment can be almost identical, as is the risky meaning of the diagnosis for recruited individuals. In most cases, members of the original disease, like the new predisease group, do not suffer symptoms referable to the disease. It also should not be surprising that the resulting larger group of people attached to the diabetes or hypertension class of diseases coalesce into one large market for new preventive drugs and other interventions (Rosenthal 2006).
New Ways of Conceptualizing Efficacy
The emergence of risky chronic disease is predicated on highly subjective ways of evaluating efficacy. For example, individual compliance with screening and diagnostic tests, a prerequisite for recruiting individuals into risk states, depends on individual judgments that these tests “work.” Throughout the twentieth century, men and women wrestled with different disease prevention messages, such as the one to examine the body for dangerous signs of cancer and to seek medical treatment without delay if something suspicious is found. Later in the century, similar individual decisions about efficacy had to be made about cancer screening programs like Pap smears and mammography. In both these cases, efficacy was often understood in highly individual and psychological terms. These early detection and screening programs largely “worked” by giving individuals a way to assert some control over their fears of cancer (Aronowitz 2001).
Such social efficacy calculations also help explain data suggesting that many Americans today who have experienced false positive cancer screening tests generally do not feel harmed or become skeptical of the screening enterprise. Instead, they typically feel more invested in the screening paradigm. One interpretation is that being given a cancer diagnosis and then having it taken away is experienced as a victory over cancer, leading to a greater sense of control over cancer and fears of cancer (Schwartz et al. 2004).
Risk-reducing pharmaceuticals similarly promise to eliminate or control the fears, discomfort, and hassles associated with risk. This rationale is not often trumpeted and, if made explicit, is combined with more objective claims of efficacy against disease.
Whether asserting control over fear or for other purposes, we have become accustomed to accepting the efficacy of many interventions as reducing the probability of this or that bad outcome. In the last years of his life, my father suffered from memory loss, confusion, and disorientation. Fearful that he would hurt himself and others, we sent him to a clinic that specialized in evaluating mental decline among the elderly. After a series of neuropsychological and radiological evaluations, he was diagnosed with Alzheimer's disease, told to stop driving, and prescribed Aricept. Obtaining a diagnosis and a prescription is the expected outcome of a medical encounter, but it obscures the historically new prominence of the risk calculus lying behind so many decisions in chronic disease. Like so many other drugs for chronic disease aimed at positively influencing the natural history of the disease, evidence from clinical trials of Aricept showed some statistically significant advantage in symptoms for people taking the drug. No one's Alzheimer's disease got better; rather, the rate of decline was, on average, less steep for people taking the medicine than for those taking a placebo. The individual taking Aricept would never experience efficacy in the way that a person taking a pain reliever, a curative cancer treatment, or an antibiotic that led to symptom improvement would. Instead, efficacy is a promise of a positive deviation from a projected downhill trajectory. This kind of efficacy calculus has become commonplace and has eased the acceptance of many risk interventions and the risk states they target.
Riskiness Induced by Intense Diagnostic Testing and Treatment
Finally, the chronic disease experience is risky because of the intensity of modern medical intervention, both diagnostic and therapeutic. Increased diagnostic testing in the course of caring for patients has led to an ever-expanding web of putative associations between different objective markers of clinical variation (blood tests, X-rays, etc.) and the probabilities of different diseases. Residents and interns in American hospitals walk around in white coats weighted down with manuals and crib sheets that contain lists of possible outcomes of every conceivable abnormal blood test, X-ray, EKG pattern, urine test, and the like. This intense testing contributes to the web of knowledge discussed earlier and also is the entry point for the career of the patient with the newly discovered abnormality (adapted from Goffman 1961).
The parallel role of therapeutic interventions is illustrated by the creation of an entirely new major category of disease experience, that of cancer survivorship. Cancer has long been understood and experienced as an encounter with increasing dangers to health, culminating in pain, wasting, and death. As such, it has been both greatly feared and subject to medical and popular routines aimed at evading this or that outcome. In these respects, it is the ultimate risky disease, putting patient and doctor in real and potential conflict with an enfolding, devastating narrative. Over the past decades, with a generation of cancer survivors outliving the immediate threat of the disease, knowledge of the risks throughout one's lifetime—emanating from both the natural history of cancer and different modalities of treatment—has exploded. The dimensions of this transformation are huge, in part because the numbers of people who constitute the class of cancer survivors is growing. According to government estimates, the number of “cancer survivors” has risen from slightly more than 2 million in 1971 to more than 10 million in 2005 (Ries et al. 2008; for an interesting discussion of what he characterizes as a “remission society,” see also Frank 1995).
In one sense, cancer survivorship is the kind of direct and successful consequence of intervening in the natural history of disease discussed earlier. But in many other ways, cancer survivorship has been dominated by the possible long-term consequences of treatment as much as, or more than, by the transformed disease itself.
It is not surprising, therefore, that one of the twenty-eight general disease management categories listed in a highly regarded contemporary medical text is cancer survivorship (Rakel and Bope 2007). For cancer survivors, disease management includes surveillance for twenty-four different late effects known to be associated with nine common classes of chemotherapy. For example, patients who have received anthracycline antibiotics (a common chemotherapy class that includes drugs such as doxorubicin, given to breast cancer patients) in the past should be evaluated yearly for their risk of developing cardiac toxicity, along with a careful heart-oriented history and physical as well as a test of heart muscle function (echocardiograms or nuclear medicine tests of pump function) and an EKG.
Similar screening for serious late effects follows from knowledge that chemotherapy and radiation can cause endocrine dysfunction (e.g., adrenal insufficiency from cranial radiation), neurological disorders (e.g., neuropathy), lung disease, kidney disease, and hearing problems. Perhaps most feared are new cancers caused by earlier cancer treatment. In addition to these mechanisms, transfusions often given during treatment may lead to chronic infectious disease (e.g., hepatitis C), and steroids may lead to cataracts and other sequelae. Survival itself puts people at risk of delayed and/or chronic psychiatric disorders, especially depression. Recommendations to screen and watch for all these late complications create a formidable challenge to the care and peace of mind of cancer survivors—all in addition to fears of and actions to be taken to survey the body for and ultimately prevent or treat early any recurrence of the original cancer.
For many people who experience other highly intervened-in chronic diseases that are not as deadly as untreated cancer but that require constant treatment, the risks associated with medications mean worry, screening, and lifestyle modification. Today, many patients with severe rheumatoid arthritis take drugs like Plaquenil, Methotrexate, and Enbrel to influence positively the natural history of disease. Plaquenil is an antimalarial drug that is believed to have a modifying effect on the disease. Unfortunately, there is a much-feared rare side effect of retinal damage. Out of concern for this complication, patients are told to get baseline and routine examinations by an ophthalmologist. Methotrexate is a folate antagonist often used as a chemotherapeutic agent against cancer. It also is believed to have a disease-modifying effect on rheumatoid arthritis. Among other side effects, it can be toxic to the liver. Because of that, doctors frequently order liver function blood tests and warn patients to not drink alcohol excessively or to do anything else that might further compromise the liver. Enbrel is a highly innovative, recombinant DNA product containing two immunologically active proteins. Unfortunately, the same immune-modifying effect that helps stabilize or modify an autoimmune disease process like rheumatoid arthritis also can modify the host's immune response to infection, making patients especially vulnerable to some infectious diseases. As a result, patients are told to stop Enbrel at the first sign of an infectious disease, to take special precautions to avoid infection, and to start antibiotics if they develop a cough, fever, and so forth. Very recently, an increased risk of cancer has been reported associated with the general class of immune-modifying drugs used to modulate the course of chronic inflammatory conditions like rheumatoid arthritis, greatly adding to this already highly risky chronic disease experience (Pollack 2008a).
Consequences and Implications
The transformed chronic disease experience and its convergence with the growing numbers of people recruited into risk states have contributed to a great deal of improvement in individual and population health, for example, the dramatic survival benefits of insulin treatment for children with type 1 diabetes mellitus and the role played by the diabetes risk continuum in rationalizing some public health efforts against childhood obesity. At the same time, some unsettling and generally underappreciated consequences might be subjected to more clinical and policy reflection and response.
Chronic disease today entails a great deal of expectant treatment and surveillance for other diseases and complications. Physicians routinely prescribe inhaled steroids to prevent asthma exacerbations and lipid-lowering drugs, beta blockers, and aspirin to prevent a second heart attack. Belief in the efficacy of such secondary prevention at the same time serves as an incentive to make more and earlier diagnoses of the condition that is the object of secondary prevention efforts. For example, belief in the efficacy of early intervention drives the earlier diagnosis of childhood autism and the growing number of children diagnosed with Asperger's syndrome. This interaction between the intense secondary prevention activity and the expansion of disease diagnoses and the changed character of the chronic disease experience has not been widely appreciated.
The intensity of testing, anticipatory, or expectant treatment and the belief that such maneuvering is consequential have led to greater efforts at disease management. The idea is that there are enough predictable tests, preventive maneuvers, surveillance, and other routines tied to specific conditions that management systems—case workers, patient education materials, reminder systems—will keep patients healthier and reduce costs, especially by avoided or deferred hospital admissions. These elements constitute the career of the bureaucratic patient (see Rosenberg 2002, 2003, 2009).
In asthma, for example, patients have been urged to develop “action plans” in cooperation with their doctors that specify detailed, individualized plans for adjusting medications and seeking medical care in response to changes in symptoms, signs, and home technical monitoring (e.g., spirometry). Such plans also list environmental and other individual triggers of asthma exacerbations. Such detailed, explicit management can profoundly change the asthma experience. Among other effects, they increase the nodal points at which the clinical situation needs to be assessed and management decisions must be made.
For many patients, the experience of chronic disease is not dominated by symptoms of the pathological processes but by reading the body for signs of future problems, negotiating different secondary prevention measures, and making decisions about the future. The diagnosis of type 2 diabetes mellitus is often based on laboratory abnormalities alone. Many other patients with symptoms of excessive thirst and urination will become asymptomatic soon after diagnosis and the beginnings of lifestyle change and/or medical treatment. But asymptomatic does not mean that they have no experience of disease. Patients will understand that they are at higher risk of heart disease and will need to be screened aggressively for known heart disease risk factors. They are likely to pay special attention to any chest pains as potential angina pectoris. They not only are urged to get nutritional counseling and diabetes education but also are likely to be screened regularly for different diabetes complications: kidney disease, eye problems, and so forth. Medicines carry side effects—especially hypoglycemia—that need monitoring and attention. Many new medicines and interventions are both promoted and developed and then reported in the health and business pages of daily newspapers and on local and national news. Patients closely follow the significant media coverage of the many controversies over efficacy and the inevitable side effects that follow from mass use and study.
As a growing fraction of the chronic disease experience becomes secondary prevention and surveillance rather than the experience of symptoms, and more of the disease population is asymptomatic or minimally symptomatic from the disease itself, the disease experience also becomes more uniform, and thus an individual's illness experience becomes more legible to third parties and others. These transformations rationalize and permit more external control of decision making such as practice guidelines and protocols. Explicit evidence-based evaluation of particular products and practices, an important and necessary policy response, often means less attention to aspects of the disease experience that are not uniform or predictable enough to be managed by protocols and in standard ways. Given the limits of our interest and material resources, we focus on what is legible and measurable, for example, measuring the hemoglobin A1C rates or the use of secondary preventive asthma medications, rewarding and punishing providers and health systems on the basis of their compliance with these measures, but ignoring less legible but consequential idiosyncratic practices and outcomes.
The convergence of risk and disease has had more subtle effects on individual decision making, important to these individuals but also having widespread effects. There has been an understandable expansion of decision-making patterns or styles routinely used by people with symptomatic, serious disease to individuals whose illness experience falls at different points on the risk continuum. For example, some women with metastatic breast cancer have opted for treatments that medical evidence suggests are not in aggregate beneficial, like the use of highly toxic chemotherapy followed by bone marrow transplant. Faced with the near certainty of advancing disease and death, it is understandable that some women choose to bet against the odds, hoping their one roll of the dice will lead to survival. They may also want to avoid regretting later that they did not do everything possible to avert advancing disease and death.
These “playing by the law of small numbers” and “anticipated regret” heuristics have become increasingly operative in decisions about risk (Aronowitz 2007). For example, some women and doctors invoke these heuristics to explain their support for screening mammography for women under fifty, despite data indicating no or minimal overall benefit and considerable financial and personal costs. They fear cancer so much that gambling against the odds seems reasonable. Women may also anticipate the regret they would have if they later developed breast cancer and had not availed themselves of screening.
The recent report of rising rates of contralateral prophylactic mastectomies in the United States indicates that the impact of this converged decision making is significant. The number of women diagnosed with cancer in one breast who had a prophylactic mastectomy in the unaffected breast rose from 1.8 percent in 1998 to 4.5 percent in 2003 (Tuttle et al. 2007). Although accurate statistics are not yet available, there is reason to believe that rates of prophylactic mastectomies for women “merely” at high risk for breast cancer, whether identified as such by genetic screening or on other grounds, have been climbing along with the rates for women with diagnosed breast cancer.3 In other words, the converged experience is reflected in the parallel decision making of those people who are at risk for disease and those who have manifest yet very different stages of disease.
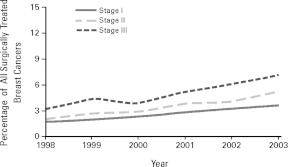
Neither improvements in surgery nor new biomedical insights are, in my view, by themselves driving such rapid change. It is revealing that the incidence of prophylactic surgery for women with breast cancer increased for women at every stage (see Figure 1), at an almost identical slope.4 Decision making about prophylactic surgery does not appear to have been based solely on straightforward calculations of altered probabilities of future cancer among women at different degrees of risk and disease. From a rational decision-making perspective, we would expect that women at a lower risk of recurrence would have a lower rate of change, since they have less to gain from this severely mutilating operation (their absolute rates are, in fact, lower). The fact that women of all stages share equally in the increasing rate of prophylactic surgery suggests that they are being exposed to some common external influence. Some observers have pinpointed the reasons for this increase, such as the use of more sensitive diagnostic and screening technologies (e.g., breast MRIs), which I am sure are operative (Pollack 2008b). But also important is the way that people at different points in the actively constructed risk continuum experience risk in similar ways and use similar decision-making strategies and styles, relatively autonomous from the objective probabilities of bad outcomes.
Figure 1.
Trends in the Proportion of All Surgically Treated Patients Who Underwent Contralateral Prophylactic Mastectomy (CPM-A) by Cancer Stage at Diagnosis
Note: The Cochran-Armitage tests for trend for CPM-A rates overall and by stage were significant (p < .001).
Source: T.M. Tuttle, E.B. Habermann, E.H. Grund, T.J. Morris, and B.A. Virnig, Increasing Use of Contralateral Prophylactic Mastectomy for Breast Cancer Patients: A Trend toward More Aggressive Surgical Treatment, Journal of Clinical Oncology (published online, October 22, 2007). Reproduced with permission of the Journal of Clinical Oncology.
Although I would not want to second-guess any particular individual's decision, I find troubling the rapid and uniform rise in prophylactic surgery at all points in the risk continuum. Clinical or policy responses should take into account the active role of knowledge production about risk and risk-reducing practices in generating fear and overselling, in my estimation, the efficacy of current risk-reducing efforts. Blurring the boundary between risk and disease in decision making is facilitated by the way that we name and classify risk and disease. Many clinicians now recognize that a pathological diagnosis after breast biopsy of Lobular carcinoma in situ is, in essence, the discovery of an underlying state of risk but that the cancer terminology, along with its embodied character, makes it much more frightening and encourages decision-making styles typically used in symptomatic and more advanced cancer. This semantic slippage and other negative aspects of the converged disease experience, such as the overselling of fear and fear-reducing interventions by the pharmaceutical industry and others, might be mitigated by more critical attention to the way we define, name, and classify cancer and other diseases.
The convergence of risk and disease has also led to a larger and more highly mobilized disease/risk population with expanded markets for interventions and greater clout for disease advocates. Earlier I cited the enlarged market for medications constituted by combining people with prehypertension and frank hypertension and prediabetes and diabetes. The larger size of the risk/disease population can lead to more visible and effective disease advocacy, which can then bring greater political pressure for funding basic and clinical research, which itself can contribute—via expanded definitions of risk and disease—to increases in the mobilized population. Again, this is most visible in the rapid growth in breast cancer advocacy in the United States over the past few decades, but similar trends can be seen in other risk/disease populations, such as the one formed by a convergence of obesity and type 2 diabetes. As these highly mobilized, converged risk/disease populations are often constituted by the marketing of tests and products, often by players with narrow economic interests (pharmaceutical companies and doctors influenced by them), we need a more vigorous and skeptical response to the active attempts to converge individuals at different points in the risk continuum into large markets for interventions and products.
The converged experience of risk and disease also has led to dramatic shifts in the perceived severity and spectrum of the disease, with ripple effects on how people experience and understand their illness. The expanded risk continuum makes people with a poor prognosis and rapidly advancing disease much more of a minority than in the past and makes the public face of some transformed diseases seem healthier and in general helps put a veneer of optimism onto the expanded group's identity. One of my students with long-standing type 1 diabetes has been upset by media reports that a famous actress, previously a spokesperson for the disease, had been able to wean herself off insulin. She suspected, rightly or wrongly, that the actress's original diagnosis was due to the expansion of the type 1 diabetes disease category, in ways that I have discussed throughout this article. My student worried that the glib media message of self-cure and control created impossible expectations for most type 1 diabetic patients and undermined public appreciation of the serious challenges that type 1 diabetics face. In the many instances in which the experience of highly symptomatic individuals are drowned out by the expanded risk/disease continuum, it might be appropriate to uphold or reinvent more categorical distinctions between risk and highly symptomatic disease.
One last consequence of the convergence of risk and disease is the proliferation of interventions promising both risk reduction and efficacy against symptomatic disease. Before data from the Women's Health Initiative appeared, many women were encouraged to take sex hormones both to control menopausal symptoms and to reduce the risk of chronic disease later in life. Menopause had been constructed partly as a symptomatic condition and partly as another “at risk” state. The bundling of risk reduction and disease treatment also is evident in the market niche of many new drugs whose sole or main selling point is the promise to put patients at less risk from particular side effects than competing drugs do. For example, the Cox-2 inhibitor class of nonsteroidal anti-inflammatory drugs, like Vioxx and Celebrex, were promoted as being as efficacious as existing pain relievers and anti-inflammatory drugs but putting patients at less risk of gastrointestinal bleeding. Their remarkable market success followed from this combination of efficacy against disease and symptoms and promise to be less risky than competing drugs. But risk reduction is subject to an “easy come, easy go” principle. Knowledge of imposed risks can quickly undermine a drug's risk-reducing rationale. As quickly as the Cox-2 inhibitors dominated the anti-inflammatory market, so did this market share collapse with evidence that they increased cardiovascular risk. It is hard to promote the efficacy of a risk-modifying intervention as reducing fear and uncertainty when the very same intervention causes fear and uncertainty.
Combining risk reduction with symptomatic relief is a subspecies of the larger way that risk reduction has permeated not just disease prevention for the healthy but the experience and management of existing disease. Bundling risk reduction and symptom relief may be a good thing in itself, but in many instances, it is part of a problematic, self-reinforcing cycle of fear promotion followed by the marketing of tests and products that promise some means to reassert control over fear.
These cycles of risk production and risk reduction in our primary and secondary prevention efforts have financial and psychological costs. Should we have a higher bar for accepting new practices and products whose primary goal is to reduce the risk of other practices and products? Recent arguments about the cost-effectiveness of new HPV vaccines show that the substantial savings might ensue not so much from the reduction of morbidity and mortality from cervical cancer and other HPV-related diseases but instead from reduced HPV-related Pap smear abnormalities and the expensive and intrusive workups they trigger. This is a real and important saving, but there is also something futile and problematic about this kind of meta-efficacy in which risk interventions reduce the costs and harms of other risk interventions, especially if such practices become dominant in our clinical and public health work.
Conclusion
I have emphasized the generally underappreciated and often unsettling consequences of the transformed chronic disease experience and its convergence with the experience of risk. We cannot set back the clock, nor would we want to. But we can do more to reduce the financial costs, disturbance to peace of mind, and distractions from other health goals that are the downsides of the converged experience.
Many of the changes that I have described are the result of one or another form of knowledge production, especially research into the natural history of disease and the construction of new risk states within existing chronic disease. Additional influences have been secondary or spillover effects of the ways that we diagnose and treat chronic disease. Yet neither this knowledge production nor these late effects of existing clinical practices are typically understood as central issues in policy analysis or response.
Our current policies generally respond to different risk-reducing interventions—screening or diagnostic tests, preventative medications, and lifestyle changes—one at a time, in isolation from one another, and only after they have a foothold in clinical practice. Policymakers generally do not ask, for example, why some risks and not others are researched and promoted. We do not generally evaluate the cumulative effects of different prevention practices or the ways that new treatments and surveillance regimes expand and legitimate the risk states they target. We have also placed much less evidence-based scrutiny on the surveillance of existing disease and “secondary prevention” compared with primary prevention. Current cost-effectiveness analyses of, say, a new asthma or diabetes diagnostic test or medication, aimed at reducing the impact of existing disease, however evidence based, do not typically capture the cumulative effects on the peace of mind or the work of patienthood of many such practices or measure the impacts of the expanding scope of disease, such as increased fear.
The transformed disease experience suggests a different kind of evaluation and policy response from the present status quo, one that examines the “upstream” processes resulting in knowledge production about risks and new preventive practices and products (Aronowitz 2006). Our policies need to evaluate and regulate the processes by which risks are named, identified, and researched; how the demand for intervention is produced; and what the many spillover effects are of our risk interventions.
We might, for example, expand the current regulatory oversight of direct-to-consumer advertising of prescription drugs to include weighing the impact of creating much larger markets composed of both the “at risk for” and the already diseased. We might insist that marketers maintain a stricter boundary between disease and risk in labeling and advertising. In funding research and formulating best clinical practices, we could respond more skeptically to research, product development, and calls for clinical and behavioral change that assume an unmitigated good from “early” intervention in disease. Such scrutiny already exists in oversight bodies like the U.S. Preventive Task Force when it evaluates claims for primary prevention. But there is much less awareness when evaluating “risk-reducing” interventions against existing disease.
It is, of course, difficult to measure consequences such as fear, disturbance to peace of mind, and the work of patienthood and to balance these effects against the health benefits of new knowledge and practices. But focusing only on what is easy to measure and value will not banish the challenges posed by the converged experience of risk and chronic disease.
Acknowledgments
This article was funded in part from an Investigator Award in Health Policy from the Robert Wood Johnson Foundation and grant 1G13 LM009587-01A1 from the National Library of Medicine, the National Institutes of Health, and the Department of Health and Human Services. The views expressed in any written publication, or other media do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S. government. Charles Rosenberg and David Asch read and provided useful comments on an early draft of this article.
Endnotes
Elsewhere I have argued that medicalization and social constructionist studies should more comprehensively account for the ways that different disease names, categories, and classifications materially impact the disease experience of individuals and populations (Aronowitz 2008).
My focus on the risky nature of chronic disease is related to Feudtner's concept of a transmuted chronic disease. Feudtner stressed the shift from the experience of a stable, external, and specific “natural history” to something more dynamic, individual, and negotiated. For the subset of patients with a chronic disease diagnosis for whom an “early” diagnosis and medical intervention has rendered them at least temporarily asymptomatic, there has been a paradoxical return to a stable, although more anticipated than experienced, natural history.
I have been unable to date to find good (as in population-based) data on temporal trends in prophylactic bilateral mastectomy rates among women without breast cancer. One study used a database of women who had mutations for BRCA1 and BRCA2 and reported that American women had the highest rates of prophylactic surgery (36.3 percent) among the nine industrialized countries they compared (Metcalfe et al. 2008).
In stage 1 breast cancer, the cancer has not spread beyond the breast and is no more than 2 cm wide; in stage 2, the cancer is between 2 and 5 cm wide; and in stage 3, the cancer is larger than 5 cm and has spread to the lymph nodes or local tissues.
References
- Armstrong D. The Rise of Surveillance Medicine. Sociology of Health and Illness. 1995;17(3):393–404. [Google Scholar]
- Aronowitz RA. Do Not Delay: Breast Cancer and Time, 1900–1970. The Milbank Quarterly. 2001;79(3):355–86. doi: 10.1111/1468-0009.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronowitz RA. Situating Health Risks. In: Burns R, Stevens R, Rosenberg C, editors. American Health Care History and Policy: Putting the Past Back In. New Brunswick, N.J.: Rutgers University Press; 2006. pp. 153–65. [Google Scholar]
- Aronowitz RA. Unnatural History: Breast Cancer and American Society. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Aronowitz RA. Framing Disease: An Underappreciated Mechanism for the Social Patterning of Health. Social Science & Medicine. 2008;67(1):1–9. doi: 10.1016/j.socscimed.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Barsky A. Worried Sick: Our Troubled Quest for Wellness. Boston: Little, Brown; 1988. [Google Scholar]
- Beck U. Risk Society: Towards a New Modernity. London: Sage; 1992. [Google Scholar]
- Conrad P. The Medicalization of Society: On the Transformation of Human Conditions into Treatable Disorders. Baltimore: Johns Hopkins University Press; 2007. [Google Scholar]
- Davis JE. How Medicalization Lost Its Way. Society. 2006;43(6):51–56. [Google Scholar]
- Feudtner C. Disease in Motion: Diabetes History and the New Paradigm of Transmuted Disease. Perspectives in Biology and Medicine. 1996;39(winter):158–70. doi: 10.1353/pbm.1996.0027. [DOI] [PubMed] [Google Scholar]
- Frank AW. The Wounded Storyteller: Body, Illness, and Ethics. Chicago: University of Chicago Press; 1995. [Google Scholar]
- Giddens A. The Consequences of Modernity. Stanford, Calif.: Stanford University Press; 1990. [Google Scholar]
- Goffman E. Asylums: Essays on the Social Situation of Mental Patients and Other Inmates. Garden City, N.Y.: Doubleday; 1961. [Google Scholar]
- Greene J. Prescribing by Numbers. Baltimore: Johns Hopkins University Press; 2007. [Google Scholar]
- Klawiter M. Risk, Prevention and the Breast Cancer Continuum: The NCI, the FDA, Health Activism and the Pharmaceutical Industry. History and Technology. 2002;18:309–53. [Google Scholar]
- Kramer PD. Listening to Prozac. New York: Penguin; 1993. [Google Scholar]
- Metcalfe KA, Birenbaum-Carmeli D, Lubinski J, Gronwald J, Lynch H, Moller P, Ghadirian P, et al. International Variation in Rates of Uptake of Preventive Options in BRCA1 and BRCA2 Mutation Carriers. International Journal of Cancer. 2008;122(9):2017–22. doi: 10.1002/ijc.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Digestive Diseases Information Clearing House. Chronic Hepatitis C: Current Disease Management. 2006. Available at http://digestive.niddk.nih.gov/ddiseases/pubs/chronichepc/index.htm accessed April 1, 2009. [Google Scholar]
- Novas C, Rose N. Genetic Risk and the Birth of the Somatic Individual. Economy and Society. 2000;29:485–513. [Google Scholar]
- Pollack A. FDA Reviews Arthritis Drugs for Links to Cancer. New York Times. 2008a June 5. [Google Scholar]
- Pollack A. Study Links Rise in Mastectomies to M.R.I. Detection. New York Times. 2008b May 16. [Google Scholar]
- Rakel R, Bope E. Conn's Current Therapy. New York: Saunders; 2007. [Google Scholar]
- Ries L, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, Mariotto A, Feuer EJ, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2002. Bethesda, Md.: National Cancer Institute; 2005. Available at http://seer.cancer.gov/csr/1975_2002/ based on November 2004 SEER data submission, posted to the SEER website, 2005 (accessed July 25, 2008) [Google Scholar]
- Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, et al., editors. SEER Cancer Statistics Review, 1975–2005. Bethesda, Md.: National Cancer Institute; 2008. Available at http://seer.cancer.gov/csr/1975_2005/ based on November 2007 SEER data submission, posted to the SEER website, 2008 (accessed April 1, 2009) [Google Scholar]
- Rosenberg CE. The Tyranny of Diagnosis: Specific Entities and Individual Experience. The Milbank Quarterly. 2002;80(2):237–60. doi: 10.1111/1468-0009.t01-1-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg CE. What Is Disease? In Memory of Oswei Temkin. Bulletin of the History of Medicine. 2003;77:491–505. doi: 10.1353/bhm.2003.0139. [DOI] [PubMed] [Google Scholar]
- Rosenberg CE. Managed Fear. The Lancet. 2009;373:802–3. doi: 10.1016/s0140-6736(09)60467-0. [DOI] [PubMed] [Google Scholar]
- Rosenthal E. Drugs Can Prevent Diabetes in Many at High Risk, Study Suggests. New York Times. 2006 September 17. [Google Scholar]
- Schwartz L, Woloshin S, Fowler FJ, Welch HG. Enthusiasm for Cancer Screening in the United States. Journal of the American Medical Association. 2004;291(1):71–78. doi: 10.1001/jama.291.1.71. [DOI] [PubMed] [Google Scholar]
- Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing Use of Contralateral Prophylactic Mastectomy for Breast Cancer Patients: A Trend toward More Aggressive Surgical Treatment. Journal of Clinical Oncology. 2007 doi: 10.1200/JCO.2007.12.3141. October 22 (published online). [DOI] [PubMed] [Google Scholar]