Abstract
Males with fragile X syndrome are at risk for significant cognitive and behavioral deficits, particularly those involving executive prefrontal systems. Disruption of the cholinergic system secondary to fragile X mental retardation protein deficiency may contribute to the cognitive-behavioral impairments associated with fragile X. We measured choline in the dorsolateral prefrontal cortex of 9 males with fragile X syndrome and 9 age-matched typically developing controls using 1H magnetic resonance spectroscopy. Right choline/creatine was significantly reduced in the fragile X group compared to controls. In controls, both left and right choline was significantly positively correlated with intelligence and age was significantly negatively correlated with left choline. There were no correlations in the fragile X group. Subjects with fragile X syndrome participating in a pilot open-label trial of donepezil, an acetylcholinesterase inhibitor, demonstrated significantly improved cognitive-behavioral function. Studies utilizing biochemical neuroimaging techniques such as these have the potential to significantly impact the design of treatment strategies for fragile X syndrome and other genetic disorders by helping identify neurochemical targets for intervention as well as serving as metrics for treatment efficacy.
Keywords: fragile X syndrome, choline, donepezil, executive function
INTRODUCTION
Fragile X syndrome (FRAX) is the most common heritable cause of developmental disability. FRAX results from a mutation of the FMR1 gene on the X chromosome that interferes with transcription of FMR1 into mRNA and, therefore, synthesis of the fragile X mental retardation protein (FMRP) [Garber et al., 2006]. FRAX is associated with a profile of cognitive-behavioral deficits predominantly related to executive function and adaptive behaviors [Reiss and Dant, 2003]. Neuroimaging studies have repeatedly demonstrated abnormalities in prefrontal cortex of individuals with FRAX and have shown that lower FMRP is correlated with impaired performance and abnormal prefrontal brain activation during executive tasks [Menon et al., 2004].
Disruption of the cholinergic system may contribute to executive dysfunction and fragile X syndrome (FRAX) has been linked to the cholinergic system by several lines of evidence. First, FMR1 is highly expressed in cholinergic neurons of the nucleus basalis during early neurodevelopment [Abitbol et al., 1993], Second, cholinergic pathways in the basal forebrain and hippocampus, two areas of abnormal development or function in FRAX [Greicius et al., 2004; Reiss and Dant, 2003], are known to subserve several cognitive functions that are significantly affected in FRAX including executive attention, learning and memory [Sarter et al., 2003]. Third, examination of the FRAX knockout mouse has indicated aberrant cholinergic function in the subiculum, a limbic region involved in learning and memory [D’Antuono et al., ]. Finally, recent evidence from the FRAX drosophila model suggest that cholinergic pathways may help mediate certain features of FRAX, possibly including aspects of brain morphology [Chang et al., 2008]. However, no data currently exist regarding in vivo neurometabolite levels in this important neurogenetic condition.
We therefore measured bilateral dorsolateral prefrontal cortex (DLPFC) levels of Choline in 9 males with FRAX and 9 age-matched typically developing males using using 1H magnetic resonance spectroscopy (1H MRS). Given our previous findings of reduced neurofunctional activity in cholinergic brain regions among individuals with FRAX [Greicius et al., 2004; Reiss and Dant 2003], we hypothesized that Choline levels would be lower in subjects with FRAX compared to controls.
MATERIALS AND METHODS
Participants
We prospectively recruited 9 males with FRAX (mean age = 18.8 ± 4.2) and 9 age-matched typically developing control males (mean age = 19.2 ± 4.0, p = .87). Males with FRAX possessed the FMR1 full mutation as indicated by standard Southern blot and polymerase chain reaction performed following FMR1-specific probe hybridization [Oostra and Willemsen 2001]. Males with FRAX were recruited from our participant registry of families who expressed interest in our research. Controls were recruited via web and flyer postings and were selected to match one-to-one with the FRAX males in terms of age (within 6 months), zip code and ethnicity. All participants were excluded for MR contraindications. Controls were excluded for any history of neurological or psychiatric disorder using standard screening instruments. The human subjects committee at Stanford University School of Medicine approved the protocols used in this study.
Magnetic Resonance Imaging
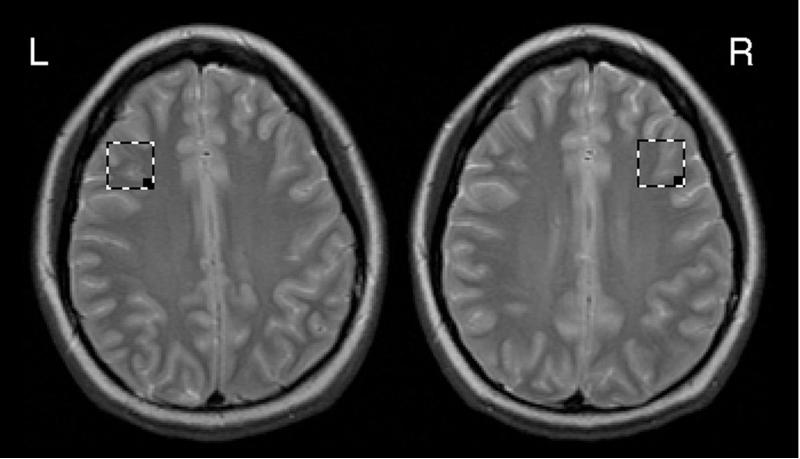
All imaging scans were obtained with a GE 3.0T scanner (GE medical systems, Milwaukee). A T2-weighted gradient echo spiral pulse sequence (TR = 3000 ms, TE = 30 ms, flip angle = 89° slice thickness = 4mm, 0.5mm skip, field of view = 200 mm, 18 slices and inplane spatial resolution = 3.125 mm) was used to prescribe a 2 × 2 × 2 cm voxel in the DLPFC, from the first axial slice above the lateral ventricles. The voxel was placed above the lateral ventricles, immediately anterior to a line drawn between the anterior aspects of the lateral ventricles, and as far lateral as possible while remaining in the cerebrum and visually maintaining approximately equal parts of gray and white matter (Fig 1).
Figure 1.
Illustration of voxel placement on the T2 scan.
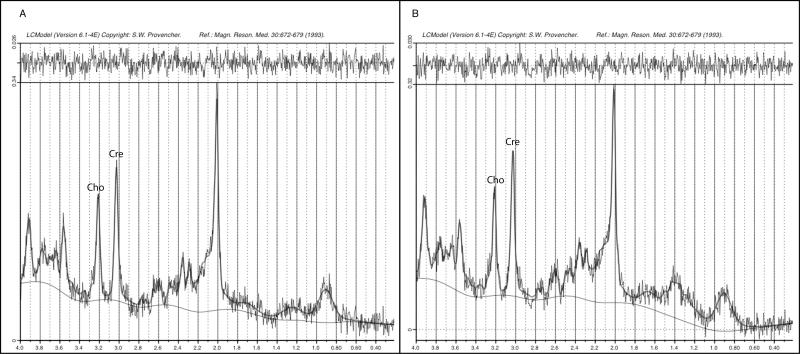
1H MRS data from the voxel regions were acquired using point-resolved spectroscopy with a TR/TE of 2000/26ms and 32 averages. Water suppression was achieved using CHESS pulses. The fully automated LC Model [Provencher, 2001] was used to analyze MRS spectra with eddy current correction and water scaling. Spectra exclusion criteria included full width at half maximum greater than 0.1 ppm or signal-to-noise ratio less than 3, however, none required exclusion (Fig 2). Absolute and ratio data for Cho (glycerophosphocholine + phosphocholine peak) were compared between the two groups using Mann Whitney analysis. Absolute creatine (Cre, creatine + phosphocreatine peak) data also were compared between groups using Mann Whitney analysis to ensure the validity of the Cho/Cre ratio data. Although LC Model output automatically includes several other metabolites, we only analyzed Choline for this study given our specific a priori hypotheses regarding Choline in FRAX and because our small sample size would lack power for multiple comparisons.
Figure 2.
Example 1H MRS spectra for (A) male with FRAX and (B) control.
A rater blind to subject diagnosis placed a 2 × 2 × 2 cm voxel on the T2 scan in the same anatomic location of the MRS voxel. The rater then segmented the T2 scan into cerebral spinal fluid (CSF), gray and white matter using the brain extraction and segmentation tools of the FMRIB Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl) and calculated the percentage of each tissue type within the voxel for each subject. The ratios of gray and white matter in the left and right voxels were calculated for each subject. Between group analysis of these ratios was conducted using Mann Whitney which revealed no significant differences (Table I).
Table I.
Demographic, DLPFC voxel composition and metabolite data for all males with fragile X syndrome (FRAX) and controls. Data are show as mean (standard deviation).
| FRAX | Controls | Test | p | |
|---|---|---|---|---|
| Age | 18.82 (4.2) | 19.16 (4.0) | F = .03 | .87 |
| IQ | 51.86 (10) | 114.25 (11) | F = 133 | < .0001 |
| Medications % | ||||
| Stimulants | 33 | 0 | ||
| SSRI’s | 33 | 0 | ||
| Atypical antipsychotic | 44 | 0 | ||
| MRS voxel composition | ||||
| Left gray/white | 1.2 (.45) | 1.2 (.37) | U = 32 | 1.0 |
| Right gray/white | 1.1 (.55) | 1.2 (.46) | U = 24 | .44 |
| Metabolite levels | ||||
| Left Cho/Cre | .248 (.02) | .257 (.01) | U = 26 | .11 |
| Left Cho | 1.43 (.29) | 1.40 (.13) | U = 33 | .24 |
| Right Cho/Cre | .223 (.03) | .275 (.02) | U = 7.0 | .001 |
| Right Cho | 1.26 (.15) | 1.38 (.18) | U = 24 | .06 |
| Left Cre | 9.21 (2.6) | 10.14 (1.7) | U = 26 | .22 |
| Right Cre | 8.69 (1.6) | 9.23 (2.4) | U = 29 | .34 |
IQ = intellectual function
SSRI = selective serotonin reuptake inhibitor
MRS = magnetic resonance spectroscopy
DLPFC = dorsolateral prefrontal cortex
Cho = choline [glycerophosphocholine and phosphocholine (GPC+PCh)]
Cre = creatine [creatine and phosphocreatine (Cr+PCr)]
Intellectual Function
Intellectual function (IQ) scores were measured using the Wechsler scales of intelligence [Wechsler, 1999]. Analysis of variance (ANOVA) was used to determine group differences in IQ and two-tailed Spearman rho correlation was used to explore relationships between Choline and IQ.
Medication
Medications were classified into three groups: 1-stimulants (Ritalin, Dexedrine, Adderall), 2- selective serotonin reuptake inhibitors (Paxil, Lexapro, Fluoxetine), and 3- atypical antipsychotics/anticonvulsant (Risperdal, Abilify, Depakote). Subjects received a dummy code of 1 or 0 for each medication group. The association of medication group with Choline was assessed in the FRAX group using multiple regression.
Drug Trial
We initiated a 6 week, open-label drug trial to investigate whether enhancing cholinergic activity with donepezil, an acetylcholinesterase inhibitor, has a beneficial effect on cognitive-behavioral function in FRAX. After a baseline assessment of cognitive-behavioral function, 8 subjects with FRAX (6 males, 2 females, ages 14-44) began a 3 week trial of 5mg donepezil daily. They were then re-tested before switching to a 10mg dose for 3 weeks, after which the assessments were completed again. The assessment battery consisted of the Contingency Naming Task (CNT) to measure working memory and mental flexibility [Kirk et al., 2005], the Hopkins Verbal Learning Test (HVLT) to assess auditory memory [Morey et al., 2003], the Aberrant Behavior Checklist (ABC), a standardized rating scale developed to measure pharmacologic effects on problem behaviors in individuals with mental retardation [Berry-Kravis et al., 2008], and the Achenbach Child and Adult Behavior Checklists (CBCL, ABCL) to assess general cognitive-behavioral status [Hatton et al., 2002]. Alternate forms of the measures were used when available. One subject did not receive the HVLT and another subjects’ ABC and CBCL data were excluded from the analyses due to invalid profiles (parent ratings at baseline were too low to meet study behavioral impairment criteria). Repeated measures analysis of variance with LSD post hoc comparisons between the three time points (baseline, 5mg and 10mg) were used to determine the effects of the donepezil trial and dosage on cognitive-behavioral function. There was no overlap in the subjects who completed the MRI scans and those who participated in the drug trial due to the timing, specifications and resources of each study.
RESULTS
Males with FRAX demonstrated significantly lower IQ scores (p < .0001, Table I) significantly, lower right Cho/Cre ratio and a trend for lower absolute right Cho in the DLPFC compared to controls. Cre and MRS voxel gray/white tissue ratios were not significantly different between groups (Table I). There were no significant correlations between Cho levels, IQ, age or medication in the FRAX group. In controls, both left and right Cho were significantly positively correlated with IQ (left: r = .74, p - .04; right: r = .80, p =.02), and age was significantly negatively correlated with left Cho (-.72, p = .03). However, only the correlation between right Cho and IQ was significantly different between the groups (Fisher’s z = 1.8, p = .03).
All recruited subjects completed the donepezil trial. Side effects were very minimal during the 6 week trial. None of the participants experienced side effects during the 3 weeks on 5mg. Two participants reported side effects at 10mg. One endorsed feeling dizzy and short of breath. Examination of the subject did not reveal increased respiratory rate or neurological symptoms. Nevertheless, after consultation with the study-physician, the dose was lowered to 5mg for the remainder of the trial during which time the symptoms subsided. The other experienced nausea at the dose switch which subsided after 3 days and, thus, continued on the 10mg for the remainder of the trial with no further side effects. Subjects demonstrated significantly increased CNT scores (p = .05) and significantly decreased ABC Total (p = .002), Hyperactivity (p =.04) and Irritability (p = .009) scores as well as significantly decreased CBCL/ABCL Attention Problems (p = .009) scores. These improvements appeared to be primarily related to the 5mg dosage as post hoc tests indicated no significant differences between the 5mg and 10mg time points (Table II).
Table II.
Cognitive-behavioral data for participants of the donepezil trial. F and p values are for repeated measures omnibus test. Post hoc tests comparing the 3 time points indicated significant cognitive-behavioral improvements between baseline and the 5mg dosing as well as baseline and the 10mg dosing. There were no significant changes in cognitive-behavioral performance between 5mg and 10mg trials. An increase in score for the CNT and HVLT signify improvement while a decrease in score for the ABC and CBCL/ABCL indicate improvement.
| Measure | N | Baseline | 5 mg (3 wks) | 10 mg (6 wks) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | F | p | ||
| CNT | 8 | 12.60 | 8.20 | 19.60* | 7.59 | 19.09 | 10.13 | 3.71 | .051 |
| HVLT | 7 | 22.29 | 6.08 | 21.57 | 8.44 | 19.00 | 6.08 | .632 | .548 |
| ABC Total | 7 | 28.14 | 11.67 | 16.14* | 9.08 | 12.14* | 7.20 | 11.17 | .002 |
| ABC Hyperactivity | 7 | 7.86 | 5.46 | 4.57* | 3.55 | 3.57 | 2.94 | 4.21 | .041 |
| ABC Irritability | 7 | 7.43 | 5.06 | 3.00* | 3.16 | 2.57* | 2.37 | 7.06 | .009 |
| CBCL/ABCL | 7 | 12.43 | 3.65 | 8.43* | 4.12 | 7.71* | 4.23 | 7.11 | .009 |
significantly different from baseline (p ≤ 0.05)
CNT: Continuous Naming Test
HVLT: Hopkins Verbal Learning Test
ABC: Aberrant Behavior Checklist
CBCL/ABCL: Child/Adult Behavior Checklist (Achenbach) Attention Problems
DISCUSSION
To our knowledge, this is the first study of fragile X syndrome (FRAX) to examine in vivo choline (Cho) levels in the human prefrontal cortex using magnetic resonance spectroscopy (MRS). Our findings contribute further information regarding the neurobiologic mechanisms involved in DLPFC development and executive function in FRAX. Specifically, FMRP is believed to mediate several receptor systems important for cognitive function. Determining the downstream effects of FMRP deficiency on cholinergic receptor systems in humans provides direction for potential pharmacologic treatments for cognitive impairments associated with FRAX.
Of particular interest to the area of potential therapeutics for FRAX, the 1H MRS Cho peak is composed of acetylcholine synthesis precursors as well as Cho [Kantarci et al., 2004]. Our finding of decreased Cho in males with FRAX is consistent with a hypothesis of functional disruption of cholinergic systems secondary to reduced FMRP. A recent study utilizing a rat model of basalocortical atrophy and cholinergic dysfunction suggests that the use of an acetylcholinesterase inhibitor (i.e. donepezil) may improve basocortical cholinergic function through alteration of cholinergic synaptic plasticity [Ginestet et al., 2007]. Thus, the use of such medications may represent another viable, syndrome-specific treatment for FRAX.
Consistent with this premise, our pilot open-label trial of donepezil in individuals with FRAX demonstrated positive preliminary results. Specifically, subjects with FRAX enrolled in the trial demonstrated significantly improved executive function and significantly decreased problem behaviors, primarily associated with the 5mg dosage, suggesting a potential dose-response effect. Donepezil did not appear to have an effect on verbal memory in our small sample. Larger, randomized clinical trials are needed to test the efficacy of donepezil in treating cognitive-behavioral impairments associated with FRAX.
Increased Cho levels were associated with increased IQ in healthy males but no such correlation was observed in males with FRAX. Although DLPFC function is highly correlated with IQ [Kane and Engle 2002], more specific measures of executive function may be required to elucidate the relationships between Cho levels and cognitive outcome in FRAX. Additionally age was negatively associated with left Cho/Cre in controls. This is consistent with a previous study of healthy children and adults examining the Cho/Cre ratio in the frontal cortex [Hashimoto et al., 1995]. Other studies involving much older individuals indicate that all metabolites, including Cho, may remain stable with age [Haga et al., 2007]. Our results suggest the importance of examining adolescents and young adults separately from older individuals.
In conclusion, elucidating the neurobiology associated with reduced FMRP expression with in vivo neuroimaging has the potential for guiding development of disease-specific pharmacological interventions [Bear, 2005; Dolen and Bear, 2005; Yan et al., 2005] and for providing a potential metric for measuring treatment response. Our findings suggest that Cho dysfunction is related to FMRP deficiency in males with FRAX. Accordingly, further studies utilizing techniques such as 1H MRS could potentially contribute significantly to future intervention planning. However, we note that our results are derived from a preliminary study based on a small sample of males with FRAX. We also cannot rule out confounding effects of medication on Cho levels in FRAX [Bertolino et al., 2001; Streeter et al., 2005]. Additionally, a third group of individuals with idiopathic developmental delay IQ-matched to the FRAX group would help determine if our results are specific to FRAX rather than to individuals with low IQ. Further studies are needed to determine the nature of the association between biological differences observed with neuroimaging and the core cognitive and behavioral features of FRAX.
ACKNOWLEDGMENTS
This work was supported by NIH grants MH50047 (ALR) and a gift from the Canel family to support fragile X syndrome research. The authors wish to thank Natalee Maynes, Ed.M. for her assistance with participant recruitment, Nancy Adleman, Ph.D. candidate, for her help with the scanning protocol and Asya Karchemskiy, M.S. for her work on the voxel composition analysis.
REFERENCES
- Abitbol M, Menini C, Delezoide AL, Rhyner T, Vekemans M, Mallet J. Nucleus basalis magnocellularis and hippocampus are the major sites of FMR-1 expression in the human fetal brain. Nature genetics. 1993;4:147–153. doi: 10.1038/ng0693-147. [DOI] [PubMed] [Google Scholar]
- Bear MF. Therapeutic implications of the mGluR theory of fragile X mental retardation. Genes Brain Behav. 2005;4:393–398. doi: 10.1111/j.1601-183X.2005.00135.x. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Sumis A, Hervey C, Nelson M, Porges SW, Weng N, Weiler IJ, Greenough WT. Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome. J Dev Behav Pediatr. 2008;29:293–302. doi: 10.1097/DBP.0b013e31817dc447. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Callicott JH, Mattay VS, Weidenhammer KM, Rakow R, Egan MF, Weinberger DR. The effect of treatment with antipsychotic drugs on brain N-acetylaspartate measures in patients with schizophrenia. Biol Psychiatry. 2001;49:39–46. doi: 10.1016/s0006-3223(00)00997-5. [DOI] [PubMed] [Google Scholar]
- Chang S, Bray SM, Li Z, Zarnescu DC, He C, Jin P, Warren ST. Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat Chem Biol. 2008;4:256–263. doi: 10.1038/nchembio.78. [DOI] [PubMed] [Google Scholar]
- D’Antuono M, Merlo D, Avoli M. Involvement of cholinergic and gabaergic systems in the fragile X knockout mice. Neuroscience. 2003;119:9–13. doi: 10.1016/s0306-4522(03)00103-9. [DOI] [PubMed] [Google Scholar]
- Dolen G, Bear MF. Courting a cure for fragile X. Neuron. 2005;45:642–644. doi: 10.1016/j.neuron.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Garber K, Smith KT, Reines D, Warren ST. Transcription, translation and fragile X syndrome. Curr Opin Genet Dev. 2006;16:270–275. doi: 10.1016/j.gde.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Ginestet L, Ferrario JE, Raisman-Vozari R, Hirsch EC, Debeir T. Donepezil induces a cholinergic sprouting in basocortical degeneration. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2007.04497.x. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Boyett-Anderson JM, Menon V, Reiss AL. Reduced basal forebrain and hippocampal activation during memory encoding in girls with fragile X syndrome. Neuroreport. 2004;15:1579–1583. doi: 10.1097/01.wnr.0000134472.44362.be. [DOI] [PubMed] [Google Scholar]
- Haga KK, Khor YP, Farrall A, Wardlaw JM. A systematic review of brain metabolite changes, measured with (1)H magnetic resonance spectroscopy, in healthy aging. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Tayama M, Miyazaki M, Fujii E, Harada M, Miyoshi H, Tanouchi M, Kuroda Y. Developmental brain changes investigated with proton magnetic resonance spectroscopy. Dev Med Child Neurol. 1995;37:398–405. doi: 10.1111/j.1469-8749.1995.tb12023.x. [DOI] [PubMed] [Google Scholar]
- Hatton DD, Hooper SR, Bailey DB, Skinner ML, Sullivan KM, Wheeler A. Problem behavior in boys with fragile X syndrome. Am J Med Genet. 2002;108:105–116. doi: 10.1002/ajmg.10216. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Petersen RC, Boeve BF, Knopman DS, Tang-Wai DF, O’Brien PC, Weigand SD, Edland SD, Smith GE, Ivnik RJ, Ferman TJ, Tangalos EG, Jack CR., Jr 1H MR spectroscopy in common dementias. Neurology. 2004;63:1393–1398. doi: 10.1212/01.wnl.0000141849.21256.ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk JW, Mazzocco MM, Kover ST. Assessing executive dysfunction in girls with fragile X or Turner syndrome using the Contingency Naming Test (CNT) Dev Neuropsychol. 2005;28:755–777. doi: 10.1207/s15326942dn2803_2. [DOI] [PubMed] [Google Scholar]
- Menon V, Leroux J, White CD, Reiss AL. Frontostriatal deficits in fragile X syndrome: relation to FMR1 gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3615–3620. doi: 10.1073/pnas.0304544101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey CE, Cilo M, Berry J, Cusick C. The effect of Aricept in persons with persistent memory disorder following traumatic brain injury: a pilot study. Brain Inj. 2003;17(9):809–15. doi: 10.1080/0269905031000088586. [DOI] [PubMed] [Google Scholar]
- Oostra BA, Willemsen R. Diagnostic tests for fragile X syndrome. Expert review of molecular diagnostics. 2001;1:226–232. doi: 10.1586/14737159.1.2.226. [DOI] [PubMed] [Google Scholar]
- Provencher S. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR in Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Dant CC. The behavioral neurogenetics of fragile X syndrome: analyzing gene-brain-behavior relationships in child developmental psychopathologies. Dev Psychopathol. 2003;15:927–968. doi: 10.1017/s0954579403000464. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Givens B. Attentional functions of cortical cholinergic inputs: what does it mean for learning and memory? Neurobiology of learning and memory. 2003;80:245–256. doi: 10.1016/s1074-7427(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Streeter CC, Hennen J, Ke Y, Jensen JE, Sarid-Segal O, Nassar LE, Knapp C, Meyer AA, Kwak T, Renshaw PF, Ciraulo DA. Prefrontal GABA levels in cocaine-dependent subjects increase with pramipexole and venlafaxine treatment. Psychopharmacology (Berl) 2005;182:516–526. doi: 10.1007/s00213-005-0121-5. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) Harcourt Assessment; San Antonio, TX: 1999. [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]