Abstract
Glutamate signaling in the nucleus accumbens influences reinstatement of previously extinguished cocaine-seeking behavior in rats. Whether or not region specific glutamate signaling in the nucleus accumbens contributes to reinstatement of cocaine-seeking behavior is not known. We investigated whether directly stimulating ionotropic glutamate receptors (GluR’s) within the nucleus accumbens core or shell would differentially influence renewed cocaine-seeking behavior following extinction training. We also tested the hypothesis that GluR 1 subunit (GluR1) containing alpha-amino-3-hydroxy-5-methylisoxazole-4-proprionic acid (AMPA) receptors in the nucleus accumbens core and not the shell regulate reinstatement of previously extinguished cocaine-seeking behavior. Microinjection of AMPA into the nucleus accumbens shell and the nucleus accumbens core dose-dependently elicited significant cocaine-seeking behavior. Administration of antisense oligonucleotides (AS) directed against GluR1 subunit mRNA into the core and shell disrupted AMPA- and cocaine-primed reinstatement— with the most pronounced effects seen in the nucleus accumbens shell. These results demonstrate that GluR’s in the nucleus accumbens core and shell influence AMPA- and cocaine-primed reinstatement, yet the nucleus accumbens shell exerts a prepotency over the nucleus accumbens core.
Keywords: Rat, self-administration, relapse, intracranial, extinction, AMPA, GluR1
1. Introduction
Relapse to cocaine intake of following abstinence is a problematic obstacle in treatment of cocaine addiction (Kreek and Koob 1998). The role of glutamate signaling in reinstatement of cocaine-seeking behavior in preclinical models of cocaine relapse is emerging (Baker et al 2003; Cornish et al., 1999; Kruzich and Xi 2006; Sun et al., 2005; Suto et al., 2004). Inhibition of GluR’s in the ventral tegmental area dampens cocaine-primed reinstatement of previously extinguished cocaine-seeking behavior (Sun et al., 2005). Direct stimulation of GluRs in the nucleus accumbens (Cornish et al., 1999; Suto et al., 2004) and more specifically nucleus accumbens core (Kruzich and Xi, 2006) leads to significant reinstatement of previously extinguished cocaine-seeking behavior in rats. While it has been demonstrated that dopamine receptors in the nucleus accumbens shell and not the nucleus accumbens core influence cocaine-primed reinstatement (Anderson et al 2006; Schmidt et al 2006), determination of whether or not glutamate signaling in the nucleus accumbens shell is also involved in reinstatement of cocaine-seeking behavior has not been demonstrated.
In the present study we hypothesized that stimulation of GluR’s in the nucleus accumbens core and not shell would lead to significant reinstatement of previously extinguished cocaine-seeking behavior. This hypothesis was based on a prior study showing inactivation of the nucleus accumbens shell with GABA-A and GABA-B agonists does not disrupt cocaine-primed reinstatement in rats (McFarland and Kalivas 2001). We also sought to test the hypothesis that the specific ionotropic GluR subtype, type 1 (GluR1), regulates AMPA- and cocaine-primed reinstatement of cocaine-seeking behavior. We investigated GluR1 subunit because prior studies demonstrated robust increases of GluR1 subunits and not GluR2/3 expression in the nucleus accumbens of rats displaying behavioral sensitization to cocaine (Churchill et al. 1999; Boudreau et al. 2007). Currently, there are no commercially available specific GluR1 subunit agonists or antagonists. We therefore targeted GluR1 subunit receptor mRNA with antisense oligonucleotides (AS) in order to decrease GluR1 subunit protein expression in the nucleus accumbens core or shell of the nucleus accumbens.
2. RESULTS
2.1. Experiment #1: Dose-dependent AMPA priming
2.1.1. Histology of subjects used for data analysis
Figure 1 depicts representative photomicrographs from animals with appropriate cannula placement in the nucleus accumbens core (Figure 1A and B) and nucleus accumbens shell (Figure 1C and D). The group numbers used for statistical analyses were: nucleus accumbens core 0.3 nmol/side n=7, nucleus accumbens core 0.6 nmol/side n=7, nucleus accumbens shell 0.3 nmol/side n=8, and nucleus accumbens shell 0.6 nmol/side n=6.
Figure 1.
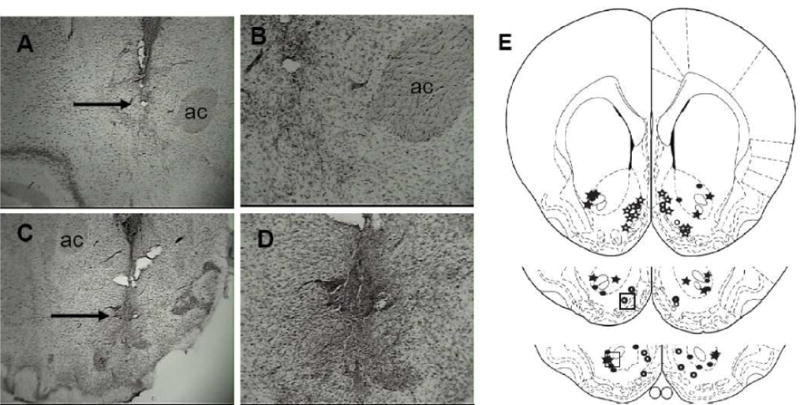
Cannula placement from representative nucleus accumbens core and shell rats in the AMPA-priming dose response study. 4x magnification of the: A) Core and C) Shell. 10x magnification of the same location in the: B) Core and D) Shell which are highlighted in the boxes in the cartoons depicted in E (based on Paxinos and Waston, 1998). The arrows in Figures 1A and 1C are pointing at the tips of the injection needles. Closed circles = 0.3 nmol/side core (n=7), open circles = 0.3 nmol/side shell (n=8), closed stars = 0.6 nmol/side core (n=7), open stars = 0.6 nmol/side shell (n=6). ac=anterior commissure.
2.1.2. Active Lever Responding
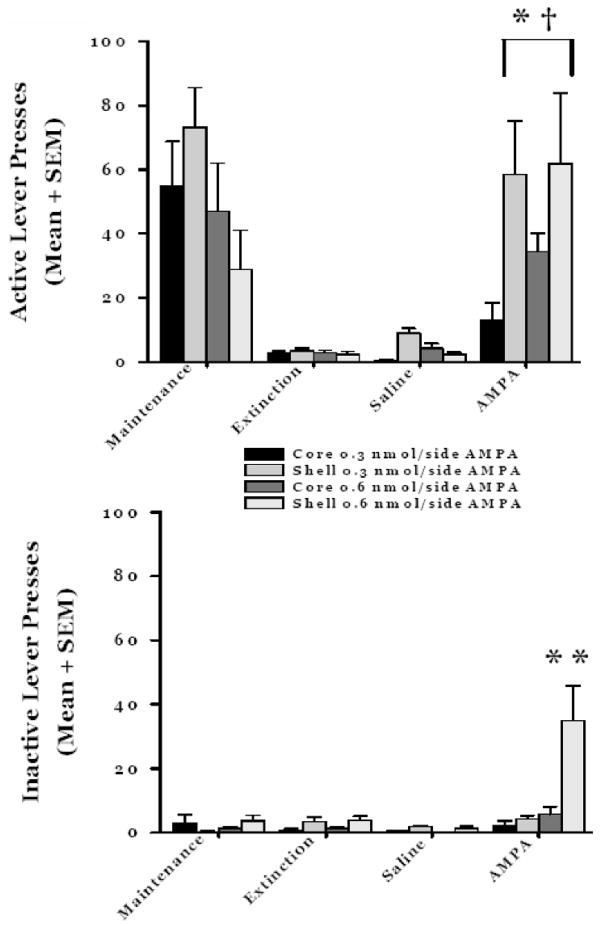
Figure 2 depicts the behavioral data for experiment #1. There was not an overall significant effect of treatment group on AMPA-primed reinstatement (F(3,26) = 2.10; p>0.1 2). There was a significant effect of test phase on responding (F(3,78) = 30.4; p<0.001). The rats emitted significantly more active lever responses during maintenance and AMPA test sessions compared to the last extinction session and the saline priming test session (p<0.05 for all comparisons). There was a significant group x test session interaction for active lever responding (F(9,78) = 2.3; p<0.05). Within the various groups, the rats challenged with 0.3 nmol/side AMPA (low dose) in the shell emitted the most responses during maintenance compared to all other phases (p<0.05 for all comparisons). The 0.3 nmol/side AMPA shell rats emitted significantly more responses during the maintenance phase compared the last day of extinction and the saline challenge (p<0.05 for all comparisons). Unlike the 0.3 nmol/side AMPA core group, the 0.3 nmol/side AMPA shell rats emitted significantly more responses following AMPA compared to extinction and saline phases (p<0.05). Response levels did not differ between the maintenance phase following the AMPA challenge for the 0.3 nmol/side AMPA shell group (p<0.2). The rats in the 0.6 nmol/side core and shell AMPA groups emitted significantly more responses during the maintenance and AMPA test sessions compared to the extinction and saline challenge sessions (p<0.05 for all comparisons). Response levels during maintenance and the AMPA challenge did not differ for the 0.6 nmol/side AMPA core rats (p=0.3), but the high dose shell rats emitted more responses during the AMPA challenge session compared to the maintenance session (p<0.05).
Figure 2.
Cocaine self-administration and AMPA-primed reinstatement. Top. Active lever responses. There were no differences in behavioral output for Shell or Core groups (F(1,57) = 0.77; p>0.05). There were differences in responding according to the particular stage of the experiment (F(3,57) = 27.13; p<0.001). The 0.3 nmol/side shell (n=8), 0.6 nmol/side shell (n=6), and 0.6 nmol/side core (n=7) rats emitted significantly more lever responses during the AMPA prime compared to extinction and saline priming test sessions (*p<0.05 for all comparisons). The 0.3 nmol/side shell, 0.6 nmol/side shell, and 0.6 nmol/side core rats emitted significantly more lever responses during the AMPA prime than did the 0.3 nmol/side core (n=7) group (†p <0.05 for all comparisons).
Bottom. Inactive lever responses. There was a significant group difference in response output on the inactive lever (F(1,57) = 24.74; p<0.001), significant differences in responding across the various test sessions (F(3,57) = 17.96; p<0.001) and a significant test session x group interaction (F(3,57) = 13.54; p<0.001). The 0.6 nmol/side Shell group rats emitted more responses than the either Core group and the 0.3 nmol/side Shell group during the AMPA-primed reinstatement test session (**p<0.05).
For the between groups comparisons of responding across the various test phases, several significant differences were found. The rats in the 0.3 nmol/side shell group emitted more responses during the maintenance phase than the rats in the 0.6 nmol/side core and shell groups (p<0.05 for each comparison). There were no group differences in response magnitude for the extinction and saline phases (p>0.5 for all comparisons). For the AMPA test session, rats administered 0.3 nmol/side and 0.6 nmol/side AMPA into the shell emitted significantly more responses compared to rats that received 0.3 nmol/side into the core (p<0.05 for all comparisons). There was a significant trend for the rats from 0.3 nmol/side shell group to respond more than rats that received 0.6 nmol/side AMPA in the core (p=0.06). The rats injected with 0.6 nmol/side AMPA in the shell emitted significantly more responses than the rats injected with 0.6 nmol/side AMPA into the core (p<0.05). Response output did not differ between 0.3 nmol/side shell rats and 0.6 nmol/side shell rats (p=0. 81).
2.1.3. Inactive lever presses
There were significant differences between the groups for inactive lever responding (F(3,26) = 11.01; p<0.001). Rats from the 0.6 nmol/side shell group responded significantly more on the inactive lever than all other groups (p<0.05 for all comparisons). A significant effect of test phase was also determined (F(3,78) = 16.9; p<0.001); significant levels of responding on the inactive lever occurred during the AMPA challenge compared to all other phases (p<0.05 for all comparisons). The test phase x group interaction was significant (F(9,78) = 8.0; p<0.05). For the within group comparison, only the 0.6 nmol/side shell group demonstrated an elevation of responding on the inactive lever during the AMPA challenge compared to all other test phases (p<0.05 for all comparisons). For the between group analyses, the groups did not differ in responding during the maintenance, extinction, or saline phases of this experiment (p>0.3 for all comparisons). However, the 0.6 nmol/side shell rats emitted significantly more responses following the AMPA challenge compared to all other groups (p<0.05 for all comparisons).
2.2. Experiment #2: Involvement of Nucleus Accumbens Core and Shell GluR1 subunits in AMPA- and Cocaine-primed Reinstatement
2.2.1. Timeline
Figure 3 depicts the timeline for the procedures utilized in experiment #2.
Figure 3.

Timeline for Experiment #2. The order of experimental procedures for the GluR1 subunit antisense experiment. Animals were pretreated with AS, Scramble, or Saline for 2-days prior to the first test session (Prime 1). No AS, Scramble, or Saline was administered during the test session. The AS, Scramble, or Saline regimen was started again the next day following the first test session. The “primes” were AMPA (0.6 nmol/side) and cocaine. Each animal received an AMPA prime during one test session and cocaine priming in the other test session. The testing order was counterbalanced; some animals received AMPA during the first test session and cocaine during the second test session. For other rats, the opposite order was used. Each animals was primed with AMPA and cocaine just one time.
2.2.2. Flourescein-labeled oligonucleotide histology
Figure 4 depicts the spread of flourescein-labeled oligonucleotides under 10x magnification. The animals were killed 6-hrs following the microinjections. The majority of fluorescent staining was located in the specific target region. There was some avoidable spread into adjacent regions. However, the concentration (intensity of labeling) of flourescein-labeled oligonucleotides in adjacent tissue was extremely low relative to the target regions.
Figure 4.

Flourescein labeled oligonucleotide uptake. Photomicrographs showing tract damage and oligonucleotide (1 nmol) spread 6-hr following injection. Left 10x magnification under light microscopy, Middle fluorescence microscopy at 10x magnification, Right a cartoon depicting the spread of fluorescent marker. The dotted line depicts the tract left by the injection needle and the box represents the area where the most intense labeling was seen utilizing fluorescence microscopy.
2.2.3. Western blot analysis of GluR1 subunit protein expression
Figure 5 shows representative Western blots of tissue samples obtained from Saline (SAL), antisense (AS) or scrambled (SCR) oligodeoxynucleotide injected rats. Tissue was analyzed for GluR1 subunit and actin abundance. Densitometric analysis of GluR1 subunit/actin ratio showed a 34.6% decrease in GluR1 subunit abundance in the antisense group compared that of saline and scrambled treated rats.
Figure 5.
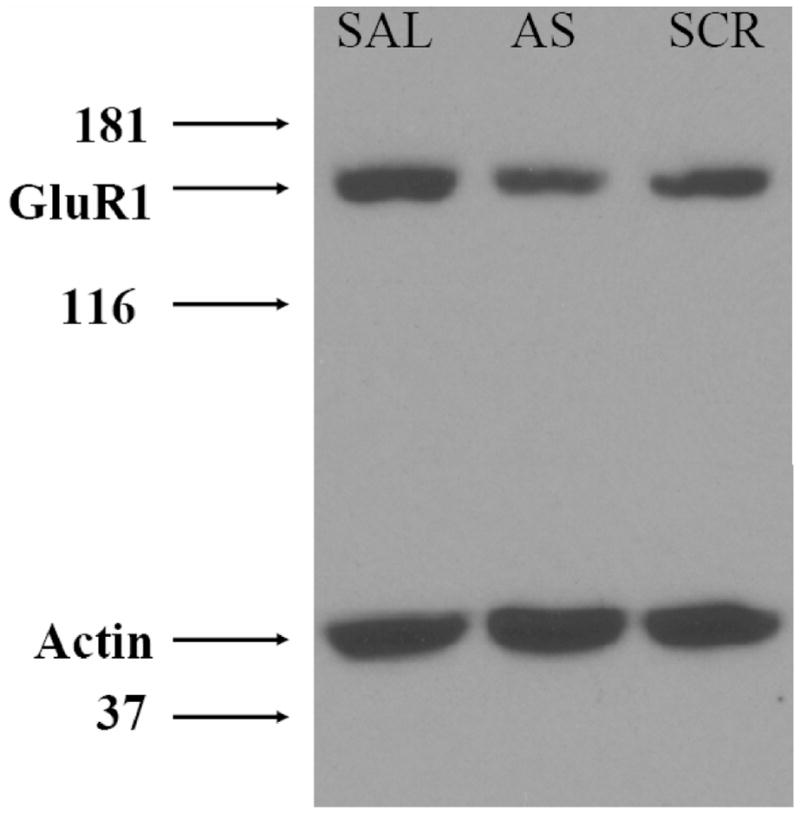
A Representative Immunoblot of GluR1 subunit expression in the nucleus accumbens following repeated administration of GluR1 subunit anitsense. Adminstration of GluR1 subunit antisense was associated with a 36% decrease in GluR1 subunit protein expression compared to saline (SAL) and scramble (SCR) treated controls. This particular immunoblot was from a nucleus accumbens core subject.
2.2.4. Histology of subjects used for behavioral data statistical analysis
Figure 6 depicts representative photomicrographs from animals with appropriate cannula placement in the nucleus accumbens core (Figure 6A and B) and nucleus accumbens shell (Figure 6C and D). The group numbers used for statistical analyses were: saline pretreated nucleus accumbens core n=7, saline pretreated nucleus accumbens shell n=7, scramble pretreated nucleus accumbens core n=6, scramble (SCR) pretreated nucleus accumbens shell n=6, antisense (AS) pretreated nucleus accumbens core n=5, AS pretreated nucleus accumbens shell n=7.
Figure 6.
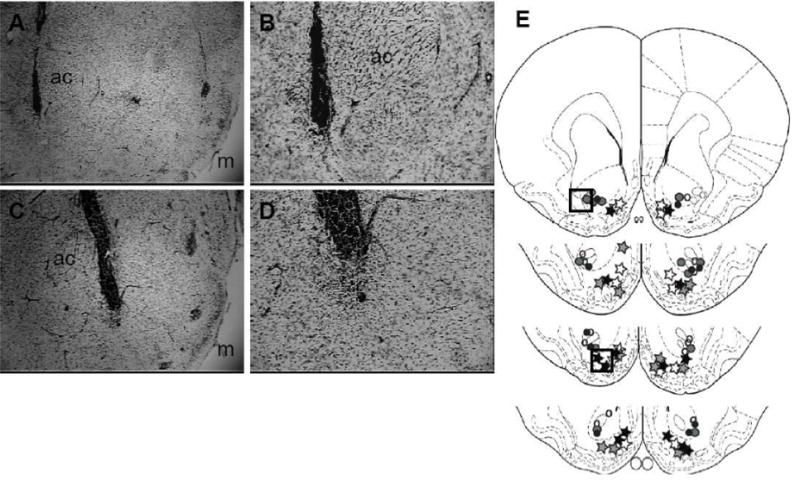
Cannula placement from representative nucleus accumbens core and shell rats in the GluR1 subunit antisense study. 4x magnification of the: A) Core and C) Shell. 10x magnification of the same location in the: B) Core and D) Shell which are highlighted in the boxes in the cartoons depicted in E. The total number of subjects per group was: nucleus accumbens core SAL n=7 (open circles), nucleus accumbens core SCR n=6 (grey circles), nucleus accumbens core AS n=5 (filled circles), nucleus accumbens shell SAL n=7 (open stars), nucleus accumbens shell SCR n=6 (grey stars) and nucleus accumbens shell AS n=7 (closed stars). ac=anterior commissure; m=medial aspect of the brain.
2.2.5 Active Lever Responding
There was an overall significant effect of “group” on active lever responding (F(5,32) = 7.80; p <0.001; Figure 7, Top) . The core and shell AS groups responded significantly less on the active lever than the Scramble and Saline groups (p <0.05 for each comparison). There was a significant effect of test phase (F(3,96) = 39.51; p <0.001). The majority of responses were emitted during the maintenance phase compared to all other phases (p <0.05 for all comparisons). The lowest total number of responses was emitted during the extinction phase (p <0.05 for all comparisons). There was a significant group x testing phase interaction (F(15, 96) = 4.15).
Figure 7.
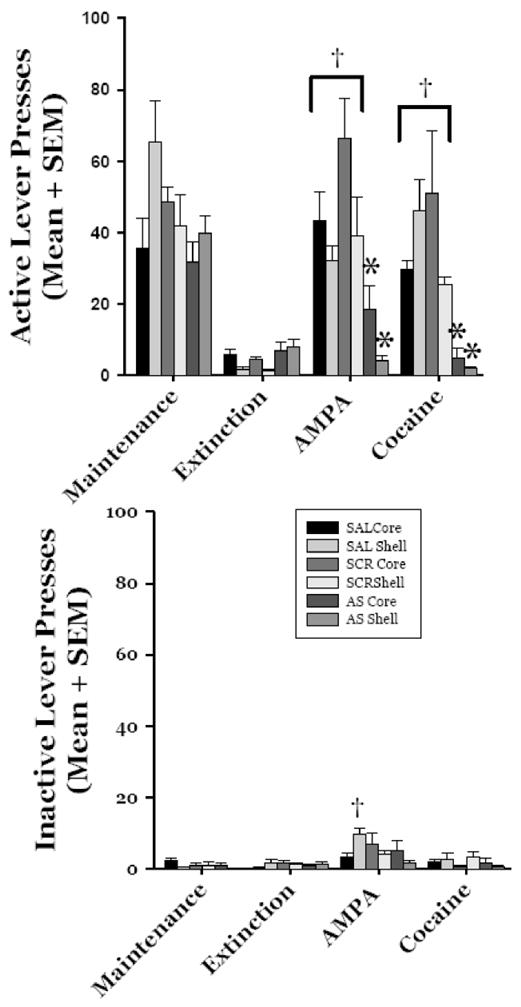
GluR1 subunit and cocaine- and AMPA-primed reinstatement. Top. Active lever responses. GluR1 subunit antisense significantly decreased responding on the active lever following AMPA- (0.6nmol/side) and cocaine-priming (10 mg/kg/ip) compared to SCR and SAL groups (*p <0.05 for all comparisons). All of the SAL and SCR groups demonstrated a significant reinstatement of responding compared to extinction response levels following the AMPA and cocaine challenges (†p <0.05 for all comparisons). Bottom. Inactive lever responses. 0.6 nmol/side in the nucleus accumbens shell led to a significant increase in inactive lever responding in the SAL pretreated group compared to extinction levels of responding (†p <0.05). Please see RESULTS for complete description of test results.
Rats pretreated with saline in the nucleus accumbens core emitted significantly fewer responses during the extinction phase compared to all other phases (p <0.05 for all comparisons); response levels demonstrated during the maintenance, cocaine, and AMPA challenge did not differ for the rats pretreated with saline in the nucleus accumbens core (p >0. 1 for all comparisons). Rats pretreated with saline in the nucleus accumbens shell emitted the fewest number of responses during the extinction phase compared to all other testing conditions (p <0.05 for all comparisons). Surprisingly, the nucleus accumbens shell saline group responded significantly more during the maintenance phase compared to all other phases (p <0.05 for all comparisons). Careful analysis of the data from each subject in the nucleus accumbens shell saline pretreated group yielded no outliers (individual subject response levels above or below 2 standard deviations from the group average). Response levels during the cocaine and AMPA test sessions did not differ for the nucleus accumbens shell saline pretreated group (p =0. 11).
The nucleus accumbens core Scramble rats and the nucleus accumbens shell Scramble rats emitted significantly fewer responses during the extinction phase compared to all other test phases (p <0.05 for both sets of comparisons). Both Scramble groups emitted similar levels of responses during the maintenance, cocaine challenge, and AMPA challenge phases (p>0. 1 for all comparisons).
The nucleus accumbens core AS group emitted significantly more responses during the maintenance phase compared to the extinction and cocaine test phases (p<0.05). Response levels demonstrated during the maintenance and AMPA phases did not significantly differ for the nucleus accumbens core AS group (p=0.21). The nucleus accumbens shell AS group responded more during the maintenance phase compared to the extinction, cocaine challenge, and AMPA challenge phases (p<0.05 for all comparisons).
The nucleus accumbens shell Saline group responded significantly more during the maintenance phase than the: nucleus accumbens core saline and AS groups (p<0.05 for both comparisons) and the nucleus accumbens shell Scramble and AS groups (p<0.05 for both comparisons). The nucleus accumbens shell Saline group emitted significantly more responses during maintenance than the: nucleus accumbens core Saline, nucleus accumbens core AS, nucleus accumbens shell Scramble, and nucleus accumbens shell AS groups (p<0.05 for all comparisons). There were no group differences in responding during the extinction phase (p>0.05 for all comparisons).
Significant group differences in active lever responding following the AMPA challenge (0.6 nmol/side) were found. The nucleus accumbens core Saline group emitted significantly more responses following AMPA than nucleus accumbens core AS and nucleus accumbens shell AS groups (p<0.05 for both comparisons). The nucleus accumbens shell Saline group responded more following AMPA compared to the nucleus accumbens shell AS group (p<0.05). The nucleus accumbens core Scramble group emitted significantly more active lever responses following AMPA than the nucleus accumbens core Saline and AS groups (p<0.05 for both comparisons) and all of the nucleus accumbens shell treatment groups following the AMPA challenge (p<0.05 for all comparisons). The nucleus accumbens shell Scramble group emitted more responses following the AMPA challenge compared to the nucleus accumbens shell AS and nucleus accumbens core AS groups (p<0.05 for all comparisons). The nucleus accumbens shell AS group emitted significantly less responses following AMPA compared to both sets of Saline and Scramble treated groups from both brain areas (p<0.05 for all comparisons). The nucleus accumbens core AS and shell AS groups did not differ in responding following the AMPA challenge (p>0.05).
The groups differed in response output during the cocaine challenge. Specifically, injection of AS into either the nucleus accumbens core or shell significantly reduced responding compared to the Saline and Scramble pretreated groups from both nucleus accumbens regions (p<0.05 for all comparisons). Unexpectedly, the nucleus accumbens shell Saline and nucleus accumbens core Scramble groups emitted significantly more responses during the cocaine challenge than all other groups (p<0.05 for all comparisons). The response levels demonstrated by the nucleus accumbens shell Saline and nucleus accumbens core Scramble groups did not differ during cocaine challenge test session. The nucleus accumbens core saline and nucleus accumbens shell Scramble groups did not differ in response output following the cocaine challenge. Table 1 depicts the active lever responding and bodyweights from all subjects during intracranial treatments of the various constructs during extinction. These data are reported in order to demonstrate that AS did not disrupt basal response rate or basic drive reduction behavior such as food consumption in the home cage.
TABLE 1.
Behavioral output and body weights assessed during intracranial administration of antisense, scramble construct, and saline during the experiment.
| Responses during construct treatment: Mean (SEM) | % increase in body weight (extinction day 6 vs. last injection: Mean (SEM)) | |
|---|---|---|
| Saline Core | 4.094(0.720) | + 4.047 (0.42) |
| Saline Shell | 5.655(0.779) | +4.051(0.67) |
| Scramble Core | 5.857(0.890) | +4.436(0.44) |
| Scramble Shell | 4.679(1.058) | +3.63 (0.544) |
| Antisense Core | 3.375(1.206) | +4.834 (0.62) |
| Antisense Shell | 3.938(0.823) | +3.632(0.62) |
There were no significant differences in response rates (F=1.1; p=0.34) or body weights (F=0.7; p=0.64) during intracranial administration of any of the constructs. These data demonstrate that differences in behavior during AMPA and cocaine priming were due to the direct effects of the constructs on GluR1 expression and not rate decreasing or anorexic effects of antisense.
2.2.6 Inactive Lever Responding
There was not an overall significant effect of “group” on inactive lever responding (F(5,32) = 2.38; p >0.05; Figure 7 Bottom). There was a significant effect of “test phase” on inactive lever response levels (F(3,96) = 24.03; p <0.001). Significantly more responses were emitted on inactive levers during the AMPA test phase compared to all other phases (p <0.05 for all comparisons). Response output did not statistically differ across maintenance, extinction, or cocaine test phases (p >0.05 for all comparisons). There was a significant group x test phase interaction (F(15, 96) = 2.57; p <0.01). Post-hoc comparisons revealed that rats in the nucleus accumbens shell Saline group emitted significantly more responses on the inactive lever during the AMPA test phase compared to all other groups (p <0.05) accept the nucleus accumbens core Scramble group (p =0.07). All other groups did not differ in inactive lever responses across all the various test phases.
3. DISCUSSION
The present study demonstrates that the nucleus accumbens core and shell play significant roles in AMPA- and cocaine-primed reinstatement—with the shell showing a level of ascendancy that was not anticipated. This study also supports very recent findings showing the specific role of GluR1 subunit in the nucleus accumbens shell in reinstatement of cocaine-seeking behavior and a partial role of GluR1 subunits in the nucleus accumbens core in AMPA-primed reinstatement (Anderson et al 2008).
The nucleus accumbens is parceled into 3-major regions: the rostral pole, the lateral core, and the medial shell (Zahm and Brog 1992). An elegant study demonstrated that the nucleus accumbens core mediates the goal-directed and contingency based behavioral output of cocaine seeking behavior, whereas the nucleus accumbens shell mediates the “stimulant” or “excitatory” characteristics of cocaine (Ito et al 2004). The dopaminergic innervation of the nucleus accumbens shell has received extensive investigation in active cocaine self-administration (e.g. Caine et al 1995; Lecca et al. 2007) and cocaine-primed reinstatement (e.g. Anderson et al. 2003). Injection of dopamine receptor antagonists into the nucleus accumbens shell shifts the cocaine self-administration dose-response curve to the right (Caine et al 1995) and reduces cocaine-primed reinstatement (Anderson et al. 2003). Our present findings identify a role of the nucleus accumbens shell in AMPA- and cocaine-primed reinstatement of cocaine-seeking behavior.
In the present study, direct stimulation of ionotropic glutamate receptors in the nucleus accumbens core led to robust and dose-dependent reinstatement of cocaine-seeking behavior, in agreement with previous reports (Cornish et al., 1999; Kruzich and Xi 2006; Suto et al., 2004). AMPA administered into the nucleus accumbens shell resulted in significant elevation ofresponding at a much lower dose compared to the nucleus accumbens core (Figure 2). Administration of 0.6 nmol/side AMPA was necessary for evoking significant responding in the nucleus accumbens core group. The shell animals demonstrated a prepotency in sensitivity to AMPA compared to the core group; significant reinstatement was demonstrated following 0.3 nmol/side AMPA in the shell and not the core. The higher concentration of AMPA (0.6 nmol/side) led to a generalized stimulant response in the shell group—as seen by the significant increase in inactive lever responses in both experiments. Perhaps excitatory neurotransmission in the more limbic shell acts to stimulate (Ito et al. 2004) and drive behavioral output that is mediated through the motor circuitry influenced nucleus accumbens core (Groenewegen et al 1990; Ito et al 2004; Kalivas and Volkow 2005; Zahm and Brog 1992). Indeed, a hypothesis regarding potential “reentrant loops” between the nucleus accumbens core and shell has been posited (van Dongen et al 2005). At the very least, this body of data and the present study further supports the seminal hypothesis that the nucleus accumbens serves as a dynamic limbic/motor interface in the brain (Mogenson et al 1980).
Maximal behavioral output following 0.6 nmol/side AMPA into the shell differed between experiment #1 and #2. The inactive lever responding following AMPA administration into the shell also differed across experiments. Possible explanations for differences in responding could be attributed to increased mechanical damage to the brain due to additional injections for experiment #2, pernicious chance, or some combination of both factors. Nevertheless responding was significantly increased in both shell groups following AMPA across both experiments.
Cocaine priming was robust in the saline pretreated groups in experiment #2. The overall conclusion we can make is relative to its saline control counterpart in the same sub-nucleus of the nucleus accumbens, AS decreased cocaine-primed reinstatement. The identified differences in total behavioral output for the control groups seen in experiment #2 were unexpected. Possible explanations could be differences in mechanical damage, differential and site-specific influences of nonsense sequence (Scramble groups) or some unidentified environmental influence. However our data are in agreement with an earlier study suggesting a specific role of GluR’s in cocaine-primed reinstatement (Cornish and Kalivas 2000; Anderson et al. 2008).
Despite our current findings and others (Anderson et al. 2008), we must mention McFarland and Kalivas (2001) reported that inactivation of neural transmission within the nucleus accumbens shell with a combination of muscimol and baclofen failed to disrupt cocaine-primed reinstatement of cocaine-seeking behavior. Despite this, two previous studies from this same group support our present results (Cornish et al. 1999; Cornish and Kalivas 2000). In these corroborating studies, glutamate signaling in the nucleus accumbens core and shell was manipulated during cocaine- and AMPA-primed reinstatement of cocaine-seeking behavior. Cornish and Colleagues reported that microinjection needle tips were located in both the nucleus accumbens core and shell, and no mention of site specific differences in response output following manipulations in either structure were mentioned (Cornish et al. 1999; Cornish and Kalivas 2000). Differences in training (there was no set extinction period for McFarland and Kalivas, 2001 versus our set criteria), and priming methods (baclofen and muscimol versus AMPA and GluR1 subunit AS) may account for disparities between McFarland and Kalivas (2001) and others (e.g. Anderson et al. 2003; Anderson et al. 2008; Cornish et al. 1999; Cornish and Kalivas 2000; Ito et al. 2004) including our present findings (Figure 7) demonstrating that the nucleus accumbens shell does indeed drive cocaine-seeking behavior. Future studies should be conducted to determine if the influence of GluR1 AS in the nucleus accumbens core and shell is specific to just cocaine, or would also generalize to other classes of abuse drugs such as opiates and renewed food-seeking behavior following extinction training.
4. EXPERIMENTAL PROCEDURES
4.1 Experimental Animals and Surgery
Male Fischer 344 (F344) rats (Harlan, Indianapolis, IN, USA) were used in this experiment. Animals weighed on average 275 g at the beginning of the experiment and were individually housed and maintained on a 12-hr light/dark cycle (lights on 0700 hrs) in a temperature and humidity controlled vivarium. Rats were kept at 90% of their individual ad libidum weights during food training and sessions 1–7 of cocaine self-administration by feeding them approximately 20 g of food/day. Following self-administration session 7, rats received 30 g of food each day for the remainder of the experiment based on Kruzich and Xi (2006). All protocols were approved by the Institutional Animal Care and Use Committee at the Medical College of Georgia, and were in compliance with “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003).
We anesthetized rats with a combination of 90 mg/kg ketamine and 2 mg/kg xylazine via an intraperitoneal (ip) injection. Rats were implanted with SILASTIC (Dow Corning, Midland MI, USA) catheter (ID = 0.21 mm, OD = 0.94 mm) catheters and indwelling guide cannula (Plastics One, Roanoke, VA, USA) according to previously described methods (Kruzich and Xi, 2006). The bilaterally implanted 26-g guide cannulae were aimed at the lateral nucleus accumbens core (Anterior +1.2, Lateral = +2.5, Ventral = −5.4, or medial shell (Anterior = +1.3, Lateral = +2.5, Ventral = −6.1 at a 10 angle) relative to the skull surface and bregma (Paxinos and Watson, 1998). The cannulae were secured by imbedding jeweler screws into the skull and covering the cannulae and screws with dental acrylic. Anesthesia was boosted intravenously (iv) by slowly infusing 0.2 ml of a 20 mg/ml concentration of ketamine. Dummy cannulae were placed into the intracranial guide cannula following surgery. Rats received 0.1 ml/intramuscular cefazolin (100 mg/ml), a broad spectrum antibiotic, prior to surgery. Antibiotics were not used following surgery. Rats received 0.1 ml of sterile 1 00U/ml heparinized saline solution daily following self-administration sessions for the remainder of the experiment. All rats received a minimum of 7-days of recovery from surgery.
4.2 Chemicals
Cocaine HCl (cocaine) and AMPA were purchased from Sigma-Aldrich (St Louis, MO, USA) and pentobarbital sodium was purchased from Ovation Pharmaceuticals, Inc. (Deerfield, IL). Flourescein-labeled randomized phosphorothioate oligos (FITC-oligos), GluR1 subunit antisense (AS), GluR1 subunit scramble sequence control (Scramble), and AS vehicle (Saline) were purchased from Biognostik (Göttingen, Germany).
4.3 Apparatus
Lever training and cocaine self-administration experiments were conducted in 16 operant conditioning chambers (Coulbourn Instruments, Allentown, PA, USA). Each chamber was housed in a sound-attenuating cubicle. The chambers had two retractable levers at the front, a removable pellet hopper, and a house light located outside the chamber. Intravenous cocaine was delivered through one channel of 2-channel liquid swivels (Instech, Plymouth Meeting, PA, USA) by selectable speed infusion pumps (model A73-02-SEL, Razel Scientific Instruments, St. Albans, VT, USA). The behavioral programs, pumps, and data collection were controlled and collected by a PC clone computer (Colbalt, Allentown, PA, USA) that ran Graphic State Notation 3.0 software (Coulbourn Instruments, Allentown, PA, USA).
4.4 Procedure
4.4.1 Food Training
Rats were food-restricted to approximately 90% of their free-feeding weights. Rats were then trained to lever press for 45-mg food pellets (Bio-Serv, Frenchtown, NJ, USA) during a 1-hr training session according to a continuous reinforcement schedule. Rats responded for food for 5-sessions. Our criterion for successful acquisition of operant lever pressing was successfully earning a minimum of 100 food pellets in a 1-hr session. We removed the food hopper from the particular rat’s chamber when it met our lever pressing criterion.
4.4.2 Cocaine Self-administration
After surgical recovery, rats received access to cocaine during 2-hour self-administration sessions 7-days a week. Lever pressing on the active cocaine lever was reinforced according to a fixed ratio-1 (FR-1) schedule of reinforcement with a 10-sec timeout. A reinforced response resulted in a 5-sec infusion of cocaine (0.5 mg/kg/iv in a volume of 0.05 ml) plus a 5-sec timeout. Responses emitted during the infusion or timeout or on the inactive lever resulted in no programmed consequences, but were recorded. In order to account for the dead volume of the catheter and to avoid passively infusing cocaine to load catheters at the beginning of the self-administration sessions, the first reinforced infusion lasted 9-sec plus a 1-sec time out, resulting in a full catheter and an infusion bolus in the rats’ bloodstream of 0.05 ml. The rats self-administered cocaine for at least 14 sessions. Our criteria for successful cocaine self-administration were: a minimum of 10 infusions/session, at least 85% of responses were emitted on the active lever, and intake could not vary by over 20% the last 3-days of self-administration.
4.4.3 Extinction
Following cocaine self-administration, all rats underwent 6 extinction sessions across consecutive days. During extinction sessions, responses on the previously active lever resulted in no programmed consequences, but were recorded. After completing 6 extinction sessions (responding had to be at or below 20% of self-administration levels), rats were prepared for the pharmacological reinstatement procedures. To habituate animals to the priming regimens, rats received an ip injection of saline, and an intracranial injection of saline (AMPA vehicle) or AS vehicle (saline) prior to beginning extinction sessions 5 and 6.
4.4.4 Intracranial Infusion
The procedures for intracranial injections are fully explained elsewhere (Kruzich and Xi, 2006). Briefly, 33-gauge needles that extended 2-mm beyond the 26-gauge guide cannulae were gently inserted just prior to all test sessions. The infusion volume for AMPA (0.5μl/side) was delivered over 2-minutes and the infusion bolus for oligonucleotides (AS, Scramble or Vehicle;1.0 μl/side) was delivered over 5-minutes by an infusion pump (model PHD 2000, Harvard Apparatus, Holliston, MA, USA). The needles were left in place for 1-min following the infusions in order to allow diffusion of drug/constructs from the needle tips.
4.4.5 Antisense Oligonucleotides
We used antisense oligonucleotides targeting the GluR1 subunit receptor subunit mRNA (AS; sequence 5′-TGA GAG CTT CCT GTA GTT -3′) designed by Biognostik (Göttingen, Germany). The sequences were based on GenBank accession number NM_03 1608. The sequences were further validated by use of R.A.D.A.R. (Rational Algorithmic Design of Antisense Reagents) software (Biognostic, Göttingen, Germany) and immunohistochemistry. Lyophilized AS was dissolved in sterile saline and adjusted to a final concentration of 1 nmol/1 l. We injected 1 l of Vehicle, Scramble (5′-ACT ACT ACA CTA GAC TAC - 3′), or AS into the nucleus accumbens core or shell of rats per day over 4-days (2 days in a row, one day off for testing, then another 2 days in a row). Each injection was slowly infused over 5-min to reduce mechanical tissue damage. Immediately following the last testing session, the rats were anesthetized with intravenous pentobarbital (50 mg/ml) and transcardially perfused with 10% formalin for histology of needle placement. Non-perfused brains from a small number of rats (n=3) from Vehicle, Scramble, and AS groups were harvested and prepared on ice for Western Blotting for quantification of GluR1 subunit protein expression.
4.4.6 Tracing AS uptake with FITC-labelled oligonucleotides
A frequent and justified concern regarding intracranial injections is the spread of administered constructs. We addressed this issue in a separate set of animals (n=4) injected with flourescein-labeled randomized phosphorothioate oligos (FITC-oligos). Rats were injected with FITC - oligos into the nucleus accumbens core (n=2) or shell (n=2) in a total volume of 1 -l/side over 5-minutes (Biognostik, Göttingen, Germany). Approximately 6-hrs later, the approximate time of optimal cellular uptake reported by the manufacturer, rats were euthanized by rapid decapitation and brains were harvested on ice. Brains were blocked and submerged in embedding media and rapidly frozen at - 8 0 C . Ten micron sections were made with a cryostat and sections were thaw mounted onto glass slides and fixed in 5% paraformaldehyde and then dehydrated in a graded series of alcohol. Sections were then viewed with a fluorescence microscope (Carl Zeiss, Thornwood, NY).
4.4.7 AMPA-Primed Reinstatement
Rats were bilaterally pretreated with intracranial physiological saline 1-day prior to receiving AMPA. During the AMPA-primed reinstatement session, rats received an intracranial injection of AMPA—0.0, 0.3, or 0.6 nmol/side. The doses are based on a previous study where we thoroughly investigated the dose-dependency of AMPA-primed reinstatement in F344 rats (Kruzich and Xi 2006). Responding during this test session resulted in no programmed events but was recorded.
4.4.8 Cocaine-Primed Reinstatement
Rats received 10.0 mg/kg/ip cocaine (10 mg/ml) at the beginning of an extinction session. Responding resulted in no infusions or programmed consequences for the duration of the 2-hr test session. This priming dose was selected based on our previous work with F344 rats and cocaine-priming (Kruzich and Xi 2006).
4.4.9 Western Blot Analysis
Nucleus accumbens tissue surrounding the microinjection site was dissected from 1 mm slices immediately following the last behavioral session with use of a brain matrix (Braintree Scientific, Braintree, MA). The tissue was homogenized in RIPA buffer and protein content of samples was analyzed using BCA kit (Pierce, Rockford, IL). 15 μg of protein samples were resolved on 10% PAGE-gels and the blots were sequentially probed with GluR1 subunit (rabbit polyclonal, Upstate Cell Signaling, Lake Placid, NY) and -Actin (goat polyclonal, Sigma, St. Louis, MO) antibodies. Appropriate HRP-conjugated secondary antibodies were used to detect the protein bands. Densitometric analysis of bands corresponding to these two proteins was performed by Scion Image software after subtracting the background intensities. Ratio of GluR1 subunit to actin was analyzed to control for gel loading variability.
4.4.10 Histological Verification of Injection Needle Placement
When testing ended, animals were anesthetized with 0.5 ml iv of 50 mg/ml pentobarbital. Rats were transcardially perfused with an ice-cold saline rinse followed by a 10% formalin fixative. Brains were harvested and kept in 10% formalin at 4 C for a minimum of 4-days. Coronal sections (50 m) were made with a vibratome (The Vibratome Company, St. Louis, MO, USA). Sections were mounted and dried on gelatinized slides and stained with 0.2% cresyl violet. Slides were then coded to conceal subject identity for determination of cannulae placement by an investigator (PJK) unaware of the individual subject’s reinstatement data and intended cannula placement.
4.5 Data Analysis
The average number of active lever presses emitted during the last self-administration session, the number of responses emitted during extinction session 6, the saline prime (experiment #1), the cocaine prime (experiment #2) and the AMPA prime (experiments #1 and #2) were analyzed with separate 2-way repeated measures analysis of variance (ANOVA) with nucleus accumbens core or shell groups and session as the variables. If a significant ANOVA was determined, post-hoc comparisons utilizing the Fisher LSD test were performed. Significance was set at p < 0.05. If cannula placement was determined to be outside of the intended region, the data from that animal were not used.
Acknowledgments
This research was funded by the State of Georgia (PJK).
References
- Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:132–138. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Schmidt HD, Pierce RC. Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core, of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacology. 2006;31:1452–1461. doi: 10.1038/sj.npp.1300922. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Heinrichs SC, Coffin VL, Koob GF. Effects of the dopamins D-1 antagonist SCH 23390 microinjected into the accumbens, amygdale or striatum on cocaine self-administration in the rat. Brain Res. 1995;692:47–56. doi: 10.1016/0006-8993(95)00598-k. [DOI] [PubMed] [Google Scholar]
- Churchill L, Swanson CJ, Urbina M, Kalivas PW. Repeated cocaine alters glutamate receptor subunit levels in the nucleus accumbens and ventral tegmental area of rats that develop behavioral sensitization. J Neurochem. 1999;72:2397–2403. doi: 10.1046/j.1471-4159.1999.0722397.x. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93:1359–1367. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierrke DF, Cha J, Pierce RC. Behavioral and molecular evidence that increased glutamate transmission through AMPA receptors in both the core and shell of the nucleus accumbens promotes the reinstatement of cocaine seeking. Society for Neuroscience Abstracts. 2006:767.9. [Google Scholar]
- Groenewegen HJ, Berendse HW, Wolters JG, Lohman AH. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Prog Brain Res. 1990;85:95–116. doi: 10.1016/s0079-6123(08)62677-1. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Xi J. Different patterns of pharmacological reinstatement of cocaine-seeking behavior between Fischer 344 and Lewis Rats. Psychopharmacology (Berl) 2006;187:22–29. doi: 10.1007/s00213-005-0264-4. [DOI] [PubMed] [Google Scholar]
- Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di Chiara G. Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Psychopharmacology (Berl) 2007;191:653–667. doi: 10.1007/s00213-006-0496-y. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academy Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- Paxinos G, Watson D. The rat brain in stereotaxic coordinates. New York: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behaviour in the rat. Eur J Neurosci. 2006;23:2 19–228. doi: 10.1111/j.1460-9568.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- Sun W, Akins CK, Mattingly AE, Rebec GV. Ionotropic Glutamate Receptors in the ventral tegmental area regulate cocaine-seeking behavior in rats. Neurosphychopharmacology. 2005;30:2073–2081. doi: 10.1038/sj.npp.1300744. [DOI] [PubMed] [Google Scholar]
- Suto N, Tanabe LM, Austin JD, Creekmore E, Pham CT, Vezina P. Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacology. 2004;29:2149–2159. doi: 10.1038/sj.npp.1300533. [DOI] [PubMed] [Google Scholar]
- Van Dongen YC, Deniau JM, Pennartz CM, Galis-de Graaf Y, Voorn P, Thierry AM, Groenewegen HJ. Anatomical evidence for direct connections between the shell and core subregions of the rat nucleus accumbens. Neuroscience. 2005;136:1049–1071. doi: 10.1016/j.neuroscience.2005.08.050. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience. 1992;50:751–67. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]