Abstract
The Aedes albopictus Aa23 cell line, which is persistently infected with Wolbachia pipientis strain wAlbB, tends to grow as aggregated clusters of cells that are difficult to disperse for conventional quantification based on cell number. We used A. albopictus C7-10 cells to validate conversion of methylthiazole tetrazolium (MTT) to a colored formazan product with respect to incubation time, cell number over a 40-fold range, and metabolic activity as cells enter stationary phase. Using this assay, we showed that the doubling time of Aa23 cells increases from about 45 h early after plating to more than 70 h as the cells reach stationary levels. Growth of Aa23 cells proceeds at similar rates in the presence or absence of tetracycline concentrations that decrease the abundance of Wolbachia. Insofar as the MTT assay reflects mitochondrial function, our results indicate that, in Aa23 cells, abundance of intracellular Wolbachia has no measurable effect on mitochondrial activity in the presence of tetracycline.
Keywords: Insect cell cultures, Cell quantification, Mosquito cell lines, Formazan, Wolbachia
Introduction
The Wolbachia-infected mosquito cell line, Aa23, established from infected Aedes albopictus (Houston strain) embryos (O’Neill et al. 1997), provides an important tool for understanding metabolic interactions between an obligate intracellular bacterium and its mosquito host cell. Growth of Aa23 cells proceeds by formation of cell clusters that detach from the substrate as floating aggregates of tightly adherent cells, which attain sizes easily visible to the naked eye. Because these clusters are impossible to dissociate for routine quantification, we investigated other options for quantitative comparisons between infected cells and uninfected cells generated by treatment with the broad-spectrum antibiotic, tetracycline. Tetracycline has been used for a number of years to eliminate Wolbachia infections from diverse insects (Stouthamer et al. 1999), as well as from Aa23 cells (O’Neill et al. 1997).
Assays based on conversion of methylthiazole tetrazolium [MTT; 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide] to a colored formazan product were of particular interest because they are rapid and convenient and require little manipulation of cells (Mosmann 1983). To the extent that the MTT assay reflects mitochondrial function (reviewed by Berridge et al. 2005), the assay further provided the opportunity to explore possible long-term differences in mitochondrial metabolism resulting from use of tetracycline to eliminate Wolbachia. Based on studies with Drosophila simulans, Ballard and Melvin (2007) suggested that, in infected flies, absorption of tetracycline by Wolbachia protected mitochondria from deleterious effects of the antibiotic.
We standardized the MTT assay with A. albopictus C7-10 cells, which grow as uniformly attached monolayers easily dispersed by simple pipetting for counting with a Coulter electronic cell counter. With adjustment of MTT assay parameters relative to conditions described by Mosmann (1983), C7-10 cells showed excellent correlation of formazan product with cell numbers measured electronically. When we applied the assay to Aa23 cells, doubling times and levels of formazan product were comparable in the presence or absence of tetracycline. These results indicate that, at levels used to eliminate Wolbachia from cultured cells, tetracycline has no detectable effect on mitochondrial metabolism.
Materials and Methods
Cells and culture conditions
Mosquito cells were maintained in Eagle’s minimal medium supplemented with 5% (E-5 medium; C7-10 cells) or 20% (E-20 medium; Aa23 cells) fetal bovine serum as described previously (Shih et al. 1998; Fallon 2008). Cultures were grown at 28°C in a 5% CO2 atmosphere. Tetracycline stock (1 mg/ml) was prepared in water, filter-sterilized, and diluted into the culture medium to a final concentration of 10 μg/ml. C7-10 cells were resuspended by pipetting, diluted in isotonic saline, and counted using a Coulter electronic cell counter.
With Aa23 cells, plating of replicate cultures was optimized by treating the cells either as attached monolayers or as resuspended pellets after centrifugation, with medium containing 0.25% trypsin–ethylenediaminetetraacetic acid (Invitrogen Corp., Carlsbad, CA) for 10 min at room temperature. Serum-containing medium was added to inactivate trypsin, and the cells were passed through a 40-μm nylon strainer (BD-Falcon), counted using a Coulter electronic cell counter, and diluted to 1×105 cells per milliliter in a single batch of E-20 medium to provide sufficient material for an entire experiment. After distributing tetracycline-free cells (2 ml per 35-mm plate), tetracycline was added to the remaining medium at a final concentration of 10 μg/ml, and remaining cells were plated. At regular intervals, MTT assays were performed, and formazan pellets were stored at −20°C.
MTT assays
MTT ([3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide, cell culture tested]; Sigma-Aldrich Corporation, St. Louis, MO) was dissolved in phosphate-buffered saline (PBS; Dulbecco and Vogt 1954) at a final concentration of 5 mg/ml and sterilized by filtration through a 45-μm filter. MTT (100 μl) was added to cells maintained in 2 ml of medium in 35-mm culture dishes, and the cells were returned to the incubator for 1 h. Cells were directly resuspended in the culture medium using a Pasteur pipette and transferred to a 2-ml microcentrifuge tube. A purple pellet was collected by centrifugation at 10,000 rpm for 4 min. The supernatant was aspirated and discarded; pellets were stored at −20°C or processed immediately in acid isopropanol (0.04 N HCl in isopropanol).
Acid isopropanol (1 ml) was added to pellets, and the MTT formazan reaction product was leached from the pellet by incubation at 50°C for 15 to 30 min, with occasional vortexing. When the pellet lost color, tubes were centrifuged at 10,000 rpm for 4 min to clarify the supernatant by pelleting white cellular debris. The supernatant was read in a spectrophotometer at 570 nm, against an acid isopropanol blank. The conclusions drawn from each figure were verified in a series of experiments done with several independent batches of cells over a period of several months. Data shown in Figs. 2, 3, 4, and 5 are representative experiments, each with a single batch of cells. Values indicate averages, and error bars (Cumming et al. 2007) indicate maximum variation (5%) between replicates. On logarithmic scales, error bars are obscured by the data points.
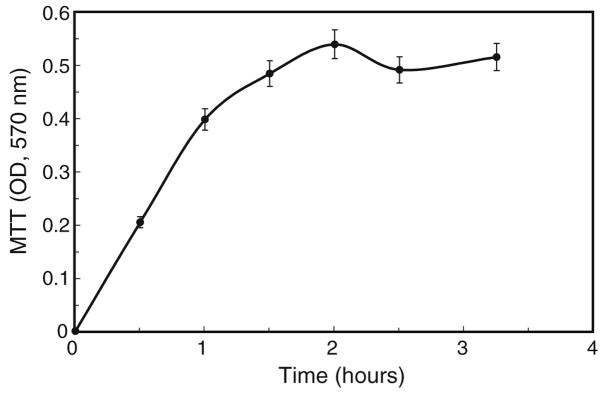
Figure 2.
Time course for MTT treatment. Cells (2.6×105 per 35-mm dish) were incubated with 100 μl of MTT (5 mg/ml) in 2 ml of culture medium, under standard growth conditions, for times ranging up to 3.5 h. Values are averages, ± 5%.
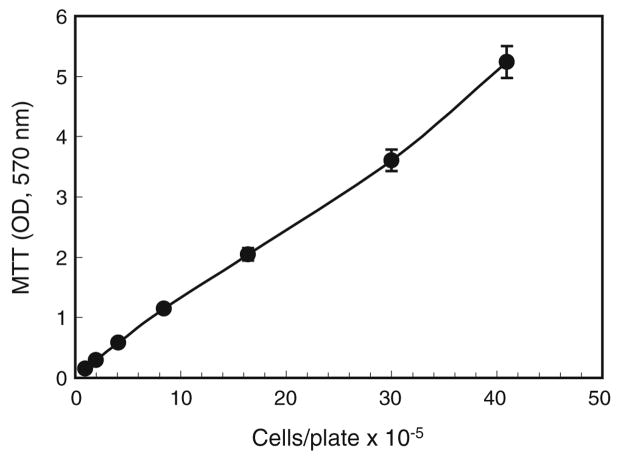
Figure 3.
MTT conversion as a function of cell numbers. A. albopictus C7-10 cells were plated in E-5 medium by twofold serial dilution. After 36 h, replicate plates were assayed by MTT conversion to formazan product during 1 h at 28°C, 5% CO2. OD570 readings were plotted against cell counts determined with a Coulter electronic cell counter. Values are averages from duplicate plates; error bars show ±5%. Samples with OD readings above 2 were diluted to accommodate the linear range of the spectrophotometer.
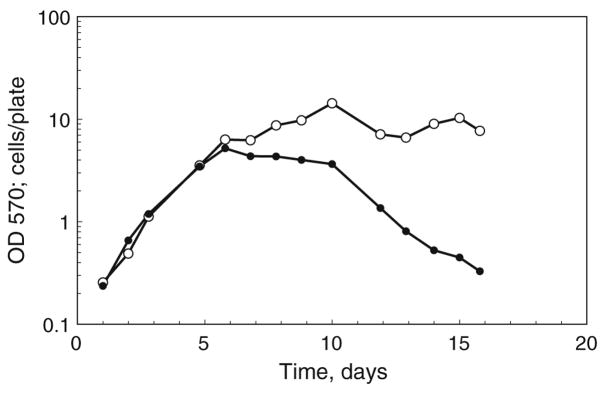
Figure 4.
MTT conversion and cell counts based on daily sampling. At daily intervals, two of four replicate plates were incubated with MTT for 1 h, and resulting pellets were stored at −20°C. The remaining plates were counted using a Coulter electronic cell counter (open circles). After 2 wk, all of the pellets (closed circles) were dissolved in acid isopropanol and read at 570 nm in a single assay. On the logarithmic scale, error bars are obscured by the data points.
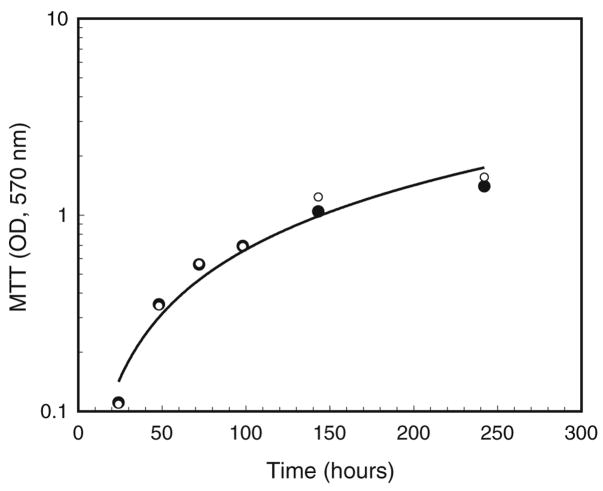
Figure 5.
Comparison of cell growth in the presence and absence of tetracycline. Cells were seeded at approximately 2×105 per 35-mm plate in E-20 medium, allowed to grow at 28°C, 5% CO2, and assayed with MTT for 1 h at the indicated time points. Solid circles indicate Wolbachia-infected cells grown in the absence of tetracycline; the R2 value for the computer-generated trendline was 0.950. Open circles (note superimposition of some points) indicate cells grown in the presence of tetracycline from the time of plating. The R2 value for these data was 0.961.
Fluorescent antibody staining
Freshly prepared cells were collected onto glass slides using a Cytospin centrifuge (Shandon, Inc., Pittsburgh, PA), dried briefly in air, fixed in acetone for 10 min, and dried. The sample was hydrated in PBS for 5 min at room temperature, followed by a 15 min incubation in blocking solution (1% bovine serum albumin in PBS). Blocking solution was replaced with monoclonal anti-heat-shock-protein 60 (HSP60), clone LK2 (Sigma, St. Louis, MO, product # H3524) diluted 1:100 in PBS, and the sample was incubated at 4°C overnight in a humidified chamber. Primary antibody was removed by two successive 10-min rinses in PBS. Secondary antibody (goat anti-mouse-IgG fluorescein isothiocyanate, human-adsorbed; Santa Cruz BioTechnology Inc., product # sc-2010; Santa Cruz, CA) was diluted 1:100 and incubated for 1–3 h at room temperature in the dark. Slides were rinsed with PBS three times for 10 min each and photographed under phase-contrast and fluorescence microscopy.
Results
Cells that grow as adherent clusters are difficult to quantify
We standardized the MTT assay using A. albopictus C7-10 cells, which grow as confluent monolayers that are easily dispersed by gentle pipetting and can be counted with high accuracy using a hemocytometer or with an electronic cell counter. Our goal was to develop an assay that could accurately quantify Aa23 cells, which are persistently infected with Wolbachia pipientis (O’Neill et al. 1997). During culture from low cell densities, Aa23 cells gradually form nonuniform clusters of tightly adherent cells that eventually float in the culture medium (Fallon 2008). These multicellular aggregates were easily visible by the eye after MTT staining (Fig. 1, arrow). By microscopic examination, cells appeared to be uniformly stained through the interior of the cluster.
Figure 1.
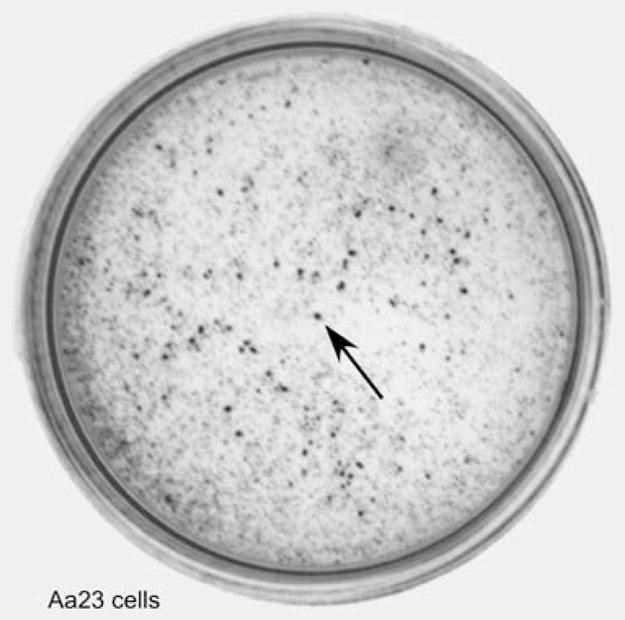
Appearance of Aa23 cells. Cells were trypsinized, strained, and plated at 2×105 cells per 35-mm plate. After several days, MTT was added to the medium, and the cells were photographed on a lightbox without magnification. The arrow indicates a cluster of the tightly adherent cells that accumulate during cell growth.
Standardization of the MTT assay with A albopictus C7-10 cells
We began this study by investigating the dynamics of MTT conversion to formazan product by A. albopictus C7-10 mosquito cells as a function of time. For use with microtiter plates, Mosmann (1983) suggested an incubation time of 4 h, with 0.1 volume of MTT added to the culture medium. We typically seed mosquito cells at 2×105 cells in 2 ml of medium per 35-mm plate and allow cells to grow until they reach or exceed confluence, depending on experimental design. Under these conditions, conversion of MTT to colored product was linear for about 1.5 h when 5 mg/ml MTT (100 μl) was added directly to 2 ml of medium (Fig. 2). After incubation under normal culture conditions (28°C, 5% CO2) for the indicated time, we resuspended the cells and collected the cell pellet by centrifugation. The supernatant was aspirated and discarded. As the incubation time increased beyond 2 h, the cell pellet became less compact, making aspiration of the medium less reliable. Control samples, incubated on ice to decrease metabolic activity, gave OD570 readings of 0.046 and 0.052 after 4 h.
Using smaller numbers of cells in microtiter plates, Mosmann (1983) terminated the MTT reaction by adding acid isopropanol directly to the culture medium. After mixing to dissolve the formazan crystals, samples were read at a test wavelength of 570 nm and a reference wavelength of 630 nm. When we used this protocol with cells in 35-mm plates, we noted that the resuspended cellular debris contributed unacceptable levels of turbidity to the sample, making spectrophotometric measurements less reliable over the range of cell concentrations used in our studies.
We found that the formazan product could be recovered by centrifugation. After aspiration of the supernatant, the purple pellet was dissolved in 1 ml of acid isopropanol by heating at 50°C with occasional vortexing. Once the color had leached from the pellet, samples were clarified by centrifugation, and the colored supernatant was transferred to a cuvette and read against an acid isopropanol blank at 570 nm. Note that, in our protocol, the cell culture medium was discarded.
To test the linearity of the reaction as a function of cell number, we assayed a twofold serial dilution of cells 48 h after plating. Cell number was obtained by counting replicate plates in a Coulter electronic cell counter. MTT conversion was proportional to cell numbers ranging from 1×105 cells per 35-mm plate to 4×106 cells per plate (Fig. 3). Note that, when readings exceeded the linear range of the spectrophotometer, the samples were diluted with acidified isopropanol, and values shown in Fig. 2 were adjusted for the dilution factor.
The MTT assay reflects metabolic activity in stationary-phase cells
Finally, to evaluate sequential samples taken over a series of days, we stored the formazan cell pellets at −20°C following daily sampling over a period of 2 wk. After collecting all of the samples, results from the MTT assay (Fig. 4, closed circles) were plotted together with counts from replicate samples determined with a Coulter electronic cell counter (Fig. 4, open circles). Over the first 5 d of growth, cell counts were proportional to formazan product, as would be expected from Fig. 3. As the cells reached the stationary phase of growth, cell counts based on physical particles remained high, while cell metabolism, as measured by formazan product, gradually decreased. In previous studies, we noted that, when plated in medium containing serum, C7-10 cells remain structurally intact and 80–90% viable, for up to 2 wk after plating (Schwientek et al. 2002). It is not surprising that, without replenishment of the medium, metabolic activity per cell decreases substantially, as shown by the MTT assay. In this respect, the MTT assay provides additional information that is not obvious on the basis of cell counts alone.
Effects of tetracycline on Wolbachia-infected cells
As shown in Fig. 1, Aa23 cells tend to aggregate as they grow, making quantification difficult. In an earlier study, we estimated cell counts on the basis of nuclei released from cells after treatment with NP-40. Although we saw no apparent difference between growth patterns of Wolbachia-infected and tetracycline-cured cells (Fallon 2008), the counts varied considerably, relative to counts with C7-10 cells. An important motivation for the present study was to identify a more accurate method for comparing growth of cultures that were less uniform than those of C7-10 cells. Over the course of an experiment, Aa23 cells ranged from attached cells to floating clusters, typically with mixtures of both in a single plate. In addition, while this work was in progress, Ballard and Melvin (2007) called into question the use of tetracycline to cure cells of Wolbachia, based on potential toxicity of tetracycline to mitochondria.
During the period of active cell growth, MTT assays gave nearly identical values for Aa23 cells maintained in the presence or absence of tetracycline, suggesting that, at 10 μg/ml, the antibiotic has little effect on growth of these cells (Fig. 5). The increase in Aa23 cell number was fastest immediately after plating, with a calculated doubling time of about 45 h at 0.3–0.6 OD570, increasing to about 70 h at 0.5–1.0 OD570. In medium containing 20% fetal bovine serum, Aa23 cell numbers continued to increase gradually to an OD570 of 2.2 or about 2.2×106 cells per plate, while C7-10 cells typically reached a maximum density of 6–8× 106 cells per plate in 5% serum. Thus, despite being provided with a richer medium, the growth rate of Aa23 cells was substantially slower than that of C7-10 cells, which have a generation time of 10–12 h when the cells are cycling most rapidly (Gerenday and Fallon 1996).
To verify that numbers of Wolbachia were in fact lower in tetracycline-treated cells, we collected cells by centrifugation onto slides, which were stained with an antibody to human HSP60 protein as described by Ferree et al. (2005), who used this reagent to stain Wolbachia in egg chambers of Drosophila melanogaster. Figure 6A,B shows fluorescence and phase-contrast photographs of a representative area of Aa23 cells grown without tetracycline, while panels Fig. 6C,D show cells grown in the presence of tetracycline. Although the distribution of Wolbachia among individual cells is variable (O’Neill et al. 1997) and tetracycline does not completely eliminate Wolbachia within a single passage (see O’Neill et al. 1997; Fallon 2008), fluorescent staining of the cells grown in the presence of tetracycline was substantially reduced, relative to cells grown in its absence. Giemsa-stained samples showed comparable reductions in Wolbachia abundance in tetracycline-treated cells but required visualization using a ×100 objective, under oil.
Figure 6.
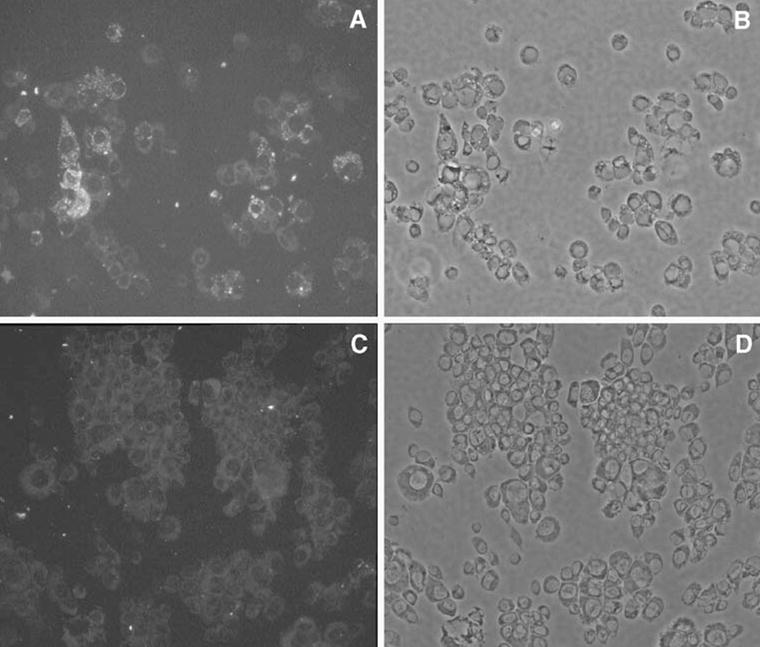
Immunohistochemical staining of Wolbachia. Cells were collected onto slides and stained with a monoclonal antibody to human HSP60 as detailed in the “Materials and Methods.” (A) and (B) show cells seeded at 2× 105 per 35-mm plate and grown in the absence of tetracycline for 2 wk, while (C) and (D) show cells maintained in tetracycline. Photographs were taken with a ×20 objective. (A) and (C) show fluorescence; (B) and (D) show corresponding phase-contrast images.
Discussion
When grown in tissue culture flasks, insect cell lines exhibit diverse morphologies, ranging from relatively uniform monolayers to clustered cell aggregates or even hollow floating vesicles (Fallon and Kurtti 2005). As cultures mature over several days, cell properties may change in response to nutrient conditions or experimental treatment as population growth in a confined space progresses from exponential to stationary phase. Because quantification of cell growth using standard approaches is less rigorous when cells cannot be uniformly dispersed, we sought alternative assays to examine metabolic properties of A. albopictus Aa23 cells, which develop tightly adherent cell clusters. Because Aa23 cells are persistently infected with W. pipientis (O’Neill et al. 1997), they provide an important in vitro system for probing metabolic interactions between an obligate intracellular bacterium and its insect host cell.
With Aa23 cells, we used trypsin and cell filtration to produce uniform replicates of starting cultures. As growth proceeds, MTT allows a more accurate estimation of Aa23 cell number than does enumeration of released nuclei (see Fallon 2008) or direct counting of cells after a second round of trypsinization. Based on the MTT assay, we show that growth of Aa23 cells slows down as cell numbers accumulate but is not measurably affected by relative levels of Wolbachia, which are reduced in the presence of tetracycline. Likewise, cured Aa23 cell populations that result from longer-term treatment with tetracycline, followed by polymerase-chain-reaction-based verification of the absence of Wolbachia, are microscopically similar to infected cells and continue to grow by gradual formation of solid floating cell aggregates (Fallon 2008). In ongoing studies, we anticipate that the sensitivity of MTT-based assays, coupled with use of radiolabeled precursors, will facilitate our efforts to understand the nature of the host cell interaction with Wolbachia.
MTT-based assays were developed as an alternative to the use of radiolabeled substrates to evaluate cell survival–proliferation of immune–responsive mammalian cells (Mosmann 1983) and had been used in a wide variety of applications (Berridge et al. 2005). For use with mosquito cells, we standardized the assay using the A. albopictus C7-10 cell line, which represents a clonal population of cells with uniform growth characteristics that make it ideally suited for quantification using a hemocytometer or electronic cell counter. Direct addition of MTT to the culture medium requires minimal disruption of the cells, and cells can metabolize MTT regardless of their state of attachment to the substrate or aggregation with one another.
During exponential-phase growth, we found excellent agreement between cell number using the MTT assay and cell counts. However, the MTT assay has increased sensitivity over cell counts when metabolic activity, rather than physical particles, may be the more important parameter. In complementary studies, we have shown, for example, that the MTT assay reflects decreased metabolic activity in cells treated with ultraviolet light or sodium azide. Controls using cells on ice show acceptably low background. To extend the present work with insect cell lines, we have explored the suitability of the MTT assay with dissected mosquito tissues. Although we have not rigorously quantified studies with tissues, the assay appears promising, both for visualizing tissue structure and for quantitative estimation of relative metabolic activities.
Acknowledgments
This work was supported by National Institutes of Health grant AI 070913 and by the University of Minnesota Agricultural Experiment Station, St. Paul, MN.
References
- Ballard JWO, Melvin RG. Tetracycline treatment influences mitochondrial metabolism and mtDNA density two generations after treatment in Drosophila. Insect Mol Biol. 2007;16:799–802. doi: 10.1111/j.1365-2583.2007.00760.x. [DOI] [PubMed] [Google Scholar]
- Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev. 2005;11:127–152. 11004–7. doi: 10.1016/S1387–2656(05). [DOI] [PubMed] [Google Scholar]
- Cumming G, Fidler F, Vaux DL. Error bars in experimental biology. J Cell Biol. 2007;177:7–11. doi: 10.1083/jcb.200611141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R, Vogt M. Plaque formation and isolation of pure lines with poliomyelitis virus. J Exp Med. 1954;99:167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon AM. Cytological properties of an Aedes albopictus mosquito cell line infected with Wolbachia strain wAlbB. In Vitro Cell Dev Biol Anim. 2008;44:154–161. doi: 10.1007/s11626-008-9090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon AM, Kurtti TJ. Cultured cells as a tool for analysis of gene expression. In: Marquardt WC, editor. Biology of disease vectors. 2. Elsevier; New York: 2005. pp. 539–549. [Google Scholar]
- Ferree PM, Frydman HM, Li JM, Cao J, Wieschaus E, Sullivan W. Wolbachia utilizes host microtubules and dynein for anterior localization in the Drosophila oocyte. PLoS Pathogens. 2005;12:E14. doi: 10.1371/journal.ppat.0010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerenday A, Fallon AM. Cell cycle parameters in Aedes albopictus mosquito cells. In Vitro Cell Dev Biol Anim. 1996;32:307–312. doi: 10.1007/BF02723064. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. 90303–4. doi: 10.1016/0022-1759(83). [DOI] [PubMed] [Google Scholar]
- O’Neill SL, Pettigrew MM, Sinkins SP, Braig HR, Andreadis TG, Tesh RB. In vitro cultivation of Wolbachia pipientis in an Aedes albopictus cell line. Insect Mol Biol. 1997;6:33–39. doi: 10.1046/j.1365–2583.1997.00157.x. [DOI] [PubMed] [Google Scholar]
- Schwientek MS, Higgins LA, Fallon AM. Cultured Aedes albopictus mosquito cells accumulate elongation factor 1-α (EF-1α) during serum starvation. Insect Biochem Mol Biol. 2002;32:1055–1063. doi: 10.1016/S0965-1748(02)00043–7. [DOI] [PubMed] [Google Scholar]
- Shih KM, Gerenday A, Fallon AM. Culture of mosquito cells in Eagle’s medium. In Vitro Cell Develop Biol Anim. 1998;34:629–630. doi: 10.1007/s11626-996-0010-1. [DOI] [PubMed] [Google Scholar]
- Stouthamer R, Breeuwer JAJ, Hurst GDD. Wolbachia pipientis: Microbial manipulator of arthropod reproduction. Annu Rev Microbiol. 1999;53:71–102. doi: 10.1146/annurev.micro. 53.1.71. [DOI] [PubMed] [Google Scholar]